Abstract
Introduction
Frontal fibrosing alopecia (FFA) is a cicatricial alopecia typically occurring in post-menopausal women. The etiology and pathophysiology of FFA is poorly understood but thought to be immune mediated. This study aims to further explore the extent of fibrosis and the inflammatory microenvironment by characterizing Langerhans cells (LCs), helper T cells, cytotoxic T cells, and B cells near hair follicles in FFA.
Methods
11 paraffin-embedded tissues from patients with a clinical and histopathologic diagnosis of FFA were selected for immunohistochemical studies using CD3, CD4, CD8, CD1a, and CD20. The lymphocytes and LCs were counted around involved follicles. The CD4/CD8 T lymphocyte ratios were calculated and compared to the CD4/CD8 T lymphocyte ratios in uninvolved areas.
Results
On histopathologic review, at least 35% of follicles in each case were affected by the disease with concentric perifollicular fibrosis and a perifollicular lichenoid lymphocytic infiltrate around the infundibuloisthmic portion of the hair follicle. There was an increase of perifollicular LCs (mean of 18, standard deviation of 5.5) and intrafollicular LCs (mean of 14, standard deviation of 4.3) in involved follicles compared to uninvolved follicles (p<0.0001). The involved follicles also showed a relative decrease in the CD4/CD8 ratio indicating increased numbers of CD8+ T cells; a finding distinct from the CD4-predominant population in uninvolved follicles (p<0.0001).
Conclusion
The inflammatory features of FFA demonstrate a CD8-biased T cell infiltrate with increased numbers of LCs in the infundibuloisthmic region. The increased LCs may represent an aberrant immune reaction promoting a CD8+ T cell response.
Introduction
Frontal fibrosing alopecia (FFA) was first described in 1994 as a progressive frontal recession with a fibrosing alopecia pattern, generally occurring in post-menopausal women but also in pre-menopausal women and men.1 Clinically, FFA involves the frontal hairline with frequent associated loss of eyebrows and body hair on arms.2–4 Early FFA may demonstrate normal hair density with pruritus, perifollicular erythema and scaling. In later stages, FFA demonstrates alopecia with loss of follicular ostia and pale skin without sclerosis or induration.2, 4 Follicles along the transition zone between affected and unaffected scalp show perifollicular erythema and hyperkeratosis as the disease progresses posteriorly. Terminal, intermediate and vellus hairs may be affected, often termed the “follicular triad”, and the latter may present as red or skin-colored papules on the temples or glabella.4–6 However, often the triad is only identified in early disease and lost in progressive disease due to the location past the hairline. 1, 6
Histologically, the affected follicles are involved by a dense lichenoid inflammatory infiltrate around the isthmus and infundibular regions of the hair follicles that does not extend into the hair bulb.1, 6 The composition of the infiltrate has been described as lymphohistiocytic with a CD4+ T lymphocyte bias.1 The inflammation is associated with a concentric perifollicular lamellar fibrosis.1, 6 The interfollicular epidermis is usually spared.
FFA has been postulated to be a variant of lichen planopilaris (LPP).7 Lichen planopilaris is a chronic inflammatory disorder that leads to disfiguring and permanent hair loss.1, 7–9 Classic LPP demonstrates alopecic patches on the vertex and parietal scalp that coalesce into larger patches and may involve the entire scalp.7 The patches may be associated with erythematous macules and papules with attenuated follicles, hyperkeratosis around the base of the hair and follicular hyperkeratotic plugs.10, 11 The inflammation leads to follicular destruction with loss of ostia and scarring.12 Histologically, these lesions manifest a lichenoid lymphohistiocytic infiltrate around follicles similar to the pattern seen in FFA.8, 12, 13
As LPP is thought to be an immune-mediated reaction, several studies have characterized the composition of the inflammatory infiltrate. In LPP, the inflammatory infiltrate, has demonstrated an increase in Th1-biased cytotoxic T cell (CD8+) cells involving affected follicles.13, 14 In contrast, FFA appears to manifest predominantly an infiltrate with a majority of CD4+ T cells. 8, 12, 13 However, recent studies have not commented on the composition of the lymphocytic infiltrate in FFA. To determine the composition of the inflammatory infiltrate in FFA, we determined the numbers of Langerhans cells (LCs), helper T cells, cytotoxic T cells and B cells around involved and uninvolved follicles.
Materials and Methods
In this retrospective study, cases between January 2012 and December 2014 with a clinical and histologic diagnosis of FFA were identified from the database in the Division of Dermatopathology, Department of Dermatology, at the Hospital of the University of Pennsylvania. No patient-identifying information was used as part of this study; performance of the study was approved under IRB protocol 808225. A total of 11 cases were identified with appropriate preclinical diagnosis, female gender, age range, and histopathologic confirmation of FFA. De-identified clinical images were available from a referring practice on a subset of patients. No male patients were identified. The original hematoxylin-eosin (H&E) stained sections of all cases were re-reviewed and diagnoses were confirmed by (JTS and SM). Of the 11 cases selected, nine cases had sufficient inflammation on deeper sectioning for analysis.
Immunohistochemistry of formalin fixed paraffin embedded tissue was performed on a Leica Bond-IIITM instrument using the Bond Polymer Refine Detection System (Leica Microsystems AR9800). The sections underwent deparaffinization and heat-induced antigen retrieval with epitope retrieval-1 (Leica Microsystems AR9640) at 60℃. Hydrogen peroxide was added to block endogenous peroxidase. The primary antibodies for CD3 (Leica, NCL-L-CD3-565, 1:50 dilution), CD4(Leica, NCL-L-CD4-368, 1:50 dilution), CD8(Leica, NCL-L-CD8-4B11, 1:50 dilution), CD20(Leica, NCL-L-CD20-L26, 1:50 dilution), and CD1a (Cell Marque, CD1a(MTB1) Ref#101M-17, RTU) were applied and incubated, followed by ACE chromagen (DAKO) to visualize the complex. The slides were then cover-slipped with aqueous permount (DAKO). Positive and negative stained control tissues were provided for all cases. A Verhoef-Van Gieson (VVG) stain was performed for each case.
Analysis
The biopsies analyzed were processed using the HoVert technique.15 H&E sections were analyzed for follicular inflammation. The percentage of affected follicles and the location of the infiltrate (superficial as infundibulum and isthmus, while deep as hair stem and bulb) were noted. An affected follicle is defined as a follicle with a lymphocytic infiltrate producing vacuolar changes in the outer root sheath and necrotic keratinocytes. Uninvolved hair follicles with dermal resident immune cells served as negative controls.16 The number of CD3, CD4, CD8 and CD1a positive cells were counted independently in three affected and unaffected follicles per case by two pathologists (JTS and SM) without greater than 10% variation. Joint review was used to resolve discrepancies greater the 10%. The average number of cells, when values differed, was used to calculate values. A radius of approximately 0.25 mm from the center of the follicular canal defined the zone for counting. The average number of cells for each marker was determined for affected and unaffected follicles in each biopsy. The CD4/CD8 T lymphocyte ratios were calculated in each case and compared to the CD4/CD8 T lymphocyte ratios in uninvolved areas. The numbers of CD20 positive B lymphocytes were also assessed. Statistical analysis was performed using ANOVA and the Student’s t-test with XLSTAT, statistical software and data analysis in Excel. Mean values and variance for each category were calculated. P values of <0.05 was considered statistically significant.
Results
All nine cases of FFA were female patients and 89% of the patients were post-menopausal, with a mean age of 58 and a range of 42-76 (Figure 1). The majority (89%) of patients had frontal scalp involvement while 11% of patients had temporal scalp involvement (Figure 2). Clinically, the patients presented with classical frontal band of hair loss with associated eyebrow loss.
Figure 1.
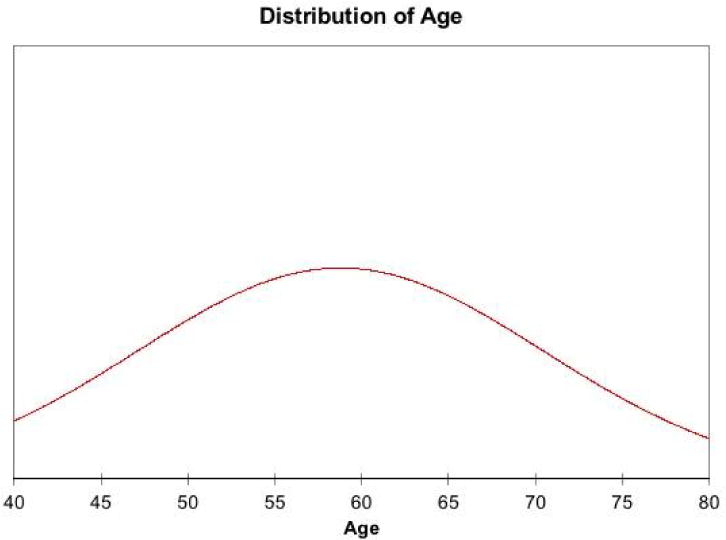
Histogram of age range of patients with frontal fibrosing alopecia.
Figure 2. Representative clinical images of patients with FFA.
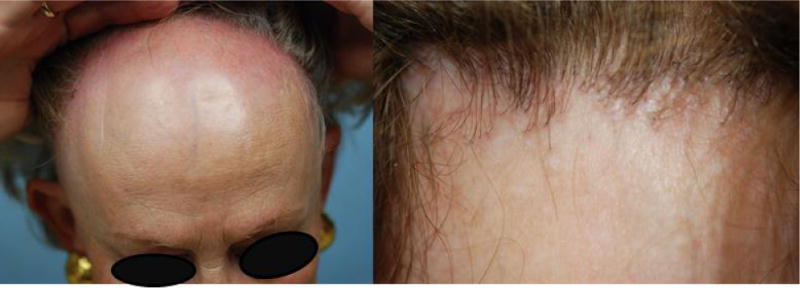
The patients demonstrate a receding hair-line associated with loss of follicular ostia and erythema. Thinning of the lateral eyebrows is present.
Each case had at least 35% of follicles affected by the disease, with an average of 49% follicular involvement (Figure 3). The majority (78%) had only superficial involvement (infundibulum and isthmus); however, two cases had both superficial and deep (stem and bulb) follicular involvement (Table 1). The involved follicles demonstrated concentric, perifollicular fibrosis associated with a perifollicular lichenoid lymphocytic infiltrate. The majority of inflammation was present around the infundibulo-isthmic portion of the hair follicle. The infiltrates largely spare the interfollicular epidermis. On VVG stains, the perifollicular inflammation is associated with a loss of elastin fibers, seen on both vertical and transverse sections (Figure 4).
Figure 3. Percentage of inflammed follicles in biopsies.
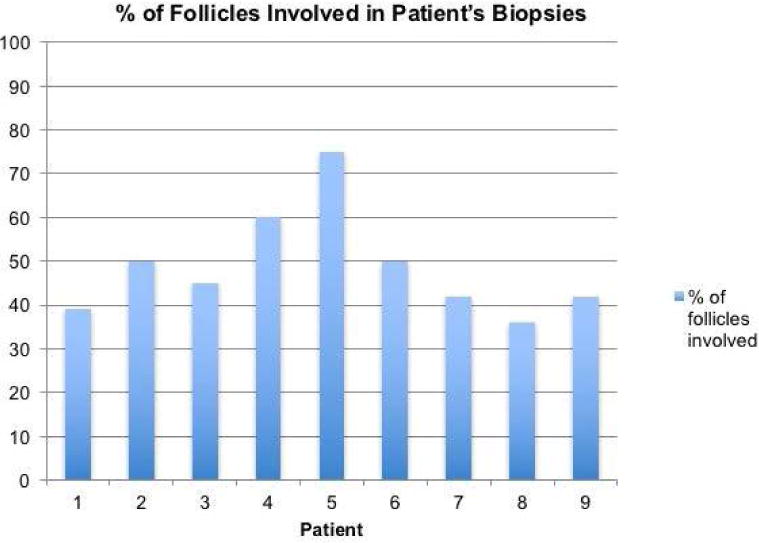
In all nine cases, at least 35% of total follicles were involved by the disease process with an average follicular involvement of 49%.
Table 1.
Clinical and histological characteristics of FFA cases.
| Case | Sex | Age | Site | Total follicles | Affected Follicles | Location of Involvement |
|---|---|---|---|---|---|---|
| 1 | Female | 50 | Left frontal | 28 | 39% | Superficial |
| 2 | Female | 75 | Left frontal | 16 | 50% | Superficial |
| 3 | Female | 55 | Frontal | 31 | 45% | Superficial |
| 4 | Female | 50 | Frontal | 15 | 60% | Superficial |
| 5 | Female | 42 | Left Temple | 4 | 75% | Superficial |
| 6 | Female | 55 | Right frontal | 20 | 50% | Superficial & Deep |
| 7 | Female | 52 | Right frontal | 12 | 42% | Superficial & Deep |
| 8 | Female | 52 | Left frontal | 14 | 36% | Superficial |
| 9 | Female | 75 | Frontal | 19 | 42% | Superficial |
Figure 4. Decreased peri-follicular elastin.
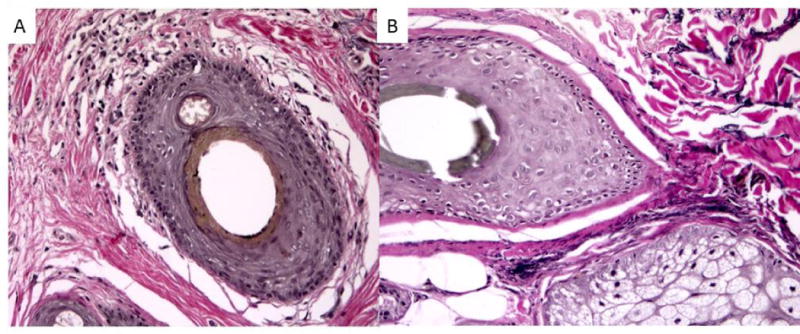
Loss of elastin fibers around involved follicles in FFA (A) when compared to uninvolved follicles (B), Verhoeff-Van Gieson stain, 200x magnification.
The CD1a-stained sections show a perifollicular and interfollicular pattern of LCs (Figure 5). There is an increase of LCs in the perifollicular inflammatory infiltrate (mean of 18, standard deviation of 5.5) and within the outer root sheath (mean of 14, standard deviation of 4.3) when compared with those of uninvolved follicles (p<0.0001) (Figure 6). The increase in CD1a+ cells was also noted in the 2 cases with a deeper dermal infiltrate.
Figure 5. Composition of FFA inflammatory infiltrate.
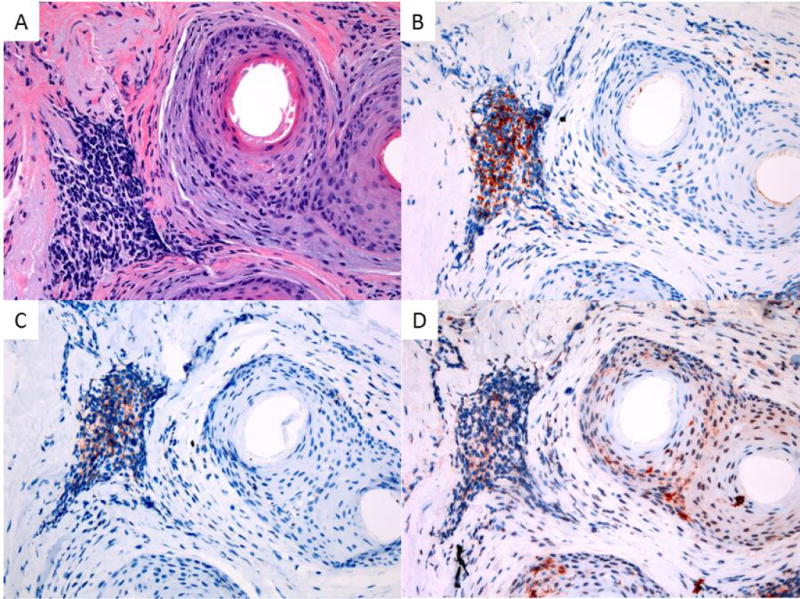
Lymphocytic and LC infiltrate in FFA illustrating CD3 positive T cells with a CD8 predominance and increased perifollicular and interfollicular LCs: A – H&E 200x magnification, B – CD3 IHC 200x magnification, C – CD8 200x magnification, D – CD1a 200x magnification.
Figure 6. Density of Langerhans cells in FFA.
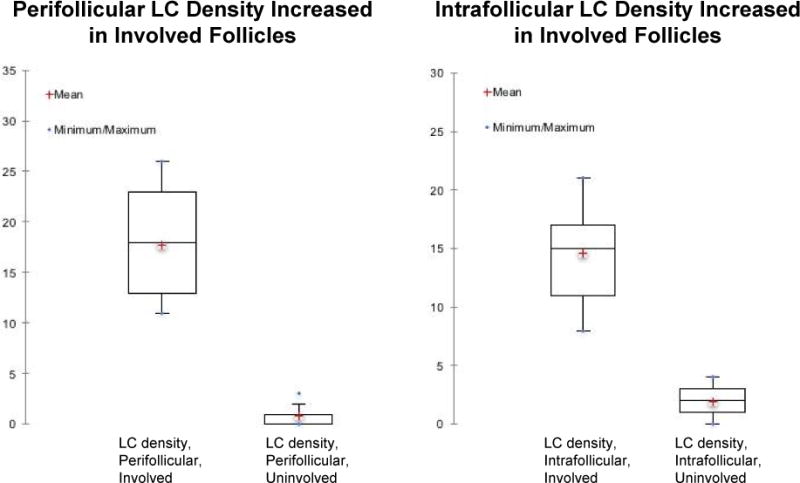
The density of peri- and intrafollicular Langerhans cells (LC) was elevated in involved follicles.
The perifollicular and lichenoid inflammatory infiltrate consists of macrophages, T cells, and focally scattered CD20 positive B cells. The B cells tend to be more perivascular than perifollicular in location (data not shown). CD3 highlights a predominantly T cell infiltrate with both CD4 and CD8 positive cells (Figure 6). For the CD4/8 ratio, the involved follicles show a relative increase in CD8 positive T cells, significantly distinct from the CD4- predominant population in uninvolved follicles in the same biopsies (p<0.0001). The mean CD4/8 ratio in involved follicles is 0.629, 95% confidence interval of 0.446-0.811, while the CD4/8 ratio in uninvolved follicles’ mean is 3.372, 95% confidence interval of 3.177-3.567 (Figure 7). Uninvolved follicles contained rare perifollicular and intrafollicular inflammatory cells.
Figure 7.
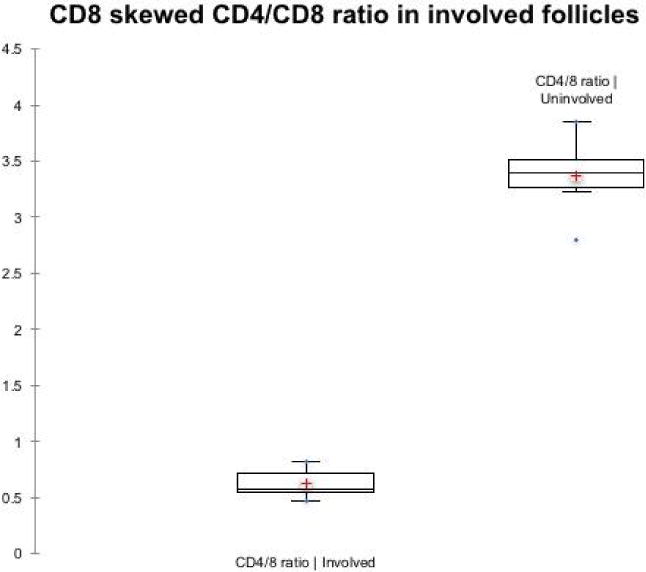
The CD4/CD8 ratios show a CD8-predominant distribution in involved follicles. The CD4/CD8 ratios were calculated for involved and uninvolved follicles. The mean and standard deviation are shown.
Discussion
Human hair follicles contain lymphocytes and LCs to promote immune surveillance and to protect against infections.17, 18 In terminal anagen follicles, the ‘bulge’ region is crucial for preserving human hair since follicle stem cells reside in this region.19 If the bulge stem cells are injured and lost due to inflammation, follicular scarring eventually occurs.12, 20
The composition of the inflammatory infiltrate in FFA and the mechanism of follicular destruction are not well defined. The inflammatory infiltrate in our series of FFA biopsies predominantly involved the infundibulum and isthmus portions of the follicle, rarely involving the lower follicle and bulb, as previously described.1, 8 In general, the inflammation in scarring alopecia, especially LPP, involves the infundibuloisthmic regions of the follicle.13, 14, 20 In our study, the majority of involved follicles in FFA demonstrate inflammation in the bulge region, with the exception of the two cases which also showed involvement of the lower follicle.
On VVG elastin staining, involved follicles in FFA show a perifollicular loss of elastin fibers associated with the fibrosis. The loss of elastin staining also has been shown in LPP, folliculitis decalvans and discoid lupus.21, 22
Similar to LPP, the inflammatory cell infiltrate in FFA is characterized by an increase in the percentage of CD8+ T cells and is a statistically significant reversal of the typical CD4:CD8 ratio which is approximately 2:1.18 Interestingly, the uninvolved follicles in the series of FFA biopsies demonstrated an increased CD4:CD8 ratio of >3:1. This finding may be due to the prominent migration of CD8+ T cells out of uninvolved regions of the biopsy into involved regions.
LCs typically reside in the epidermis representing approximately 2-4% of total epidermal population.23 In hair follicles, LCs are present in the outer root sheath, especially in the bulge region and can be highlighted with CD1a immunohistochemical stain.24, 25 Biologically, these cells play important roles in antigen presentation and the innate immunity in skin.16, 17 There can be alterations in the number and distribution of the LCs in a variety of inflammatory disorders such as pseudolymphomatous folliculitis (PLF). Studies have described increased perifollicular CD1a positive dendritic cell clusters in PLF, in contrast to unremarkable skin with a predominantly perivascular distribution of and peripheral T cell lymphoma with no consistent pattern of distribution or increase in density.26, 27 In mice depleted of LCs, inflammation induces a rapid repopulation of LCs which requires chemokine expression by the hair follicular keratinocytes.28
In primary alopecia, especially LPP, the LCs are depleted but these cells are hypothesized to have a role in antigen presentation in early stages of the disease that leads to the cytotoxic immune response involving a CD8-rich T cell infiltrate.19 Our study shows a significant increase in perifollicular and interfollicular LCs. This sustained increase in LCs may represent a pathophysiologic feature of FFA suggesting an immunologic mechanism involving recruitment of LCs. The sustained elevation of LCs may also be related to the progressive nature of FFA.
The analysis of FFA specimens demonstrates a infundibuloisthmic lymphocytic infiltrate with an elevated CD4/CD8 ratio similar to that seen in LPP. However, unlike LPP, elevated numbers of LCs are persistent in infundibuloisthmic region in FFA. Perhaps, the increased LCs may be a component of an immune reaction that leads to a CD8+ T cell response. As with LPP, the inciting antigen or cause of immune disruption in FFA requires further study.
Acknowledgments
Tissue acquisition, processing and immunohistochemical staining was provided by the University of Pennsylvania, Skin Biology and Diseases Resource-based Center supported by NIAMS P30 AR069589.
References
- 1.Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Archives of dermatology. 1994;130:770–4. [PubMed] [Google Scholar]
- 2.Banka N, Mubki T, Bunagan MJK, McElwee K, Shapiro J. Frontal fibrosing alopecia: a retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. International journal of dermatology. 2014;53:1324–30. doi: 10.1111/ijd.12479. [DOI] [PubMed] [Google Scholar]
- 3.Pirmez R, Donati A, Valente NS, Sodre CT, Tosti A. Glabellar red dots in frontal fibrosing alopecia: a further clinical sign of vellus follicle involvement. The British journal of dermatology. 2014;170:745–6. doi: 10.1111/bjd.12683. [DOI] [PubMed] [Google Scholar]
- 4.Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. The British journal of dermatology. 2009;160:75–9. doi: 10.1111/j.1365-2133.2008.08861.x. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Pestana A, Tuneu A, Lobo C, Ormaechea N, Zubizarreta J, Vildosola S, Del Alcazar E. Facial lesions in frontal fibrosing alopecia (FFA): Clinicopathological features in a series of 12 cases. Journal of the American Academy of Dermatology. 2015;73:987.e1–6. doi: 10.1016/j.jaad.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Miteva M, Tosti A. The follicular triad: a pathological clue to the diagnosis of early frontal fibrosing alopecia. The British journal of dermatology. 2012;166:440–2. doi: 10.1111/j.1365-2133.2011.10533.x. [DOI] [PubMed] [Google Scholar]
- 7.Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. The British journal of dermatology. 2010;163:1296–300. doi: 10.1111/j.1365-2133.2010.09965.x. [DOI] [PubMed] [Google Scholar]
- 8.Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. Journal of the American Academy of Dermatology. 1997;36:59–66. doi: 10.1016/s0190-9622(97)70326-8. [DOI] [PubMed] [Google Scholar]
- 9.Meinhard J, Stroux A, Lunnemann L, Vogt A, Blume-Peytavi U. Lichen planopilaris: Epidemiology and prevalence of subtypes - a retrospective analysis in 104 patients. J Dtsch Dermatol Ges. 2014;12:229–35, -36. doi: 10.1111/ddg.12264. [DOI] [PubMed] [Google Scholar]
- 10.Tan E, Martinka M, Ball N, Shapiro J. Primary cicatricial alopecias: clinicopathology of 112 cases. Journal of the American Academy of Dermatology. 2004;50:25–32. doi: 10.1016/j.jaad.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Nayar M, Schomberg K, Dawber RP, Millard PR. A clinicopathological study of scarring alopecia. The British journal of dermatology. 1993;128:533–6. doi: 10.1111/j.1365-2133.1993.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohyama M. Primary cicatricial alopecia: recent advances in understanding and management. The Journal of dermatology. 2012;39:18–26. doi: 10.1111/j.1346-8138.2011.01416.x. [DOI] [PubMed] [Google Scholar]
- 13.Harries MJ, Meyer K, Chaudhry I, Kloepper EJ, Poblet E, Griffiths CE, Paus R. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231:236–47. doi: 10.1002/path.4233. [DOI] [PubMed] [Google Scholar]
- 14.Mobini N, Tam S, Kamino H. Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. Journal of cutaneous pathology. 2005;32:675–9. doi: 10.1111/j.0303-6987.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen JV, Hudacek K, Whitten JA, Rubin AI, Seykora JT. The HoVert technique: a novel method for the sectioning of alopecia biopsies. Journal of cutaneous pathology. 2011;38:401–6. doi: 10.1111/j.1600-0560.2010.01669.x. [DOI] [PubMed] [Google Scholar]
- 16.Bos JD, Zonneveld I, Das PK, Krieg SR, van der Loos CM, Kapsenberg ML. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. The Journal of investigative dermatology. 1987;88:569–73. doi: 10.1111/1523-1747.ep12470172. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, Takigawa M, Paus R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. The Journal of investigative dermatology. 2008;128:1196–206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 18.Christoph T, Muller-Rover S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, Ruckert R, Paus R. The human hair follicle immune system: cellular composition and immune privilege. The British journal of dermatology. 2000;142:862–73. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- 19.Cotsarelis G. Epithelial stem cells: a folliculocentric view. The Journal of investigative dermatology. 2006;126:1459–68. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 20.Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. The American journal of pathology. 2010;177:2152–62. doi: 10.2353/ajpath.2010.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinkus H. Differential patterns of elastic fibers in scarring and non-scarring alopecias. Journal of cutaneous pathology. 1978;5:93–104. doi: 10.1111/j.1600-0560.1978.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 22.Elston DM, McCollough ML, Warschaw KE, Bergfeld WF. Elastic tissue in scars and alopecia. Journal of cutaneous pathology. 2000;27:147–52. doi: 10.1034/j.1600-0560.2000.027003147.x. [DOI] [PubMed] [Google Scholar]
- 23.Igyarto BZ, Kaplan DH. The evolving function of Langerhans cells in adaptive skin immunity. Immunol Cell Biol. 2010;88:361–5. doi: 10.1038/icb.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxicol Pathol. 1996;48:175–81. doi: 10.1016/S0940-2993(96)80039-X. [DOI] [PubMed] [Google Scholar]
- 25.Jimbow K, Sato S, Kukita A. Langerhans’ cells of the normal human pilosebaceous system. An electron microscopic investigation. The Journal of investigative dermatology. 1969;52:177–80. doi: 10.1038/jid.1969.26. [DOI] [PubMed] [Google Scholar]
- 26.Arai E, Okubo H, Tsuchida T, Kitamura K, Katayama I. Pseudolymphomatous folliculitis: a clinicopathologic study of 15 cases of cutaneous pseudolymphoma with follicular invasion. Am J Surg Pathol. 1999;23:1313–9. doi: 10.1097/00000478-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Gerami P, Guitart J. The spectrum of histopathologic and immunohistochemical findings in folliculotropic mycosis fungoides. Am J Surg Pathol. 2007;31:1430–8. doi: 10.1097/PAS.0b013e3180439bdc. [DOI] [PubMed] [Google Scholar]
- 28.Murphy GF, Katz S, Kligman AM. Topical tretinoin replenishes CD1a-positive epidermal Langerhans cells in chronically photodamaged human skin. Journal of cutaneous pathology. 1998;25:30–4. doi: 10.1111/j.1600-0560.1998.tb01686.x. [DOI] [PubMed] [Google Scholar]


