ABSTRACT
Objectives: Patients with rheumatoid arthritis (RA) are at an increased risk of Pneumococcal infections. Immunogenicity and persistence of a prime-boost revaccination strategy using 13-valent/23-valent anti-pneumococcal vaccines was evaluated in patients with RA treated by Methotrexate (MTX) and anti-TNF.
Method: Twenty-four patients with RA received one dose of PCV13 (Prevenar13®; Pfizer) followed two months later by one dose of PPV23 (Pneumovax®, Merck). Concentrations of IgG specific for 7 serotypes common to both vaccines and 3 uncommon serotypes, included only in the PPV23 were measured by ELISA and Opsonophagocytic Assay (OPA) at baseline and after 4, 12 and 24 months post-vaccine.
Results: Similar percentages of protection were found at 4 months (63% vs. 55%), 12 months (54% vs. 50%) and 24 months (52% vs. 55%) for the 7 common and 3 uncommon serotypes when antibody titers were assayed by ELISA. Based on functional antibody measurements by OPA, a decrease of protected patients was observed 24 months after vaccine with only 19% of patients protected compared to 29% at baseline.
Conclusion: Although the combined pneumococcal revaccination strategy induces good protection in the short term in RA patients, this protection does not persist beyond two years with levels of functional antibody decreasing below pre-vaccine levels. We did not observe a higher efficacy of the conjugate vaccine compared to the polysaccharide vaccine. Our results clearly question the advantage of the prime-boost strategy as it highlight the possible hyporesponse induced by PPV23 against the immune response elicited by the primo-injection of the PCV13 vaccine.
KEYWORDS: Rheumatoid arthritis, Pneumococcal vaccines, Methotrexate, anti-TNF, ELISA, OPA
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory joint disease, affecting approximately 1% of the population worldwide. Patients with rheumatoid arthritis (RA) are at an increased risk of some serious vaccine-preventable infections.17-3 Pneumococcal infections are one of the most prevalent vaccine-preventable infectious diseases that have been associated with increased hospitalizations and/or death among patients with RA.4,5 Nowadays, two types of pneumococcal vaccines are available: a 23-valent polysaccharide vaccine and conjugated vaccines with serotypic coverage from 7 to 13. The 23-valent pneumococcal polysaccharide vaccine (PPV23) has been licensed in 1983 and has a large serotypic coverage with 23 pneumococcal serotypes. This vaccine is less immunogenic than the newly developed conjugated vaccines (PCV10, PCV13).6 These new vaccines due to T-cell dependent immune responses induce high level of memory B-cells to trigger an immunological memory.7
Although a class of biologic disease-modifying anti-rheumatic drugs (bDMARDs) has significantly improved the treatment of RA, these drugs are immunomodulator and thus associated with an increased risk of several types of infections.8 and with a potential impact on vaccine efficacy.9,10 In line with these observations, a lower vaccine-specific IgG levels has been observed following pneumococcal vaccination in arthritis patients treated with methotrexate compared to those on TNF inhibitors, to those without DMARD and to healthy controls.11-13 With respect to non-TNF biologics, rituximab or abatacept but not tocilizumab may also impair antibody responses to pneumococcal conjugated vaccine.14-17 In view of these data highlighting the increased risk of infectious diseases in RA patients especially for those receiving immunosuppressive treatments and the awareness and performance of vaccinations, clear recommendations for vaccinations under the use of biological agents are needed.
Since 2013, French vaccine recommendations committee considered as the best strategy for immune-suppressed patients, previously vaccinated by one dose of PPV23, a revaccination three years after by a prime-boost strategy consisting in a primo-vaccination by PCV13 (Prevenar13®) followed by a revaccination at 8 weeks by PPV23 (Pneumo23®) to enlarge the serotypic coverage.18 To our knowledge, no information was available with respect to the short (4 months) and long term (12 and 24 months) efficacy of the prime-boost strategy PCV13+PPV23 vaccines in immunocompromised patients in particularly MTX + anti-TNF-treated RA patients.
Material and methods
Study population
Twenty-four adults patients with rheumatoid arthritis (RA) treated by methotrexate (MTX) and anti-TNF were enrolled in this study for routine-vaccination against Streptococcus pneumoniae. All patients signed written informed consent. All patients received a previous vaccination by the pneumococcal polysaccharide vaccine PPV23, at least three years before enrolment. Patients were enrolled between September 2014 and September 2015 in Rheumatology B department, Cochin Hospital, Paris (Fig. 3).
Figure 3.
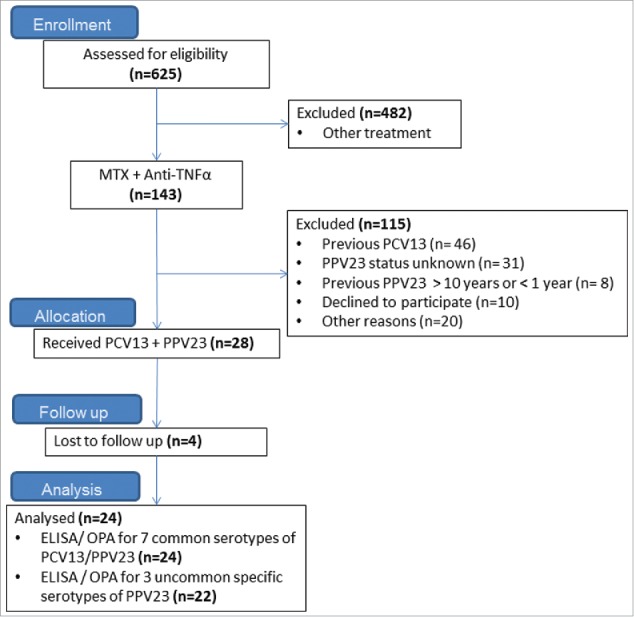
Flow chart illustrating the study population. Note: All Patients were recruited in Rhumatology B Department, Cochin Hospital, AP-HP, Paris, France.
Vaccination
RA patients were included after having received one dose of PCV13 (Prevenar13®; Pfizer) followed two months later by one dose of PPV23 (Pneumovax®, Merck) during routine-visits according to recommendations. Blood samples were obtained at baseline, 4 months, 12 months and 24 months following PCV13 immunization.
Serological evaluation
We evaluated antibody concentrations for ten serotypes (4, 6B, 9V, 14, 18C, 19F, 23F, 10A, 12F and 15B) by ELISA and seven (4, 6B, 9V, 14, 18C, 19F, 23F) by OPA. For the evaluation of the combined strategy, we selected 7 serotypes included in both vaccines (seven serotypes that were tested routinely in our laboratory: 4, 6B, 9V, 14, 18C, 19F, 23F). To see the impact of the PPV23 vaccination only, we selected 3 serotypes included only in the polysaccharide vaccine: 10A, 12F and 15B. These three serotypes were also known to be responsible of invasive pneumococcal infection in France.19
ELISA
IgG antibody concentrations for ten serotypes (4, 6B, 9V, 14, 18C, 19F, 23F, 10A, 12F and 15B) were determined using a modified enzyme linked immunosorbent (ELISA).20 previously described.21 Patient's sera were pre-absorbed with a solution containing 5 μg/ml pneumococcal C-polysaccharide (Statens Serum Institut, Copenhagen, Denmark) and 10 μg/ml serotype 22F capsular polysaccharide (American Type Culture Collection). Anti-pneumococcal antibody levels were determined in each specimen by analysis of linear regression plots compared with the reference serum (007sp) (National Institute for Biological Standards and Control (NIBSC)).
There is in the paediatric population a threshold of protection commonly admitted by 0.35 µg/ml, which serves as a reference for all pneumococcal immunogenicity studies in children. Currently, there are no validated correlates of pneumococcal vaccine protection in adults. Several studies have defined a response criteria for pneumococcal vaccine particularly adapted for evaluation of long-term protection in immunocompromised patients.22,23 Based on recommendations of the American Academy of Allergy, Asthma & Immunology and as used in previous study in RA patients, an at least two-fold increase in antibody level was an indicator of positive antibody responsive to the vaccine (i.e., ratio of post-vaccination and pre-vaccination antibody levels) and a concentration ≥ 1.3 µg/ml, an indicator of protection. Patients developing an immunological response (two-fold increased) toward at least 70% of serotypes at four months were considered as Responders. For the evaluation of the long-term protection, patients with an IgG-concentration ≥ 1.3 µg/ml for at least 70% of the serotypes were considered as Protected.
OPA
Opsonophagocytic activities (OPA) were measured for seven serotypes (4, 6B, 9V, 14, 18C, 19F, 23F) at baseline, 4 months, 12 months and 24 months by a multiplexed opsonophagocytic killing assay (MOPA), [www.vaccine.uab.edu] previously described.21 Opsonization titers (OT) were defined as interpolated reciprocal serum dilution that kills 50% of the bacteria in the assay. The assay sensitivity is the lowest dilution of sera tested (limit of detection LOD), which is normally 4 for undiluted sera, and is the same for each serotype. However, to quantify functional antibodies with more precision, the lower limit of quantification (LLOQ) was determined for each serotype-specific assay during assay validation. The LLOQs for the various serotypes were: serotype 4: 24; serotype 6B: 131; serotype 9V: 38; serotype 14: 85; serotype 18C: 47; serotype 19F: 74 and serotype 23F: 30.
For OPA titers higher than the LLOQ were considered accurate and their values were reported. Titers below the LLOQ were set to a value of 2 (half of LOD).24 In our study, patients developing an immunological response (four-fold increased) toward at least 70% of serotypes at four months were considered as Responders. For the evaluation of the long-term protection, patients with an OT ≥ LLOD for at least 70% of the serotypes were considered as Protected.
Statistics analysis
For each serotype, the geometric mean concentrations (GMC) and titers (GMT) and the corresponding 95% confidence intervals (CI) were calculated. Primary endpoint was the proportion of patients developing an immunological response toward at least 70% of serotypes at four months in ELISA. Patient characteristics were compared using Mann-Whitney's Ranksum test for quantitative variable and Fisher's exact test for categorical variable. The percentages of responders are provided together with their 95% confidence interval (95%CI). Quantitative variables were compared using Student's t-test and categorical variables were compared using the chi-square test. All tests were 2-sided at the level of 0.05. All analyses were performed using Graph Pad Prism 5.0 software (GraphPad, San Diego, CA).
Results
Pneumococcal serotype-specific Ig concentrations and determination of immune response to vaccination
Serotype-specific IgG response was evaluated for 7 common polysaccharides (4, 6B, 9V, 18C, 14, 19F, and 23F) contained in both PCV13 and PPV23 vaccine. At four months post immunization (M4), 33% of patients (8/24) were considered responders as defined by a two-fold increase in the IgG-concentration from baseline toward at least 70% of serotypes (Fig. 1). For the responders-group, geometric mean of concentrations (GMC) with 95% Confidence interval (CI) at four months increased for all serotypes compared to baseline but differences were significant for only serotypes 4 (GMC: 0.6 µg/ml (CI: 0.3–1.3) at baseline vs 1.8 µg/ml (1–3.4) at M4, p = 0.0379) and 19F (3.5 µg/ml (1.7–7.3) at baseline vs 12.8 µg/ml (3.9–41.3) at M4, p = 0.0281) (Table 1). Antibody levels decreased drastically one year after immunization as only two patients' remains responders after 12 months (8%). These patients were still responder 2 years after vaccination. Functional activity of polysaccharides specific-antibodies was tested by MOPA. Same percentages of responders were found (29% at M4, 14% at M12 and 10% at M24) indicating the production of functional antibodies (Table 2).
Figure 1.
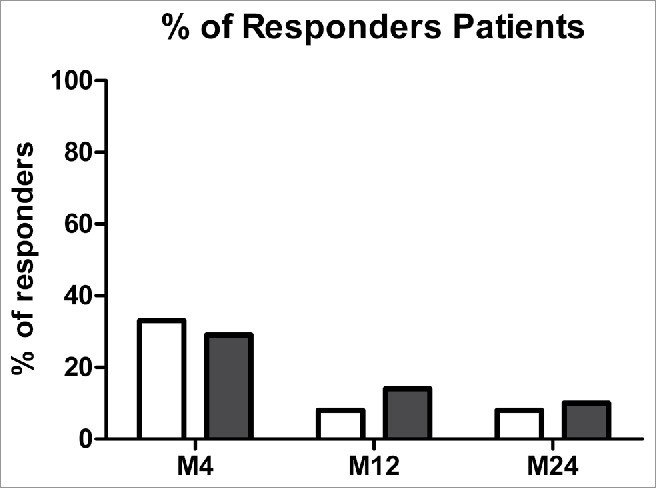
Percentages of responders patients for pneumococcal vaccine at 4, 12 and 24 months after immunization in ELISA (white) and in OPA (black). Note: Responders patients for each serotype at a specific time point were defined as a two-fold increase of IgG anti-PS compared to baseline by ELISA (in white) and a titer four-fold increase from baseline of Ig anti-PS by OPA (in black) for each serotype. Patients were assigned as responders when more than 70% of serotypes tested fit with those criteria.
Table 1.
Geometric mean (95% CI) Concentrations by ELISA of IgG Antibody at baseline, 4, 12 and 24 months after immunization.
| Serotypes | Baseline | M4 | M12 | M24 | |
|---|---|---|---|---|---|
| 4 | NR | 0.6 (0.4–0.9) | 0.9 (0.5–1.4) | 0.7 (0.4–1.2) | 0.7 (0.3–1.6) |
| R | 0.6 (0.3–1.3) | 1.8$ (1–3.4) | 1.2 (0.6–2.3) | 1.8 (0.7–4.3) | |
| 6B | NR | 1.6 (0.8–3.1) | 2 (1.1–3.8) | 1.6 (0.8–3.3) | 2.0 (0.8–4.6) |
| R | 1.9 (0.7–5.4) | 6.6 (2.3–18.3) | 3.7 (1.3–10.1) | 3.7 (1.0–14.4) | |
| 9V | NR | 1.1 (0.6–2.2) | 1.7 (0.9–3.2) | 1.5 (0.8–2.9) | 1.6 (0.6–4.5) |
| R | 2.6 (0.9–7.3) | 6.5# (2.5–17.2) | 3.6 (1.1–11.8) | 2.9 (0.5–16.3) | |
| 14 | NR | 6.3 (2.7–15) | 10 (4.5–22.1) | 8.7 (3.3–22.4) | 6.9 (1.7–28.2) |
| R | 4.4 (1.5–13) | 9.9 (3.7–26.6) | 8.4 (3.-22.6) | 8.2 (3.2–21.4) | |
| 18C | NR | 1.2 (0.7–2.1) | 2.1 (1.3–3.4) | 1.6 (0.9–2.9) | 1.8 (0.6–5.2) |
| R | 3.2# (1.4–7.3) | 9.4# (3.9–23) | 6.3# (2.6–14.9) | 2.6 (0.6–15.3) | |
| 19F | NR | 1.7 (0.9–3.1) | 2.3 (1.5–3.5) | 2 (1.1–3.6) | 3.1 (1.1–8.2) |
| R | 3.5 (1.7–7.3) | 12.8$,# (3.9–41.3) | 8.2# (2.7–24.7) | 2.5 (0.6–10.6) | |
| 23F | NR | 1.3 (0.6–3) | 1.6 (0.7–3.5) | 1.2 (0.5–2.9) | 1.2 (0.4–3.3) |
| R | 1.1 (0.4–3.4) | 3.7 (1.1–13) | 2.5 (0.8–7.7) | 1.2 (0.2–5.6) | |
| 10A | NR | 1.2 (0.3–4.9) | 1.7 (0.4–7.4) | 1.7 (0.4–7.1) | 2.0 (0.7–534) |
| R | 1.4 (0.6–3.2) | 6.7$,# (2.4–18.9) | 3.9 (1.4–10.4) | 6.2 (1.1–35.9) | |
| 12F | NR | 0.4 (0.1–1.3)) | 0.5 (0.1–1.9) | 0.6 (0.2–1.7) | 0.6 (0.2–2.1) |
| R | 0.6 (0.3–1.1) | 2.1 (0.7–6.2) | 1.4 (0.5–3.9) | 1.0 (0.4–2.6) | |
| 15B | NR | 2.8 (0.5–15.9) | 3.3 (0.5–20.1) | 3.1 (0.5–19.5) | 3.5 (0.7–18.1) |
| R | 4 (1.5–10.4) | 11.3 (3.4–38.1) | 8 (2.5–25.7) | 5.6 (1.0–32.4) |
Note: Responders at four month were defined as at least an IgG- two fold increased from baseline by ELISA. Data are in geometric means: antibody concentrations are in µg/ml (95% confidence limits). R: Reponders group, NR: Non-reponders group,
p<0.05 vs. baseline
Table 2.
Geometric mean (95% CI) Titer by OPA of Ig Antibody at baseline, 4, 12 and 24 months after immunization.
| Serotypes | Baseline | M4 | M12 | M24 | |
|---|---|---|---|---|---|
| 4 | NR | 9.8 (4.4–21.5) | 41.7 (25–69.4) | 18.7 (7.7–45.4) | 32.2 (11.8–88) |
| R | 4.2 (1.2–15.2) | 120.4 (28.8–503.2) | 42.2 (12.2–146.1) | 14.5 (3.0–71.2) | |
| 6B | NR | 31 (10.9–88.6) | 132.2 (63.8–274.2) | 96.9 (44–213.1) | 60.5 (15.7–234) |
| R | 13.9 (1.2–166.2) | 178.1 (26.1–1216) | 119.9 (27.1–530) | 29.7 (1.3–705.2) | |
| 9V | NR | 27.2 (11.1–66.6) | 58 (27.6–122) | 31.7 (11.1–90.8) | 41.9 (11.8–148.6) |
| R | 15.6 (2.5–99.2) | 79.2 (16.7–375.5) | 41.1 (6.8–248.8) | 17.2 (1.5–199) | |
| 14 | NR | 86.1 (31.3–236.6) | 145.5 (63.8–331.6) | 124 (49–314) | 59.1 (12.3–284.4) |
| R | 11.2 (1.2–102.9) | 137.6 (24.3–779.7) | 61.3 (6.3–598.5) | 25.8 (2.2–302.5) | |
| 18C | NR | 55.7 (20.4–152.3) | 89.7 (36.6–220) | 74.4 (29.1–190.2) | 51.2 (8.8–298.5) |
| R | 9.3 (1.4–61.5) | 118.1 (18.4–758.9) | 23.2 (2.2–244.8) | 13.1 (0.9–186.3) | |
| 19F | NR | 57.2 (26.5–123.3) | 188.7$ (89.5–397.5) | 125.1 (62.5–250.2) | 87.2 (18.8–405.6) |
| R | 28.5 (11.8–68.8) | 200.5$ (68.9–582.9) | 131.8$ (35.1–494.7) | 46.2 (4.5–475.8) | |
| 23F | NR | 19 (7.1–51.2) | 29.1 (9.2–91.7) | 27.8 (8.6–89.3) | 18.5 (3.2–107.5) |
| R | 5.4 (1–29) | 45.3 (7.1–287.1) | 12.6 (2.5–63.8) | 4.7 (1.1–20.7) | |
Note: Responders at four month were defined as at least a four-fold increased from baseline. Data are in geometric means: Opsonisation titer (95% confidence limits). R: Reponders group, NR: Non-reponders group,
p<0.05 vs. baseline
Serotype-specific IgG response was evaluated for 3 polysaccharides (10A, 12F and 15B) contained only in PPV23 but not in PCV13 vaccine in order to evaluate the specific impact of the PPV23 vaccination performed two months after the PCV13 vaccine. Because of lack of sera, two patients could not be tested for these three uncommon serotypes. Four months post immunization, 36% of patients (8/22) were considered responders (Fig. 1). Interestingly, only 4 of them were responders for the 7 common serotypes tested. For the responders-group, geometric mean of concentrations at four months increased for all serotypes compared to baseline but differences was significant for only serotype 10A (1.4 µg/ml (0.6–3.2) at baseline vs 6.7 µg/ml (2.4–18.9) at M4, p = 0.0148). One year after immunization, half of the responders at M4 (18%) had enough antibodies to be still considered as responders. The decrease of antibody levels was stable since these 4 patients were still responders 2 years after vaccination.
Evaluation of protective level of Pneumococcal serotype-specific Ig after immunization
For the evaluation of the protection, a second criterion was applied where an IgG-concentration ≥ 1.3µg/ml in ELISA and ≥Lower limits of quantifications (LLOQ) in OPA for at least 70% of the serotypes was required to be considered protected.22
At baseline, in ELISA, 33% of patients were considered protected for the 7 common serotypes and 32% of patients for the 3 uncommon serotypes. After vaccination, protection for the 7 common reached 63% of patients versus 55% for the 3 uncommon. Despite a more severe decrease 12-month after immunization for the 7 common serotypes (54% of patients protected compared to 63% at M4 versus 50% for the 3 uncommon compared to 55% at M4), the protection remains stable over the years, with similar percentage of protection 2 years after vaccination (52% for the 7 common serotypes versus 55% for the 3 uncommon serotypes), indicated no higher efficacy of the PCV13 for long term protection on the PPV23.
Although the Opsonophagocytic Assay showed similar percentage of patients protected at baseline and four months after vaccination (respectively 29% and 71%), protection did not stay stable over the time as found for ELISA, and decreased drastically 2 years post immunization with only 19% patients still protected in OPA, a rate inferior than baseline (29%)(Fig. 2).
Figure 2.
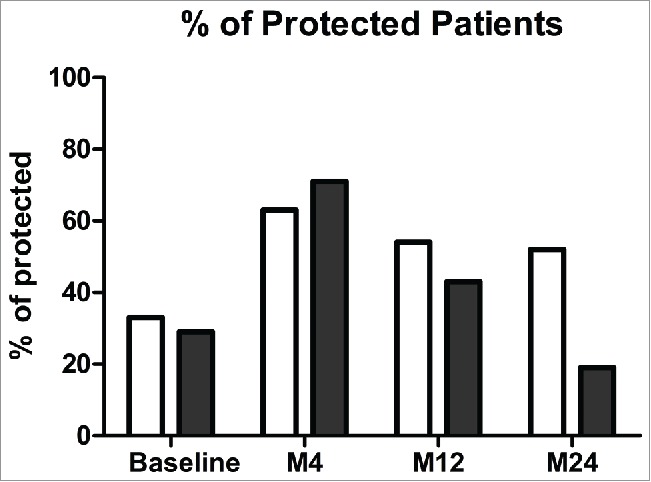
Percentages of protected patients for pneumococcal vaccine at baseline, 4, 12 and 24 months after immunization in ELISA (white) and in OPA (black). Note: Protected patients for each serotype at a specific time point were defined as at least a concentration of IgG anti-PS ≥ 1.3µg/ml in ELISA (in white) and a titer of Ig anti-PS ≥ LLOQ in OPA (in black) for each serotype. Patients were assigned as protected when more than 70% of serotypes tested fit with those criteria.
Population data
Twenty-four patients with RA were recruited for the study (Fig. 3). All patients were treated with combined Methotrexate (MTX) + anti-TNF. The time of introduction of MTX treatment was similar in Protected patients and Non Protected patients with medians respectively at 11.7 years for the Non Protected patients and 10.5 years for the Protected patients. No differences were observed in the time of introduction of anti-TNF treatment with medians at 5.8 years for Non Protected patients and 6.2 years for the Protected patients. The median age of the study population was 62.5 years (range 32–71) with 18 females (75%) and 6 males (25%) (Table 3). There were no differences between the median age and gender of the Protected patients compared to the Non Protected patients. Previous pneumococcal vaccination times were similar in both groups with medians respectively at 5.25 years for Non Protected and 5.11 years for the Protected patients. Interestingly, the presence of Rheumatoid Factor (RF) in sera of more Protected patients compared to Non-Protected patients (respectively 79% versus 56%) seems to have a positive impact in protection. In opposite, presence of antibody anti-citrullinated protein (CCP) seems to be a negative indicator for protection, as we found more antibody anti-CCP in Non-Protected patient's sera patients (78% versus 64% for Protected patients). There were no significantly differences observed between the two groups for the other biological or clinical data tested (ESR, CRP, IgG, IgA, IgM, DAS28). None of our patients developed any type of pneumococcal disease during the 24 months of the study.
Table 3.
Comparison of Demographic and disease status pre-vaccination for the Protected and Non Protected Patients.
| Protected | Non Protected | ||
|---|---|---|---|
| Characteristic | (n = 15) | (n = 9) | p |
| Age; years, median SD (range) | 56 (32–71) | 64 (50–70) | 0.2959 |
| Sex; % female | 73 | 78 | 0.5108# |
| DAS28 | 2.29 | 2.79 | 0.2866 |
| RF titer; mg/l, median | 31.67 | 16 | 0.6818 |
| RF; % of positive | 79* | 56 | 0.0008# |
| anti-CCP titer; median | 71.62 | 50.75 | 0.8981 |
| anti-CCP; % of positive | 64* | 78 | 0.0423# |
| ESR; mm/hour, median | 13.76 | 7.9 | 0.5906 |
| CRP; mg/l, median | 2.83 | 1.65 | 0.0628 |
| IgG | 11.19 | 10.77 | 0.5928 |
| IgA | 2.18 | 2.55 | 0.3672 |
| IgM | 1.03 | 1.28 | 0.5931 |
| Previous PPV23 vaccination; years, median | 5.11 | 5.25 | 0.5144 |
Note: comparison of biological values for Protected patients group versus non-Protected patients group at baseline. DAS28 = Disease Activity Score; ESR = Erythocyte Sedimentation Rate; CRP = C Reactive Protein, Ab anti-CCP = Anti–citrullinated protein antibody, PPV23 = 23-valent anti-pneumococcal vaccine. # referred to Fisher's exact test; others p values were calculated by Mann-Whitney's Ranksum test
Discussion
Here, we evaluated in a pilot study the immunogenicity of the prime-boost revaccination strategy using PCV13 vaccination followed by PPV23 vaccination 8 weeks later in 24 patients with Rheumatoid Arthritis (RA) treated by Methotrexate (MTX) and anti-TNF. The persistence of a protective immunity for two years after revaccination was also evaluated in our population.
We chose to evaluate RA patients treated with MTX because this drugs is the most common DMARD used first line.25 and because a lower vaccine-specific IgG levels has been observed following pneumococcal vaccination in arthritis patients treated with methotrexate alone or in combination with anti-TNF compared to those on TNF inhibitors alone, to those without DMARD and to healthy controls.11,12,26
Based on recommendations of the American Academy of Allergy, Asthma & Immunology.22 and as used in previous study evaluating pneumococcal vaccine in RA patients, an at least two-fold increase in antibody level was an indicator of positive antibody responsive to the vaccine and an antibody concentration ≥ 1.3 µg/ml as protection. Patients developing an immunological response toward at least 70% of serotypes were considered as responders and patients developing an immunological protection toward at least 70% of serotypes were considered as protected. In contrast with previous studies evaluating pneumococcal vaccine in RA patients that evaluated the serologic response by testing antibodies concentrations against 2 serotypes (6B and 23F) included in both PCV13 and PPV23, we have tested antibodies concentrations against ten different polysaccharides. Seven were contained in both PCV13 and PPV23 vaccine and three only contained in PPV23. The lack of control groups is a limitation of our study that is counterbalanced by the large number of anti-PS antibodies tested here that allows us to compare in a same group of immunocompromised patients the antibody response and protection elicited by the combination of PCV13 and PPV23 vaccine against the seven common serotype versus the response elicited by the PPV23 alone using the 3 uncommon serotype.
Four months post immunization, we observed 33% of responders patients to the seven common serotypes and 36% to the three uncommon serotypes included in the PPV23 only (10A, 12F and 15B). Our results are in line with previous observation evaluating PPV23 and PCV7 vaccine. Indeed, the percentage of responder evaluated by a two-fold increase in antibody concentration versus baseline were 40% for serotype 23F and 20% for serotype 6B upon PCV7 vaccine.11 and 53% for 23F and 45% for 6B after PPV23 vaccination in patients treated with MTX+anti-TNF.12 However, no significant difference in the efficacy of both vaccines could be observed.27 This conclusion is consistent with studies in immunocompetent patients.28,29 who found no differences between the combined strategy versus PCV alone in healthy adults aged 50–80. Moreover, pneumococcal vaccines elicit a poor antibody response in RA patients compared to healthy patients. Indeed, Melmed et al found 84% of responders to one dose of PPV23 in healthy controls.30 and different studies showed percentage of responders after one injection of PCV7 from 72% to 84% depending on the serotype.31,32
Interestingly, more patients positive for anti-cyclic citrullinated peptide (CCP) antibody were found in our non-protected compared to protected patients. In opposite, more protected patients were found positive for rheumatoid factor (RF) than the non-protected patients. Anti-CCP antibody positivity is an established diagnostic factor for severe disease activity and joint damage and a prognostic factor for aggressive disease in rheumatoid arthritis (RA) wich is not the case for RF. Thus, the association of poor vaccine response in patients positive for anti-CCP may be related to the less immunogenicity of the vaccine in the more severe and agressive forme of RA.33,34
To our knowledge, this is the first study describing the long-term evolution of the anti-polysaccharides antibody response after pneumococcal vaccination especially in the case of a prime boost strategy. If the percentage of protection is quite equivalent for the seven common and the three uncommon serotype, the more intense drop in antibody response for the seven common serotype compared to the three uncommon may suggest a negative impact of the PPV23 vaccine on the PCV13 response. Inferior rate found in OPA at 2 years post-immunization compared to baseline (19% at 24 months versus 29% at baseline) improves this hypothesis of a negative impact. Such phenomenon has been extensively described and called hyporesponsiveness and may be due to the toxicity of polysaccharides contained in PPV23 vaccine against anti-PS memory B cells generated after PCV13 vaccination.35,36
The prime boost strategy combining conjugated and unconjugated pneumococcal vaccine is recommended for immunocompromised patients and thus particularly indicated in RA patients treated with MTX because of the poor immunogenicity of both type of pneumococcal vaccine in this case. Our results clearly question the advantage of the prime-boost strategy versus PPV23 or PCV13 alone as it highlight the possible hyporesponse induced by PPV23 against the immune response elicited by the primo-injection of the PCV13 vaccine.
Disclosure of potential conflicts of interest
OL participated in industry-sponsored clinical trials that included Pneumococcal vaccines. The other authors declare no conflict of interest for this manuscript.
Acknowledgments
The authors thank Maryse Mezieres (Service de Rhumatologie B, Cochin, Paris, France) for trial monitoring and handling.
Grant support
No funding
References
- 1.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. PMID:12355475. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira I, Isenberg D. Vaccines and biologics. Ann Rheum Dis. 2014;73:1446–54. doi: 10.1136/annrheumdis-2014-205246. PMID:24845388. [DOI] [PubMed] [Google Scholar]
- 3.De Keyser F. Choice of biologic therapy for patients with rheumatoid arthritis: the infection perspective. Curr Rheumatol Rev. 2011;7:77–87. doi: 10.2174/157339711794474620. PMID:22081766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Assen S, Elkayam O, Agmon-Levin N, Cervera R, Doran MF, Dougados M, Emery P, Geborek P, Ioannidis JPA, Jayne DRW, et al.. Vaccination in adult patients with auto-immune inflammatory rheumatic diseases: A systematic literature review for the European League Against Rheumatism evidence-based recommendations for vaccination in adult patients with auto-immune inflammatory rheumatic diseases. Autoimmun Rev. 2011;10:341–52. doi: 10.1016/j.autrev.2010.12.003. PMID:21182987. [DOI] [PubMed] [Google Scholar]
- 5.Van Assen S, Agmon-Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, Emery P, Geborek P, Ioannidis JPA, Jayne DRW, et al.. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414–22. doi: 10.1136/ard.2010.137216. PMID:21131643. [DOI] [PubMed] [Google Scholar]
- 6.Dinleyici EC. Current status of pneumococcal vaccines: lessons to be learned and new insights. Expert Rev Vaccines. 2010;9:1017–22. doi: 10.1586/erv.10.86. PMID:20822344. [DOI] [PubMed] [Google Scholar]
- 7.Aliberti S, Mantero M, Mirsaeidi M, Blasi F. The role of vaccination in preventing pneumococcal disease in adults. Clin Microbiol Infect. 2014;20:52–8. doi: 10.1111/1469-0691.12518. PMID:24410778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF Antibody Therapy in Rheumatoid Arthritis and the Risk of Serious Infections and Malignancies: Systematic Review and Meta-analysis of Rare Harmful Effects in Randomized Controlled Trials. JAMA. 2006;295:2275. doi: 10.1001/jama.295.19.2275. PMID:16705109. [DOI] [PubMed] [Google Scholar]
- 9.McMahan ZH, Bingham III CO. Effects of biological and non-biological immunomodulatory therapies on the immunogenicity of vaccines in patients with rheumatic diseases. Arthritis Res Ther [Internet]. 2014. [cited 2017May29]; 16. Available from: http://arthritis-research.biomedcentral.com/articles/10.1186/s13075-014-0506-0. doi: 10.1186/s13075-014-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahier J-F, Moutschen M, Van Gompel A, Van Ranst M, Louis E, Segaert S, Masson P, De Keyser F. Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatology. 2010;49:1815–27. doi: 10.1093/rheumatology/keq183. PMID:20591834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapetanovic MC, Saxne T, Sjöholm A, Truedsson L, Jönsson G, Geborek P. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology. 2006;45:106–11. doi: 10.1093/rheumatology/kei193. PMID:16287919. [DOI] [PubMed] [Google Scholar]
- 12.Kapetanovic MC, Roseman C, Jönsson G, Truedsson L, Saxne T, Geborek P. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum. 2011;63:3723–32. doi: 10.1002/art.30580. PMID:21834061. [DOI] [PubMed] [Google Scholar]
- 13.Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis: immune response to influenza and pneumococcal vaccines in ra. Arthritis Care Res. 2014;66:1016–26. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 14.Crnkic Kapetanovic M, Saxne T, Jönsson G, Truedsson L, Geborek P. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R171. doi: 10.1186/ar4358. PMID:24286269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori S, Ueki Y, Akeda Y, Hirakata N, Oribe M, Shiohira Y, Hidaka T, Oishi K. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann Rheum Dis. 2013;72:1362–6. doi: 10.1136/annrheumdis-2012-202658. PMID:23345600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Migita K, Akeda Y, Akazawa M, Tohma S, Hirano F, Ideguchi H, Kozuru H, Jiuchi Y, Matsumura R, Suematsu E, et al.. Effect of abatacept on the immunogenicity of 23-valent pneumococcal polysaccharide vaccination (PPSV23) in rheumatoid arthritis patients. Arthritis Res Ther [Internet]. 2015. [cited 2017May29]; 17. Available from: http://arthritis-research.com/content/17/1/357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, Gomez-Reino J, Soma K, Mebus C, Wilkinson B, et al.. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75:687–95. doi: 10.1136/annrheumdis-2014-207191. PMID:25795907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haut Conseil de la Santé Publique. HSCP Avis relatif aux recommandations de la vaccination pour les adultes et les enfants âgés de plus de 2 ans à risque d'infection invasive à pneumocoque. [Internet] 2013. Available from: http://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr = 355.
- 19.Varon E, Janoir C. Rapport d'acitivité 2016 - Epidémiologie 2015 [Internet]. 2016. Available from: http://cnr-pneumo.com/docs/rapports/CNRP2016.pdf.
- 20.Wernette CM, Frasch CE, Madore D, Carlone G, Goldblatt D, Plikaytis B, Benjamin W, Quataert SA, Hildreth S, Sikkema DJ, et al.. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10:514–9. PMID:12853378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabar S, Groh M, Bahuaud M, Le Guern V, Costedoat-Chalumeau N, Mathian A, Hanslik T, Guillevin L, Batteux F, Launay O. Pneumococcal vaccination in patients with systemic lupus erythematosus: A multicenter placebo-controlled randomized double-blind study. Vaccine [Internet]. 2017. [cited 2017August24]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S0264410X17310204. doi: 10.1016/j.vaccine.2017.07.094. [DOI] [PubMed] [Google Scholar]
- 22.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, De La Morena M, Kumararatne D, Harville TO, Hesterberg P, Koleilat M, et al.. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130:S1–24. doi: 10.1016/j.jaci.2012.07.002. PMID:22935624. [DOI] [PubMed] [Google Scholar]
- 23.Lopez B, Boucher A, Bahuaud M, Mortuaire G, Melliez H, Launay D, Terriou L, Wemeau-Stervinou L, Wallaert B, Faure K, et al.. Specific Polysaccharide Antibody Deficiency Revealed by Severe Bacterial Infections in Adulthood: A Report on 11 Cases. Clin Infect Dis. 2017;65:328–31. doi: 10.1093/cid/cix284. PMID:28379361. [DOI] [PubMed] [Google Scholar]
- 24.Juergens C, Patterson S, Trammel J, Greenberg D, Givon-Lavi N, Cooper D, Gurtman A, Gruber WC, Scott DA, Dagan R. Post Hoc Analysis of a Randomized Double-Blind Trial of the Correlation of Functional and Binding Antibody Responses Elicited by 13-Valent and 7-Valent Pneumococcal Conjugate Vaccines and Association with Nasopharyngeal Colonization. Clin Vaccine Immunol. 2014;21:1277–81. doi: 10.1128/CVI.00172-14. PMID:24990907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J, Bombardier C, Carmona L, van der Heijde D, Bijlsma JWJ, et al.. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68:1086–93. doi: 10.1136/ard.2008.094474. PMID:19033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapetanovic MC, Nagel J, Nordström I, Saxne T, Geborek P, Rudin A. Methotrexate reduces vaccine-specific immunoglobulin levels but not numbers of circulating antibody-producing B cells in rheumatoid arthritis after vaccination with a conjugate pneumococcal vaccine. Vaccine. 2017;35:903–8. doi: 10.1016/j.vaccine.2016.12.068. PMID:28081972. [DOI] [PubMed] [Google Scholar]
- 27.Kapetanovic MC, Roseman C, Jönsson G, Truedsson L. Heptavalent pneumococcal conjugate vaccine elicits similar antibody response as standard 23-valent polysaccharide vaccine in adult patients with RA treated with immunomodulating drugs. Clin Rheumatol. 2011;30:1555–61. doi: 10.1007/s10067-011-1856-5. PMID:21956234. [DOI] [PubMed] [Google Scholar]
- 28.Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. The Immunogenicity of 7‐Valent Pneumococcal Conjugate Vaccine versus 23‐Valent Polysaccharide Vaccine in Adults Aged 50–80 Years. Clin Infect Dis. 2009;49:1318–25. doi: 10.1086/606046. PMID:19814624. [DOI] [PubMed] [Google Scholar]
- 29.Ridda I, MacIntyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, McIntyre PB. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27:1628–36. doi: 10.1016/j.vaccine.2008.11.098. PMID:19100304. [DOI] [PubMed] [Google Scholar]
- 30.Melmed GY, Agarwal N, Frenck RW, Ippoliti AF, Ibanez P, Papadakis KA, Simpson P, Barolet-Garcia C, Ward J, Targan SR, et al.. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–54. doi: 10.1038/ajg.2009.523. PMID:19755964. [DOI] [PubMed] [Google Scholar]
- 31.Sinisalo M, Vilpo J, Itälä M, Väkeväinen M, Taurio J, Aittoniemi J. Antibody response to 7-valent conjugated pneumococcal vaccine in patients with chronic lymphocytic leukaemia. Vaccine. 2007;26:82–7. doi: 10.1016/j.vaccine.2007.10.053. PMID:18053620. [DOI] [PubMed] [Google Scholar]
- 32.Crum‐Cianflone NF, Huppler Hullsiek K, Roediger M, Ganesan A, Patel S, Landrum ML, Weintrob A, Agan BK, Medina S, Rahkola J, et al.. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among hiv‐infected adults. J Infect Dis. 2010;202:1114–25. doi: 10.1086/656147. PMID:20795819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Linden MPM, Batstra MR, Bakker-Jonges LE, Foundation for Quality Medical Laboratory Diagnostics, Detert J, Bastian H, Scherer HU, Toes REM, Burmester G-R, Mjaavatten MD, et al.. Toward a data-driven evaluation of the 2010 American College of Rheumatology/European League Against Rheumatism criteria for rheumatoid arthritis: Is it sensible to look at levels of rheumatoid factor? Arthritis Rheum. 2011;63:1190–9. doi: 10.1002/art.30200. PMID:21538311. [DOI] [PubMed] [Google Scholar]
- 34.Lamerato L, Price K, Szymialis R, Eaddy M, Ogbonnaya A, Shih H-C, Ahmad H. Comparative evaluation of treatment patterns and healthcare utilization of newly-diagnosed rheumatoid arthritis patients by anti-cyclic citrullinated peptide antibody status. J Med Econ. 2018;21(3):231–240. doi: 10.1080/13696998.2017.1391819. PMID:29027497. [DOI] [PubMed] [Google Scholar]
- 35.Bjarnarson SP, Benonisson H, Del Giudice G, Jonsdottir I. Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness. PLoS ONE. 2013;8:e72588. doi: 10.1371/journal.pone.0072588. PMID:24069152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clutterbuck EA, Lazarus R, Yu L-M, Bowman J, Bateman EAL, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al.. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific b cells. J Infect Dis. 2012;205:1408–16. doi: 10.1093/infdis/jis212. PMID:22457293. [DOI] [PMC free article] [PubMed] [Google Scholar]


