ABSTRACT
Introduction: China has used 3 different mumps-containing vaccines (MuCV) since 1990: monovalent mumps vaccine, measles–mumps (MM) vaccine, and measles–mumps–rubella (MMR) vaccine, and one dose MuCV (using MMR at 18 months) has been included in the EPI since 2007. MuCV effectiveness has been of concern following large-scale mumps outbreaks. In 2015, an outbreak of mumps occurred in a primary school, which allow us assess vaccine effectiveness of different MuCVs.
Method: All children in the school were studied as a retrospective cohort. Vaccination histories and case information were obtained from vaccination records and clinic/hospital logs. Parental questionnaires were used to confirm students' illnesses and calculate attack rate (AR). VE was assessed using the formula, VE = (AR in unvaccinated students- AR in the vaccinated students) / (AR in unvaccinated students). VEs of different type of MuCV were compared.
Results: In total, 283 students were identified as clinical mumps among the 2370 students, and 1908 students were included for MuCV VE assessment. 213 (including 21 [8.9%] patients) were 2-dose MuCV recipients (AR: 9.9%), 1165 (including 123 [51.9%] patients) were 1-dose recipients (AR: 10.6%), and 530 (including 93 [39.2%] patients) were unvaccinated (AR: 17.5%). VE was 44% for 2 doses and 40% for one dose. For one-MuCV-dose students, estimated mumps VE was 63% for vaccinated within 3 years (between vaccination and this outbreak); 50% for vaccinated within 3 to 5 years; and 34% for vaccinated more than 5 years. Comparing VE by vaccine type and 5-year interval since vaccination, VE for MMR was 60%, which was consistently higher than VE for monovalent mumps vaccine (22%) and MM (2%).
Conclusion: This outbreak was associated with low and declining 1-dose MuCV effectiveness. China's immunization program should evaluate the potential of a 2-dose MMR schedule to adequately control mumps.
KEYWORDS: Mumps, vaccine, effectiveness, schedule, China
Introduction
Mumps is an infectious disease caused by mumps virus that usually presents with fever and parotid gland swelling; vaccination with mumps vaccine (MuV) the most effective way to prevent mumps. In 1977, the United States Centers for Disease Control and Prevention recommended MuV for routine immunization of children, and after 12 years, the number of reported cases had declined by over 99%.1-3 In 1990, the China Food and Drug Administration licensed a live, attenuated mumps vaccine that was made using the S79 strain of mumps vaccine virus, which had been derived through further attenuation of the Jeryl Lynn strain used in the U.S.-licensed vaccine.4
Since 1990, China has used 3 different mumps-containing vaccines (MuCV): monovalent mumps vaccine, measles–mumps (MM) vaccine, and measles–mumps–rubella (MMR) vaccine. However, mumps vaccines were not included in the Expanded Program on Immunization (EPI), implying that parents had to pay out-of-pocket for mumps vaccine. In 2007, China's EPI system replaced monovalent measles vaccine with MMR for the second routine dose of measles vaccine, targeting 18–24 months old children. Since the first dose of measles-containing vaccine is given as measles-rubella vaccine, the EPI system supports a 1-dose mumps vaccination strategy.5 Despite introduction into EPI for routine use and the rapid attainment of high MMR vaccination coverage levels, over 479,518 mumps cases were reported in 2012, with incidence rates as high as 35.6/100,000 and most outbreaks occurring among school-age children.6
The effectiveness of a vaccine (VE) is critically important for developing and monitoring an immunization strategy. Mumps VE assessments have varied widely in China,7 and the VEs of different MuCVs have been of concern following large-scale use of 3 different MuCVs. In 2015, an outbreak of mumps occurred in a primary school in Anhui province in which different MuCVs had been in use. In September 2015, we conducted a retrospective investigation to evaluate the effectiveness of different MuCVs, and to provide evidence for mumps vaccination strategy in China. We report the results of our investigation and our program recommendations.
Results
Outbreak description
In total, 283 clinical mumps cases were identified in this outbreak; all cases were among students, 6–15 years of age, none of whom had a history of having mumps disease. Illness onsets were between December 1, 2014 and September 20, 2015. The overall attack rate was 10.50% (283 of 2,695 students). Clinical features were able to be documented in 272 (96.11%) cases, and among these cases, all had parotid or other salivary gland swelling and pain (by case definition); and 61.76% had a temperature ≥38C. Other symptoms were rare and included one child with orchitis, one child with pancreatitis, and one child with hearing loss.
Cases were distributed in 33 (97.06%) of the 34 school classes. There was no difference in attack rate between male (11.47%) and female students (9.41%) (χ2 = 3.0325, P = 0.0816). Isolation (school exclusion) was implemented for 198 (70.32%) cases. The shortest isolation period was 2 days; 65.06% were isolated for less than 9 days.
The epidemic curve showed a small peak in January – before winter vacation. When school resumed the case count increased and peaked in July. Among the cases, 237 (83.75%) provided vaccination certificates; 122 (43.11%) were confirmed to have received 1 dose of MuCV; and 22 (7.77%) had 2 doses of MuCV. No outbreak response immunization was conducted. Figure 1 shows the epidemic curve by the number of MuCV doses administered.
Figure 1.
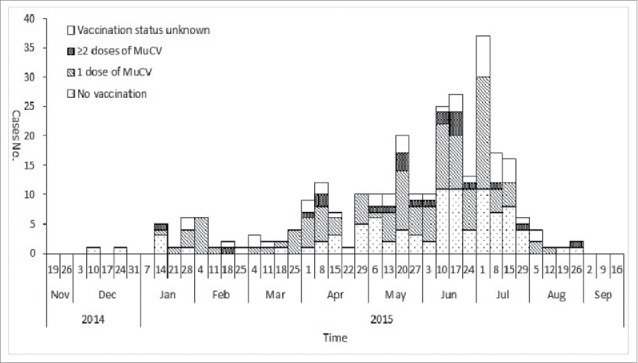
Distribution of mumps cases by onset date in a mumps outbreak in Anhui province, China, 2014–2015 (n = 283).
Vaccine effectiveness
We included 2303 (97.17%) students in Grade 1 through Grade 5 in the VE determination. Among these students, 114 were excluded because they had a history of mumps illness, and 281 students were excluded because of unknown immunization history. We categorized the remaining 1908 students into vaccinated and unvaccinated groups based on vaccination certificate review (Fig. 2). Among these students, the overall coverage was 72% (1378 of 1908).
Figure 2.
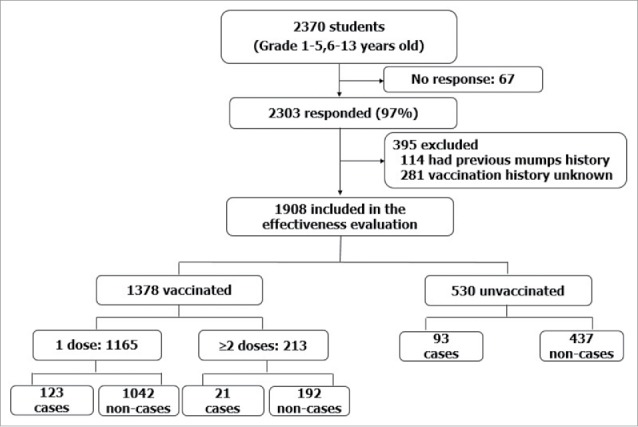
Inclusion, exclusion, and vaccination status of subjects in mumps vaccine effectiveness assessment.
The attack rate (AR) among unvaccinated students (17.5%) was higher than among the one-MuCV-dose group (10.6%) and the ≥2-dose group (9.9%). The estimated VE for one dose of MuCV was 40% (95% CI: 23%-53%), and the estimated VE for ≥2 dose MuCV was 44% (95% CI: 12%-64%). There was no statistically significant difference (χ2 = 0.09, P > 0.05) between 1- and 2- dose recipients. (Table 1.)
Table 1.
MuCV effectiveness in a mumps outbreak in Anhui province, China, 2014–2015.
| Vaccination status | cases | Exposed persons | AR (%) | RR* (95%CI) | VE (%,95%CI) |
|---|---|---|---|---|---|
| Un-vaccinated | 93 | 530 | 17.5 | Ref | Ref |
| 1 dose | 123 | 1165 | 10.6 | 0.60(0.47,0.77) | 40(23,53) |
| ≥ 2 doses | 21 | 213 | 9.9 | 0.56(0.36,0.88) | 44(12,64) |
RR: relative risk
The one-MuCV-dose students were categorized into 3 groups by the interval, in years, between vaccination and the start of the outbreak. Estimated mumps VE for children vaccinated within 3 years of the outbreak was 63% (95% CI: 0%-88%); VE for children vaccinated within 3 to 5 years of the outbreak was 50% (95% CI: 26%-66%); and VE for children vaccinated more than 5 years prior to the outbreak was 34% (95% CI: 14%-50%). (Table 2.)
Table 2.
MuCV effectiveness by time since vaccination among students receiving 1 MuCV dose prior to a mumps outbreak in Anhui province, China, 2014–2015.
| Years since vaccination | Cases | Exposed persons | AR (%) | RR (95%CI) | VE (%,95%CI) |
|---|---|---|---|---|---|
| Un-vaccinated | 93 | 530 | 17.5 | Ref | Ref |
| <3 years | 3 | 46 | 6.5 | 0.37(0.12,1.13) | 63(0,88) |
| 3-5 years | 30 | 339 | 8.8 | 0.50(0.34,0.74) | 50(26,66) |
| >5 years | 90 | 780 | 11.5 | 0.66(0.50,0.86) | 34(14,50) |
Among the 1165 one-MuCV-dose students, 386 received monovalent mumps vaccine, 115 received MR, and 664 received MMR. For monovalent mumps vaccine, VE was 20% (95% CI: 0–41%); for MM vaccine, VE was 1% (95% CI: 0–36%); and for MMR, VE was 58% (95% CI: 42%-70%). Because VE varied by the number of years since vaccination, we calculated VE by vaccine type and interval since vaccination. VE for MMR administered more than 5 years before the outbreak was 60% (95% CI: 38%-75%), which was consistently higher than VE for monovalent mumps vaccine (VE = 22%, 95% CI: 0–43%) and MM vaccine (VE = 2%, 95% CI: 0–38%) administered at least 5 years earlier. (Table 3.)
Table 3.
MuCV effectiveness by vaccine type and interval since vaccination among 1 dose MuCV students in a mumps outbreak in Anhui province, China, 2014–2015.
| Vaccine type | Years since vaccination | Cases | Exposed persons | AR (%) | RR (95%CI) | VE (%,95%CI) |
|---|---|---|---|---|---|---|
| Un-vaccinated | 93 | 530 | 17.5 | Ref | Ref | |
| Monovalent mumps vaccine | ≤5 years | 3 | 11 | 17.3 | 1.55(0.58,4.15) | 0(-315,-42) |
| >5 years | 51 | 375 | 13.6 | 0.78(0.57,1.06) | 22(0,43) | |
| MM | ≤5 years | 2 | 11 | 18.2 | 1.04(0.29,3.68) | 0(0,71) |
| >5 years | 18 | 104 | 17.3 | 0.98(0.62,1.56) | 2(0,38) | |
| MMR | ≤5 years | 28 | 363 | 7.7 | 0.44(0.29,0.65) | 56(35,71) |
| >5 years | 21 | 301 | 7.0 | 0.40(0.25,0.62) | 60(38,75) |
Discussion
We have shown that in this outbreak setting, mumps-containing vaccine effectiveness was low, and varied by interval since vaccination and type of vaccine (monovalent mumps, MM, and MMR vaccines). VE was highest (56% to 60%) for MMR vaccine regardless of interval since vaccination, but VE was insignificant for MM and monovalent mumps vaccine.
The only outbreak control measure that was taken for this outbreak was case isolation by exclusion from school. No outbreak response immunization was conducted, which allowed the calculation of vaccine effectiveness. Overall mumps vaccination coverage was modest at 72%; half of the cases were documented to have received MuCV at least 2 weeks prior to the outbreak. Modest MuCV vaccination coverage with a partially effective vaccine enabled the outbreak to occur.
Seroconversion, efficacy, and effectiveness measure different aspects of the ability of a vaccine to protect a child. Mumps seroconversion rates, measured by enzyme-linked immunosorbent assay, after 1 dose of MMR II (the vaccine used in the U.S.) were consistently high (97.7-100%) in studies conducted between 1988 and 2009.8 We found differences by vaccine types and years since vaccination in this outbreak investigation, which is consistent with literature showing waning immunity to mumps vaccines.9
The VE of mumps vaccines has been shown to be variable. Field studies showed VE estimates (62%–85%)10 to be lower than vaccine efficacy in clinical trials. North American and some European countries have reported the effectiveness of Jeryl Lynn strain mumps vaccine in their countries to be 79% (62%-91%).11 Systematic reviews of Jeryl Lynn strain MMR vaccine have shown ≥1 dose effectiveness of 69–81% at preventing clinical illness among children and adolescents.12
A meta-analysis of vaccine effectiveness of mumps-containing in China showed that the overall VE for MuCV (either one dose or two doses) was 85% (95% CI: 76–90%) for cohort studies, but that VE was lower in school (69%) than in day care (88%) settings, possibly related to a shorter interval since vaccination.7 MuCV effectiveness in our study was similar to monovalent mumps vaccine effectiveness (49%) measured in Jiangsu, China.13 In another case-control study in Guangzhou, China, the overall VE for 1 dose of mumps vaccine, irrespective of the manufacture, was 53.6% (95% CI: 41.0-63.5%) among children aged 8 months to 12 years from 2006 to 2012.14
Although some of the VE confidence intervals in our study were wide due to relative small sample sizes, the point estimates suggest some additional questions about these vaccines. Mumps VE for MMR vaccine, at 58%, appears better than mumps VE for monovalent mumps and MM vaccines at 1% to 20%. These differences in VE may be related to differences in vaccine viral titers. The “China Requirements for Biological Products” states that the mumps viral titer for mumps vaccines must at least 3.7 lg CCID50/dose.15 The mumps vaccine virus titer in monovalent mumps vaccine and MM were standardized at 3.7 lg CCID50/dose, but the Chinese MMR standardized the mumps virus titer at 4.3 lg CCID50/dose in order to reduce potential interference of mumps vaccine virus with measles and rubella vaccine viruses. The relation between mumps vaccine virus titer and effectiveness should be explored further.
We also showed that MuCV effectiveness decreased with increasing intervals since vaccination, a finding consistent with studies showing waning immunity. Effectiveness of 1 dose of mumps vaccine was shown to decline from 96% (95% CI: 81%–99%) among 2-year-olds to 66% (95% CI: 30%–83%) among 11- to 12-year-olds. Effectiveness of 2 doses declined from 99% (95% CI: 97%–99.5%) among 5- to 6-year-olds to 86% (95% CI: 74%–93%) among 11- to 12-year-olds (p<0.001 for 1 or 2 doses).10 The lower vaccine effectiveness we observed may be related to the type of MuCV the students received. We showed that MMR VE was greater than VEs of monovalent mumps vaccine and MM vaccine, and we found that 93.24% of students vaccinated >5 years received monovalent mumps vaccine or MM, while 94.2% of students vaccinated ≤5 years received MMR.
Limitations
Our study has several limitations. This was a retrospective study of an outbreak, and as such, additional laboratory testing to determine new infections was not possible and recall bias cannot be controlled for. An estimated 20–30% of mumps infections maybe asymptomatic,16,17 and it was not possible to identify asymptomatic cases. All cases were diagnosed by clinical symptoms without laboratory confirmation.
Immunization status was unknown for 281 subjects (12.8%) in this study. Classification of vaccinated or unvaccinated persons into the unknown group can influence VE estimates. However, the impact of the unknown group is relative small in our study. If cases for whose vaccination status was unknown were all vaccinated, the estimates of vaccine effectiveness would be lower. For example, the vaccine effectiveness for 1 dose would decrease from 40% to 37% if all cases with unknown vaccine status were unvaccinated. For ≥2 doses VE would decrease from 44% to 42%.
Due to small numbers of students receiving 2 MuCV doses, and to the non-experimental nature of the investigation, it was not possible to determine the relative effectiveness of various 2-dose schedules.
Program implications
We have provided additional data to be considered in refinement of China's mumps control and prevention policy. The low VE seen in monovalent mumps and MM vaccines suggests the need to further evaluate these vaccines for continued inclusion in the EPI system as the vaccines are currently constituted. It is unlikely that we will be able to adequately control mumps with the current vaccines and the 1-dose MuCV vaccination policy. As of 2014, 121 countries have introduced MMR into their national immunization programs.18 Most countries use, and WHO recommends, a 2-dose MuCV policy. One consideration for China's EPI system is to use 2 doses of MMR vaccine instead of one dose of measles-rubella vaccine and one dose of MMR vaccine. We think that additional studies are necessary to improve mumps immunization strategy, and that these studies need to include aspects of the vaccines and the vaccination schedule.
Methods
Setting
The school with the outbreak was a primary school with 34 classes in grades 1 through 5 that had 2,695 students enrolled. All students were day students; the school did not have a cafeteria, and students ate lunch at home. The school had 1 part-time doctor. In 2015, the school was on winter vacation from February 11 through March 4, and summer vacation was July 1 through August 31.
Case definition
We defined a mumps case as a student having unilateral or bilateral parotid or other salivary gland swelling and pain, lasting 2 or more days, with onset between December 1, 2014 and September 20, 2015. All cases were diagnosed by clinical criteria without laboratory confirmation, and no mumps virus genotype information was obtained during this outbreak investigation.
Case finding
To identify cases, investigators reviewed medical records in school clinics and absentee records kept by teachers. We surveyed parents of all students with a standardized, written questionnaire to determine demographic characteristics, MuCV vaccination and mumps illness history, and information on symptoms and isolation if the student was a case in the outbreak. Parents were interviewed by telephone to obtain information that was missing or unclear in the written questionnaire.
Vaccination status
Students’ vaccination certificates were obtained during the field investigation. MuCV vaccination histories were built with vaccination certificates kept by parents and verified with vaccination records kept by immunization clinics. Vaccination histories included numbers of doses, types of MuCV, and dates of vaccination. We considered the vaccination history as unknown if no vaccination record was available. To be considered vaccinated, a student had to have an official-certificate-verified vaccination.
Statistical analyses
We used a retrospective cohort method to compare the attack rate (AR) of vaccinated students with the AR of unvaccinated students, and we estimated MuCV effectiveness using the formula:
Students with mumps illness history prior to this outbreak and students with unknown vaccination history were excluded. Students with mumps onset within two weeks of vaccination were considered unvaccinated.
Data were collected using Microsoft Office Excel (version 2010) and were analyzed using SPSS for Windows, version 17.0 (SPSS Inc., USA). Differences between proportions were calculated using Chi-square tests. All comparisons were 2-tailed, and a p-value <0.05 was considered significant.
Ethical considerations
This investigation was approved by the Ethical Review Committee of Chinese Center for Disease Control and Prevention (Approval Notice No. 201502), recognizing that the rights and the welfare of the subjects are adequately protected and that the potential risks are outweighed by the potential benefits of the investigation. In the written questionnaire for parents of the students, we informed parents of the objectives of this study, and obtained written consent prior to interview. Individual identifying data were not retained in analytic data sets.
Funding Statement
Funding to support the activities described in this article came from The Project Sponsored by the Young Scholar Scientific Research Foundation of China CDC (Project No.: 2015A103).
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank the staff at county, prefecture, and province level Centers for Disease Control and Prevention in Anhui Province for their assistance with this investigation. We also acknowledge Dr. Lance Rodewald for his review on this manuscript.
Authors' contributions
Dr. Chao Ma, Lixin Hao, and Yan Liu designed the study and directed its implementation, including quality assurance and control. Dr. Haimei Jia, Jihai Tang, Ying Su, and Wei Qin played important role in the field investigation. Chao Ma and Yan Liu, Haimei Jia designed and implemented the study's analytic strategy. Dr. Huaqing Wang helped supervise the field activities and helped review the manuscript. Chao Ma and Yan Liu lead this work and they contributed equally to this work.
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Chinese Center for Disease Control and Prevention.
References
- 1.Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, Hunt K, Finley CG, Leschinsky DP, O'Keefe AL. Recent resurgence of mumps in the United States. N Engl J Med. 2008;358:1580–9. doi: 10.1056/NEJMoa0706589. PMID:18403766 [DOI] [PubMed] [Google Scholar]
- 2.CDC Impact of vaccines universally recommended for children–United States, 1990–1998. MMWR. 1999;48:243–8. [PubMed] [Google Scholar]
- 3.CDC Current Trends Mumps – United States, 1985–1988. MMWR. 1989;38:101–5. PMID:2915643 [PubMed] [Google Scholar]
- 4.Wenyuan Z, Haiping C, Jiping S. Immunoprophylaxis of infectious disease. People's Medical Publishing House, Beijing. 2010:175. [Google Scholar]
- 5.Ma C, An Z, Hao L, Cairns KL, Zhang Y, Ma J, Cao L, Wen N, Xu W, Liang X. Progress toward measles elimination in the People's Republic of China, 2000–2009. J Infect Dis. 2011;204 Suppl 1:S447–54. doi: 10.1093/infdis/jir103. PMID:21666198 [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Hao L, Wang H. Analysis on epidemiological characteristic of mumps in China, 2010–2012. Chinese Journal of Vaccines and Immunization. 2014;20:127–31. [Google Scholar]
- 7.Wang H, Hu Y, Zhang G, Zheng J, Li L, An Z. Meta-analysis of vaccine effectiveness of mumps-containing vaccine under different immunization srategies in China. Vaccine. 2014;32:4806–12. doi: 10.1016/j.vaccine.2014.05.061. PMID:25000591 [DOI] [PubMed] [Google Scholar]
- 8.Kuter BJ, Brown M, Wiedmann RT, Hartzel J, Musey L. Safety and immunogenicity of M-M-RII (Combination Measles-Mumps-Rubella Vaccine) in clinical trials of healthy children conducted between 1988 and 2009. Pediatr Infect Dis J. 2016(35):1011–20. [DOI] [PubMed] [Google Scholar]
- 9.Man W, Jin-Kou Z, Tao W, Li-Xin H, Chao M, Qi-Ru S, Hui-Ming L. Mumps-containing vaccine effectiveness during outbreaks in two schools in Guangdong, China. 2012 Western Pac Surveill Response. 2012;3:29–32. doi: 10.5365/wpsar.2012.3.4.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, Brown D, Ramsay ME. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis. 2007;13:12–7. doi: 10.3201/eid1301.060649. PMID:17370510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO The Immunological Basis for Immunization Series – Mumps. [http://www.who.int/immunization/documents/WHO_IVB_ISBN9789241500661/eng.pdf] 2010.
- 12.Demicheli V, Rivetti A, Debalini MG, Di Pietrantonj C. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev. 2012:CD004407. PMID:22336803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Ma F, Zhang L, Wang Z, Tao H. Study on the epidemiological effect of live attenuated mumps vaccine. Mod Prev Med. 2010;37:4322–3. [Google Scholar]
- 14.Fu C, Xu J, Cai Y, He Q, Zhang C, Chen J, Dong Z, Hu W, Wang H, Zhu W. Effectiveness of one dose of mumps vaccine against clinically diagnosed mumps in Guangzhou, China, 2006–2012. Hum Vaccin Immunother. 2013;9:2524–8. doi: 10.4161/hv.26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biological standardization committee of the Ministry of Health, China requirements of Biological Products. Beijing: China Population Publishing House; 2010:18. [Google Scholar]
- 16.CDC Manual for the surveillance of vaccine-preventable diseases. Atlanta, GA: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 17.Centers for Disease C, Prevention Mumps epidemic–Iowa, 2006. MMWR Morb Mortal Wkly Rep. 2006;55:366–8. PMID:16601665 [PubMed] [Google Scholar]
- 18.Ma C, Hao L, Qiru S, Wen N, Fan C. Vaccination Schedules, Reported Vaccination Coverage Rates, and Incidences of Measles, Mumps, and Rubella of the 194 Member States of the World Health Organization. 2015;21(3):251–247. Chinese Journal of Vaccines and Immunization 2015; 21:7. [Google Scholar]


