ABSTRACT
Pre-clinical models mimicking persistent hepatitis B virus (HBV) expression are seldom, do not capture all features of a human chronic infection and due to their complexity, are subject to variability. We report a meta-analysis of seven experiments performed with TG1050, an HBV-targeted immunotherapeutic,1 in an HBV-persistent mouse model based on the transduction of mice by an adeno-associated virus coding for an infectious HBV genome (AAV-HBV). To mimic the clinical diversity seen in HBV chronically infected patients, AAV-HBV transduced mice displaying variable HBsAg levels were treated with TG1050. Overall mean percentages of responder mice, displaying decrease in important clinical parameters i.e. HBV-DNA (viremia) and HBsAg levels, were 52% and 51% in TG1050 treated mice, compared with 8% and 22%, respectively, in untreated mice. No significant impact of HBsAg level at baseline on response to TG1050 treatment was found. TG1050-treated mice displayed a significant shorter Time to Response (decline in viral parameters) with an Hazard Ratio (HR) of 8.3 for viremia and 2.6 for serum HBsAg. The mean predicted decrease for TG1050-treated mice was 0.5 log for viremia and 0.8 log for HBsAg, at the end of mice follow-up, compared to no decrease for viremia and 0.3 log HBsAg decrease for untreated mice. For mice receiving TG1050, a higher decline of circulating viremia and serum HBsAg level over time was detected by interaction term meta-analysis with a significant treatment effect (p = 0.002 and p<0.001 respectively). This meta-analysis confirms the therapeutic value of TG1050, capable of exerting potent antiviral effects in an HBV-persistent model mimicking clinical situations.
KEYWORDS: AAV-HBV mouse model, chronic hepatitis B, immunotherapeutic, meta-analysis, TG1050
Introduction
Over two billion people have been infected by HBV worldwide and approximately 257 million are currently chronically infected and at risk of developing cirrhosis and hepatocellular carcinoma.2 Current therapies include nucleos(t)ide analogues (NUC) targeting the inhibition of viral replication and pegylated-IFNα. Despite controlling HBV replication and improving liver histology in most patients, a complete HBV cure is seldom achieved (3–5% of treated patients), leading to costly and lifelong treatments. Novel therapies increasing the cure rate are urgently needed3 and should lead to functional cure, defined as loss of HBsAg with or without the appearance of anti-HBs antibodies. Functional cure might be achieved without true eradication of cccDNA and is associated with persistent suppression of viremia and sustained immune control.
Numerous cohort studies have demonstrated the role of strong, multispecific, sustained HBV-specific T cells, in particular CD8+ ones, in HBV control and resolution of infection.4 This recognized correlate of protection has triggered the development of T cell based immunotherapeutic approaches aiming at improving HBV cure. TG1050 is an immunotherapeutic based on a non-replicative human adenovirus and encodes for a large fusion protein comprising modified HBV Core, Polymerase and two HBV Envelope domains. It was shown to induce HBV-specific T cells both in HBV-free mice and in HBV-persistent mouse models and to exert antiviral effects (i.e. both on HBV viremia and circulating levels of HBsAg).1 With the objective to further support clinical development of TG1050, and in particular in view of the high heterogeneity of chronically infected HBV patients in terms of circulating HBsAg levels,5 we have chosen to perform seven independent experiments that have included AAV-transduced mice displaying a broad range of circulating HBsAg levels before treatment with TG1050, from approximately 100 to 174000 ng/mL. We then conducted a meta-analysis of the activity of TG1050 in the AAV-HBV persistent mouse model gathering these 7 experiments. Meta-analyses published in preclinical settings, especially in animal models of chronic infection, are rare but provide a very powerful tool to validate observations made in models displaying important heterogeneity. This meta-analysis showed that following TG1050 administration the observed significant decrease in viral titers (quadratic regression and interaction term analysis) is associated with a higher percentage of responder mice and an earlier time to response (TTR). No significant impact of HBsAg level at baseline on TG1050 efficacy was found.
Material and methods
The HBV persistent mouse model used is based on a recombinant AAV expressing a full-length infectious genome of HBV which when injected intravenously results in hepatic expression of all HBV antigens and release of infectious particles in mice sera for several months.6 Immune tolerance to HBV antigens is observed in this model mimicking to some extent chronic HBV infection. Overall experimental conditions for the testing of TG1050 in the AAV model have been described by Martin et al.1 Differences between the seven independent experiments are presented in Table 1. Briefly, female mice of the C57BL/6J strain (Charles River Laboratories, France) were injected intravenously with the AAV-HBV6 (produced by Plateforme de Thérapie Génique, INSERM U1089, France) at a dose of 5 × 1010 vg/mouse. They were then either left untreated or treated with TG1050 (2 × 109 vp/injection/mouse; 3 sub-cutaneous injections 1 week apart, 1st injection taking place between 32 to 40 days post AAV-HBV injection). At least 3 time points were monitored for viral parameters after last TG1050 administration. Each experiment included 9 to 15 mice per group. To homogenize the study groups in each experiment, HBsAg titers before treatment were used to allocate mice to study groups displaying then comparable median HBsAg titers and standard deviation. Circulating HBsAg levels and viremia were determined during the whole duration of the studies as described by Martin et al.1 All protocols were conducted with the formal approval of the local animal care committees. This study was performed in compliance with the EU directive 2010/63/UE of 22nd September 2010 and the French décret n° 2013–118 of 1st February 2013 was applied. All mandatory animal welfare, laboratory health and safety procedures were complied with in the course of conducting any experimental work reported in this paper.
Table 1.
Study groups. Experimental conditions are detailed in the Materials and Methods section. Each experiment included 9 to 15 mice per group, which were sacrificed 11–15 weeks post AAV-HBV injection. At least 3 time points after TG 1050 administration were monitored for viral parameters. Mean HBsAg levels at baseline for each study group are shown (with standard deviation).
| Experiment |
N° of mice / group |
TG1050 dose (vp/inj/mouse) |
Days of TG1050 treatment |
Data points post treatment |
Analysis up to Day |
Mean HBsAg level at baseline in ng/mL (Standard Deviation) |
| A | 12 | 2E+9 | 32, 39, 46 | 5 | 88 | 19887 (12417) |
| 12 | — | — | 19495 (11857) | |||
| B | 12 | 2E+9 | 34, 41, 48 | 3 | 80 | 37513 (31531) |
| 11 | — | — | 37518 (28100) | |||
| C | 10 | 2E+9 | 32, 39, 46 | 5 | 98 | 34733 (15414) |
| 10 | — | — | 32441 (19730) | |||
| D | 10 | 2E+9 | 32, 39, 46 | 5 | 98 | 23221 (14248) |
| 9 | — | — | 26013 (13089) | |||
| E | 10 | 2E+9 | 40, 47, 54 | 3 | 81 | 51499 (51446) |
| 10 | — | — | 46540 (40588) | |||
| F | 15 | 2E+9 | 36, 43, 50 | 3 | 76 | 40983 (26475) |
| 15 | — | — | 40876 (25911) | |||
| G | 10 | 2E+9 | 36, 43, 50 | 5 | 104 | 28166 (21438) |
| 10 | — | — | 27078 (17751) |
A mouse was considered as “Responder” if it presented an HBsAg or DNA decrease or both (Fig. 1A) higher than 0.5 log from baseline value (at D28) for two or more time points during the follow-up time (consecutive or not). This definition was derived from our earlier experiments performed in the model and was found to reflect accurately antiviral responses (DNA decrease and/or HBsAg decrease), in comparison with spontaneous variation of antiviral parameters typically observed in non-treated animals. The percentage of responders was calculated for each experiment and the mean percentage of responders was then calculated for each treatment group and compared with a non-parametric Wilcoxon-Mann-Whitney test. Odd-ratios were calculated with a stratified logistic regression (experiments as strata).
Figure 1.
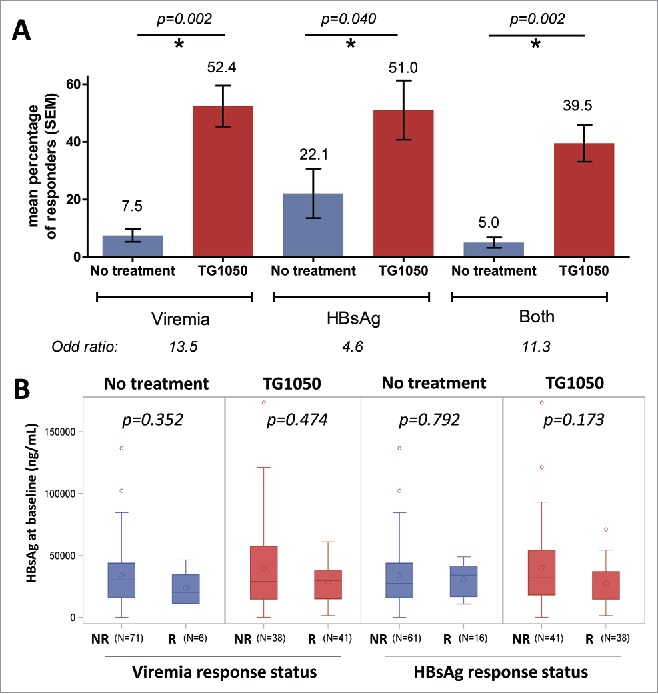
Responder mice were defined as a mouse displaying a decrease in HBV-DNA (viremia), circulating HBsAg levels or both together higher than 0.5 log from baseline value for two or more time points during the study. (A) Overall response rate in seven experiments (mean percentage from 7 individual experiments). P-values for responders in TG1050 treated (red) and not-treated (blue) mice are shown above the graph. The increase in the chance (odd-ratio) to present a response (decrease in viremia, HBsAg or both) when treated with TG1050 is shown below the graph. (B) Circulating HBsAg levels before treatment in non-responder (NR) and responder mice (R), for viremia (left) or HBsAg (right). Values of individual mice from all 7 seven experiments are analyzed together. N is the number of mice analyzed in each bar. The median is indicated by a horizontal line, the mean by a big circle. P-values for responders versus non-responders are shown above the plots.
Among TG1050 treated and untreated mice, HBsAg levels before start of treatment were compared between responder and non-responder mice (for both viremia and HBsAg) using a non-parametric Mann-Whitney test (Fig. 1B).
The continuous evolution over time of viremia and HBsAg level was evaluated with a global repeated mixed model including the seven experiments and considering the following covariates: Time, Treatment, interaction between Time and Treatment and the DNA or HBsAg value at baseline as fixed effects and the Experiment as random effect (Fig. 2A). As a decline in viremia and HBsAg was observed in untreated mice (model characteristics), the objective was to obtain a significant interaction term showing a higher decline in mice receiving TG1050. A meta-analysis was performed by estimating for seven experiments the interaction term Time*Treatment and the associated standard error. The meta-analysis used a fixed effect model weighting estimation with the inverse-variance7 (Fig. 2B).
Figure 2.
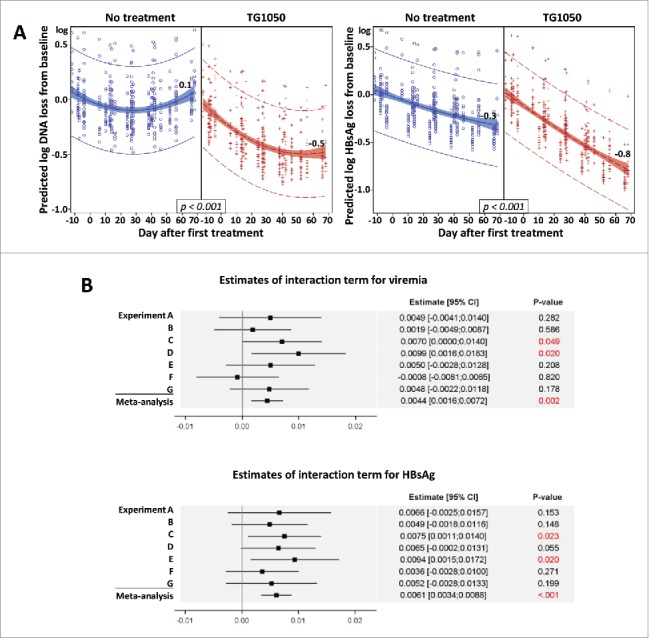
(A) Global mixed model analysis. Quadratic regression on predicted loss from baseline. Each blue circle (no treatment) or red plus (TG1050 treatment) represents the predicted DNA (left) or HBsAg loss (right) from baseline of one mouse at one time point. Quadratic regression with 95% confidence intervals are shown, as well as predicted log loss for viremia and HBsAg at D68 after first treatment (or corresponding day in untreated groups). (B) Estimates of interaction term (indicating a different evolution over time according to treatment received) for viremia (top) and HBsAg (bottom) are shown for 7 individual experiments (A-G) and together (meta-analysis), analyzed using the fixed effect model and weighting estimation with the inverse-variance. Estimates are shown by a square with 95% confidence interval (line). P-values are given for each experiment and for meta-analysis.
Time-To-Response (TTR) was defined as the time between the first day of TG1050 administration (or the corresponding day in the untreated groups) and the time of response (defined as the second time point presenting a decrease superior to 0.5 log), TTR curves were compared with a stratified Log-rank test. If a mouse did not present a response, TTR was censored at the last blood sample measurement. A stratified Cox model (experiments as strata) was done to estimate the Hazard Ratio (HR) and 95% confidence interval (Fig. 3).
Figure 3.
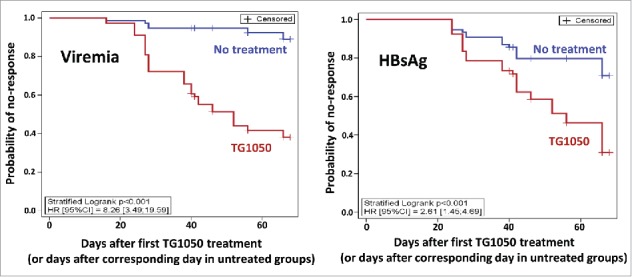
Time-To-Response with a stratified Log-rank test. The probability of no-response is shown for viremia (left) and HBsAg (right), for untreated mice (blue) or TG1050 treated mice (red).
A p-value below 5% was considered as significant. Statistical analyses were done with SAS version 9.4 and R version 3.2.2.
Results and discussion
Seven experiments (Table 1) were analyzed to evaluate TG1050 efficacy as stand-alone treatment in an AAV-HBV animal model which displays heterogeneous levels of circulating HBsAg. All analyses were based on monitoring of both HBsAg and viremia during the whole duration of the various experiments (ranging from 76 to 104 days). Pre-treatment HBsAg levels varied widely between mice, ranging approximately from 100 to 174000 ng/mL. The mean proportion of responder mice was significantly higher in mice treated with TG1050 than in untreated mice (Fig. 1A): for viremia, 52.4% of responders versus 7.5% (p-value = 0.002) and for HBsAg, 51% versus 22.1% (p-value = 0.040), respectively. A combined response was defined as a response for both DNA and HBsAg and a significantly higher percentage of responder mice for both viral parameters was also obtained for mice treated with TG1050 (39.5%) compared to untreated ones (5%, p-value = 0.002). With a stratified logistic regression, mice receiving TG1050 were found to have 13.5 (associated 95% CI for odd-ratio was [5.1;35.9]) and 4.6 (associated 95% CI for odd-ratio was [2.0;10.4]) more chances to present a response for viremia and HBsAg, respectively (p-values < 0.001, not shown).
Interestingly, HBsAg levels detected before treatment in mice responding to TG1050 administration (decrease in viremia, Fig. 1B left, or decrease in HBsAg level, Fig. 1B right) are comparable to HBsAg levels detected before treatment in mice which did not display any response to TG1050 administration (p-values > 0.05). This observation indicates that, at least in the mouse model used here, TG1050 is able to exert an antiviral activity independently of pre-treatment HBsAg levels, in particular that it is able to induce a decrease in viral parameters even in mice displaying high level of antigenemia. Although at that stage the observation is made in a mouse model, capacity of TG1050 to trigger an antiviral response in settings that may display higher tolerance level (linked to higher circulating HBsAg levels8,9) such as can be encountered in some patients is encouraging. Chronically infected HBV patients display heterogeneous circulating HBsAg levels. The levels vary strongly for patients in different phases of HBV-infection but also for patients attributed to the same phase. Jaroszewski et al. showed median HBsAg levels in patients in the low-replicative phase of 1230 IU/mL and of 90881 IU/mL for patients in the immune tolerant (non-inflammatory) phase.5 For the only immune-mediated therapy used today, peg-IFNα, studies are controversial. Some studies show an impact of baseline HBsAg level on response to IFN therapy in CHB patients (response to therapy being higher when baseline HBsAg is low),10,11 while others do not show any correlation.12,13 These discordant observations may be attributed to other confounding factors such as baseline level of HBV DNA and/or pre-existing T cell based immunity. The preclinical HBV models reflect this discordance. Backes et al.14 show the impact of high antigenemia on therapeutic vaccination in transgenic mice. We could not detect a statistical significant impact of the antigenemia in our study including 7 experiments and 156 mice in total. This might be due to the model, as tolerance is induced differently in transgenic mice and in the AAV-model. High antigenemia might refer to HBeAg14 or HBsAg. Finally, the therapeutic treatment possibly plays an important role on detected immune responses, as a modified treatment in Backes et al. resulted in the appearance of HBV-specific T cells and anti-HBs antibodies even in transgenic mice with high antigenemia. Clinical studies with TG1050 combined with a careful evaluation of baseline viral parameters or pre-existing HBV immunity will help in validating the predictive value of observations made in the AAV-HBV model and define lack of impact or impact of baseline HBsAg level on response to TG1050 treatment.
We further performed a global mixed model analysis including all experiments to study evolution of viral parameters over time. A significant interaction between Treatment and Time was found for both viremia and HBsAg (both p-values were <0.001) revealing that decrease of viremia and HBsAg over time was higher in mice treated with TG1050. Overall, no significant impact of HBsAg and DNA level at baseline on TG1050 antiviral activity was found, suggesting that baseline levels of these two parameters did not influence TG1050 induced decline in viremia or HBsAg levels over time. A quadratic regression on predicted loss from baseline is presented in Fig. 2A. At the end of mice follow-up (68 days after the first TG1050 administration) the mean predicted decrease for TG1050 treated and untreated mice was 0.48 log versus 0.07 log and 0.79 log versus 0.32 log for viremia and HBsAg, respectively. This result was confirmed with the meta-analysis of interaction terms (Treatment and Time). Forest plots in Fig. 2B showed that a significant interaction term was found for some experiments (statistical power limited for each experiment due to small number of mice) but all interaction terms (except experiment F for viremia) presented the same trend. By combining these results to increase the statistical power of the analysis, a significant interaction term was found by the meta-analysis for both viremia and HBsAg (p-values were 0.002 and <0.001, respectively), confirming that viremia and HBsAg decrease over time was significantly higher in mice treated by TG1050. Publication bias was assessed with funnel plot representation and asymmetry was not detected using Egger's test and trim and fill method (not shown), which validates the performed meta-analysis.
As shown in Fig. 3, Time-To-Response (TTR, see definition within Material et Method section) was found to be significantly shorter in mice treated with TG1050 for both viremia and HBsAg (stratified log-rank p-values were both <0.001) with median TTR of respectively 52 and 56 days after first TG1050 administration whereas median TTR was never reached for untreated mice. It showed that even if some untreated mice presented decline in viremia or HBsAg level, the decrease was significantly quicker for mice treated with TG1050. The Hazard Ratio (HR) obtained with stratified Cox model were 8.3 (associated 95%CI [3.5;19.6]) and 2.6 (associated 95%CI [1.5;4.7]) for viremia and HBsAg, respectively.
TG1050 is currently under clinical development (phase 1) in HBV chronically infected patients treated by antivirals (NCT02428400). We have shown in earlier pre-clinical studies, that TG1050 has the ability to induce robust HBV-specific T cell responses in naïve mice comparable to those observed in resolving patients15 as well as functional T cells in liver of HBV persistent mice. In the AAV-HBV model, TG1050 can exert antiviral effects both on the circulating HBV DNA and HBsAg.1 Preclinical in vivo models are inherently heterogeneous. The HBsAg levels in individual mice vary largely in general in HBV mouse models, as in the AAV-HBV model. To consolidate and expand earlier observations, we performed several additional experiments to evaluate TG1050-induced antiviral effects over a large range of baseline HBsAg level such as expected to be encountered during clinical development and used them to realize a meta-analysis. Preclinical animal experiments are typically confounded by pressures to reduce the number of animals (ethical, cost, practical and time reasons). These limitations have led to an increasing number of reported meta-analyses applied to multiple, repeated protocols and studies with the aim to reach unbiased, consolidated interpretation although publication bias which is essential to control was rarely assessed.16 This preclinical meta-analysis shows, to our knowledge for the first time, the heterogeneity of several experiments in an AAV model. It allows to compare the antiviral effects of TG1050 between individual experiments. Furthermore, it allows to strongly validate the efficacy of TG1050 over a very broad range of pre-treatment HBsAg levels in individual mice. Analyses performed here demonstrate without ambiguity the antiviral efficacy of TG1050 and the significant impact of its administration on HBV viremia and circulating HBsAg. This antiviral activity is very encouraging, especially since monitoring took place at relatively early time points after treatment (max 68 days). In a handful of experiments that lasted longer (more than 16 weeks post-initial TG1050 administration), we did observe mice displaying anti-HBsAg antibody seroconversion17 (results were not included here due to limited number of such experiments).
A number of HBV-specific immuno-therapeutics have been developed over the years and have so far resulted in limited or no efficacy.3 NASVAC, based on recombinant HBsAg and HBV Core proteins, showed some therapeutic effects on HBV-DNA but limited effect on HBsAg when administered in HBV chronically infected patients in a phase 3 study.18 Similarly, the YIC vaccine, based on immune complexes composed of HBsAg and anti-HBsAg antibodies did not outperform the control group in a phase 3 study.19 In a phase 2 study, no significant reduction in circulating HBsAg levels in virally suppressed chronic HBV patients treated with GS-4774 (recombinant yeast encoding Core, X and HBsAg proteins) was achieved.20 The HBV DNA-based therapeutic vaccine (encoding for Pre-S2-HBsAg) developed by Institut Pasteur was also unsuccessful in a phase 2 study in HBV chronically infected patients under antiviral treatment.21 All these vaccines have relied on platforms not qualified for their capacity to induce strong T cell based immunity, with the exception of the DNA vaccine but which was administered by simple intramuscular route (no electroporation). To our knowledge, TG1050 is the only HBV-specific immunotherapeutic based on a viral vector under clinical development.3 In contrast, a few therapeutic vaccine candidates using viral vectors coding for HBV antigens showed promises in different preclinical models. For example, Kosinska et al.22 showed in the chronically infected woodchuck model that a combination treatment of NUC with a DNA prime/adenovirus boost regimen led to a strong reduction in viral load, WHsAg decrease and immunological responses. In another study, an adjuvanted protein prime with a MVA boost led to the appearance of HBV-specific T cells and anti-HBs antibodies at day 6 post boost even in high-antigenemic HBV-transgenic mice.14
TG1050 differentiates itself from these vaccines by being the only one displaying a complex antigenic mix including the near full-length polymerase organized in a large single polyprotein expressed by a single vector (adenovirus) known for its remarkable capacity to induce long-lasting CD8+ T cells. Anti-HBV-polymerase specific T cells were identified in some HBV resolved patients23 and it was shown very recently that these T cells might play a role as potential biomarker associated with control of chronic HBV infection without ALT flares in patients undergoing NUC discontinuation.24 TG1050 encodes also for modified HBV core. Despite the implication of HBeAg in tolerance induction, the presence of circulating HBeAg in a similar model6 and the similarity of the protein sequence of HBeAg and HBV core, TG1050 induces anti-HBc antibodies17 and functional core-specific T cells in the AAV-HBV model.1 The expected mechanism of action of TG1050 is induction of cellular immunity, particularly HBV-specific CD8+ T cells, capable of exerting antiviral functions via direct cytolysis of infected cells and/or production of inhibitory cytokines.25 Experiments are in progress to effectively demonstrate such MOA and more specifically the role of various immune cell populations in TG1050-induced antiviral activity observed in the AAV-HBV model.
The meta-analysis performed here is based on experiments in which TG1050 is administered as stand-alone. It would be interesting to evaluate TG1050 in combination settings with NUC, at both the pre-clinical and clinical level. NUC treatment has been described to partially restore HBV immune responses in chronically infected patients.26,27 Experiments in the AAV-HBV model in combination with NUC may further enhance antiviral effects of TG1050 compared to stand-alone treatment. Other combinations of TG1050 with currently developed novel anti-HBV therapeutics would obviously also deserve investigation.
In conclusion, the current analysis supports development of TG1050 in chronically infected patients displaying a wide range of circulating HBsAg.
Disclosure of potential conflicts of interest
All authors are employees of Transgene SA.
References
- 1.Martin P, Dubois C, Jacquier E, Dion S, Mancini-Bourgine M, Godon O, Kratzer R, Lelu-Santolaria K, Evlachev A, Meritet JF, et al.. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice. Gut. 2015;64:1961–71. doi: 10.1136/gutjnl-2014-308041. PMID:25429051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization http://www.who.int/mediacentre/factsheets/fs204/en/. 2017.
- 3.Brahmania M, Feld J, Arif A, Janssen HL. New therapeutic agents for chronic hepatitis B. Lancet Infect Dis. 2016;16:e10–21. doi: 10.1016/S1473-3099(15)00436-3. PMID:26795693. [DOI] [PubMed] [Google Scholar]
- 4.Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–68. doi: 10.1038/nm.3251. PMID:23836236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, et al.. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514–22. doi: 10.1016/j.jhep.2010.01.014. PMID:20207438. [DOI] [PubMed] [Google Scholar]
- 6.Dion S, Bourgine M, Godon O, Levillayer F, Michel ML. Adeno-associated virus-mediated gene transfer leads to persistent hepatitis B virus replication in mice expressing HLA-A2 and HLA-DR1 molecules. J Virol. 2013;87:5554–63. doi: 10.1128/JVI.03134-12. PMID:23468504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Statistical Software. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 8.Kondo Y, Ninomiya M, Kakazu E, Kimura O, Shimosegawa T. Hepatitis B surface antigen could contribute to the immunopathogenesis of hepatitis B virus infection. ISRN Gastroenterol. 2013;2013:935295. PMID:23401786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu D, Liu L, Yang D, Fu S, Bian Y, Sun Z, Zhang L, Peng H, Fu YX. Clearing persistent extracellular antigen of hepatitis B Virus: An immunomodulatory strategy to reverse tolerance for an effective therapeutic vaccination. J Immunol. 2016;196:3079–87. doi: 10.4049/jimmunol.1502061. PMID:26936879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen GY, Zhu MF, Zheng DL, Bao YT, Wang J, Zhou X, Lou GQ. Baseline HBsAg predicts response to pegylated interferon-alpha2b in HBeAg-positive chronic hepatitis B patients. World J Gastroenterol. 2014;20:8195–200. doi: 10.3748/wjg.v20.i25.8195. PMID:25009392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li MH, Zhang L, Qu XJ, Lu Y, Shen G, Wu SL, Chang M, Liu RY, Hu LP, Li ZZ, et al.. Kinetics of hepatitis B surface antigen level in chronic hepatitis B patients who achieved hepatitis B surface antigen loss during Pegylated Interferon Alpha-2a treatment. Chin Med J (Engl). 2017;130:559–65. doi: 10.4103/0366-6999.200554. PMID:28229987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rijckborst V, Hansen BE, Cakaloglu Y, Ferenci P, Tabak F, Akdogan M, Simon K, Akarca US, Flisiak R, Verhey E, et al.. Early on-treatment prediction of response to peginterferon alfa-2a for HBeAg-negative chronic hepatitis B using HBsAg and HBV DNA levels. Hepatology. 2010;52:454–61. doi: 10.1002/hep.23722. PMID:20683945. [DOI] [PubMed] [Google Scholar]
- 13.Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology. 2010;52:1251–7. doi: 10.1002/hep.23844. PMID:20830787. [DOI] [PubMed] [Google Scholar]
- 14.Backes S, Jager C, Dembek CJ, Kosinska AD, Bauer T, Stephan AS, Dišlers A, Mutwiri G, Busch DH, Babiuk LA, et al.. Protein-prime/modified vaccinia virus Ankara vector-boost vaccination overcomes tolerance in high-antigenemic HBV-transgenic mice. Vaccine. 2016;34:923–32. doi: 10.1016/j.vaccine.2015.12.060. PMID:26776470. [DOI] [PubMed] [Google Scholar]
- 15.Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, Larrubia JR, Webster GJ, McMichael AJ, Ferrari C, et al.. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117:1386–96. doi: 10.1016/S0016-5085(99)70289-1. PMID:10579980. [DOI] [PubMed] [Google Scholar]
- 16.Korevaar DA, Hooft L, ter Riet G. Systematic reviews and meta-analyses of preclinical studies: publication bias in laboratory animal experiments. Lab Anim. 2011;45:225–30. doi: 10.1258/la.2011.010121. PMID:21737463. [DOI] [PubMed] [Google Scholar]
- 17.Lélu K, Evlachev A, Kratzer R, Dion S, Mancini-Bourgine M, Godon O, Schmitt D, Dubois C, Méritet JF, Schlesinger Y, et al.. TG1050, a novel immunotherapeutic to treat chronic hepatitis B, can control HBsAg and provoke HBsAg seroconversion in HBV-persistent mouse models. 50th Annual meeting of the European Association for the Study of the Liver (EASL) Vienna, Austria: J Hepatol 2015:S187–S931. [Google Scholar]
- 18.Akbar S, Mishiro S, Mahtab MA, Rahman S, Aguilar J. A phase III clinical trial with a therapeutic vaccine containing both HBsAg and HBcAg administered via both mucosal and parenteral routes in patients with chronic hepatitis B. Poster presented at AASLD Liver Meeting, 2013. https://liverlearning.aasld.org/aasld/2013/thelivermeeting/35877/sheikh.mohammad.fazle.akbar.a.phase.iii.clinical.trial.with.a.therapeutic.html. [Google Scholar]
- 19.Xu DZ, Wang XY, Shen XL, Gong GZ, Ren H, Guo LM, Sun AM, Xu M, Li LJ, Guo XH, et al.. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol. 2013;59:450–6. doi: 10.1016/j.jhep.2013.05.003. PMID:23669281. [DOI] [PubMed] [Google Scholar]
- 20.Lok AS, Pan CQ, Han SH, Trinh HN, Fessel WJ, Rodell T, Massetto B, Lin L, Gaggar A, Subramanian GM, et al.. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol. 2016;65:509–16. doi: 10.1016/j.jhep.2016.05.016. PMID:27210427. [DOI] [PubMed] [Google Scholar]
- 21.Godon O, Fontaine H, Kahi S, Meritet JF, Scott-Algara D, Pol S, Michel ML, Bourgine M. Immunological and antiviral responses after therapeutic DNA immunization in chronic hepatitis B patients efficiently treated by analogues. Mol Ther. 2014;22:675–84. doi: 10.1038/mt.2013.274. PMID:24394187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosinska AD, Zhang E, Johrden L, Liu J, Seiz PL, Zhang X, Ma Z, Kemper T, Fiedler M, Glebe D, et al.. Combination of DNA prime–adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog. 2013;9:e1003391. doi: 10.1371/journal.ppat.1003391. PMID:23785279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, Williams R, Dusheiko G, Bertoletti A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78:5707–19. doi: 10.1128/JVI.78.11.5707-5719.2004. PMID:15140968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bert N, Rivino L, Gill U, Cheng Y, Kunasegaran K, Tan D, Koh S, Becht E, Hansi N, Foster G, et al.. An immunological biomarker to predict hepatic flares upon NUC therapy discontinuation in chronic hepatits B. J Hepatol. 2016;64:S164–S5 doi: 10.1016/S0168-8278(16)01672-X. [DOI] [Google Scholar]
- 25.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. PMID:12477811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boni C, Laccabue D, Lampertico P, Giuberti T, Vigano M, Schivazappa S, Alfieri A, Pesci M, Gaeta GB, Brancaccio G, et al.. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–73 e9. doi: 10.1053/j.gastro.2012.07.014. PMID:22796241. [DOI] [PubMed] [Google Scholar]
- 27.Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology. 2015;61:712–21. doi: 10.1002/hep.27323. PMID:25048716. [DOI] [PMC free article] [PubMed] [Google Scholar]


