Abstract
Immune thrombocytopenia (ITP) is an acquired autoimmune bleeding disorder which presents with isolated thrombocytopenia and risk of hemorrhage. While most children with ITP promptly recover with or without drug therapy, ITP is persistent or chronic in others. When needed, how to select second-line therapies is not clear. ICON1, conducted within the Pediatric ITP Consortium of North America (ICON), is a prospective, observational, longitudinal cohort study of 120 children from 21 centers starting second-line treatments for ITP which examined treatment decisions. Treating physicians reported reasons for selecting therapies, ranking the top three. In a propensity weighted model, the most important factors were patient/parental preference (53%) and treatment-related factors: side effect profile (58%), long-term toxicity (54%), ease of administration (46%). possibility of remission (45%), and perceived efficacy (30%). Physician, health system, and clinical factors rarely influenced decision-making. Patient/parent preferences were selected as reasons more often in chronic ITP (85.7%) than in newly diagnosed (0%) or persistent ITP (14.3%, p=0.003). Splenectomy and rituximab were chosen for the possibility of inducing long-term remission (p<0.001). Oral agents, such as eltrombopag and immunosuppressants, were chosen for ease of administration and expected adherence (p<0.001). Physicians chose rituximab in patients with lower expected adherence (p=0.017). Treatment choice showed some physician and treatment center bias. This study illustrates the complexity and many factors involved in decision-making in selecting second-line ITP treatments, given the absence of comparative trials. It highlights shared decision-making and the need for well-conducted, comparative effectiveness studies to allow for informed discussion between patients and clinicians.
Keywords: Immune thrombocytopenia, Decision making, Children, Rituximab, Thrombopoietin Receptor Agonists, Immunosuppression
Introduction
Immune thrombocytopenia (ITP) is an uncommon hematologic condition during childhood. Despite presenting with severe thrombocytopenia, only 0.4–0.6% of affected children will have intracranial hemorrhage (ICH) and <3% will have other severe (non-ICH) bleeding at diagnosis.[1, 2] The majority (70–80%) will experience complete resolution of their disorder within 12 months from diagnosis [3, 4]. Many such children can be safely managed initially with close observation, with the decision to initiate front-line therapy with corticosteroids, anti-D immunoglobulin, or intravenous immunoglobulin (IVIG) based on signs or symptoms of mucocutaneous bleeding or bleeding risk [5, 6].
There has been extensive controversy regarding how to manage newly diagnosed children with ITP. The indications, timing, and choice of second-line treatment in children with ITP, when needed, are more complex and highly variable among treating physicians [7]. Among many options are splenectomy, rituximab, oral immunosuppressive agents, thrombopoietin receptor agonists (TPO-RA), and intermittent first-line treatments (e.g., IVIG or corticosteroids). Each differs in potential short and long-term efficacy and side effect profiles. Additional considerations include patient age, different routes of administration, dietary considerations, monitoring, and costs, which in turn influence patient, family, and physician preference. Therefore, selection of a specific second-line treatment for a child with ITP is complex and ideally would be individualized.
In shared decision-making, health care decisions are made together by the patient and/or caregiver along with the medical provider. The goal is to fully engage in an informed consent model of care by improving communication between providers and their patients or caregivers. One recent study suggested a lack of shared decision-making in the care of children with ITP [8]. Focus groups have revealed that parents of children with ITP commonly have feelings of anxiety and confusion and feel they had little choice in the decision for treatment. Physicians also have their own biases, which influence their decision-making [8–10]. A few studies have attempted to address the importance of decision modeling and shared decision-making for first-line ITP therapy [7], but factors involved in decision-making around second-line therapies for children with ITP have not been studied.
ICON1 was subsequently designed as a prospective, observational, longitudinal cohort study of children starting second-line treatments for ITP to be conducted by the Pediatric ITP Consortium of North America (ICON) [11]. In this report, the factors physicians prioritize when selecting second-line treatments for individual patients were explored.
Methods
ICON1 Study Design
Prior to study initiation, a focus group was convened to determine which factors would be ascertained in assessment of physician decision-making. This focus group, consisting of 13 investigators with expertise in ITP, designed a conceptual model (Supplemental Figure 1), which was then used in ICON1. The process by which the conceptual model was derived is described in the Supplemental materials.
ICON1 is a longitudinal observational cohort of 120 children with ITP requiring second-line treatments. Participants were enrolled from 2013–2015 at 21 centers following local IRB approval. All participants had their caregivers provide consent for participation in the study. Enrollment requirements included: age 1–17 years and starting a second-line treatment (i.e., all treatments except observation, IVIG, corticosteroids or anti-D immunoglobulin). Patients with secondary ITP were included except for Evans syndrome with prior or ongoing autoimmune hemolytic anemia. Baseline and follow-up demographic and clinical characteristics were recorded, including disease duration, response to prior treatments, and platelet counts.
Hematologists at each ICON site were invited by site investigators to participate in the study. By submitting completed patient forms, physicians thus provided their own consent for information in the forms to be used to understand physician choice. The physician primarily involved in the treatment decision completed the questionnaire after the visit in which the treatment decision was made. The questionnaire listed all the factors outlined by the focus group, and physicians selected all relevant factors and ranked their top 3 factors. Physicians recorded the impact of ITP on the patient’s HRQoL using a 5 point scale.
Statistical Methods
Data were entered into a REDCap database, which was exported to SAS for analysis [12]. Frequencies and means were computed for descriptive purposes. Fisher’s exact test was used to compare treatment choice on categorical variables. Patient age was compared between treatment choices using the Kruskal-Wallis test. Treatments were selected for comparison if they were used in at least 15 evaluable patients in the study cohort. Oral immunosuppressant agents (n=19) were grouped for comparison and included 6-mercaptopurine (n=13), azathioprine (n=1), mycophenolate (n=3), and sirolimus (n=2). To better understand reasons for treatment choice, physicians were asked to select and rank the top three reasons for their treatment choice. A weighted summary of reasons for treatment choice was created by giving the top reason a weight of three, the second reason a weight of two, and the third reason a weight of one. These weights were used to determine the most important reasons for treatment choice. Distribution of treatment choice between centers was restricted to five centers with adequately large sample sizes (n ≥ 8 patients) and was compared using Fisher’s exact test.
Results
ICON1 Demographics
One hundred twenty patients were enrolled in the study (Table 1); the majority (85%, n=102) had primary ITP [13]. The types of secondary ITP included: Evans syndrome (n=9), underlying immunodeficiencies (n=5), rheumatologic conditions (n=3), and inflammatory bowel disease (n=1). The median age was 11.7 years (range 1.2–17.8 years). The majority of children had chronic ITP (n=64, 53%) but a significant proportion were newly diagnosed (n=19, 16%). Children had received a median of 3 prior treatments (range 0–8) with 47 (39%) patients having received at least one prior second-line treatment, including rituximab (n=12, 10%), romiplostim (n=11, 9%), eltrombopag (n=10, 8%), 6-mercaptopurine/azathioprine (n=6, 5%), and/or splenectomy (n=3, 3%). Two patients had no prior treatments and were monitored with observation only.
Table 1.
Demographic Features of ICON1 participants
| n=120 | ||
|---|---|---|
|
| ||
| Age at Enrollment (y); Median (range) | 11.7 (1.2–17.8) | |
|
| ||
| Primary ITP N (%) | 102 (85%) | |
| Secondary ITP | 18 (15%) | |
|
| ||
| Platelet Count in Prior Month (%, total) | ||
| <10×109/L | 62 (52%) | |
| 10–19×109/L | 26 (22%) | |
| 20–29×109/L | 13 (11%) | |
| ≥30×109/L | 14 (12%) | |
| Unknown | 5 (4%) | |
|
| ||
| Phase of ITP | Newly Diagnosed | 19 (16%) |
| Persistent | 37 (31%) | |
| Chronic | 64 (53%) | |
|
| ||
| Number of Prior Treatments* | Median (range) | 3 (0–8) |
| Number of Prior First Line Treatments | 2 (0–5) | |
| Number of Prior Second Line Treatments | 0 (0–5) | |
|
| ||
| Number with no Prior First-Line Treatments | 2 (1.6%) | |
| Number with no Prior Second-Line Treatments | 73 (61%) | |
|
| ||
| Prior Treatments (%, total) | ||
| IVIG | 115 (96%) | |
| Prednisone | 94 (78%) | |
| Anti-D globulin | 27 (23%) | |
| Rituximab | 17 (14%) | |
| Romiplostim | 12 (10%) | |
| 6-Mercaptopurine/Azathioprine | 9 (8%) | |
| Eltrombopag | 8 (7%) | |
| Splenectomy | 6 (5%) | |
| Dapsone | 5 (4%) | |
| Mycophenolate | 3 (3%) | |
Number reflects absolute number of different treatments and does not account for repeated courses of treatments.
Patients were treated by physicians who were a median of 8 years (range 1–44 years) from completion of fellowship. The majority of treating physicians were seeing >20 patients per year with ITP.
At enrollment, patients were started on the following second-line treatments: rituximab (43/120, 36%), romiplostim (31/120, 26%), eltrombopag (20/120, 17%), oral immunosuppressant agents (19/120, 16%), splenectomy (4/120, 3%), and dapsone (3/120, 3%). Treatment groups were not different with regard to the number of patients with chronic ITP (p=0.97) or by those who had been treated previously with second-line treatments compared with those who had never received a second-line treatment (p=0.10).
Overall Reasons for Selecting Treatments
In the non-weighted analysis, the most important factors guiding treatment decisions were patient and parental preference (53%) and treatment-related factors, including the side effect profile (58%), long-term toxicity (54%), ease of administration (46%), possibility of remission (45%), and efficacy (30%). Physician factors, such as experience and citing published guidelines, rarely influenced decision-making with only 9% of physicians giving published guidelines as a reason for choice of therapy. Additionally, 38% of physicians did not endorse any patient clinical factors (i.e., frequency of bleeding, expected compliance, response to other therapies, age, comorbidities) as key in their decision-making. Health system factors, such as insurance approval or distance from the closest medical center, rarely were chosen as having an impact on treatment choice.
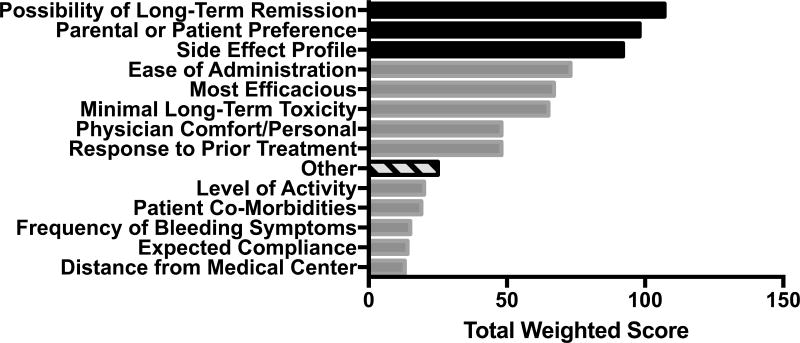
In the propensity weighted analysis, the most commonly selected factors included possibility of long-term remission, parental and patient preference, side effect profile, and ease of administration (Figure 1).
Figure 1.
Overall highest ranked reasons for treatment choice. The top 3 reasons are highlighted in black and are all related to either medication characteristics or family preferences.
The most common other reasons (indicated with hash marks) included: relevant other medical problems (n=6), fatigue (n=5), menstrual bleeding (n=2), surgical procedure (n=2).
Patient and parent preferences were reasons for choosing a treatment more often in patients with chronic ITP (85.7%) than in those with newly diagnosed (0%) or persistent (14.3%, p=0.003). The potential for long-term remission as a reason for selecting a treatment tended to be lower in newly diagnosed patients (16.7%) but was not significantly different than patients with persistent (45.8%) or chronic ITP (37.5%, p=0.16). Long-term remission was not selected more often in patients with primary versus secondary ITP (44% vs. 50%, p=0.80).
Reasons for Selecting Specific Treatments
Certain treatments tended to be favored for specific reasons (Figure 2). A significant determinant of choosing splenectomy or rituximab (p<0.001) was the possibility of long-term remission. This reason was endorsed in 100% and 91% of cases respectively when splenectomy or rituximab was chosen. Oral agents, such as eltrombopag and immunosuppressant agents, were significantly more likely to be chosen due to ease of administration and expected adherence (p<0.001); this reason was endorsed in 95% of those starting eltrombopag and 84% of those starting oral immunosuppressants. Physicians indicated expected efficacy as a reason more frequently for romiplostim (61%) as compared to eltrombopag (30%; p<0.001) and were more likely to choose rituximab in patients in whom there was lower anticipated adherence (p=0.017).
Figure 2.
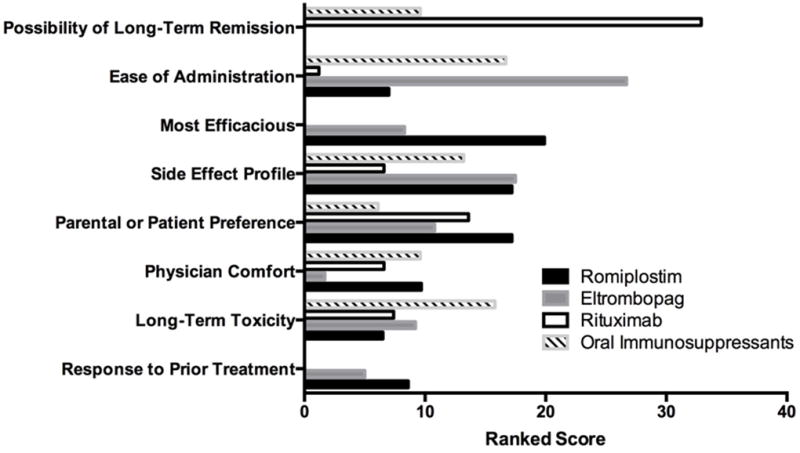
Ranked reasons for physician choice by second-line therapy. Individual therapies were examined and weighted scores were assigned to reasons for physician choice.
Scores in this figure are re-scaled so that equally long bars indicate that the reason was equally ranked for each treatment. Reasons that were less important are excluded from the figure.
Among the most frequent reasons for favoring an individual treatment, the specific treatment related factors were major determinants (Table 2). For rituximab, 93% of physicians endorsed at least one treatment factor; for oral immunosuppressants, romiplostim, and eltrombopag, 100% of physicians endorsed at least one treatment factor. Although treatment related factors were the major determinants, the specifics of the factors differed among them. For romiplostim, the side effect profile (68%) was the most important treatment factor. For rituximab, the possibility of cure was a major treatment factor (91%), whereas for oral immunosuppressant agents and eltrombopag, ease of administration (84% and 95%, respectively) was most important. The oral treatments, eltrombopag and oral immunosuppressants, were also selected for similar patient-centered reasons, including patient adherence and preference.
Table 2.
Most Frequent Determinants of Treatment Choice in Selecting Second-line ITP Therapies
| Treatment | Reasons for Selecting a Specific Treatment | Number of Times cited (%) |
Top ranked reasons |
|---|---|---|---|
|
| |||
| Rituximab n=43 | Possibility of long-term remission | 39(91%) | 1 |
| Parental or Patient preference | 23(53%) | 2 | |
| Physician Comfort / Experience with the treatment | 22(51%) | 5* | |
| Minimal long-term toxicity | 20(47%) | 4 | |
| Side effect profile | 17(40%) | 5* | |
| Response to Prior Treatments | 14(33%) | 3 | |
|
| |||
| Oral Immunosuppressants n=19 | Ease of administration | 16(84%) | 1 |
| Minimal long-term toxicity | 14(74%) | 2 | |
| Physician Comfort / Experience with the treatment | 12(63%) | 5 | |
| Side effect profile | 11(58%) | 3 | |
| Expected Compliance | 11(58%) | ||
| Parental or Patient preference | 8 (42%) | ||
| Possibility of long-term remission | 7(37%) | 4 | |
|
| |||
| Romiplostim n=31 | Side effect profile | 21(68%) | 2* |
| Perceived Efficacy | 19(61%) | 1 | |
| Parental or Patient preference | 18(58%) | 2* | |
| Physician Comfort / Experience with the treatment | 17(55%) | 3 | |
| Minimal long-term toxicity | 14(45%) | ||
| Expected Compliance | 13(42%) | ||
| Ease of administration | 11(35%) | 5 | |
| Response to Prior Treatments | 10(32%) | 4 | |
|
| |||
| Eltrombopag n=20 | Ease of administration | 19(95%) | 1 |
| Side effect profile | 14(70%) | 2 | |
| Minimal long-term toxicity | 11(55%) | 4 | |
| Parental or Patient preference) | 11(55%) | 3 | |
| Expected Compliance | 10(50%) | ||
| Level of activity | 7(35%) | ||
| Response to Prior Treatments) | 7(35%) | ||
| Physician Comfort / Experience with the treatment | 7(35%) | ||
| Perceived Efficacy | 6(30%) | 5 | |
tied ranking
In all patients undergoing splenectomy (n=4), this option was selected both due to the possibility of long-term remission and patient or provider preference. Those patients who underwent splenectomy had a median age of 16.2 years (range 15–17 years) and 75% had chronic ITP. These patients had received a median of 1 prior second-line treatments (range 1–2), which included rituximab (n=3) and eltrombopag (n=1). One splenectomized patient had no prior second-line treatments.
Physician/Patient Factors and Treatment Choice
There was no relationship between the age of the patient and the treatment selected (p=0.20). The experience of the physician, in terms of the number of years in practice, also did not correlate with the second-line treatment chosen (p=0.25).
There was a tendency for individual physicians to select certain treatments. When restricting the analysis to the five highest enrolling centers (n=61; range 8–17 patients per center), there was an association between treatment choice and treatment center (p=.008). In particular, rituximab was selected for at least half the patients at two centers, while romiplostim was selected for more than half of the patients at two other centers.
While low patient HRQoL may have been a reason second-line treatment was initiated, the physician report of the impact of ITP on patient HRQoL did not differ by treatment at the time of treatment selection (p=0.169). Although when asked to supply reasons for selecting particular therapies, physicians ranked patient adherence as an important reason, physician report of patient adherence did not actually correlate with any particular second-line treatment choice (p=0.29). In addition, the worst non-skin bleeding in the previous week did not correlate with the choice of treatment (p=0.73).
Discussion
Management of childhood ITP, particularly when persistent or chronic, can be challenging. The process of selecting a second-line treatment is complex with many issues affecting the hematologist’s choice of therapy for a given patient. ITP decisions may readily change in the course of treatment and are influenced by a variety of factors. The study described here is the first to assess physician treatment decisions in second-line therapy for pediatric ITP. The result was that patient preference and physician perception of treatment characteristics are the primary drivers of treatment choice.
Individual treatments were most often chosen for similar reasons. For example, clinicians chose splenectomy and rituximab because of the possibility of long-term remission desired by the patient and family. The most robust data regarding long term remission is available for splenectomy, a longstanding historical treatment for ITP, for which there is some confidence in its efficacy and long-term remission rates [14–17]. Nevertheless, only 6 of the 120 patients in this study had already undergone splenectomy at the time of enrollment and just 4 additional patients underwent the procedure during the study. This presumably represents the trend in both children and adults to defer or avoid splenectomy in favor of medical therapies, given the concerns about long-term sequelae of splenectomy [18, 19]. Rituximab, on the other hand, is a relatively newer treatment for ITP, and the long-term remission data are not as robust [20]. While initial response rates were encouraging with rituximab, long-term remission at 5 years now appears to be much lower (20–30%) and consistent with expected spontaneous remission rates [20]. Based on the ICON1 data, the perceived long term efficacy of rituximab among pediatric hematologists and patients may be higher than that demonstrated in clinical trials.
Eltrombopag was approved for children ≥1 year of age in 2015, and romiplostim, although used off-label, is not yet approved for use in pediatric ITP. Most patients in this study were enrolled during 2013 to 2015 before eltrombopag was FDA approved for children. As expected, long-term remission was not prioritized with regard to selecting TPO-RA therapy. Data about potential efficacy and side effects with these agents is evolving [21–24]. However, the TPO-RAs were also selected for a perceived reduction of toxicity, presumably because they avoid immunosuppression. While there has been some evolution of data over the course of this study, in general, physician practice did not change and prescribing patterns were similar over time.
Practice varied by center, which may reflect the practice of single physicians at these centers, physicians’ perception of efficacy and potential side effects of individual treatments, or dissemination of novel information about current treatments for ITP. Although physicians reported that they did not select treatments based on their own comfort level, these center specific practices suggest that physician factors and institutional biases do influence treatment selection. While parent and patient preference is a primary reason for selecting a treatment, this is often strongly influenced by the treating physician’s experience and the manner in which information is presented to families [25, 26]. The lack of comparative effectiveness studies for evidence-based decision-making increases the potential for physician and center-specific biases.
Study data suggest that the selection of individual treatments did not correlate with the physician’s perception of the effect of ITP on patient HRQoL. In most patients starting a second-line treatment, ITP has a significant negative effect on HRQoL. However, given the lack of data about differences among treatments in improving patient HRQoL, it would have been surprising if the physician’s assessment of the patient’s baseline HRQoL did correlate with treatment selection. As HRQoL and fatigue measures are integrated into trials and more is learned about the impact of treatment on HRQoL, patient baseline HQoL may factor into decision making in selecting specific treatments. Age, a factor that may play an important role in determining whether or not to initiate treatment to raise the platelet count, did not influence the type of therapy selected.
This study highlights several important points regarding second-line agent use. First, many patients will receive second-line therapy prior to being diagnosed with chronic ITP. However, a number of treatments have only been studied in children with chronic ITP, and their efficacy and benefits earlier in the disease are less understood. Second, guidelines with low-grade recommendations resulting from a lack of randomized trials have resulted in physicians relying more on personal preferences and individual therapy characteristics. This emphasizes the need for clinical trials to define patient characteristics carefully and to collect data on patient-related endpoints similarly to allow for comparison of endpoints even if a given study is not conducted in a randomized fashion. The physicians who participated in this study also have a specific interest in ITP which may have influenced their reliance on guidelines compared with other practitioners. Furthermore, guidelines should be routinely updated to reflect emerging evidence. Lastly, health-system factors in this study did not seem to impact decision-making; however, this may not be the case in many countries where access to certain treatments is more limited.
This study has several limitations. Some physician’s decisions were represented multiple times at certain centers. Since we did not capture data to discern between participating physicians, we cannot be certain whether patients from a site are managed by the same physician. Second, physicians ranked the top three factors in selecting an individual treatment. These factors are likely not independent and accounting for the interaction between factors is difficult to model. Therefore, treatment decision making may be much more complex than our model, and the model used may not adequately reflect the influence of all the factors that helped to guide the decision. In particular, certain factors may be taken for granted, consciously or unconsciously, from previous experience with the patient, disease, or treatment. Additionally, physicians are influenced by the expected results of treatment, both efficacy and toxicity, and these may change over time as new information and treatments become available. Nevertheless, although center-specific preferences were clear, the centers with >15 enrolled participants had variability in the treatment selected.
This study illustrates the complexity of decision making in the absence of comparative trials. Perhaps even more importantly, it also emphasizes the need for ongoing clinical research to better define populations of ITP patients most likely to benefit from specific interventions and to compare treatments in these populations. This study also underscores the need for a shared decision-making model to ensure a comprehensive consideration of all factors important to both physicians and their patients and families. Fully incorporating and individually prioritizing the key factors for a given patient requires extensive discussion to fully inform and engage the patient in the treatment decision. Finally, by describing our collective experience, the authors anticipate that this study may serve as a basis for the design of clinical trials which will adequately address knowledge gaps and inform patient and clinician decision-making.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the research coordinators, nurses, hematologists, patients, and their families who participated in the ICON1 study.
Footnotes
Conflicts of Interest:
EJN: Consultancy and Advisory Board for Novartis and Genentech. JMD: Consultancy and honoraria from Sanofi-Genzyme. JRR: Advisory Board for Novartis. YDP: Consultancy for Novartis. MPL: Consultancy for Novartis, Baylor, Sysmex, and EBSCO. RJK: Consultancy for Amgen Inc., Hoffman-La Roche LTD and Speaker for Octapharma AG, Baxalta, and Biogen Canada Limited. JBB : Advisory Board and Research support from Amgen and Novartis. The remaining authors have no relevant conflicts of interest.
References
- 1.Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13:457–464. doi: 10.1111/jth.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neunert CE, Buchanan GR, Imbach P, et al. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 2008;112:4003–4008. doi: 10.1182/blood-2008-03-138487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhne T, Buchanan GR, Zimmerman S, et al. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. The Journal of pediatrics. 2003;143:605–608. doi: 10.1067/s0022-3476(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 4.Imbach P, Kuhne T, Muller D, et al. Childhood ITP: 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS) Pediatric blood & cancer. 2006;46:351–356. doi: 10.1002/pbc.20453. [DOI] [PubMed] [Google Scholar]
- 5.Bolton-Maggs PH, Dickerhoff R, Vora AJ. The nontreatment of childhood ITP (or "the art of medicine consists of amusing the patient until nature cures the disease") Seminars in thrombosis and hemostasis. 2001;27:269–275. doi: 10.1055/s-2001-15256. [DOI] [PubMed] [Google Scholar]
- 6.Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 7.Neunert CE, Bright BC, Buchanan GR. Severe chronic refractory immune thrombocytopenic purpura during childhood: a survey of physician management. Pediatric blood & cancer. 2008;51:513–516. doi: 10.1002/pbc.21621. [DOI] [PubMed] [Google Scholar]
- 8.Beck CE, Boydell KM, Stasiulis E, et al. Shared decision making in the management of children with newly diagnosed immune thrombocytopenia. Journal of pediatric hematology/oncology. 2014;36:559–565. doi: 10.1097/MPH.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann HP, Dambita N, Buchanan GR, et al. Decision modeling of disagreements: pediatric hematologists' management of idiopathic thrombocytopenic purpura. Medical decision making : an international journal of the Society for Medical Decision Making. 2011;31:805–815. doi: 10.1177/0272989X11400417. [DOI] [PubMed] [Google Scholar]
- 10.Vesely SK, Buchanan GR, Adix L, et al. Self-reported initial management of childhood idiopathic thrombocytopenic purpura: results of a survey of members of the American Society of Pediatric Hematology/Oncology, 2001. Journal of pediatric hematology/oncology. 2003;25:130–133. doi: 10.1097/00043426-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Neunert C, Despotovic J, Haley K, et al. Thrombopoietin Receptor Agonist Use in Children: Data From the Pediatric ITP Consortium of North America ICON2 Study. Pediatric blood & cancer. 2016 doi: 10.1002/pbc.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 14.Vianelli N, Palandri F, Polverelli N, et al. Splenectomy as a curative treatment for immune thrombocytopenia: a retrospective analysis of 233 patients with a minimum follow up of 10 years. Haematologica. 2013;98:875–880. doi: 10.3324/haematol.2012.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz J, Leber MD, Gillis S, et al. Long term follow-up after splenectomy performed for immune thrombocytopenic purpura (ITP) American journal of hematology. 2003;72:94–98. doi: 10.1002/ajh.10253. [DOI] [PubMed] [Google Scholar]
- 16.Kuhne T, Blanchette V, Buchanan GR, et al. Splenectomy in children with idiopathic thrombocytopenic purpura: A prospective study of 134 children from the Intercontinental Childhood ITP Study Group. Pediatric blood & cancer. 2007;49:829–834. doi: 10.1002/pbc.21108. [DOI] [PubMed] [Google Scholar]
- 17.Kojouri K, Vesely SK, Terrell DR, et al. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623–2634. doi: 10.1182/blood-2004-03-1168. [DOI] [PubMed] [Google Scholar]
- 18.Boyle S, White RH, Brunson A, et al. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood. 2013;121:4782–4790. doi: 10.1182/blood-2012-12-467068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghanima W, Godeau B, Cines DB, et al. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second-line treatment. Blood. 2012;120:960–969. doi: 10.1182/blood-2011-12-309153. [DOI] [PubMed] [Google Scholar]
- 20.Patel VL, Mahevas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119:5989–5995. doi: 10.1182/blood-2011-11-393975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussel JB, de Miguel PG, Despotovic JM, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. The Lancet Haematology. 2015;2:e315–325. doi: 10.1016/S2352-3026(15)00114-3. [DOI] [PubMed] [Google Scholar]
- 22.Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;386:1649–1658. doi: 10.1016/S0140-6736(15)61107-2. [DOI] [PubMed] [Google Scholar]
- 23.Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388:45–54. doi: 10.1016/S0140-6736(16)00279-8. [DOI] [PubMed] [Google Scholar]
- 24.Bussel JB, Hsieh L, Buchanan GR, et al. Long-term use of the thrombopoietin-mimetic romiplostim in children with severe chronic immune thrombocytopenia (ITP) Pediatric blood & cancer. 2015;62:208–213. doi: 10.1002/pbc.25136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherr KA, Fagerlin A, Wei JT, et al. Treatment Availability Influences Physicians' Portrayal of Robotic Surgery During Clinical Appointments. Health Commun. 2017;32:119–125. doi: 10.1080/10410236.2015.1099502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sah S, Fagerlin A, Ubel P. Effect of physician disclosure of specialty bias on patient trust and treatment choice. Proc Natl Acad Sci U S A. 2016;113:7465–7469. doi: 10.1073/pnas.1604908113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.