Abstract
The ATP-binding cassette sterol transporter Abcg5/g8 is Lith9 in mice and two gallstone-associated variants in ABCG5/G8 have been identified in humans. Although ABCG5/G8 plays a critical role in determining hepatic sterol secretion, cholesterol is still secreted to bile in sitosterolemic patients with a defect in either ABCG5 or ABCG8 and in either Abcg5/g8 double or single knockout mice. We hypothesize that in the defect of ABCG5/G8, an ABCG5/G8-independent pathway is essential for regulating hepatic secretion of biliary sterols, which is independent of the lithogenic mechanism of the ABCG5/G8 pathway. To elucidate the effect of the ABCG5/G8-independent pathway on cholelithogenesis, we investigated the biliary and gallstone characteristics in male wild-type, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice fed a lithogenic diet or varying amounts of cholesterol, treated with an LXR agonist, or injected intravenously with [3H]sitostanol- and [14C]cholesterol-labeled HDL. We found that ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice displayed the same biliary and gallstone phenotypes. Although both groups of knockout mice showed a significant reduction in hepatic cholesterol output compared to wild-type mice, they still formed gallstones. The LXR agonist significantly increased biliary cholesterol secretion and gallstones in wild-type, but not ABCG5(−/−)/G8(−/−) or ABCG8 (−/−) mice. The 6-hour recovery of [14C]cholesterol in hepatic bile was significantly lower in both groups of knockout mice than in wild-type mice and [3H]sitostanol was detected in wild-type, but not ABCG5(−/−)/G8(−/−) or ABCG8 (−/−) mice. We conclude that the ABCG5/G8-independent pathway plays an important role in regulating biliary cholesterol secretion, the transport of HDL-derived cholesterol from plasma to bile, and gallstone formation, which works independently of the ABCG5/G8 pathway. Further studies are needed to observe if this pathway is also operational in humans.
Keywords: bile salts, cholesterol crystallization, Lith gene, lithogenic bile, mucin
INTRODUCTION
Supersaturated bile is an essential prerequisite for the precipitation of solid cholesterol monohydrate crystals and the formation of cholesterol gallstones (1). It has been established that the ATP-binding cassette (ABC) sterol transporters G5 and G8 encoded by the ABCG5 and ABCG8 genes are located primarily on the canalicular membrane of hepatocytes and the apical membrane of enterocytes and play a key role in hepatic secretion and intestinal absorption of cholesterol (2–5). The Abcg5/g8 has already been identified as the mouse Lith9 by quantitative trait locus (QTL) linkage analysis (6). Subsequently, the ABCG5/G8 was found to be associated with gallstone formation in patients and two gallstone-associated variants in ABCG5/G8 (ABCG5-R50C and ABCG8-D19H) were identified not only in Germans and Chileans, but also in Chinese and Indians (7–12). These findings indicate the importance of ABCG5/G8 as LITH9 in the pathogenesis of gallstones not only in mice, but also in humans.
Sitosterolemia is a rare autosomal, recessively inherited lipid metabolic disorder that is caused by mutations in either ABCG5 or ABCG8 (13–16). It is characterized mainly by increased absorption of cholesterol and sitosterol and reduced secretion of these sterols into bile (17–19). In patients with sitosterolemia, the intestinal absorption of cholesterol is augmented by ~30%, from ~46% to ~60%; however, the intestinal absorption of sitosterol is dramatically increased by ~800%, from <5% to ~45% (20–23). Moreover, several human studies on biliary lipid secretion found that hepatic cholesterol secretion is markedly reduced and hepatic secretion of sitosterol and other plant sterols is almost totally inhibited. As a result, these patients often display hypercholesterolemia, tendon and tuberous xanthomas, premature development of atherosclerosis, and abnormal hematologic and liver function test results. To explore the mechanism underlying the effect of ABCG5/G8 on biliary sterol secretion, we quantified biliary cholesterol and sitostanol secretion for 6 hours in ABCG8 (−/−) mice. We found that mass transport rate of [3H]sitostanol from plasma HDL into bile was significantly faster than that of [14C]cholesterol in wild-type (WT) mice; however, reduced amounts of [14C]cholesterol and no [3H]sitostanol were detected in bile of ABCG8 (−/−) mice (24). These results clearly demonstrated that the deletion of the Abcg8 gene alone significantly reduces but does not eliminate hepatic cholesterol secretion. Consistent with the human results, these mouse data strongly suggest that an ABCG5/G8-independent pathway could also be involved in hepatic cholesterol secretion. However, it is still unknown whether this pathway has a crucial effect on the regulation of hepatic cholesterol secretion and whether it plays a pivotal role in the formation of cholesterol gallstones in the lithogenic state.
In the current study, we investigated whether the targeted disruption of the Abcg5/g8 genes or the Abcg8 gene alone protects against the formation of cholesterol gallstones in gallstone-susceptible C57BL/6J mice fed a lithogenic diet for 56 days. It was surprising to find that although the prevalence of gallstones was significantly reduced in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice, many classical parallelogram-shaped cholesterol monohydrate crystals and gallstones were still found in these mice during the 56-day period of the lithogenic diet feeding. Our results provide clear evidence to support a novel concept that the ABCG5/G8-independent pathway is essential for regulating hepatic cholesterol secretion in the absence of ABCG5/G8 and also plays a determinant role in gallstone formation in mice.
MATERIALS AND METHODS
Genetically modified mice and diets
The breeding pairs of ABCG5(−/−)/G8(−/−) mice on a C57BL/6J genetic background were purchased from the Jackson Laboratory (Bar Harbor, ME). The generation of ABCG8 (−/−) mice on a C57BL/6J background has been described elsewhere (24, 25). The wild-type (WT) mice displayed normal Abcg5 and Abcg8 expression on the same C57BL/6J background and were a gallstone-susceptible strain. The breeding colonies of all these mice have been established in-house and they were bred to generate male mice for the studies. For gallstone study, mice at 8–10 weeks of age were fed a lithogenic diet containing 1% cholesterol, 0.5% cholic acid, and 15% butter fat for 56 days. To study the effect of the ABCG5/G8-independent pathway on biliary cholesterol secretion, mice were fed normal rodent chow (Harlan Teklad F6 Rodent Diet 8664, Madison, WI) containing trace (<0.02%) amounts of cholesterol, or the chow diet supplemented with 0.5% or 1% cholesterol for 14 days. To explore whether the induction of Abcg5/g8 by the liver X receptor (Lxr) increased biliary cholesterol secretion and gallstone formation, mice were given by gavage the synthetic LXR agonist T0901317 (Cayman Chemical, Ann Arbor, MI) at 0 or 20 mg/day/kg body weight and fed the lithogenic diet for 56 days. All mice were housed in a temperature-controlled vivarium (22±1°C) on a 12/12 h light/dark cycle (lights on at 0600 h). All procedures were in accordance with current NIH guidelines and were approved by the Institutional Animal Care and Use Committee of Harvard University.
Microscopic studies of gallbladder bile and gallstones
After mice were fasted overnight and anesthetized with pentobarbital, cholecystectomy was performed. Gallbladder bile was collected before (day 0, on chow) and at frequent intervals after feeding the lithogenic diet for 14, 28, and 56 days for cholesterol crystallization and gallstone studies (n=20 for the 56-day group and n=5 per group for each of other time points). The entire gallbladder bile was examined by phase contrast and polarizing light microscopy for the presence of mucin gel, liquid crystals, solid cholesterol crystals, sandy stones, and gallstones according to previously established criteria (26). The images of cholesterol monohydrate crystals and gallstones were analyzed by a Carl Zeiss Imaging System (Carl Zeiss Microimaging GmbH, Göttingen, Germany).
Additional methods are described in the Supporting Materials and Methods.
RESULTS
Lipid composition and CSI values of gallbladder bile
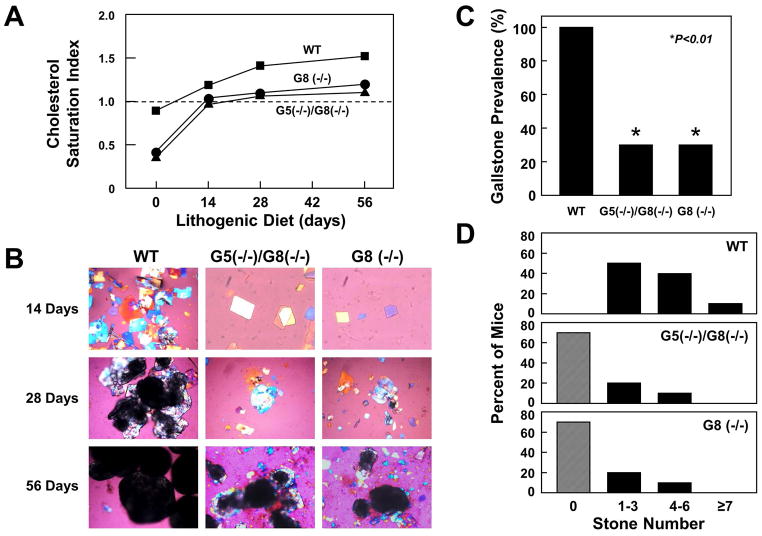
Figure 1A shows changes in bile CSI values as a function of days on the lithogenic diet in WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice before (day 0, on chow) and during feeding the lithogenic diet for 56 days. Although CSI values were increased progressively in three groups of mice and reached supersaturation after 14 days of feeding, they were markedly higher in WT mice than in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice.
Figure 1.
(A) CSI values of pooled gallbladder bile as a function of days on the lithogenic diet in WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice. (B) Photomicrographs of cholesterol monohydrate crystal and gallstone formation observed in mice after 14, 28, and 56 days on the lithogenic diet. Notably, although ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice displayed significantly slower crystallization, growth, and agglomeration of cholesterol monohydrate crystals compared to WT mice, they still formed gallstones. All magnifications are 800x by polarizing light microscopy, except for the panels (400x) of day 28 for WT mice and of day 56 for ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice, and the panel (200x) of day 56 for WT mice. (C) Gallstone prevalence was significantly higher in WT mice than in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice and (D) the frequency of stone number was assessed in these mice after 56 days on the lithogenic diet.
Prevalence rates and characteristics of gallstones
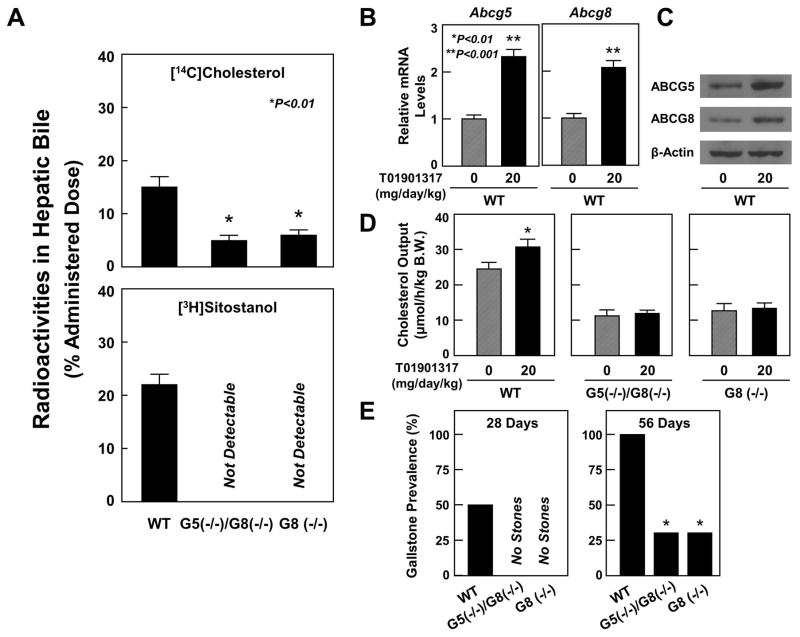
Figure 1B exhibits representative photomicrographs of amorphous mucin gel, liquid crystals, cholesterol monohydrate crystals, and gallstones as observed by polarizing light microscopy during gallstone formation in gallbladder bile of WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice. At 56 day on the lithogenic diet, gallbladders of WT mice formed a thick layer of sticky mucin gel interspersed with numerous solid plate-like cholesterol monohydrate crystals, as well as aggregated and fused liquid crystals. In contrast, gallbladders of ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice contained a thin layer of mucin gel, aggregated and fused liquid crystals, and cholesterol monohydrate crystals. Figure 1C shows that gallstone prevalence in WT mice (100%) is significantly higher than that in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice (30%). As shown in Figure 1D, assessment of gallstone frequency in WT mice find that the number distribution fell between 1 and 3 in 50% of the mice and between 4 and 6 in 40% of the mice, while 10% of the mice formed more than 7 stones. Moreover, 20% of ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice had 1–3 stones and 10% of them harbored 4–6 stones. Of special note, 70% of ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice did not form gallstones in the gallbladder.
Phase analysis of gallbladder bile during cholesterol crystallization
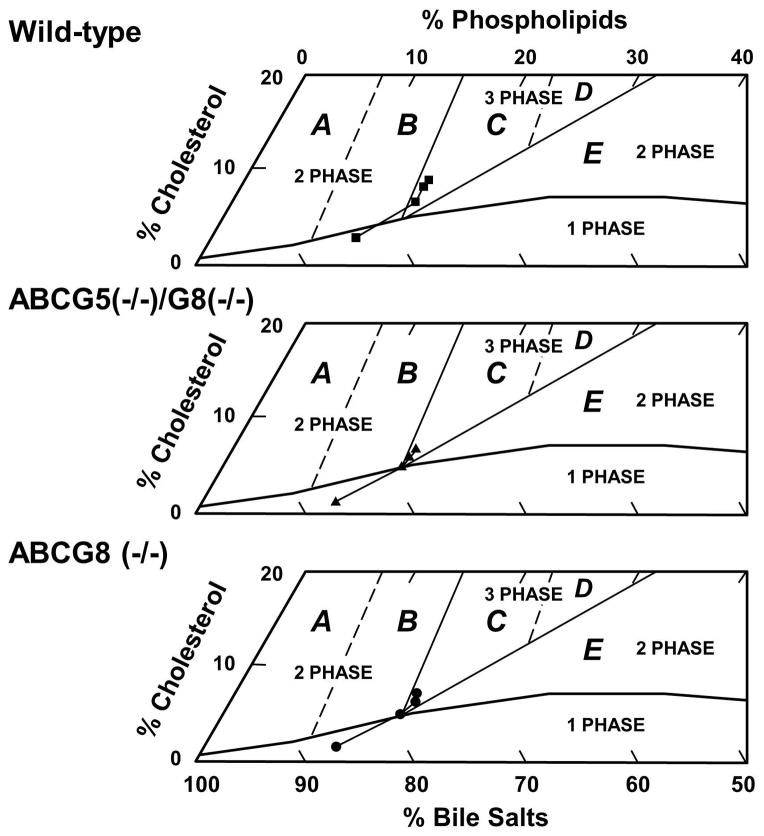
For phase analysis, three truncated phase diagrams were created for pooled gallbladder bile of WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice during the 56-day period of the lithogenic diet feeding (Figure 2). At day 0 on chow, relative biliary lipid composition of bile was located within the micellar zone in three groups of mice. By phase analysis, the bile consisted of only unsaturated micelles (27), showing that gallbladder bile was unsaturated. In the lithogenic state, relative lipid composition of pooled gallbladder bile gradually moved upward and to the right of the phase diagrams in all the three groups of mice, which was caused by an increase in cholesterol and phospholipid concentrations and a decrease in bile salt concentrations. After 14 days of feeding, relative biliary lipid composition directly entered region C from the micellar zone in these mice, indicating that the bile became supersaturated. Notably, relative biliary lipid composition was much higher in WT mice than in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice. By phase analysis, the bile was composed of three phases, namely solid cholesterol crystals, liquid crystals, and saturated micelles. As expected, liquid crystals invariably preceded cholesterol monohydrate crystals during crystallization (27).
Figure 2.
Relative lipid composition (moles per 100 moles) of pooled gallbladder bile (n=5 per group) at each time point (0, 14, 28, and 56 days on the lithogenic diet) is plotted on condensed phase diagrams in which regions A to E with different crystallization sequences have been described elsewhere (27). With passage of time, relative biliary lipid composition of pooled gallbladder bile gradually moves upward and to the right and enters the crystallization region C from the micellar zone in three groups of mice. This indicates that gallbladder bile reaches supersaturation not only in WT mice, but also in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice after 14 days on the lithogenic diet.
Biliary lipid secretion and bile flow
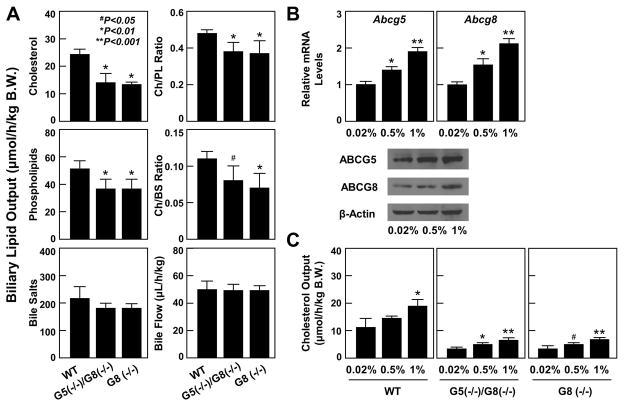
Figure 3A shows biliary lipid output in mice during the first hour after interruption of the enterohepatic circulation. Biliary cholesterol and phospholipid output was significantly higher in WT mice than in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice. Biliary bile acid output was also higher in the former than in the latter two groups of mice, but the difference did not reach statistical significance. The ratios of cholesterol to phospholipids and to bile salts were significantly higher in WT mice than in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice, indicating that bile cholesterol saturation was significantly higher in the former than in the latter. At 56 days on the lithogenic diet, bile flow rate was determined during the first hour of hepatic bile collection, thus avoiding appreciable perturbation of the enterohepatic circulation of bile salts. Bile flow rates were comparable among WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice.
Figure 3.
(A) Hepatic output of biliary cholesterol and phospholipids, but not bile salts, as well as ratios of cholesterol/phospholipids and cholesterol/bile salts were significantly reduced in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice compared to those in WT mice fed the lithogenic diet for 56 days. Bile flow was comparable among three groups of mice. (B) There was a dose-dependent increase in mRNA levels and protein concentrations of ABCG5 and ABCG8 in WT mice in response to an increase in dietary cholesterol. (C) Hepatic cholesterol secretion was increased in a dose-dependent manner not only in WT mice, but also in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice although biliary cholesterol output was lower in the latter than in the former. Abbreviations: BS, bile salts; Ch, cholesterol; PL, phospholipids.
The effect of the ABCG5/G8-independent pathway on hepatic cholesterol secretion was further examined in mice fed varying amounts (0.02%, 0.5%, or 1%) of dietary cholesterol for 14 days. The mRNA levels and protein concentrations of ABCG5 and ABCG8 were augmented in WT mice when dietary cholesterol was increased (Figure 3B), coupled with a dose-dependent increment in hepatic cholesterol output (Figure 3C). Although ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice displayed significantly lower hepatic cholesterol secretion compared to WT mice, they also showed hepatic cholesterol output in a dose-dependent manner. This indicates that the ABCG5/G8-dependent pathway plays a role in regulating hepatic cholesterol secretion in mice in response to an increase in dietary cholesterol.
Phase analysis of hepatic bile in the lithogenic state
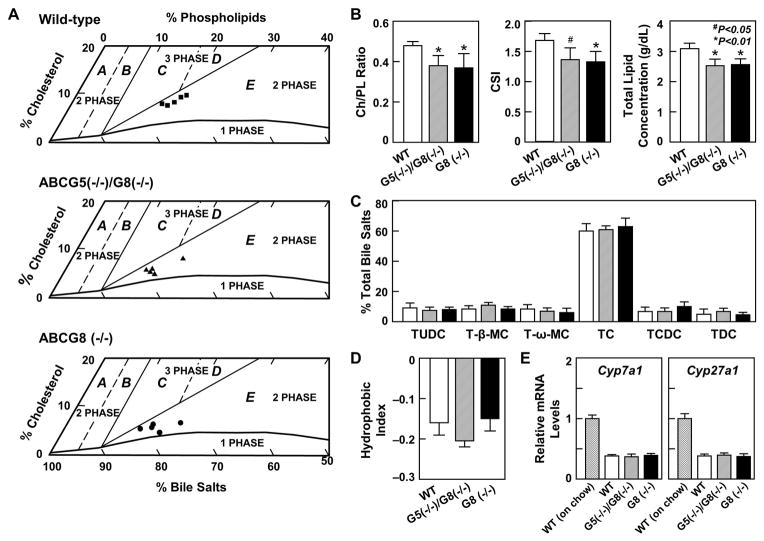
For phase analysis in WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice after 56 day of the lithogenic diet feeding (Figure 4A), relative lipid composition of individual hepatic bile was plotted in a similar fashion using an appropriate micellar phase boundary and cholesterol crystallization pathways for dilute (3 g/dL) taurocholate-rich bile (27). Notably, biliary lipid composition of all hepatic bile was plotted within the crystallization pathway denoted region E, in which only liquid crystals but not solid cholesterol crystals occurred at equilibrium. These liquid crystals consist mainly of multilamellar vesicles (27). Because the ratios of cholesterol to phospholipids were significantly lower in these knockout mice compared to WT mice (Figure 4B), this indicated that biliary vesicles contained less cholesterol in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice. These results were consistent with lower CSI values in these knockout mice than those in WT mice. Because of a reduction in biliary cholesterol and phospholipid secretion, total lipid concentration was also lower in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice than in WT mice.
Figure 4.
(A) Relative lipid composition of individual hepatic bile (n=5 each per group) is plotted on condensed phase diagrams for average total lipid concentration of 3 g/dL. This system exhibits the same physical states at equilibrium as for gallbladder bile, but all crystallization pathways are shifted to left with decreases in total lipid concentration and the one-phase micellar zone becomes smaller (27). These changes generate new condensed phase diagrams, with enlarged region E. Relative lipid composition of all hepatic bile is located in region E, where the bile is composed of liquid crystals and saturated micelles but not solid cholesterol crystals at equilibrium. After 56 days of the lithogenic diet feeding, (B) cholesterol/phospholipid ratios, CSI values, and total lipid concentrations of hepatic bile were significantly reduced in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice compared to those in WT mice. (C) Bile salt species of hepatic bile and (D) hydrophobic index of bile salt pool, as well as (E) expression of hepatic Cyp7a1 and Cyp27a1 were comparable among three groups of mice. See text for further details. Abbreviations: Ch, cholesterol; CSI, cholesterol saturation index; PL, phospholipids.
Bile salt species in hepatic bile
We further examined whether the lack of ABCG5/G8 has an effect on biliary bile salt species by HPLC. All bile salts in hepatic bile were taurine conjugated with a similar distribution of bile salt composition among three groups of mice (Figure 4C), regardless of whether Abcg5/g8 or Abcg8 was deleted. Hydrophobicity indexes of bile salt pools (Figure 4D) in bile and expression of Cyp7a1 and Cyp27a1 (Figure 4E) that encode two rate-limiting enzymes for regulating the classical and the alternative pathways of bile salt synthesis in the liver were comparable among three groups of mice. These results indicate that the deletion of the Abcg5/g8 genes or the Abcg8 gene alone did not influence bile salt metabolism in the liver and bile. Notably, expression of Cyp7a1 and Cyp27a1 was significantly reduced in mice fed the lithogenic diet compared to the chow diet because the lithogenic diet contained 0.5% cholic acid.
Effect of the ABCG5/G8-independent pathway on the transport of sterols from plasma to bile
Figure 5A exhibits that by 6 hours after the injection, the total recovery of HDL-derived [14C]cholesterol in hepatic bile is 15±2%, 5±1%, and 6±1% in WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice, respectively, indicating that the cholesterol transport from plasma to bile is significantly reduced due to the deletion of Abcg5/g8 or Abcg8. Cumulative radioactivity of sitostanol recovered in bile was 22±2% in WT mice and no sitostanol radioactivity was found in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice, showing that the sitostanol transport from plasma to bile was inhibited in mice lacking ABCG5/G8 or ABCG8. These results suggested that the ABCG5/G8-independent pathway is involved in hepatic secretion of cholesterol, but not sitostanol, derived from HDL.
Figure 5.
(A) By 6 hours after the injection of [14C]cholesterol-labeled HDL, cumulative radioactivity recovered in hepatic bile was significantly higher in WT mice than in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice. However, the HDL-derived [3H]sitostanol was found in WT, but not ABCG5(−/−)/G8(−/−) or ABCG8 (−/−) mice. The LXR agonist T0901317 significantly increased (B) mRNA levels and (C) protein concentrations of ABCG5 and ABCG8 in the liver of WT mice. The LXR agonist significantly (D) augmented hepatic cholesterol output and (E) promoted gallstone formation in WT, but not ABCG5(−/−)/G8(−/−) or ABCG8 (−/−) mice.
Effect of the LXR agonist on hepatic cholesterol secretion and gallstone formation
To explore whether LXR agonists play a role in promoting biliary cholesterol secretion and gallstone formation through the ABCG5/G8-independent pathway, we fed mice with 0 or 20 mg/day/kg of the synthetic LXR agonist T0901317 by gavage and with the lithogenic diet for 56 days. As expected, T0901317 significantly increased mRNA levels (Figure 5B) and protein concentrations (Figure 5C) of ABCG5 and ABCG8 in the liver of WT mice. After the stimulation of ABCG5/G8 by the LXR agonist, both hepatic cholesterol output (Figure 5D) and gallstone prevalence (Figure 5E) were significantly increased in WT, but not ABCG5(−/−)/G8(−/−) or ABCG8 (−/−) mice. Moreover, the size and number of gallstones were significantly increased in WT mice treated with T01901317 compared to WT mice receiving no T01901317. However, gallstone prevalence, as well as stone number and size were not influenced in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice even treated with the LXR agonist for 56 days.
Effect of the ABCG5/G8-independent pathway on gallbladder motor function
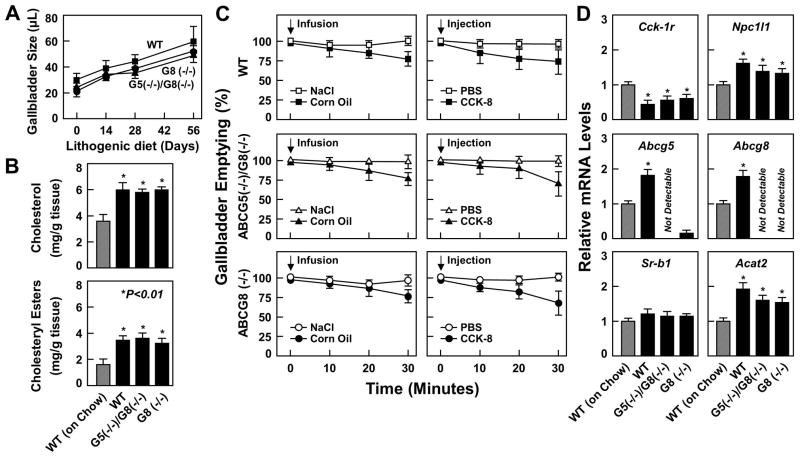
During the 56-day period of the lithogenic diet feeding, fasting gallbladder volumes were gradually increased (Figure 6A). Although fasting gallbladder sizes were larger in WT mice than in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice, the difference did not reach a statistical significance. At 56 days on the lithogenic diet, cholesterol and cholesteryl ester concentrations in the gallbladder tissues were comparable in three groups of mice; however, their concentrations were significantly higher in these mice fed the lithogenic diet compared to those in WT mice on chow (Figure 6B). As expected, 0.9% NaCl administration did not influence gallbladder emptying in all mice. Postprandial gallbladder emptying rates in response to the high-fat meal or exogenous CCK-8 stimulation were between 20% and 30% and were comparable in three groups of mice (Figure 6C), suggesting that gallbladder emptying was impaired in these mice. As shown in Figure 6D, after 56 days of the lithogenic diet feeding, expression of gallbladder Cck-1r is significantly reduced in three groups of mice compared to that in WT mice on chow. Notably, expression of gallbladder Npc1l1, Abcg5, Abcg8, and Acat2 is significantly increased in mice fed the lithogenic diet compared to that in WT mice on chow. Expression of gallbladder Sr-b1 was comparable among four groups of mice.
Figure 6.
(A) Fasting gallbladder volumes as a function of days on the lithogenic diet. (B) Cholesterol and cholesteryl ester concentrations in the gallbladder tissues after 56 days of the lithogenic diet feeding. (C) Postprandial gallbladder emptying rates in response to duodenal infusion of corn oil or exogenously administered CCK-8 via intravenous injection (as indicated by the arrows) during the early stage of gallstone formation in mice fed the lithogenic diet for 14 days. Gallbladder emptying was impaired in WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice. (D) The mRNA levels of the genes involved in gallbladder lipid metabolism and transporters. See text for further description.
DISCUSSION
Sitosterolemia is caused by a mutation in either the ABCG5 or the ABCG8 gene alone, but not in both simultaneously, and hepatic cholesterol secretion is reduced, but not completely eliminated in these patients (20–23). Further studies found by us and others (3–5, 24, 28) showed that hepatic cholesterol output is significantly reduced, but cholesterol is still secreted into bile in mice with the deletion of either Abcg5 or Abcg8 alone, or both. These results suggest that an ABCG5/G8-independent pathway could exist for regulating biliary cholesterol secretion in humans and mice. However, its pathophysiological role in gallstone formation is largely unknown. In the current study, the most important findings are that (i) the ABCG5/G8-independent pathway accounts for 30% to 40% of hepatic cholesterol output in the lithogenic state and has an effect on regulating biliary secretion of cholesterol in response to high dietary cholesterol; (ii) in the absence of ABCG5/G8, it plays a pivotal role in biliary cholesterol secretion and the pathogenesis of cholesterol gallstones; (iii) it is able to regulate hepatic secretion of HDL-derived cholesterol, but not sitostanol; and (iv) its activity in the liver is not regulated by the LXR agonist through the LXR signaling pathway.
Genetic analyses and clinical studies have found that ABCG5/G8 is LITH9 in humans and mice. In the present study, we revealed that the deletion of the Abcg5/g8 genes or the Abcg8 gene alone dramatically reduced gallstone prevalence in gallstone-susceptible C57BL/6J mice. However, during 56 days of the lithogenic diet feeding, gallbladder bile still became supersaturated with cholesterol, eventually leading to the formation of gallstones in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice. This finding indicates that the ABCG5/G8-independent pathway can play a critical role in gallstone pathogenesis, which is independent of the lithogenic mechanism of the ABCG5/G8 pathway. A careful contrast of the two pathways shows up some key differences. We found that in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice, the evolutionary sequence of cholesterol crystallization and gallstone formation was characterized by the initial supersaturation of bile with cholesterol, followed by the accumulation of excess mucin gel, the appearance of liquid crystals and solid cholesterol crystals, the growth and agglomeration of cholesterol monohydrate crystals, and the formation of sandy stones and real gallstones. Although the sequence of cholesterol crystallization and gallstone formation was basically the same in WT, ABCG5(−/−)/G8(−/−), and ABCG8 (−/−) mice, the number and size of solid cholesterol crystals and the severity of gallstone disease were dramatically reduced in both groups of knockout mice compared to WT mice.
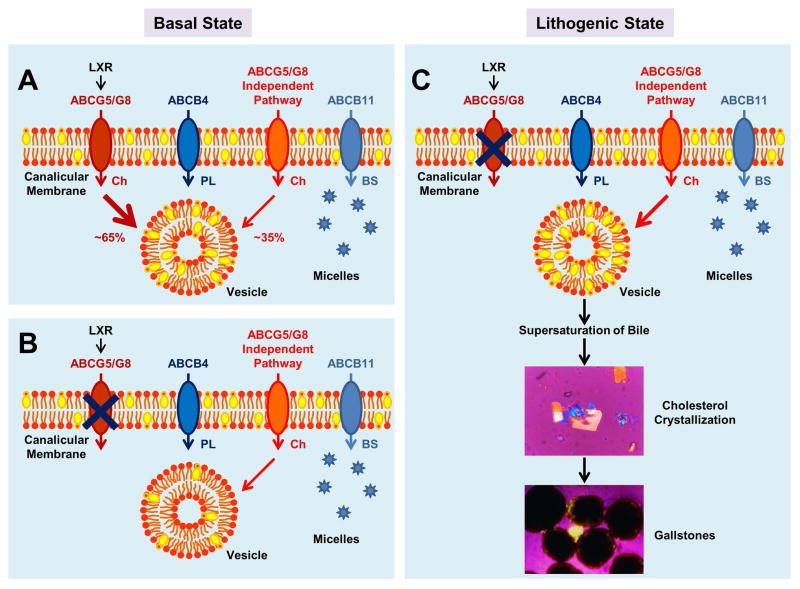
The second major difference between WT and knockout mice was the magnitude of hepatic cholesterol output. Consistent with our previous findings in chow-fed WT and ABCG8 (−/−) mice (24), the ABCG5/G8-independent pathway was also responsible for 30% to 40% of hepatic cholesterol output in these knockout mice under lithogenic diet feeding conditions (Figure 7). Moreover, when dietary cholesterol was increased from 0.02% to 1%, there was a dose-dependent increase in biliary cholesterol secretion in these knockout mice. This indicates that the ABCG5/G8-independent pathway is also involved in the regulation of biliary cholesterol secretion in mice in challenge to high dietary cholesterol, working independently of the ABCG5/G8 pathway, as both pathways may influence hepatic cholesterol secretion through different mechanisms. Furthermore, intestinal cholesterol absorption efficiency was significantly increased not only in ABCG5(−/−)/G8(−/−), ABCG5 (−/−), and ABCG8 (−/−) mice (3–5, 24, 28), but also in sitosterolemic patients (20–23). Thus, more cholesterol of intestinal origin reaches the liver for hypersecretion into bile through the chylomicron pathway in these knockout mice, thereby further enhancing cholelithogenesis. Because persistent supersaturation of bile with cholesterol, gallbladder cholesterol absorption could be enhanced so that the excess amounts of cholesterol and cholesteryl esters were accumulated in the gallbladder wall not only in WT mice, but also in these knockout mice. Thus, the cytotoxic effect of excess cholesterol on the plasma membrane of smooth muscle and excess cholesteryl esters for storage in the mucosa and lamina propria may inhibit contraction and relaxation of the gallbladder (29–31). This explains our observations that gallbladder emptying was impaired in WT, ABCG5(−/−)/G8(−/−), and ABCG5 (−/−) mice.
Figure 7.
(A) In the basal state, ~65% and ~35% of biliary cholesterol output are determined by the ABCG5/G8 and the ABCG5/G8-independent pathways, respectively. The cholesterol molecules secreted by these two pathways form biliary vesicles with phospholipids secreted by the ABCB4 transporter. Bile salts are secreted by the ABCB11 transporter, forming simple and mixed micelles with varying amounts of cholesterol and phospholipids. (B) When ABCG5/G8 in the liver is disrupted, hepatic cholesterol output is significantly reduced and the ABCG5/G8-independent pathway plays a major role in regulating biliary cholesterol secretion. Notably, the resulting vesicles contain less cholesterol. (C) In the lithogenic state, excess amounts of cholesterol are secreted to bile by the liver through the ABCG5/G8-independent pathway under conditions of disruption of ABCG5/G8, leading to supersaturated bile. These abnormalities promote cholesterol crystallization and gallstone formation in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice. Abbreviations: ABC, ATP-binding cassette (transporter); BS, bile salts; Ch, cholesterol; LXR, liver X receptor; PL, phospholipids.
It is well known that the LXR agonist T0901317 activates hepatic LXR, promoting biliary cholesterol secretion by stimulating hepatic Abcg5/g8 expression in mice (28, 32, 33). Furthermore, the activation of LXR by T0901317 sensitizes mice to lithogenic diet-induced cholesterol crystallization and gallstone formation (34). However, this was not the case in ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice. This suggests that the hepatic LXR does not have an effect on the ABCG5/G8-independent pathway for regulating biliary cholesterol secretion, which is distinct from the ABCG5/G8 pathway that is effectively regulated by the hepatic LXR through a transcriptional mechanism.
The cholesterol molecules derived from HDL, but not LDL or VLDL, are an important source for hepatic secretion into bile because HDL promotes reverse cholesterol transport from peripheral tissues to the liver where the HDL-derived cholesterol is secreted preferentially into bile (35). We found that after intravenous injection, HDL-derived [14C]cholesterol, but not [3H]sitostanol, was recovered in hepatic bile of ABCG5(−/−)/G8(−/−) and ABCG8 (−/−) mice. This indicates that the ABCG5/G8-independent pathway is also able to regulate hepatic secretion of HDL-derived cholesterol, but not sitostanol. By contrast, ABCG5/G8 is involved in the regulation of hepatic secretion of both cholesterol and plant sterols. These results are consistent with the finding in sitosterolemic patients in whom only reduced amounts of cholesterol are found in bile and hepatic secretion of plant sterols are completely inhibited, leading to a significant increase in plasma plant sterol concentrations (21).
Convincing evidence has also shown that feeding 1% diosgenin for 18 days induced ~16-fold increases in hepatic cholesterol secretion from 0.5±0.1 nmol/min/100g body weight to 8.7±0.6 nmol/min/100g body weight in chow-fed male FVB/J mice (36). Of special note is that diosgenin does not influence expression of hepatic and intestinal Abcg5/g8 (36). Although the mechanism of action of diosgenin in promoting hepatic cholesterol secretion is unknown, it is highly likely that the ABCG5/G8-independent, but not ABCG5/G8 pathway may be responsible for increased hepatic cholesterol secretion. Moreover, biliary cholesterol secretion was increased over 3-fold in rats fed 1% diosgenin for 5 days, whereas biliary phospholipid and bile salt secretion was not influenced in these rats (37). These studies strongly suggest that diosgenin, a plant-derived sapogenin structurally similar to cholesterol, may be a potential agonist for a candidate gene that is involved in the ABCG5/G8-independent pathway.
What is the candidate gene that is responsible for the ABCG5/G8-independent hepatic cholesterol secretion? Based on our findings in the current study and the results reported in the literature, we attempt to predict the potential physiological characteristic and function of the candidate gene. It is located on the canalicular membrane of hepatocytes and its primary role in the regulation of hepatic cholesterol, but not plant sterol secretion is independent of ABCG5/G8 because the candidate gene can compensate, in part, for the loss of function of ABCG5/G8, regardless of whether it is in the normal physiological state or in the lithogenic state. Its regulatory role in hepatic cholesterol secretion is not influenced by LXR through the transcriptional mechanism, which is totally different from ABCG5/G8. Of note, although the regulatory effect of ABCG5/G8 on reverse cholesterol transport is greater than that of the candidate gene, the ABCG5/G8-independent pathway plays an independent role in reverse cholesterol transport, implying that it could also be involved in the pathogenesis of atherosclerosis. We are searching for this candidate gene by genetic analysis in inbred mouse strains with varying amounts of hepatic cholesterol secretion determined by the ABCG5/G8-independent pathway.
In summary, our results clearly show that the ABCG5/G8-independent pathway plays a critical role in regulating hepatic cholesterol secretion and in determining the susceptibility to cholesterol gallstones, working independently of the ABCG5/G8 pathway. Further studies are needed to observe if this pathway is also operational in humans. Our findings suggest that both pathways are potential therapeutic targets for gallstones. Furthermore, the current study constitutes the basic framework for identifying the candidate gene that is responsible for the ABCG5/G8-independent pathway. More importantly, discovering such a gene may lead to novel strategies through modulating both the ABCG5/G8 and the ABCG5/G8-independent pathways for the prevention of cholesterol-related diseases such as cholesterol gallstones and atherosclerosis that affect millions in Westernized societies.
Supplementary Material
Acknowledgments
This work was supported in part by research grants DK73917, DK101793, and DK106249 (to D.Q.-H.W.), all from the National Institutes of Health (US Public Health Service).
Abbreviations used in this paper
- ABC
ATP-binding cassette (transporter)
- LXR
liver X receptor
- NPC1L1
Niemann-Pick C1 like 1 (protein)
- WT
wild-type
Footnotes
There is no conflict of interest to disclose for all authors.
References
- 1.Wang DQ, Afdhal NH. Gallstone Disease. In: Feldman M, Friedman LS, Brandt L, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 10. Philadelphia: Elsevier Saunders; 2014. pp. 1100–1133. [Google Scholar]
- 2.Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Mitsche MA, Lutjohann D, Cohen JC, Xie XS, Hobbs HH. Relative roles of ABCG5/ABCG8 in liver and intestine. J Lipid Res. 2015;56:319–330. doi: 10.1194/jlr.M054544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wittenburg H, Lyons MA, Li R, Churchill GA, Carey MC, Paigen B. FXR and ABCG5/ABCG8 as determinants of cholesterol gallstone formation from quantitative trait locus mapping in mice. Gastroenterology. 2003;125:868–881. doi: 10.1016/s0016-5085(03)01053-9. [DOI] [PubMed] [Google Scholar]
- 7.Grunhage F, Acalovschi M, Tirziu S, Walier M, Wienker TF, Ciocan A, Mosteanu O, et al. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 2007;46:793–801. doi: 10.1002/hep.21847. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Jiang ZY, Fei J, Xin L, Cai Q, Jiang ZH, Zhu ZG, et al. ATP binding cassette G8 T400K polymorphism may affect the risk of gallstone disease among Chinese males. Clin Chim Acta. 2007;384:80–85. doi: 10.1016/j.cca.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Kuo KK, Shin SJ, Chen ZC, Yang YH, Yang JF, Hsiao PJ. Significant association of ABCG5 604Q and ABCG8 D19H polymorphisms with gallstone disease. Br J Surg. 2008;95:1005–1011. doi: 10.1002/bjs.6178. [DOI] [PubMed] [Google Scholar]
- 10.Rudkowska I, Jones PJ. Polymorphisms in ABCG5/G8 transporters linked to hypercholesterolemia and gallstone disease. Nutr Rev. 2008;66:343–348. doi: 10.1111/j.1753-4887.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 11.Katsika D, Magnusson P, Krawczyk M, Grunhage F, Lichtenstein P, Einarsson C, Lammert F, et al. Gallstone disease in Swedish twins: risk is associated with ABCG8 D19H genotype. J Intern Med. 2010;268:279–285. doi: 10.1111/j.1365-2796.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- 12.von Kampen O, Buch S, Nothnagel M, Azocar L, Molina H, Brosch M, Erhart W, et al. Genetic and functional identification of the likely causative variant for cholesterol gallstone disease at the ABCG5/8 lithogenic locus. Hepatology. 2013;57:2407–2417. doi: 10.1002/hep.26009. [DOI] [PubMed] [Google Scholar]
- 13.Patel SB, Salen G, Hidaka H, Kwiterovich PO, Stalenhoef AF, Miettinen TA, Grundy SM, et al. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J Clin Invest. 1998;102:1041–1044. doi: 10.1172/JCI3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 15.Lu K, Lee MH, Yu H, Zhou Y, Sandell SA, Salen G, Patel SB. Molecular cloning, genomic organization, genetic variations, and characterization of murine sterolin genes Abcg5 and Abcg8. J Lipid Res. 2002;43:565–578. [PMC free article] [PubMed] [Google Scholar]
- 16.Lu K, Lee MH, Hazard S, Brooks-Wilson A, Hidaka H, Kojima H, Ose L, et al. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet. 2001;69:278–290. doi: 10.1086/321294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shulman RS, Bhattacharyya AK, Connor WE, Fredrickson DS. Beta-sitosterolemia and xanthomatosis. N Engl J Med. 1976;294:482–483. doi: 10.1056/NEJM197602262940907. [DOI] [PubMed] [Google Scholar]
- 19.Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler Thromb. 1992;12:563–568. doi: 10.1161/01.atv.12.5.563. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen TA. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur J Clin Invest. 1980;10:27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 21.Salen G, Shore V, Tint GS, Forte T, Shefer S, Horak I, Horak E, et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res. 1989;30:1319–1330. [PubMed] [Google Scholar]
- 22.Lutjohann D, Bjorkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res. 1995;36:1763–1773. [PubMed] [Google Scholar]
- 23.Gould RG, Jones RJ, LeRoy GV, Wissler RW, Taylor CB. Absorbability of beta-sitosterol in humans. Metabolism. 1969;18:652–662. doi: 10.1016/0026-0495(69)90078-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang HH, Patel SB, Carey MC, Wang DQ. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(−/−) mice. Hepatology. 2007;45:998–1006. doi: 10.1002/hep.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klett EL, Lu K, Kosters A, Vink E, Lee MH, Altenburg M, Shefer S, et al. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2004;2:5. doi: 10.1186/1741-7015-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–1411. [PubMed] [Google Scholar]
- 27.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996;37:606–630. [PubMed] [Google Scholar]
- 28.Plosch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, Van Der Sluijs F, Kema IP, et al. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 2004;126:290–300. doi: 10.1053/j.gastro.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 29.Harikumar KG, Puri V, Singh RD, Hanada K, Pagano RE, Miller LJ. Differential effects of modification of membrane cholesterol and sphingolipids on the conformation, function, and trafficking of the G protein-coupled cholecystokinin receptor. J Biol Chem. 2005;280:2176–2185. doi: 10.1074/jbc.M410385200. [DOI] [PubMed] [Google Scholar]
- 30.Desai AJ, Miller LJ. Sensitivity of cholecystokinin receptors to membrane cholesterol content. Front Endocrinol. 2012;3:123. doi: 10.3389/fendo.2012.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potter RM, Harikumar KG, Wu SV, Miller LJ. Differential sensitivity of types 1 and 2 cholecystokinin receptors to membrane cholesterol. J Lipid Res. 2012;53:137–148. doi: 10.1194/jlr.M020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 33.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 34.Uppal H, Zhai Y, Gangopadhyay A, Khadem S, Ren S, Moser JA, Xie W. Activation of liver X receptor sensitizes mice to gallbladder cholesterol crystallization. Hepatology. 2008;47:1331–1342. doi: 10.1002/hep.22175. [DOI] [PubMed] [Google Scholar]
- 35.Robins SJ, Fasulo JM. High density lipoproteins, but not other lipoproteins, provide a vehicle for sterol transport to bile. J Clin Invest. 1997;99:380–384. doi: 10.1172/JCI119170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosters A, Frijters RJ, Schaap FG, Vink E, Plosch T, Ottenhoff R, Jirsa M, et al. Relation between hepatic expression of ATP-binding cassette transporters G5 and G8 and biliary cholesterol secretion in mice. J Hepatol. 2003;38:710–716. doi: 10.1016/s0168-8278(03)00093-x. [DOI] [PubMed] [Google Scholar]
- 37.Thewles A, Parslow RA, Coleman R. Effect of diosgenin on biliary cholesterol transport in the rat. Biochem J. 1993;291:793–798. doi: 10.1042/bj2910793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.