ABSTRACT
In this study, we demonstrate, for the first time, that Saccharomyces cerevisiae-based probiotic shows an inhibitory effect on Gardnerella vaginalis infection. This effect is likely due to several actions: direct interference with adherence to vaginal tissues, inhibition of sialidase activity, reduction of vaginal epithelial exfoliation. Gardnerella vaginalis does not induce vaginal inflammation and no inflammatory cytokines were, indeed, produced, by the mouse vagina, neither by Gardnerella vaginalis and by the probiotic. Collectively, our data incite to further investigations on Saccharomyces cerevisiae probiotic as a potential prophylactic or therapeutic agent in the vaginosis caused by Gardnerella vaginalis.
KEYWORDS: probiotic, G. vaginalis, S. cerevisiae, bacterial vaginosis, sialidase, exfoliation, adherence, displacement
Introduction
Bacterial vaginosis (BV) is the most common vaginal dysbiosis in women of childbearing age [1]. It has been associated with serious health troubles including spontaneous abortion [2], pre-term birth [3], pelvic inflammatory disease [4], endometritis [5] and enhanced acquisition and transmission of some sexually transmitted agents [6] such as HIV [7].
The clinical symptoms of bacterial vaginosis (BV) include profuse vaginal discharge and a rotten fish vaginal odor. Nevertheless many women with BV remain asymptomatic [8]. This condition is, usually, associated with dramatic reduction of healthy vaginal microflora, constituted mainly by lactobacilli, particularly L. crispatus, L. jensenii and L. gasseri [9,10], related to simultaneous proliferation of anaerobic bacteria including Gardnerella vaginalis (G. vaginalis), Prevotella spp., Atopobium vaginae (A. vaginae), Bacteroides spp. and Mobiluncus spp [11]. Given the high prevalence and the associated complications, BV represents an important public health issue. However, its etiology remains, yet, unclear because of great complexity and diversity of microorganisms involved [12].
Compelling evidence shows that, among bacterial multispecies involved in BV, G. vaginalis represents a core pathogen [13]. There is consensus that BV involves the presence of a polymicrobial structured biofilm, mainly constituted by G. vaginalis, strongly adhered to vaginal epithelium [14,15]. Other features associated to persistent G. vaginalis adherence to epithelial vaginal cells, include the activity of sialidase [15], an enzyme that plays a role in the pathogenic process, and a robust epithelial exfoliation (reminiscent of clue cells). To date therapeutic strategies, available for BV, are related to antibiotic treatment with metronidazole, clindamycin or tinidazole. Metronidazole is considered the drug of choice [16]. However very high BV recurrence rates have been reported [14,17] thus highlighting that standard antibiotic therapy was not able, in many cases, to fully eradicate BV vaginal biofilms [18,19]. It is reported [14,19,20] that antibiotic resistance, biofilm-associated, is probably a major cause of treatment failure. Furthermore, the antibiotic administration may, also, cause a dysbiosis in the vaginal flora [14]. Thus, an additional or alternative therapeutic approach, which aims to restoring the healthy vaginal microbiota, is represented by the administration of probiotics, i.e. live microorganisms providing health benefits to the host [21]. Probiotics can interfere with metabolic processes of pathogens conferring some type of protection [9,22,23]. The strains mainly used as probiotics are part of the following genera: Bifidobacterium, Lactobacillus and Saccharomyces [24]. Many studies have been performed by using probiotic Saccharomyces cerevisiae (S. cerevisiae) strains on gastrointestinal tract infections, where the microbial population imbalance is evident [25]. Furthermore, it has been reported that S. cerevisiae is able to enhance the survival and therapeutic potential of probiotic L. rhamnosus [25] that is, usually, used to prevent and treat vaginal infection [26]. Recently, our group demonstrated that vaginal administration of probiotic S. cerevisiae yeast (GI) exerted beneficial therapeutic effects on vaginal candidosis [27].
The objective of the present study was to assess the ability of S. cerevisiae-based probiotic to control G. vaginalis in an experimental mouse model of vaginal infection. We, also, addressed possible mechanisms explaining the probiotic preventive and therapeutic potential.
Results
Beneficial effect of S. cerevisiae treatment on G. vaginalis infection
To analyze whether live S. cerevisiae yeast (encoded GI) was able to affect G. vaginalis growth in the mouse vagina, C57/Bl6 mice were treated with 0.5 mg/100 μl/mouse of ß-estradiol, three days prior to and on the day of intravaginal challenge with G. vaginalis. GI (108 or 109/ml) was administered intravaginally (10 μl/mouse) two days before challenge and every day, post-infection, until the end of experiment. Saline and L. crispatus (2 × 109 or 2 × 1010/ml), both 10 μl/mouse [28], were used, respectively, as negative and positive control [29–31]. After 1 and 3 days post-infection we determined G. vaginalis load in vaginal washes, vaginal tissue and uterine horns. The experimental model is outlined in Fig. 1A. The results, reported in Fig. 1B, show that GI at the dose of 10 mg/ml, was able to significantly decrease the G. vaginalis load in vaginal washes, both 1 and 3 days post challenge. GI treatment decreased of 70% and 80% of G. vaginalis CFU, 1 day and 3 days post infection, respectively (Fig. 1C). Furthemore, the reduction of CFU, 1 day after challenge, occurred in 83.3% of GI treated mice and in 66.6% of L. crispatus treated mice. This reduction was manifested in 100% of treated mice, 3 days after challenge with both treatment, GI and L. crispatus.
Figure 1.
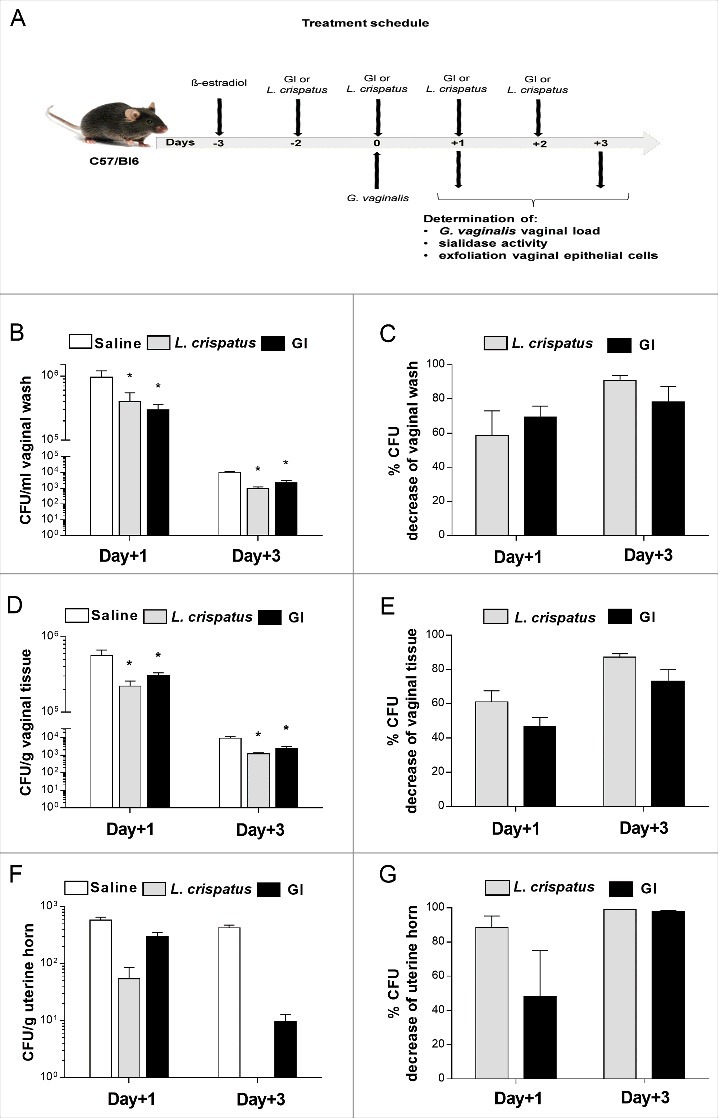
Effect of GI treatment on G. vaginalis infection. C57/Bl6 mice, under pseudoestrus condition, were treated intravaginally with 10 μl of Saline, or L. crispatus (2 × 109/ml) or GI (108/ml) two days before the challenge with G. vaginalis (5 × 107/20μl/mouse) and once a day for 3 days beginning the day of infection (A). G. vaginalis load were determined by enumerating colony forming units (CFU) in vaginal washes (B), in tissue (D) and uterine horn homogenates (F) at days 1 and 3 post-infection. Percentage of G. vaginalis CFU decrease (C, E, G) was quantified relative to G. vaginalis-infected mice treated with Saline. Data are the mean ± SEM from 2 independent experiments each with 6 mice/group. *p < 0.05 L. crispatus- or GI-treated mice vs Saline-treated mice.
G. vaginalis growth inhibition by GI was also detected in the vaginal tissue, at both days post-infection, as shown in Fig. 1, panels D, E. As for the vaginal washes, the highest inhibitory activity by GI against G. vaginalis in the vaginal tissue was observed 3 days after infection. In addition, the reduction of CFU tissue levels was observed in 100% of treated mice with GI as well as with L. crispatus, both 1 and 3 days post-infection. The higher dose of GI (109/ml) produced similar effects in clearing G. vaginalis, suggesting that 108/ml was sufficient for fighting experimental G. vaginalis infection (Supplementary Fig. 1A-D).
Given that G. vaginalis can cause ascending infections [13] we performed selected experiments to determine whether GI treatment could inhibit the colonization of uterine horns. As reported in Fig. 1F, a significant decrease of G. vaginalis load in uterine horns was observed. In particular (Fig. 1G) 1 day post-infection, the inhibition reached 50% after GI treatment. Three days after challenge G. vaginalis had been, almost, completely cleared, in all mice.
Effect of S. cerevisiae treatment on sialidase activity of G. vaginalis
The sialidase production has been associated to bacterial pathogenesis and represents a virulence factor for several pathogens such as P. aeruginosa [32], V. colerae [33], S. pneumoniae [34]. Since the clinical isolate of G. vaginalis used in our experimental model produced sialidase, we tested whether our probiotic could influence this enzymatic activity. To this purpose vaginal washes, collected from mice at days 1 and 3 post-infection, were assayed for sialidase activity (Fig. 2A–C). The results obtained show that both GI and L. crispatus were able to significantly inhibit this enzymatic activity particularly at day 1 post-infection (Fig. 2C). The administration of probiotics alone, without infection, did not produce detectable sialidase activity.
Figure 2.
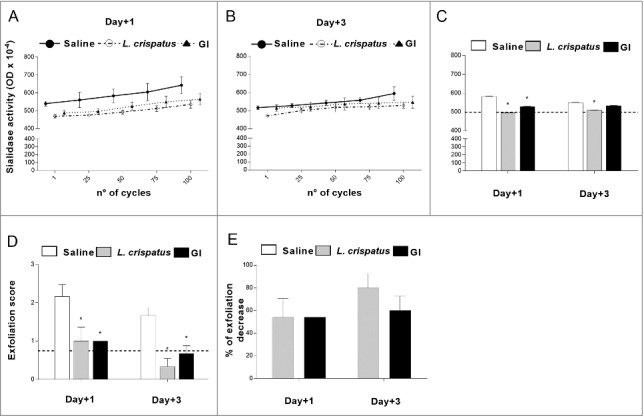
Effect of GI treatment on G. vaginalis sialidase activity and epithelial exfoliation G. vaginalis-induced. Sialidase activity and epithelial exfoliation were determined in vaginal washes of mice, treated intravaginally with 10 μl of Saline, or L. crispatus (2 × 109/ml) or GI (108/ml) and infected with G. vaginalis (5 × 107 /20 μl/mouse) as described in Materials and Methods, at days +1 and +3 post-infection. (A, B, C) Optical density, of sialidase activity, was determined as described in Materials and Methods. (A, B) Lines are representative of experiments (n = 2) with similar results. (C) Bars are the mean ± SEM from 2 independent experiments each with 6 mice/group. The dashed line represents the optical density of sialidase activity from vaginal washes of not-infected mice. *p < 0.05 L. crispatus- or GI-treated mice vs Saline-treated mice. (D) Epithelial exfoliation score has been evaluated by assigning a value from 0 to 3, with 0 = cells number < 25 and 3 = cells number > 75. The dashed line represents the exfoliation score from vaginal washes of not-infected mice. Data are the mean ± SEM from 2 independent experiments each with 6 mice/group. *p < 0.05 L. crispatus- or GI-treated mice vs Saline-treated mice. (E) Percentage of epithelial exfoliation decrease was quantified in respect to mice treated with Saline.
Effect of S. cerevisiae treatment on epithelial exfoliation G. vaginalis-induced
It has been reported that the clue cells, which are one of the key cytological features of G. vaginalis-induced BV, are the result of exfoliation of vaginal epithelium [13]. Enzymes, such as sialidase, and organic acid, produced by anaerobic microorganisms, are the potential cause of exfoliation [13]. We therefore asked whether, in our experimental model, GI treatment was able to affect the epithelial exfoliation due to G. vaginalis infection. To give a semi-quantitative perspective of this effect, we scored the degree of exfoliation with 0 being none and 3 being very robust degree of exfoliation.
As shown in Fig. 2D, GI, as well as, L. crispatus were able to significantly inhibit the G. vaginalis-induced epithelial exfoliation. This effect was, already, evident 1 day post-infection when GI, inhibited more than 50% of exfoliation process (Fig. 2E) and remained constant over the experimental time period. The administration of probiotics alone, without infection, did not produce detectable exfoliation.
Effect of S. cerevisiae treatment on immune vaginal response
To verify whether the clearance of G. vaginalis by probiotics was associated to any stimulation of immune response, the possibility that probiotics could affect the cytokine secretion in the local vaginal area was evaluated. To this end pro-inflammatory (IL-1ß and TNF-α) and anti-inflammatory (IL-10) cytokines were determined in vaginal washes at day+1 and +3 post-infection. The results show that no significant variations of cytokine levels was observed after infection with G. vaginalis respect to saline treated mice. The treatment with probiotics did not alter this condition (Fig. 3A). Moreover, histological analysis of vaginal tissue, from mice treated with probiotics alone, or infected and treated with probiotics, shows that no inflammatory cells were present in vaginal tissue in any of the histological preparations. These results confirm that, at variance with other vaginal infections [27], there is no inflammatory response in the vaginal tissue of mice challenged with Gardnerella and treated with probiotics.
Figure 3.
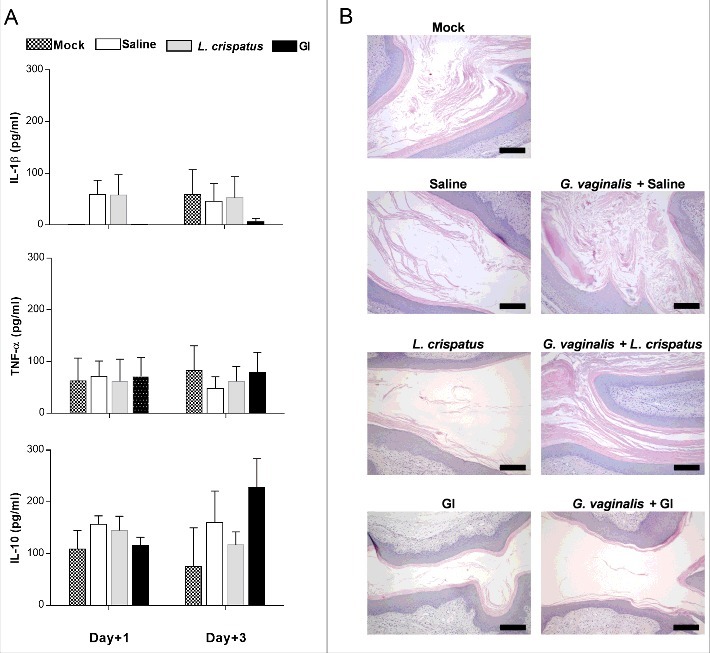
Evaluation of immune response to probiotic treatment. (A) Pro-(IL-1β and TNF-α) and anti-(IL-10) inflammatory cytokines levels have been determined in vaginal washes of mice, treated intravaginally with 10 μl of Saline, or L. crispatus (2 × 109/ml) or GI (108/ml) and infected with G. vaginalis (5 × 107/20 μl/mouse), at days +1 and +3 post-infection. Data are the mean ± SEM from 2 independent experiments each with 6 mice/group. (B) Histological inflammation was assessed by haematoxylin-eosin staining of formalin-fixed, paraffin-embedded vaginal tissue sections. Images (Bar = 200 µm, Magnification 10x) are representative of 2 separate experiments with similar results.
S. cerevisiae inhibits G. vaginalis adherence on vaginal and cervix epithelial cells
Given that immune cells do not play a role in the GI induced G. vaginalis clearance, we evaluated whether some other mechanistic effects were involved in the inhibition of G. vaginalis load. Indeed, adhesion to host cells is a critical initial step in any infectious process and in vitro models of infections have been extensively used for analyzing the interactions between non-pathogenic and pathogenic bacteria [27,29,35–37]. Therefore, we investigated whether GI was able to inhibit the G. vaginalis adhesion on epithelial cells by using an in vitro model system such as vaginal (A-431) and cervix (HeLa) epithelial cell lines. In a first series of experiments, we analyzed the capacity of GI and L. crispatus (each at two different doses) [29,31], to adhere to A-431 or HeLa cells. To this end, the cells were treated with GI or L. crispatus and, after extensive washings, colony forming units (CFU) were determined. The results reported in Fig. 4A show that both GI and L. crispatus were able to adhere, with different degrees, to vaginal and cervix epithelial cells. GI manifested a better capacity to adhere to A-431 cells than to HeLa cells, whereas L. crispatus showed an opposite behavior. Furthermore this interaction occurred in a dose dependent manner. Then the capacity of GI to compete for G. vaginalis adhesion on A-431 and HeLa cells was determined. A-431 and HeLa cells were treated with GI or L. crispatus (each at two different doses) for 4h at 37°C and, after extensive washing, G. vaginalis was added. The control was G. vaginalis adhesion only. The results reported in Fig. 4B left panel, show that a significant inhibition of G. vaginalis adhesion to A-431 and HeLa cells was observed with both doses of GI used. The inhibition of adherence reached about 40–50% for both A-431 and HeLa cells. Similar results were obtained by using of L. crispatus (Fig. 4B right panel).
Figure 4.
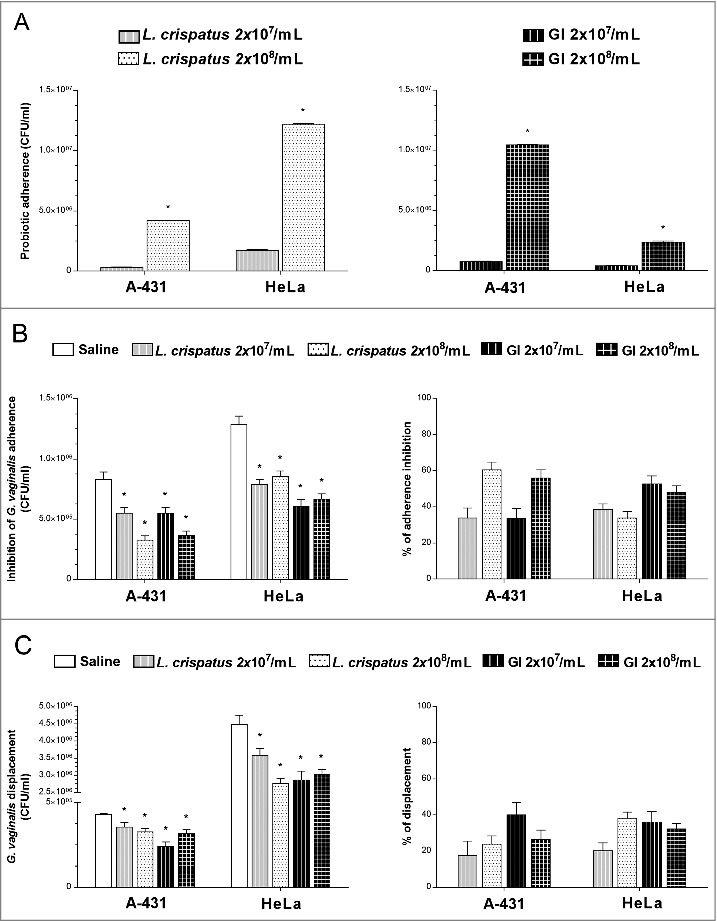
Effect of GI treatment on G. vaginalis adherence to vaginal (A-431) and cervix (HeLa) epithelial cells. (A) Adhesion of L. crispatus or GI to A-431 and to HeLa cell lines. L. crispatus or GI (both 2 × 107/ml or 2 × 108/ml) were added to monolayer of A-431 or HeLa cells for 4 h at 37°C in anaerobic conditions. After incubation, cells were washed 2 times and microorganisms adhered were quantified as number of CFU/ml. Data are the mean ± SEM from 2 independent experiments. *p < 0.05, 2 × 108/ml (L. crispatus or GI) vs 2 × 107/ml (L. crispatus or GI). (B) Interference of L. crispatus or GI on G. vaginalis initial adhesion onto A-431 and HeLa cell lines. Two inocula (2 × 107/ml or 2 × 108/ml) of L. crispatus or GI were pre-adhered to epithelial cells, as above described, and subsequently G. vaginalis (2 × 108/ml) has been added to the co-culture for 30 min at 37°C in anaerobic conditions. G. vaginalis adhered were quantified as number of CFU/ml. *p < 0.05 L. crispatus or GI vs Saline treatment. Percentage adherence inhibition was quantified in respect to Saline. (C) Reduction of G. vaginalis adherent to epithelial cells. G. vaginalis (2 × 108/ml) was incubated with the monolayers for 30 min at 37°C in anaerobic conditions. Then, non-adherent bacteria were removed by washing and probiotics (2 × 107/ml or 2 × 108/ml) were added to co-cultures for 30 min at 37°C in anaerobic conditions. G. vaginalis displacement was expressed as CFU/ml as described in Material and Methods. *p < 0.05 L. crispatus or GI vs Saline treatment.
S. cerevisiae induces the displacement of G. vaginalis adhered on vaginal and cervix epithelial cells
The capacity of GI to inhibit G. vaginalis adherence suggested the further possibility that GI could exert a displacement of pre-adhered G. vaginalis to epithelial cells. To this end the epithelial cells were treated with G. vaginalis and, after extensive washings to remove non adherent bacteria, GI or L. crispatus were added. The results reported in Fig. 4C left panel, show that a consistent amount of G. vaginalis was removed by GI. L. crispatus showed similar effect. Both doses of GI were effective in displacing G. vaginalis from epithelial and cervix vaginal cells. (Fig. 4C, right panel).
S. cerevisiae does not induce G. vaginalis co-aggregation
Another important mechanistic effect for eliminating bacteria is the capacity to aggregate pathogens. Indeed, co-aggregation is one of the mechanisms exerted by probiotics to create a competitive micro-environment around the pathogen [38,39]. In this line, GI was tested for its capacity to co-aggregate with G. vaginalis. To this purpose GI was incubated alone or mixed with G. vaginalis. Neither G. vaginalis nor GI self-aggregated at any of the tested doses. Results of co-aggregation showed that GI was unable to co-aggregate with G. vaginalis. Conversely L. crispatus was able to self-aggregate and induced co-aggregation of G. vaginalis (Fig. 5A). A representative image, demonstrating that GI was not able to self-aggregate as well as to co-aggregate G. vaginalis is reported in Fig. 5B.
Figure 5.
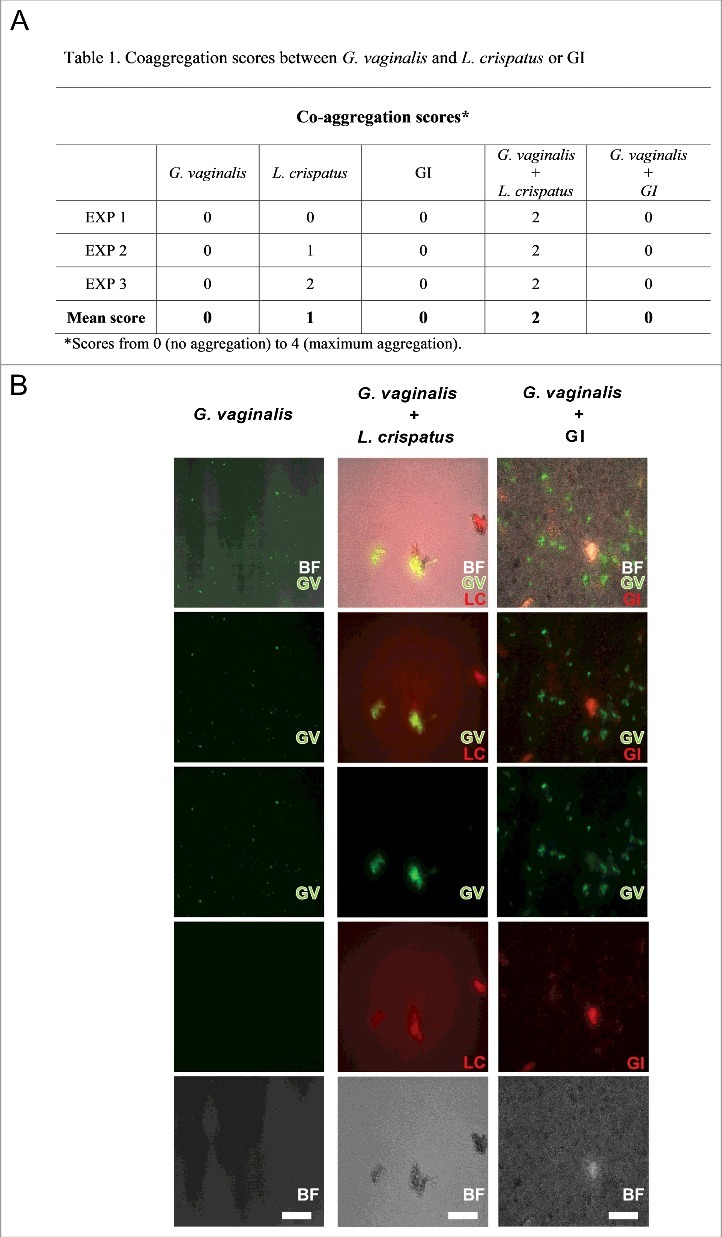
Co-aggregation between GI or L. crispatus and G. vaginalis. G. vaginalis or FITC-G. vaginalis (1 × 109/ml) in PBS were mixed with equal volume of L. crispatus or RhB-L. crispatus (1 × 109/ml) or with equal volume of GI or RhB-GI (108/ml). The samples were vortexed for at least 10 sec and incubated in a 24 well plate for 4 h at 37°C under agitation. The suspensions were, then, observed by inversion light microscopy to evaluate the aggregation degree or photographed by fluorescence microscopy. (A) Scores, from 0 (no aggregation) to 4 (maximum aggregation), and mean are shown. Data are from replicate samples of 3 different experiments. (B) Images are representative of 3 different experiments with similar results (Scale Bar = 50 µm, Magnification 20x). BF = bright field; G. vaginalis (GV) = green; L. crispatus (LC) = red.
Discussion
Bacterial vaginosis is a polymicrobial clinical syndrome in which Lactobacillus spp., major constituents of “normal vaginal microbiota”, are replaced by an overgrowth of non-beneficial anaerobic microbial species. This dysbiosis is recognized as the most common cause of abnormal vaginal discharge in women of childbearing age and it is associated with serious pregnancy-related sequelae and increased transmission of sexually transmissible infections. G. vaginalis is the most frequent microorganism isolated from vaginal fluids of women suffering from BV [40,41].
Many studies have suggested that the presence of vaginal lactobacilli may protect against BV [31,39,42–45]. The dominant Lactobacillus spp. include L. crispatus, L. gasseri and L. jensenii [46]. There is general consensus that L. crispatus inhibits G. vaginalis growth by producing lactic acid [31] and additional studies provide evidence for inhibition of G. vaginalis adherence to host cells [29,39,45,47]. Women colonized by L. crispatus show a decreased risk of developing BV [30]. For all these reasons L. crispatus was included in our experimental system as positive control.
Here we demonstrate, for the first time, that a S. cerevisiae-based probiotic shows a marked antagonistic effect against G. vaginalis colonization in vaginal environment and preclude the access of G. vaginalis to uterine horns. This is associated with inhibition of important virulence factor such as sialidase activity, with decreased exfoliation of vaginal epithelial cells and decreased adherence to them in model systems. Indeed, GI is able to adhere to vaginal epithelial cells and by these specific traits allows the inhibition of G. vaginalis adherence to EC. Nevertheless, GI not only inhibits G. vaginalis adhesion, but it is also able to displace G. vaginalis attached to EC (see mechanism of action in Fig. 6). It is well known that L. crispatus is able to inhibit the growth of G. vaginalis, however S. cerevisiae, also effective against vaginal candidosis, would represent an additional therapeutic option for preventing or curing vaginal infections.
Figure 6.
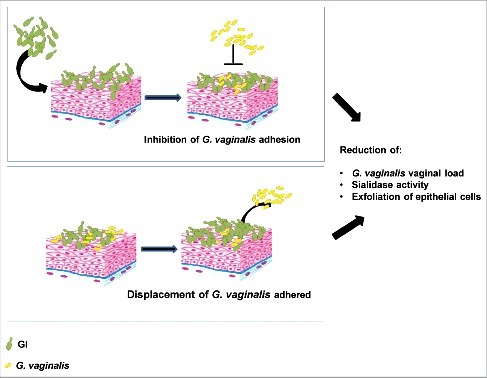
Schematic representation of GI mechanism on G. vaginalis infection. GI, by inhibition of G. vaginalis adhesion and by displacement of G. vaginalis adhered to epithelial cells, reduces G. vaginalis vaginal load and its key virulence factors.
We previously reported that the treatment with GI is beneficial during vaginal candidosis [27] and in this study we demonstrate that GI is also able to antagonize G. vaginalis infection. Indeed, by using a well-known in vivo mouse experimental model [13], we showed that intravaginal administration of S. cerevisiae-based probiotic (108/ml) was able to remove, 3 days after infection, about 80% of G. vaginalis from the mouse vagina in all the treated mice. A higher dose of probiotic (109/ml) was not more effective than the lower one reported above. Notably, no intervention of a local immune response appears to be present in the vaginal infection by G. vaginalis, confirming previous results [13]. This is in clear contrast with C. albicans infection [27], as clinically documented, and justifying the use of the mouse model as a useful simulator of human infection.
Reports have identified G. vaginalis as an etiologic agent in puerperal sepsis [48,49], endometritis and septic abortion [50]. The pathogenesis of these infections is considered to be a consequence of the microorganism spread from the vagina to the uterus and urogenital tract, due to mucosal damage during delivery. With this scientific background we determined if GI treatment affected G. vaginalis infection at the level of uterine horns. Indeed, GI significantly reduces the bacterial load and 3 days after infection, it was able to remove up to 90% of G. vaginalis infecting uterine horns. These results consistently demonstrate that GI presents a potential beneficial effect not only in vaginal infections but, also, in ascending infections and its potentially dramatic effects.
Previous investigations have shown that, in the vaginal fluid of BV patients, the levels of sialidase activity were increased compared to those detected in women with normal flora [51,52]. In addition, Gilbert et al. [13], in an in vivo experimental model, reported that the level of sialidase activity correlated with vaginal G. vaginalis titers. In our experimental model GI markedly reduced sialidase activity thereby reducing G. vaginalis virulence. Given that sialidase is an enzyme known to facilitate the destruction of the protective mucus layer on the vaginal epithelium [53] it is conceivable that GI exerts a protective effect from BV. This inhibitory effect could be due to production of GI soluble factors that degrade the enzyme and/or to direct inhibition of gene expression. These results, also, suggest that modulation of sialidase expression, by the use of appropriate probiotics or specific inhibitors could be exploited for therapeutic purposes.
A key feature used to diagnose BV is the formation of clue cells which are the result of exfoliation of vaginal epithelium. A recent paper reports that vaginal epithelial cells exfoliation occurs in an experimental model of G. vaginalis infection and in clinical specimens from women with BV [13]. It is conceivable that a poor exfoliation could be beneficial in eliminating a potential pathogen, whereas a marked exfoliation could facilitate the pathogen diffusion through adhesion to underlying tissues. In our experimental model GI strongly reduces the exfoliation induced by G. vaginalis infection likely avoiding the pathogens spread and adhesion to internal tissues.
Altogether, our data clearly demonstrate that GI has a strong capacity to fight G. vaginalis experimental infection, that its efficiency is comparable to that of L. crispatus, recognized probiotic in the treatment of BV, and that several mechanisms can contribute to this beneficial effect. The probiotic capacity, reported here, to displace adherent G. vaginalis from epithelial vaginal cells and epithelial cervix cells is of special interest for potential therapeutic purposes in humans. However we cannot exclude that other mechanisms, generated by cell-cell contact, could interfere with expression of virulence gene and/or affecting the growth conditions. To our knowledge this is the first report demonstrating that S. cerevisiae-based probiotic can exert an inhibitory effect on G. vaginalis infection. Collectively our data suggest the potential use of S. cerevisiae-based probiotic for the prophylaxis and/or treatment of bacterial vaginosis. Our results strongly encourage further studies about the capacity of this probiotic to prevent and manage urogenital tract infections in women.
Materials and methods
Study products
The product studied was provided by Lesaffre Human Care (Marcq-en-Baroeul, France). Saccharomyces cerevisiae (S. cerevisiae) live yeast (referenced GI) is a proprietary, well-characterized strain of Lesaffre, registered in the French National Collection of Cultures of Microorganisms (CNCM) under the number I-3856. The S. cerevisiae species was determined by using phenotypic (API®ID32C, Biomerieux SAS) and genotypic referenced methods (genetic amplification and sequencing of 26S DNA) [54,55]. Moreover, the strain CNCM I-3856 has been characterized by polymerase chain reaction (PCR) Interdelta typing techniques [56] and other genetic methods (e.g., complete genome sequencing).
The specification of the probiotic product is ≥5 × 109 CFU/g and the concentration of the batch used for these trials was 1 × 1010 CFU/g.
The strain of L. crispatus 33820, used in this study, was obtained from the American Type Culture Collection (ATCC).
Microbial strains and growth conditions
Sialidase-positive G. vaginalis clinical isolate was obtained from a vaginal swab from the Microbiology Unit of Santa Maria della Misericordia Hospital of Perugia. The swab was immediately used to inoculate Gardnerella selective agar (GSA) media (plates with 5% of human blood, Becton and Dickinson) and the plates were incubated anaerobically at 37°C for 24–48 hours. ß-haemolytic colonies were isolated and candidate G. vaginalis strains were identified by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF, Bruker Daltonics) mass spectrometry. A spontaneous streptomycin-resistant mutant was isolated by plating G. vaginalis on New York City III (NYC-III) agar plates +1 mg/ml streptomycin and selecting resistant colonies after incubating anaerobically at 37°C for 72 hours. Results for sialidase activity and growth curves of resistant mutant were indistinguishable from those of the clinical isolate. The G. vaginalis resistant mutant has been used for both our in vivo and in vitro experimental models. L. crispatus ATCC 33820 was grown anaerobically in de Man, Rogosa and Sharpe broth (MRS, Sigma). Before each experiment the strains were harvested by centrifugation for 5 min at 11000 rpm, washed twice with sterile phosphate-buffered saline (PBS, Life Technologies), the concentration adjusted to that desired and resuspended in the appropriate buffer.
Ethics statement
The procedures involving the animals and their care were conducted in conformity with the national and international laws and policies. All animal experiments were performed in agreement with the EU Directive 2010/63, the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes, and the National Law 116/92. The protocol was approved by Perugia University Ethics Committee for animal care and use (Comitato Universitario di Bioetica, permit number 308/2017-PR). All the animals were housed in the animal facility of the University of Perugia (Authorization number 34/2003A). Mice were acclimatized for a week before starting the experiments. 6 mice were housed in each cage and were provided with food and water ad libitum. All efforts were made to minimize suffering during experiments.
Mice
Female C57/Bl6 mice obtained from Charles River (Calco, Italy) and acclimatized for 1 week before starting experiments were used at 5 to 7 weeks of age. Animals were used under specific-pathogen free conditions that included testing sentinels for unwanted infections. According to the Federation of European Laboratory Animal Science Association standards, no infections were detected.
Culture of A-431 and HeLa cell lines
A-431 (ATCC CRL-1555) and HeLa epithelial cells (ATCC CCL-2) were cultured, at 37°C and in 5% CO2, in DMEM supplemented with 15% (vol/vol) fetal bovine serum (FBS, Life Technologies) and 1 IU penicillin/streptomycin ml−1 (Lonza). Cells were cultured, at 37°C and 5% CO2, in 24-well tissue culture plates (Iwaki) until they formed a monolayer. Before the adhesion assays, the cells were washed twice with 500 μl of sterile phosphate -buffered saline (PBS) to remove non adherent cells and culture media.
G. vaginalis infection model
A mouse model of G. vaginalis infection was previously described by Gilbert et al. [13].Mice were injected with 0.5 mg ß-estradiol in 100 μl sesame oil three days prior to and on the day of infection. A suspension of ∼ 5 × 107 CFU of G. vaginalis in 20 μl of sterile PBS was vaginally inoculated in mice anaesthetized with isoflurane. GI (108/ml = 10 mg/ml or 109/ml = 100 mg/ml) or L. crispatus (2 × 109/ml = 10 mg/ml or 2 × 1010/ml = 100 mg/ml) were administered intravaginally (10 μl/mouse) two days before challenge and once a day for 3 days beginning the day of infection.
At days 1 and 3 post-infection, the mice were sacrificed and vaginal washes were collected by flushing vaginas with sterile physiological solution. The fluid was serially diluted and plated on NYC-III agar plates +1 mg/ml streptomycin and 4 mg/L amphotericin. Colonies were, then, enumerated and expressed as CFU/ml. The percentage of CFU reduction, as consequence of treatment with probiotics, was determined by subtracting the G. vaginalis CFU of probiotics-treated mice from G. vaginalis CFU of saline-treated mice and expressed as the percentage of CFU decrease. Vaginal washes were, also, tested for sialidase activity, epithelial exfoliation and cytokines levels as described below.
In selected experiments at days 1 and 3 post-infection, the mice were sacrificed and half of the vaginas and one uterine horn from each mouse were harvested, homogenized and plated on NYC-III agar plates +1 mg/ml streptomycin and 4 mg/L amphotericin for CFU evaluation as for vaginal washes. The remaining vaginal tissue were fixed in 10% buffered formalin phosphate, embedded in paraffin, sectioned into 3 to 4 µm thick sections, and stained with H&E.
Sialidase activity assay
Sialidase activity was assessed in vaginal wash samples. Briefly, 50 μl of each vaginal wash were diluted 1:1 with working solution of Amplex Red Neuraminidase (Sialidase) Assay Kit (Thermo) and incubated at 37°C. The kinetics of the reactions were followed by measuring absorbance at 560 nm at multiple time points using a Tecan Infinite M200 plate reader.
Epithelial cell exfoliation
To assess the exfoliation of mouse vaginal epithelium, wet mounts were prepared with 5 µl of vaginal wash and visualized by phase contrast microscopy using Olympus KX31 microscope. Samples score was assigned from 0 to 3 depending on the average number of epithelial cells in microscope fields: 0 = cells number <25, 1 = cells number from 25 to 50, 2 = cells number from 50 to 75 and 3 = cells number >75 [13].
Cytokines
Supernatants of vaginal washes were collected and tested for Interleukin-1ß (IL-1ß), TNF-α, IL-6 and IL-10 levels by specific ELISAs (Thermo Fisher Scientific). Cytokine titers were calculated relative to standard curves.
Adhesion and displacement assays
Two distinct experiments were performed to study the influence of probiotics on the adhesion mechanisms of G. vaginalis to epithelial cells. First, the interference of pre-adhered probiotics, on epithelial cells, towards G. vaginalis was evaluated. To this aim, two distinct cell quantities (2 × 107/ml and 2 × 108/ml) [29] of each probiotic were added to each well of the 24-well containing the monolayers. The plates were incubated for 4 h at 37°C in anaerobic conditions. Non adherent probiotics were removed by washing with 500 μl of sterile PBS (2 times) then G. vaginalis (2 × 108/ml) [29] was incubated with the monolayers (final volume 500 µl) for 30 min at 37°C in anaerobic conditions. Each well was carefully washed (2 times) with 500 μl of sterile PBS to remove non-adherent bacteria. To evaluate CFU of adhered G. vaginalis, the medium was removed and Trypsin/EDTA solution (200 µl) was added in each well to dissociate cells [27]. Hence, the cellular suspension was serially diluted, plated onto NYC-III agar plates and incubated at 37°C for 48 h in anaerobic condition. The G. vaginalis load was quantified as the number of CFU/ml. In the second set of experiments, the ability of probiotics to displace G. vaginalis pre-adhered to monolayers was assessed. To this end, G. vaginalis (2 × 108/ml) was incubated with the monolayers for 30 min at 37°C in anaerobic conditions. Wells were washed twice with 500 μl of sterile PBS to remove non adherent bacteria, then probiotics (2 × 107/ml or 2 × 108/ml) were added to the appropriate wells for 30 min at 37°C in anaerobic conditions. Finally, each well was washed twice with sterile PBS to remove non-adherent G. vaginalis and probiotics [29]. Quantification of G. vaginalis adherent to epithelial cells was performed as above described and expressed as CFU/ml.
Co-aggregation assay
The co-aggregation assay was performed as previously described [57]. G. vaginalis cells (1 × 109/ml) were labeled with Fluorescein isothiocyanate (FITC, Sigma) at 0.1 mg/ml in PBS at room temperature (RT) for 10 min. L. crispatus (2 × 109/ml) and GI (108/ml) were labeled with Rhodamine B (0.5 mg/ml, Sigma) in PBS for 20 min at RT. Briefly, G. vaginalis or FITC-G. vaginalis (1 × 109/ml) in PBS were mixed with equal volume of L. crispatus or RhB-L. crispatus (2 × 109/ml) or with equal volume of GI or RhB-GI (108/ml). Then samples were vortexed for at least 10 sec and incubated in a 24 well plate for 4 h at 37°C under agitation. The suspensions were then observed by inversion light microscopy to evaluate the aggregation degree and scored according to the following scale: 0 = no aggregation, 1 = small aggregates comprising small visible clusters, 2 = aggregates comprising larger numbers of microorganisms, settling down to the center of the well, 3 = macroscopically visible clumps comprising larger groups which settle to the center of the well, 4 = maximum score allocated to describe a large, macroscopically visible clump in the center of the well [57]. Moreover, each fluorescent suspension was analyzed under a fluorescence microscope (Carl Zeiss).
Statistical analysis
GraphPad Prism 7.0 software was used for all statistical analysis presented. For the analysis of sialidase activity, differences between L. crispatus- or GI-treated infected mice vs saline-treated infected mice were evaluated by Mann-Whitney U-test. For the other experiments, the results were evaluated by Student's t test. Values of p < 0.05 were considered significant.
Supplementary Material
Funding Statement
This work was realized in the frame of service agreement supported by Lesaffre International.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Sobel JD. Bacterial vaginosis. Annu Rev Med. 2000;51:349–56. doi: 10.1146/annurev.med.51.1.349. PubMed PMID:10774469. [DOI] [PubMed] [Google Scholar]
- [2].Guerra B, Ghi T, Quarta S, et al.. Pregnancy outcome after early detection of bacterial vaginosis. Eur J Obstet Gynecol Reprod Biol. 2006, 128(1–2):40–5. doi: 10.1016/j.ejogrb.2005.12.024. PubMed PMID:16460868. [DOI] [PubMed] [Google Scholar]
- [3].Leitich H, Bodner-Adler B, Brunbauer M, et al.. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003, 189(1):139–47. PubMed PMID:12861153. [DOI] [PubMed] [Google Scholar]
- [4].Rothman KJ, Funch DP, Alfredson T, et al.. Randomized field trial of vaginal douching, pelvic inflammatory disease and pregnancy. Epidemiology. 2003, 14(3):340–8. PubMed PMID:12859036. [PubMed] [Google Scholar]
- [5].Jacobsson B, Pernevi P, Chidekel L, et al.. Bacterial vaginosis in early pregnancy may predispose for preterm birth and postpartum endometritis. Acta Obstet Gynecol Scand. 2002, 81(11):1006–10. PubMed PMID:12421167. [DOI] [PubMed] [Google Scholar]
- [6].Gallo MF, Macaluso M, Warner L, et al.. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemiol. 2012, 22(3):213–20. doi: 10.1016/j.annepidem.2011.11.005. PubMed PMID:22192490. [DOI] [PubMed] [Google Scholar]
- [7].Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011, 121(12):4610–7. doi: 10.1172/JCI57172. PubMed PMID:22133886; PubMed Central PMCID:PMCPMC3225992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koumans EH, Sternberg M, Bruce C, et al.. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007, 34(11):864–9. doi: 10.1097/OLQ.0b013e318074e565. PubMed PMID:17621244. [DOI] [PubMed] [Google Scholar]
- [9].Ravel J, Gajer P, Abdo Z, et al.. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011, 108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. PubMed PMID:20534435; PubMed Central PMCID:PMCPMC3063603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Backer E, Verhelst R, Verstraelen H, et al.. Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners. BMC Microbiol. 2007, 7:115. doi: 10.1186/1471-2180-7-115. PubMed PMID:18093311; PubMed Central PMCID:PMCPMC2233628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Verhelst R, Verstraelen H, Claeys G, et al.. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol. 2004, 4:16. doi: 10.1186/1471-2180-4-16. PubMed PMID:15102329; PubMed Central PMCID:PMCPMC419343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schwebke JR, Muzny CA, Josey WE. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis. 2014, 210(3):338–43. doi: 10.1093/infdis/jiu089. PubMed PMID:24511102. [DOI] [PubMed] [Google Scholar]
- [13].Gilbert NM, Lewis WG, Lewis AL. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One. 2013;8(3):e59539. doi: 10.1371/journal.pone.0059539. PubMed PMID:23527214; PubMed Central PMCID:PMCPMC3602284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Machado D, Castro J, Palmeira-de-Oliveira A, et al.. Bacterial Vaginosis Biofilms: Challenges to Current therapies and Emerging solutions. Front Microbiol. 2015;6:1528. doi: 10.3389/fmicb.2015.01528. PubMed PMID:26834706; PubMed Central PMCID:PMCPMC4718981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hardy L, Jespers V, Van den Bulck M, et al.. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS One. 2017;12(2):e0172522. doi: 10.1371/journal.pone.0172522. PubMed PMID:28241058; PubMed Central PMCID:PMCPMC5328246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prevention CfDCa Sexually transmitted Diseases treatment guideline, 2015. Atlanta, (GA): Center for Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services; 2015. [Google Scholar]
- [17].Mitchell C, Manhart LE, Thomas K, et al.. Behavioral predictors of colonization with Lactobacillus crispatus or Lactobacillus jensenii after treatment for bacterial vaginosis: a cohort study. Infect Dis Obstet Gynecol. 2012;2012:706540. doi: 10.1155/2012/706540. PubMed PMID:22693410; PubMed Central PMCID:PMCPMC3369434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Swidsinski A, Mendling W, Loening-Baucke V, et al.. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008, 198(1):97e1–6. doi: 10.1016/j.ajog.2007.06.039. PubMed PMID:18005928. [DOI] [PubMed] [Google Scholar]
- [19].Alves P, Castro J, Sousa C, et al.. Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using in an in vitro biofilm formation model. J Infect Dis. 2014, 210(4):593–6. doi: 10.1093/infdis/jiu131. PubMed PMID:24596283. [DOI] [PubMed] [Google Scholar]
- [20].Bostwick DG, Woody J, Hunt C, et al.. Antimicrobial resistance genes and Modeling of treatment failure in Bacterial Vaginosis: Clinical study of 289 Symptomatic women. J Med Microbiol. 2016;65:377–86. doi: 10.1099/jmm.0.000236. PubMed PMID:26887782. [DOI] [PubMed] [Google Scholar]
- [21].McFarland LV. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open. 2014, 25;4(8):e005047. doi: 10.1136/bmjopen-2014-005047. PubMed PMID:25157183; PubMed Central PMCID:PMCPMC4156804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hummelen R, Fernandes AD, Macklaim JM, et al.. Deep sequencing of the vaginal microbiota of women with HIV. PLoS One. 2010;5(8):e12078. doi: 10.1371/journal.pone.0012078. PubMed PMID:20711427; PubMed Central PMCID:PMCPMC2920804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sivignon A, de Vallee A, Barnich N, et al.. Saccharomyces cerevisiae CNCM I-3856 prevents colitis induced by AIEC bacteria in the transgenic mouse model mimicking Crohn's disease. Inflamm Bowel Dis. 2015;21(2):276–86. doi: 10.1097/MIB.0000000000000280. PubMed PMID:25569734. [DOI] [PubMed] [Google Scholar]
- [24].Amara AA, Shibl A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm J. 2015; 23(2):107–14. doi: 10.1016/j.jsps.2013.07.001. PubMed PMID:25972729; PubMed Central PMCID:PMCPMC4421088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lim PL, Toh M, Liu SQ. Saccharomyces cerevisiae EC-1118 enhances the survivability of probiotic Lactobacillus rhamnosus HN001 in an acidic environment. Appl Microbiol Biotechnol. 2015;99(16):6803–11. doi: 10.1007/s00253-015-6560-y. PubMed PMID:25846337. [DOI] [PubMed] [Google Scholar]
- [26].Cribby S, Taylor M, Reid G. Vaginal microbiota and the use of probiotics. Interdiscip Perspect Infect Dis. 2008;2008:256490. doi: 10.1155/2008/256490. PubMed PMID:19343185; PubMed Central PMCID:PMCPMC2662373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pericolini E, Gabrielli E, Ballet N, et al.. Therapeutic activity of a Saccharomyces cerevisiae-based probiotic and inactivated whole yeast on vaginal candidiasis. Virulence. 2017,8(1):74–90. doi: 10.1080/21505594.2016.1213937. PubMed PMID:27435998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Joo HM, Hyun YJ, Myoung KS, et al.. Lactobacillus johnsonii HY7042 ameliorates Gardnerella vaginalis-induced vaginosis by killing Gardnerella vaginalis and inhibiting NF-kappaB activation. Int Immunopharmacol. 201111(11):1758–65. doi: 10.1016/j.intimp.2011.07.002. PubMed PMID:21798373. [DOI] [PubMed] [Google Scholar]
- [29].Castro J, Henriques A, Machado A, et al.. Reciprocal interference between Lactobacillus spp. and Gardnerella vaginalis on initial adherence to epithelial cells. Int J Med Sci. 2013;10(9):1193–8. doi: 10.7150/ijms.6304. PubMed PMID:23935396; PubMed Central PMCID:PMCPMC3739018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Verstraelen H, Verhelst R, Claeys G, et al.. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol. 2009;9:116. doi: 10.1186/1471-2180-9-116. PubMed PMID:19490622; PubMed Central PMCID:PMCPMC2698831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Breshears LM, Edwards VL, Ravel J, et al.. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015;15:276. doi: 10.1186/s12866-015-0608-0. PubMed PMID:26652855; PubMed Central PMCID:PMCPMC4675025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Leprat R, Michel-Briand Y. Extracellular neuraminidase production by a strain of Pseudomonas aeruginosa isolated from cystic fibrosis. Ann Microbiol (Paris). 1980;131B(3):209–22. PubMed PMID:6781392. [PubMed] [Google Scholar]
- [33].Lipovac V, Bigalli G, Rosenberg A. Enzymatic action of sialidase of Vibrio cholerae on brain gangliosides above and below the critical micelle concentration. J Biol Chem. 1971;246(24):7641–8. PubMed PMID:5135317. [PubMed] [Google Scholar]
- [34].Scanlon KL, Diven WF, Glew RH. Purification and properties of Streptococcus pneumoniae neuraminidase. Enzyme. 1989;41(3):143–50. PubMed PMID:2542012. [DOI] [PubMed] [Google Scholar]
- [35].Campana R, Federici S, Ciandrini E, et al.. Antagonistic activity of Lactobacillus acidophilus ATCC 4356 on the growth and adhesion/invasion characteristics of human Campylobacter jejuni. Curr Microbiol. 2012;64(4):371–8. doi: 10.1007/s00284-012-0080-0. PubMed PMID:22271268. [DOI] [PubMed] [Google Scholar]
- [36].Mappley LJ, Tchorzewska MA, Cooley WA, et al.. Lactobacilli antagonize the growth, motility, and adherence of Brachyspira pilosicoli: a potential intervention against avian intestinal spirochetosis. Appl Environ Microbiol. 2011;77(15):5402–11. doi: 10.1128/AEM.00185-11. PubMed PMID:21666022; PubMed Central PMCID:PMCPMC3147436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McLean NW, Rosenstein IJ. Characterisation and selection of a Lactobacillus species to re-colonise the vagina of women with recurrent bacterial vaginosis. J Med Microbiol. 2000;49(6):543–52. doi: 10.1099/0022-1317-49-6-543. PubMed PMID:10847208. [DOI] [PubMed] [Google Scholar]
- [38].Mastromarino P, Brigidi P, Macchia S, et al.. Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J Appl Microbiol. 2002;93(5):884–93. PubMed PMID:12392537. [DOI] [PubMed] [Google Scholar]
- [39].Kaewsrichan J, Peeyananjarassri K, Kongprasertkit J. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunol Med Microbiol. 2006;48(1):75–83. doi: 10.1111/j.1574-695X.2006.00124.x. PubMed PMID:16965354. [DOI] [PubMed] [Google Scholar]
- [40].van de Wijgert JH, Borgdorff H, Verhelst R, et al.. The vaginal microbiota: what have we learned after a decade of molecular characterization?. PLoS One. 2014;9(8):e105998. doi: 10.1371/journal.pone.0105998. PubMed PMID:25148517; PubMed Central PMCID:PMCPMC4141851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Srinivasan S, Hoffman NG, Morgan MT, et al.. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. PubMed PMID:22719852; PubMed Central PMCID:PMCPMC3377712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Macklaim JM, Clemente JC, Knight R, et al.. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb Ecol Health Dis. 2015;26:27799. doi: 10.3402/mehd.v26.27799. PubMed PMID:26282697; PubMed Central PMCID:PMCPMC4539393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dover SE, Aroutcheva AA, Faro S, et al.. Natural Antimicrobials and their role in Vaginal health: A Short review. Int J Probiotics Prebiotics. 2008;3(4):219–230. PubMed PMID:20657710; PubMed Central PMCID:PMCPMC2908489. [PMC free article] [PubMed] [Google Scholar]
- [44].Homayouni A, Bastani P, Ziyadi S, et al.. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79–86. doi: 10.1097/LGT.0b013e31829156ec. PubMed PMID:24299970. [DOI] [PubMed] [Google Scholar]
- [45].Donnarumma G, Molinaro A, Cimini D, et al.. Lactobacillus crispatus L1: high cell density cultivation and exopolysaccharide structure characterization to highlight potentially beneficial effects against vaginal pathogens. BMC Microbiol. 2014;14:137. doi: 10.1186/1471-2180-14-137. PubMed PMID:24884965; PubMed Central PMCID:PMCPMC4054921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tamrakar R, Yamada T, Furuta I, et al.. Association between Lactobacillus species and bacterial vaginosis-related bacteria, and bacterial vaginosis scores in pregnant Japanese women. BMC Infect Dis. 2007;7:128. doi: 10.1186/1471-2334-7-128. PubMed PMID:17986357; PubMed Central PMCID:PMCPMC2212641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Teixeira GS, Carvalho FP, Arantes RM, et al.. Characteristics of Lactobacillus and Gardnerella vaginalis from women with or without bacterial vaginosis and their relationships in gnotobiotic mice. J Med Microbiol. 2012;61(Pt 8):1074–81. doi: 10.1099/jmm.0.041962-0. PubMed PMID:22539000. [DOI] [PubMed] [Google Scholar]
- [48].Adeniyi-Jones C, Groves DJ, Mannethu A, et al.. Hemophilus vaginalis bacteremia. Can Med Assoc J. 1980;122(4):424–6. PubMed PMID:6966179; PubMed Central PMCID:PMCPMC1801800. [PMC free article] [PubMed] [Google Scholar]
- [49].Reimer LG, Reller LB. Gardnerella vaginalis bacteremia: a review of thirty cases. Obstet Gynecol. 1984;64(2):170–2. PubMed PMID:6610845. [PubMed] [Google Scholar]
- [50].Johnson AP, Boustouller YL. Extra-vaginal infection caused by Gardnerella vaginalis. Epidemiol Infect. 1987;98(2):131–7. PubMed PMID:3493915; PubMed Central PMCID:PMCPMC2235249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Briselden AM, Moncla BJ, Stevens CE, et al.. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J Clin Microbiol. 1992;30(3):663–6. PubMed PMID:1551983; PubMed Central PMCID:PMCPMC265128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Moncla BJ, Chappell CA, Debo BM, et al.. The Effects of Hormones and Vaginal Microflora on the Glycome of the Female Genital tract: Cervical-Vaginal fluid. PLoS One. 2016;11(7):e0158687. doi: 10.1371/journal.pone.0158687. PubMed PMID:27437931; PubMed Central PMCID:PMCPMC4954690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lewis WG, Robinson LS, Gilbert NM, et al.. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem. 2013;288(17):12067–79. doi: 10.1074/jbc.M113.453654. PubMed PMID:23479734; PubMed Central PMCID:PMCPMC3636892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kurtzman CP, Robnett CJ. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol. 1997;35(5):1216–23. PubMed PMID:9114410; PubMed Central PMCID:PMCPMC232732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kurtzman CP, Robnett CJ. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek. 1998, May;73(4):331–71. PubMed PMID:9850420. [DOI] [PubMed] [Google Scholar]
- [56].ECF S. Animal feedingstuffs, CEN/TS 15790:2008. PCR typing of probiotic strains of Saccharomyces cerevisiae (yeast). 2009. PubMed PMID:ISBN 978-0-580-61806-2. [Google Scholar]
- [57].Verdenelli MC, Coman MM, Cecchini C, et al.. Evaluation of antipathogenic activity and adherence properties of human Lactobacillus strains for vaginal formulations. J Appl Microbiol. 2014;116(5):1297–307. doi: 10.1111/jam.12459. PubMed PMID:24552173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


