ABSTRACT
Patients with chronic lymphocytic leukemia (CLL) are at a high risk for infections caused by Streptococcus pneumoniae. A pneumococcal conjugate vaccine (PCV) can induce a significant antibody response for some CLL patients. In this study we investigated antibody persistence after PCV7 in patients with CLL. The study material comprised 24 patients with CLL and 8 immunocompetent controls. The median antibody concentrations five years after PCV7 were lower for six pneumococcal serotypes in patients with CLL compared to controls, but the difference was not statistically significant. Depending on the serotype, the percentage of the CLL patients with antibody levels suggested to provide protection against invasive pneumococcal disease (IPD) varied from 29 to 71% five years after vaccination. This data suggests that PCV could result in antibody persistence at least five years in CLL patients.
KEYWORDS: antibody persistence, chronic lymphocytic leukemia, pneumococcal conjugate vaccine
Chronic lymphocytic leukemia (CLL), a mature B-cell neoplasm, is the most common type of leukemia in adult Caucasians.1 CLL is associated with a significant dysfunction of the immune system that results in both quantitative and qualitative defects in innate and adaptive immune responses.2 While hypogammaglobulinemia can occur even in the early stage of disease, it usually becomes more severe during the course of the disease and at more advanced stages.3 Infections are the most common cause of mortality in CLL patients. The majority of infections are bacterial, caused by common organisms, including Streptococcus pneumoniae, Staphylococcus aureus and Haemophilus influenzae.4
Previous studies with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in patients with CLL demonstrated no antibody responses at all or only weak responses.5-8 In contrast, the immunogenicity of pneumococcal conjugate vaccine (PCV) in patients with CLL has been shown in a few earlier studies. A single dose of 7-valent pneumococcal conjugate vaccine (PCV7) given at an early stage of the CLL resulted in a significant response in almost 40% of patients.9 Furthermore, 13-valent pneumococcal conjugate vaccine (PCV13) induced at least a two-fold increase in antibody titers from baseline in 58% of previously untreated CLL patients, but an antibody concentration of 0.35 μg/ml was not used as a serological threshold for adequate response.10 Many current international guidelines recommend PCV13 for immunocompromised patients. The persistence of pneumococcal antibodies in patients with CLL has yet to be studied.
In this follow-up study, pneumococcal antibody persistence was assessed in CLL patients and immunocompetent controls at five years after one dose of PCV7 given as part of an earlier response study.9
The study population comprised 24 patients with CLL (12 males and 12 females), with a median age of 64 years (range 47–86 years) from Tampere and Turku University Hospitals. The control population comprised 8 subjects (median age 67 years, range 57–82 years, 4 males and 4 females) without any known immunological or hematological defects from Tampere University Hospital. The patients and control subjects had participated in an earlier pneumococcal vaccine response study with PCV7.9
An informed consent to participate was obtained from all patients and controls. The study was approved by the ethical board of the Pirkanmaa Hospital District and the trial was registered at http://ClinicalTrials.gov (NCT00919321).
Clinical and laboratory characteristics of the CLL patients are shown in Table 1. The disease status according to Binet classification was A (early stage of the disease) in 16, B (intermediate) in 2, and C (advanced stage) in 6 patients. A total of 16 patients had never been treated for CLL. Seven patients had suffered from severe infections (needing intravenous antibiotics or hospitalization) and six patients from mild to moderate infections (treated with oral antibiotics) during the five years since PCV7 vaccination. Only one of these infections was pneumococcal infection, i.e. pneumococcal septicemia. Hypogammaglobulinemia (S-IgG <6.77 g/l) was detected in 11 patients.
Table 1.
Clinical and laboratory characteristics of the patients with CLL.
| Character | Patients with CLL (N = 24) |
|---|---|
| Sex M/F | 12/12 |
| Age (years) | 64 (47–86) |
| Binet A/B/C | 16/2/6 |
| Past or ongoing therapy | 8 (33%) |
| Lymphocyte count (x109/l) | 24.3 (0.9–140.0) |
| Platelet count (x109/l) | 141 (38–372) |
| Hemoglobin (g/l) | 135 (81–153) |
| Neutrophil count (x109/l) | 4.0 (0.6–12.1) |
| IgG (g/l) | 7.4 (3.2–12.5) |
| IgM (g/l) | 0.3 (0.1–5.4) |
| IgA (g/l) | 0.7 (0.2–4.5) |
The concentrations of serum IgG antibody against pneumococcal capsular polysaccharides were measured by a modification of the 22F inhibition enzyme immunoassay (EIA) method as previously described.11 An antibody concentration of 0.35 μg/ml was considered as a threshold for protection against invasive pneumococcal disease (IPD), as recommended by World Health Organization WHO.12 A comparison of antibody concentrations between CLL patients and controls was performed with Fisher´s exact test.
Pneumococcal antibody concentrations four weeks and five years after the administration of PCV7 are shown in Table 2. In the CLL patients, median antibody concentrations against pneumococcal serotypes 4, 6B, 18C and 19F after five years were approximately 50% lower than those measured four weeks after vaccination. Furthermore, antibody concentrations against serotypes 9V and 23F were approximately 75% and 65% lower five years after vaccination, respectively. In contrast, the median concentration of the antibody against serotype 14 remained at a similar level over the five-year period following the vaccination. No statistically significant differences were seen in antibody concentrations between CLL patients and controls five years from vaccination. The pneumococcal antibody concentrations in controls declined by more than 50% in each serotype group. The median concentration of the antibody against serotype 9V was 95% lower in controls, as measured five years after the administration of PCV7.
Table 2.
Antibody concentrations against pneumococcal antigens of 7-valent conjugate vaccine four weeks and five years after vaccination in patients with CLL and in controls.
| Post-PCV antibody | 5 yrs post-PCV antibody | ||||
|---|---|---|---|---|---|
| level median (quartiles) |
level median (quartiles) |
||||
| Serotype | CLL | Control | CLL | Control | P-value1 |
| 4 | 0.30 (0.07–1.22) | 1.87 (0.43–6.16) | 0.15 (0.02–0.40) | 0.52 (0.13–0.90) | 0.116 |
| 6B | 0.55 (0.20–1.82) | 0.95 (0.23–14.7) | 0.29 (0.09–0.94) | 0.39 (0.06–1.24) | 1.000 |
| 9V | 1.40 (0.25–7.12) | 18.0 (0.34–63.2) | 0.33 (0.15–2.33) | 0.97 (0.17–5.06) | 0.685 |
| 14 | 0.72 (0.31–2.66) | 14.7 (1.01–20.5) | 0.73 (0.20–4.01) | 2.71 (0.45–5.83) | 0.676 |
| 18C | 1.55 (0.81–5.99) | 9.76 (4.65–81.1) | 0.73 (0.23–3.06) | 1.42 (1.00–2.47) | 0.642 |
| 19F | 2.01 (0.61–9.31) | 3.26 (1.20–51.1) | 1.07 (0.26–2.88) | 0.69 (0.31–1.56) | 1.000 |
| 23F | 1.53 (0.66–13.4) | 5.11 (0.98–23.0) | 0.51 (0.17–1.56) | 1.17 (0.11–2.23) | 1.000 |
Fisher´s exact test.
The percentages of subjects whose antibody concentrations were at a level suggested to be protective against IPD five years after the administration of PCV7 are shown in Fig. 1. In patients with CLL, these percentages varied from 29 to 71%, with the lowest proportion found against serotype 4 and the highest against serotypes 18C and 19F. In controls, the corresponding percentages ranged 50- to 87.5%, with the lowest proportion being against serotype 6B and the highest against serotype 18C.
Figure 1.
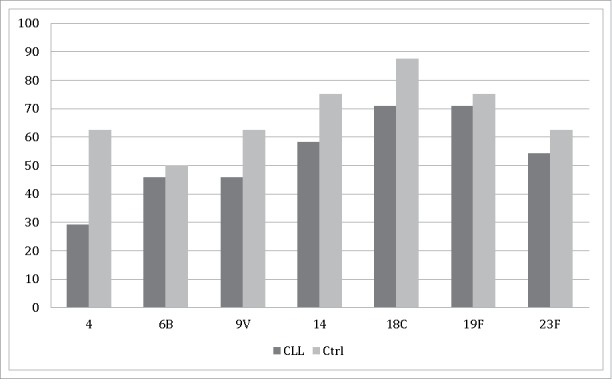
The proportions of antibody concentrations suggestive of protection (≥0.35 μg/ml) against pneumococcal antigens of 7-valent pneumococcal conjugate vaccine in patients with CLL and in controls five years after vaccine administration.
The baseline proportions of suggested protective antibody concentrations before vaccination in patients with CLL ranged 8- to 88%. The lowest baseline proportion was against serotype 4 and the highest against serotype 19F. In controls, the same percentages were 0–100%, with no protective concentrations found against serotype 4. Protective antibody concentrations against serotype 18C were observed in all controls before vaccination.9
There is no earlier data available concerning pneumococcal antibody persistence in patients with CLL. In HIV-infected children on HAART, protective antibody concentrations of ≥0.5 μg/ml persisted for longer than four years for serotypes 6B and 14 after PCV7-PCV7-PPV23 administration.13 In adults of 50 years of age and older, antibody concentrations declined over a five-year period following the administration of PCV13 for all 13 serotypes but remained higher than the levels before vaccination, except for serotype 3.14 Furthermore, in 40% of allogeneic stem cell transplant patients, who received three doses of PCV7 and one dose of PPV23 after transplantation, antibody concentrations remained at a protective level (≥0.50 μg/ml) for 8–11 years.15
In our data antibody concentrations in patients with CLL declined during the five years following a single dose of PCV7 for six serotypes and for all serotypes in healthy controls. The number of subjects was quite small, but no statistically significant difference was seen between these two groups in median antibody concentrations. There was, however, a trend toward lower antibody concentrations in CLL patients compared to controls five years after PCV7 administration for all serotypes except for serotype 19F, which was observed in higher concentrations in the patients. Currently recommended PCV13 contains six other serotypes (1, 3, 5, 6A, 7F, 19A), which are not included in PCV7. Antibody persistence in these additional serotypes remains still unclear and warrants further studies.
More than half of the CLL patients remained their antibody concentrations at protective levels for four out of seven serotypes five years after PCV administration, although the median antibody concentrations declined. Most of the patients had never been treated for CLL, which may have some impact on antibody persistence. Also, the relatively low rates of hypogammaglobulinemia and the low number of severe pneumococcal infections may contribute to better persistence of protection. Although the size of the study population was small, this follow-up data reflects the trend of the antibody persistence five years after PCV7 in patients with CLL.
Earlier data had shown a significant increase in antibody concentrations for all seven antigens in both groups after PCV7 administration.9 In this follow-up study, the median antibody concentrations in CLL patients at five years after vaccination, as compared with the baseline levels before vaccination, varied depending on the serotype. The post-vaccination antibody concentrations for serotypes 4 and 14 were 1.6- and 1.3 –times higher than the baseline, respectively. In contrast, for serotypes 6B and 18C, the median antibody concentrations declined by almost 20% during the five-year follow-up. Antibody concentrations for the other three serotypes declined to baseline levels. Whether antibody concentrations continue to decline after five years of follow-up remains unclear and warrants a longer follow-up and further studies.
In our earlier data, serotypes 14 and 6B were the most common serotypes causing IPD among patients with hematological malignancy, including CLL. Our finding of favorable persistence for serotype 14 emphasizes the importance of PCV administration for CLL patients in the early stages of the disease, whereas the decline below baseline seen for serotype 6B during the follow-up period may point to a need for a booster dose of PCV or PSV five years after the primary vaccination.
In conclusion, median antibody concentrations in patients with CLL five years after PCV7 administration varied depending on the serotype as compared to controls, and there was a trend toward lower antibody concentrations for six serotypes of PCV7. However, antibody concentrations remained at a level considered to be protective against IPD for four serotypes in more than 50% of the CLL patients. Our findings suggest that PCV given at an early stage of CLL could result in antibody persistence lasting at least five years. The effectiveness and schedule of possible booster immunization needs to be established in future studies.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Linet MS, Schubauer-Berigan MK, Weisenburger DD, Richardson DB, Landgren O, Blair A, Silver S, Field RW, Caldwell G, Hatch M, et al.. Chronic lymphocytic leukaemia: An overview of aetiology in light of recent developments in classification and pathogenesis. Br J Haematol. 2007;139(5):672–686. doi: 10.1111/j.1365-2141.2007.06847.x. PMID:18021081. [DOI] [PubMed] [Google Scholar]
- 2.Dearden C. Disease-specific complications of chronic lymphocytic leukemia. ASH Education Program Book. 2008;2008:450–456. doi: 10.1182/asheducation-2008.1.450. PMID:19074125. [DOI] [PubMed] [Google Scholar]
- 3.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–581. doi: 10.1182/blood-2015-03-567388. PMID:26084672. [DOI] [PubMed] [Google Scholar]
- 4.Morrison VA. Infectious complications of chronic lymphocytic leukaemia: Pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010;23(1):145–153. doi: 10.1016/j.beha.2009.12.004. PMID:20620978. [DOI] [PubMed] [Google Scholar]
- 5.Sinisalo M, Aittoniemi J, Oivanen P, Käyhty H, Ölander R, Vilpo J. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukaemia. Br J Haematol. 2001;114(1):107–110. doi: 10.1046/j.1365-2141.2001.02882.x. PMID:11472353. [DOI] [PubMed] [Google Scholar]
- 6.Hartkamp A, Mulder AH, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001;19(13–14):1671–1677. doi: 10.1016/S0264-410X(00)00409-6. PMID:11166890. [DOI] [PubMed] [Google Scholar]
- 7.Safdar A, Rodriguez GH, Rueda AM, Wierda WG, Ferrajoli A, Musher DM, O'Brien S, Koller CA, Bodey GP, Keating MJ. Multiple-dose granulocyte-macrophage-colony-stimulating factor plus 23-valent polysaccharide pneumococcal vaccine in patients with chronic lymphocytic leukemia: A prospective, randomized trial of safety and immunogenicity. Cancer. 2008;113(2):383–387. doi: 10.1002/cncr.23561. PMID:18470901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Velden AM, Van Velzen-Blad H, Claessen AM, Van der Griend R, Oltmans R, Rijkers GT, Biesma DH. The effect of ranitidine on antibody responses to polysaccharide vaccines in patients with B-cell chronic lymphocytic leukaemia. Eur J Haematol. 2007;79(1):47–52. doi: 10.1111/j.1600-0609.2007.00862.x. PMID:17532765. [DOI] [PubMed] [Google Scholar]
- 9.Sinisalo M, Vilpo J, Itälä M, Väkeväinen M, Taurio J, Aittoniemi J. Antibody response to 7-valent conjugated pneumococcal vaccine in patients with chronic lymphocytic leukaemia. Vaccine. 2007;26(1):82–87. doi: 10.1016/j.vaccine.2007.10.053. PMID:18053620. [DOI] [PubMed] [Google Scholar]
- 10.Pasiarski M, Rolinski J, Grywalska E, Stelmach-Goldys A, Korona-Glowniak I, Gozdz S, Hus I, Malm A. Antibody and plasmablast response to 13-valent pneumococcal conjugate vaccine in chronic lymphocytic leukemia patients-preliminary report. PLoS One. 2014;9(12):e114966. doi: 10.1371/journal.pone.0114966. PMID:25506837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simell B, Lahdenkari M, Reunanen A, Kayhty H, Vakevainen M. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin Vaccine Immunol. 2008;15(9):1391–1397. doi: 10.1128/CVI.00110-08. PMID:18596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jodar L, Butler J, Carlone G, Dagan R, Goldblatt D, Kayhty H, Klugman K, Plikaytis B, Siber G, Kohberger R, et al.. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003;21(23):3265–3272. doi: 10.1016/S0264-410X(03)00230-5. PMID:12804857. [DOI] [PubMed] [Google Scholar]
- 13.Abzug MJ, Song LY, Levin MJ, Nachman SA, Borkowsky W, Pelton SI, International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1024 and P1061s Protocol Teams . Antibody persistence and immunologic memory after sequential pneumococcal conjugate and polysaccharide vaccination in HIV-infected children on highly active antiretroviral therapy. Vaccine. 2013;31(42):4782–4790. doi: 10.1016/j.vaccine.2013.08.002. PMID:23954381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenck RW Jr., Fiquet A, Gurtman A, van Cleeff M, Davis M, Rubino J, Smith W, Sundaraiyer V, Sidhu M, Emini EA, et al.. Immunogenicity and safety of a second administration of 13-valent pneumococcal conjugate vaccine 5 years after initial vaccination in adults 50 years and older. Vaccine. 2016;34(30):3454–3462. doi: 10.1016/j.vaccine.2016.04.093. PMID:27155493. [DOI] [PubMed] [Google Scholar]
- 15.Cordonnier C, Labopin M, Robin C, Ribaud P, Cabanne L, Chadelat C, Cesaro S, Ljungman P. Long-term persistence of the immune response to antipneumococcal vaccines after allo-SCT: 10-year follow-up of the EBMT-IDWP01 trial. Bone Marrow Transplant. 2015;50(7):978–983. doi: 10.1038/bmt.2015.42. PMID:25867652. [DOI] [PubMed] [Google Scholar]


