Abstract
Objective
To determine the prevalence and epidemiologic associations of Zika Virus (ZIKV) in HIV-infected patients in Ghana, West Africa.
Methods
We examined the seroprevalence of ZIKV in HIV/HBV co-infected persons in Ghana from sera samples collected from 2012 to 2014 using ELISA assays and plaque reduction neutralization tests (PRNT).
Results
Overall, ZIKV antibody was detected in 12.9% of 236 tested samples, though the true estimate of exposure is probably less due cross-reactions with other related viruses. PRNTs were performed on a subset to provide an estimate of the frequency of false positive reaction. Dengue virus testing was also performed and antibody prevalence was 87.2%. The median CD4 count was 436 (range 2–1781 cell/mm3) and did not affect antibody results. Regional geographic ethnicity was associated with ZIKV exposure.
Discussion
Overall, these data suggest that ZIKV infection is a relatively prevalent infection in HIV-positive persons in Ghana though not as common as dengue. Further evaluation of the effect of ZIKV and HIV co-infection is warranted given the large geographical overlap of populations exposed to both viruses.
Keywords: Zika Virus, HIV, prevalence, Dengue
Introduction
Though Zika Virus has emerged as an important mosquito-borne pathogen in the Western world, there are few studies regarding the prevalence and significance of Zika Virus (ZIKV; Flaviridae, Flavivirus) in people with HIV infection. To date, there are limited case reports regarding acute ZIKV infection in those with HIV. 1,2,3 Thus, we sought to 1) determine the prevalence of ZIKV exposure in an HIV/HBV-infected cohort in Ghana, West Africa, an area where ZIKV is thought to be endemic, and 2) to identify laboratory and clinical correlates of infection in the setting of HIV-associated immunosuppression. Many viruses exhibit alterations of natural history in the setting of HIV. For example, the hepatitis C virus demonstrates increased rates of chronicity, higher viral loads and accelerated liver injury in those with HIV. Dengue virus antibody was also evaluated since it was thought to be a common infection in West Africa, and its presence could lead to false positive reactions with ZIKV antibody tests.
Methods
Clinical Samples
236 human sera infected with HIV and HBV were utilized. They were collected from a patient population who were receiving care at Korle-Bu teaching Hospital in Accra, Ghana between 2012 and 2014.4 The sample collection was part of a study of HBV treatment outcomes in the setting of HIV coinfection. Informed consent for creation of a sample repository and associated clinical data was obtained from all patients. De-identified samples were sent to the University of Cincinnati College of Medicine for Zika/dengue testing and evaluation.
Quantitative reverse transcription PCR
Viral RNA was extracted from sera using the QIAmp Ultrasense virus kit (Qiagen, Valencia, CA). RNA was quantified by qRT-PCR using a commercial kit using proprietary primers designed to amplify ZIKV RNA (MyBioSource, San Diego, CA). The kit included an internal control used during the nucleic acid extraction process to verify the presence of amplifiable RNA and to identify possible RT-PCR inhibition. Viral RNA from sera also was screened by nested RT-PCR (nRT-PCR) using degenerate primers targeting a region of the NS5 gene that is conserved among 60 different flaviviruses.5
ZIKV NS1-specific ELISA
IgM and IgG ZIKV-specific antibody responses were assessed using the Euroimmun diagnostic kit assay. Briefly, a 1:100 dilution of serum was performed in duplicate and added to pre-coated plates. The assay was performed following the manufacturer’s instructions, with photometric measurements taken at 450nm. All ZIKV IgG positive samples and a subset of ZIKV negatives were evaluated for IgG DENV-specific antibody responses using the Abcam (Abcam, Cambridge, MA) anti-dengue IgG ELISA kit.
Plaque Reduction Neutralization Test (PRNT)
Titers of neutralizing antibody to ZIKV and DENV were determined by PRNT on Vero cells (ATCC #CCL-81) with a cutoff value of 90% (PRNT90).6 Neutralization curves were generated using GraphPad Prism software, and the resulting data were analyzed by non-linear regression to estimate the dilution of serum required to inhibit 90% of infection. We considered a patient to have confirmed ZIKV exposure if ZIKV PRNT90 was at least 20, and a ratio of ZIKV PRNT90 to DENV PRNT90 titer of at least 4.
Results
During 2012–2014, 236 patients receiving care for HIV/HBV co-infection at the Korle-Bu teaching Hospital in Accra, Ghana agreed to participate in the research study. The study population was 41% male with a mean age of 40.9 years. Sixty-five percent were using antiretroviral therapy and the remainder were ART naive. The median CD4 count was 436 cells/mm3. The WHO HIV stage distribution was as follows: 1– 24%; 2 – 25%; 3 – 39%; 4 – 12%. The mean serum alanine aminotransferase (ALT) was 35 U/ml.
The samples were analyzed at the University of Cincinnati by qRT-PCR to detect ZIKV RNA and nRT-PCR to detect flavivirus RNA. No ZIKV RNA nor any flavivirus RNA was detected in any of the 236 samples.
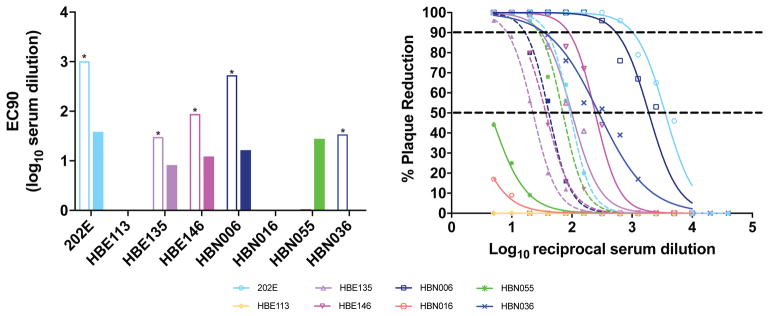
To evaluate the potential likelihood of recent and prior exposure, samples were screened for the presence of ZIKV antibody. Of the 236 samples, 29 (12.3%) were anti-ZIKV IgG positive. In contrast, DENV IgG was much more common, occurring in 87.2% of tested individuals (n = 82). In addition, 2 samples (3%) were also anti-ZIKV IgM positive, which is suggestive of a recent exposure. However, the duration of ZIKV IgM antibodies in serum remains unknown, and positive results could be confounded by cross-reactivity with related flaviviruses. Indeed, both patient samples that were ZIKV IgM positive also were DENV IgG positive; and 100% of ZIKV IgG positive samples also were DENV IgG positive. However, the Euroimmune NS-1 anti-ZIKV IgG and IgM ELISA tests have been reported to be highly specific and reliable for use in individuals with previous vaccination or flavivirus exposure. Serum panels from that study showed no cross reactivity with Chikungunya, dengue, TBE, yellow fever, or West Nile virus. 7 To further characterize the association with DENV, we performed PRNT on a subset of ZIKV positive and negative samples. This included 8 suspected ZIKV exposures, as well as 5 ZIKV negative but DENV antibody positive samples. Among the 8 suspected ZIKV positives, 5 were confirmed ZIKV positive, 1 was confirmed ZIKV negative but DENV positive, and the remaining two were both ZIKV and DENV negative (Figure 1.) In the group of DENV antibody positive, ZIKV antibody negative samples, 3 of 5 were confirmed to be DENV positive, and 1 was classified as indeterminate. The fifth sample was negative for DENV neutralization.
Figure 1.
Plaque Reduction Neutralization Test (PRNT) data against both ZIKV and DENV. Left panel: open bars are ZIKV PRNT90 titers and closed bars are DENV PRNT90 titers. Starred samples are ZIKV positive (defined as ZIKV PRNT90 of at least 20, and a ratio of ZIKV PRNT90 titer to DENV PRNT90 titer of at least 4.). Sample HBN055 is DENV positive. Right panel: Solid lines are ZIKV curves, and dashed lines are DENV curves.
The median CD4 of IgG positive patients was 466 cells/mm3. In a multivariable least squares linear regression analysis, age, gender, WHO HIV stage, CD4, serum ALT, HIV viral load and HBV viral load were not associated with ZIKV antibody positivity. In contrast, regional ethnicity (p = 0.02) appeared to be associated with ZIKV IgG positivity in the model. The highest rates were observed in the Greater Accra and Upper East regions.
Discussion
In summary, ZIKV antibody was detected in 12.3% in HIV-infected persons attending a teaching hospital HIV clinic in Accra, Ghana. Dengue virus is endemic in that region as shown by all ZIKV antibody positive persons having evidence of prior dengue infection as well. Priyamvada et al. noted high levels of cross-reaction between dengue antibodies and ZIKV, and suggested that such antibodies could modulate ZIKV infections.8 Indeed, flavivirus field research is complicated by issues of cross reactivity between many closely related viruses. Plaque reduction neutralization testing confirmed ZIKV seropositivity in 5 samples. It is most likely that the majority of subjects have been exposed to both Zika and dengue as the prevalence of dengue antibody is much higher than that observed for ZIKV antibody. The true prevalence of ZIKV in this HIV-infected population cannot be definitively determined but appears to be significant. The multivariable model identified regional ethnicity as a highly significant factor in ZIKV antibody reactivity. The highest rates were observed in those originating from the Greater Accra and Upper East regions of the country. We are unable to determine if the regional ethnicities are associated with lifestyle or environmental condition that provide greater exposure to ZIKV. Other limitations of our study include the lack of PRNT testing for ZIKV and DENV in all positive samples, as this is a labor-intensive process. The lack of detectable ZIKV viremia is reassuring, suggesting that despite frequent virus exposure, chronicity evidenced by viremia is not a key feature of infection in those with HIV. However, recent data does suggest that ZIKV does persist in protected compartments in immunocompetent patients9 and we would suspect that this might be more frequent in those with HIV.
As the ZIKV epidemic in the Americas wanes, it will become increasingly important to understand cofactors that might predispose an individual to more severe or even chronic disease. This in turn can help direct limited resources to populations most at risk. Indeed, the NIH recently initiated an international, multisite clinical study, “Prospective Cohort Study of HIV and Zika in Infants and Pregnancy” (HIV ZIP; ClinicalTrials.gov identifier NCT03263195) to enroll 2,000 pregnant women to study ZIKV/HIV co-morbidity in the continental United States, Brazil, and Puerto Rico. Still, more information is needed on the prevalence of ZIKV in Africa, because a growing body of evidence suggests that in Africa a Zika epidemic could be undetected and/or overlooked. Studies have claimed to show widespread exposure to Zika in at least 25 countries across Africa, but overall data regarding the prevalence of ZIKV infection in Africa have been limited. Herrera and colleagues reported that Zika virus IgM prevalence was 6.2% in cohorts derived from patients in Senegal and Nigeria and suggest that ZIKV has been silently circulating in West Africa for at least 20 or more years.10,11 These cohorts included over 200 HIV positive patients. The data presented herein highlight the critical need for accurate laboratory diagnostics and suggest that proper surveillance activities regarding ZIKV in sub-Saharan Africa are imperative.
In a “Hot News” editorial commentary about ZIKV in AIDS Reviews, Barreiro suggested that “exposure in patients with comorbidities and immunosuppression may unveil new and more severe clinical manifestations.”3 Therefore, comprehensive longitudinal follow-up of ZIKV cases in HIV-infected persons may yield new and valuable observations.
Acknowledgments
Source of Funding This work was supported by funding from CFAR Grant AI042853 and by a Distinguished Investigator Award from the University of Cincinnati Department of Medicine. MTA received NIH funding from R01AI132563, R56AI132563, and R21AI131454.
Footnotes
Conflicts of Interest: All authors declare no conflicts of interest.
References
- 1.Calvet GA, Filippis AM, Mendonca MC, et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. J Clin Virol. 2016;74:1–3. doi: 10.1016/j.jcv.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Penot P, Brichler S, Guilleminot J, et al. Infectious Zika virus in vaginal secretions from an HIV-infected woman, France, August 2016. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2017:22. doi: 10.2807/1560-7917.ES.2017.22.3.30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreiro P. First Case of Zika Virus Infection in a HIV+ Patient. AIDS reviews. 2016;18:112. [PubMed] [Google Scholar]
- 4.Archampong TN, Lartey M, Sagoe KW, et al. Proportion and factors associated with Hepatitis B viremia in antiretroviral treatment naive and experienced HIV co-infected Ghanaian patients. BMC infectious diseases. 2016;16:14. doi: 10.1186/s12879-016-1342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher-Sturgess SL, Forrester NL, Wayper PJ, et al. Universal primers that amplify RNA from all three flavivirus subgroups. Virology journal. 2008;5:16. doi: 10.1186/1743-422X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsey HS, Calisher CH, Mathews JH. Serum dilution neutralization test for California group virus identification and serology. Journal of clinical microbiology. 1976;4:503–10. doi: 10.1128/jcm.4.6.503-510.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2016:21. doi: 10.2807/1560-7917.ES.2016.21.16.30203. [DOI] [PubMed] [Google Scholar]
- 8.Priyamvada L, Quicke KM, Hudson WH, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A. 2016;113:7852–7. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Montalva A, Pou D, Sulleiro E, et al. Zika virus dynamics in body fluids and risk of sexual transmission in a non-endemic area. Tropical medicine & international health: TM & IH. 2018;23:92–100. doi: 10.1111/tmi.13019. [DOI] [PubMed] [Google Scholar]
- 10.Herrera BB, Chang CA, Hamel DJ, et al. Continued Transmission of Zika Virus in Humans in West Africa, 1992–2016. The Journal of infectious diseases. 2017;215:1546–50. doi: 10.1093/infdis/jix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera BB, Tsai WY, Chang CA, et al. Sustained specific and cross-reactive T cell responses to Zika and Dengue viruses NS3 in West Africa. Journal of virology. 2018 doi: 10.1128/JVI.01992-17. [DOI] [PMC free article] [PubMed] [Google Scholar]