Abstract
Objective
Children undergoing cardiopulmonary bypass (CPB) develop clinically impactful capillary leak of unclear etiology. A widely-held hypothesis that exposure of circulating cells to the CPB circuit induces the release of inflammatory mediators that act to disrupt intercellular junctions of capillary endothelial cells (ECs) inducing paracellular capillary leak either directly or through new gene expression.
Design
Cohort study.
Setting
Tertiary pediatric hospital.
Patients
Twenty children undergoing surgery with CPB for congenital heart disease. Serum was collected before CPB, two hours after CPB and eighteen hours after CBP.
Interventions
None
Measurements
We analyzed the effects of 10% patient sera on the function, structure and gene expression of cultured human dermal and pulmonary microvascular ECs. Changes in barrier function were measured using trans-endothelial electrical resistance (TEER). Associations between changes in TEER and subject characteristics were analyzed using linear mixed effects model with area under the resistance curve as outcome. Changes in junctional structure were assessed by analyzing the organization of the EC junctional proteins claudin-5 and VE-cadherin using immunofluorescence microscopy. Changes in inflammatory gene expression were measured using real time quantitative reverse transcription-polymerase chain reaction.
Main Results
All serum samples induced a transient, 120-minute increase in TEER followed by persistent loss of barrier function. Unexpectedly, sera collected post-CPB induced significantly less loss of barrier function in both dermal and pulmonary capillary EC compared to pre-CPB sera. Consistent with the TEER results, claudin-5 and VE-cadherin junctional staining showed less disruption in cultures treated with post-CPB sera. Expression of genes commonly associated with inflammation were largely unaffected by patient sera.
Conclusions
Contrary to the hypothesis, sera taken from children after CPB induces less capillary barrier disruption relative to sera taken from children before CPB and none of the sera induced significant changes in expression of inflammatory genes.
Keywords: Cardiopulmonary bypass, capillary leak, endothelial activation, endothelial dysfunction, pediatric critical care, trans-endothelial electrical resistance
INTRODUCTION
Children who are exposed to CBP during surgical repair or palliation of congenital heart disease develop post-operative capillary leak, leading to intravascular volume depletion in the setting of total body fluid overload(1). Such fluid shifts complicate post-operative fluid, diuretic and inotropic support management. These clinical effects are thought to be mediated by changes in endothelial cell (EC) barrier function, structure and gene expression, however the specific inciting factors due to CPB are unknown(2). A widely-held general explanation is that exposure of the patient’s circulating blood cells to the artificial surfaces of the bypass circuit, combined with parenchymal ischemia-reperfusion injury, induces a systemic inflammatory response that includes release of variety of soluble mediators in serum(3–14). Some of the identified mediators, such as angiopoietin-2 (angpt-2) and tumor necrosis factor (TNF), are known to act on cultured ECs to destabilize barriers, directly and via new gene expression (dependent upon the transcription factor NF-κB) (15, 16). Such studies have not been particularly revealing of pathologic changes in EC associated with post-CPB leak. Other, yet unidentified mediators with barrier stabilizing effects may be released into the circulation by CPB and may have an effect on ECs. An alternative possibility is that the soluble mediators released into serum after CPB do not play a significant role in the initiation or propagation of propagation of capillary leak. There is a significant knowledge gap between the levels of such mediators in serum and their cumulative effect on ECs and contribution of capillary leak(17). A better understanding of post-operative capillary leak may guide therapies (i.e. fluids, diuretic or inotropes) and foster the development of new therapies that target the endothelium.
Our hypothesis is that capillary leak is caused by EC changes induced by the cumulative effect of cytokines and other mediators that are released into the circulation following exposure to CPB. To test this, we treated cultured human dermal and pulmonary microvascular ECs (HDMECs and HPMECs) with sera collected from children undergoing CPB who were enrolled in a prior study. We specifically investigated changes in EC barrier function, structure and gene expression. We utilized a culture model of the capillary endothelium, as different vascular segments (i.e. arteriole, capillary, venule) respond to stimulation differently and result in variable clinical effects(18). Specifically, post-confluent HDMEC and HPMEC form claudin-5 dependent tight junctions and do not depend on adherens junctions that are characteristic of venular ECs(19). We anticipated that serum collected after CPB would induce a decrease in barrier function that would correlate with disassembly of EC junctional structure and increased inflammatory gene expression in HDMEC and HPMEC monolayers compared to sera from before CPB.
MATERIALS AND METHODS
We analyzed serum samples from a prior observational study that was conducted at Yale-New Haven Children’s Hospital from October 2013 to August 2014(20). In that study, we included twenty children aged 2 months to 17 years who underwent elective repair or palliation of their congenital heart disease under CBP. Three blood samples were obtained from each study participant. First was after line placement (T1, after anesthesia induction, before surgery); second was two hours after surgery (T2, after chest closure and modified ultra-filtration [MUF], on arrival to the pediatric intensive care unit [PICU]); and, third was eighteen hours after surgery (T3,). Samples were collected in standard red-top tubes with no anticoagulants and sera was isolated by removal of clotted blood. The samples were stored at −80° C per Yale University biological repository guidelines and had undergone a single freeze-thaw cycle prior to use in the current study. Because all samples had been previously obtained, the Human Investigations Committee at Yale University approved this study with a waiver of consent.
At our institution, CPB circuit is pump primed with heparin, methylprednisolone, and aminocaproic acid. In patients weighing <10 kg, the circuit is additionally primed with red blood cells and fresh frozen plasma. All patients underwent 10 minutes of modified ultrafiltration at the end of the surgery using a Hemocor HPH hemoconcentrator (Minntech Corporation, Minneapolis, MN, USA). Patient baseline demographics, length of Pediatric Intensive Care Unit (PICU) stay, inotropic score, total bypass time, aortic cross clamp time and duration of mechanical ventilation were provided in a de-identified database.
Laboratory Methods
Cell Isolation and Culture
HDMEC and HPMEC cultures used in this study were both isolated and cultured as previously described from de-identified and discarded human surgical specimens from Yale Department of Pathology. Their generation and use has been classified as Not Human Research by the Yale Human Investigation Committee (20, 21). Sources of surgical specimens include both males and females. Briefly, HDMECs were isolated by dermatome harvest of the superficial most 0.5 to 0.7 mm of skin, followed by enzymatic digestion (Dispase, 50 U/ml; BD Biosciences) at 37°C and culture on 10 μg/ml human plasma fibronectin-coated (Millipore) tissue culture plastic (BD Biosciences) in EGM2-MV growth medium with 10% fetal bovine serum (Lonza). HPMECs were isolated by enzymatic digestion from anatomically normal regions of discarded lung resection surgical specimens and then cultured under the same conditions as HDMECs. Primary colonies were suspended and then immunoselected for CD31 expression using commercially available magnetic bead (Miltenyi Biotec). After selection cells were serially passaged by trypsin resuspension onto 0.1% gelatin-coated (Sigma-Aldrich) tissue culture plastic (Falcon/Corning). Both HDMECs and HPMECs were assayed 24 to 36 hours post-visual confluence, at which time trans-endothelial electrical resistance (TEER) levels plateau, indicative of formation of TJs. For HDMECs cultures acclimated to human serum, cultures were thawed in media containing 50% human, 50% bovine serum for 24 hours then tested as described below in growth media made with 100% human serum (Sigma, H4522) instead of fetal bovine serum.
Assessment of Barrier Function
Trans-endothelial electrical resistance (TEER) of endothelial monolayers was assessed by electrical cell-substrate impedance sensing (ECIS, Applied Biophysics), using a 4 by 96-well reading system that allows quadruplicate replicates across all cell types to provide a real-time, non-invasive, label free and quantitative measure of endothelial barrier function(22). HDMECs or HPMECs were plated on gelatin-coated 96-well gold electrode arrays (catalog #96W20idf, Applied Biophysics) and TEER measurements were obtained daily over 3 to 5 days to monitor increasing barrier integrity until the monolayers reached a plateau that coincided with maturation of tight junctions. The TEER at the start of the experiment is referred to as the basal TEER level, and subsequent measurements are divided by the basal value to produce normalized TEER values. Normalization facilitates comparisons among experiments and cell types. HDMEC and HPMEC monolayer resistances were measured as previously described(22) with the addition of 10% patient serum or an equal volume of phosphate buffered saline (PBS, Sigma, negative control) or 1.0 ng/ml TNF in PBS (positive control). We used 10% serum as that is a standard additive to cell culture media, tolerated well by EC cultures and would provide sufficient amount of cytokine for detection of effect by TEER. Data were collected over 24 hours. To investigate the effects of higher patient serum concentrations, we added either 30% or 50% sera from 4 patients to HDMECs acclimated to human serum-based media and observed TEER changes.
Assessment of Junctional Structure
For confocal immunofluorescence microscopy analysis, confluent HDMECs and HPMECs monolayers, grown on gelatin-coated glass cover slips, were exposed to PBS or 10% patient serum for 6 hours, washed with PBS and fixed in 95% ethanol for 30 minutes at 4°C(23). For two color immunofluorescence imaging, monolayers were incubated overnight in mouse anti-claudin-5 antibody (Invitrogen #35-2500) or rabbit anti-human VE-cadherin antibody (Invitrogen #PA5-19612), diluted in TBS/0.2% triton-X-100/5% normal donkey serum. Alexa488 Donkey anti-mouse and anti-rabbit secondary antibodies (Invitrogen #A-21202, A-10040) and was used to detect primary antibody and glass coverslips were mounted for immunofluorescence analysis in ProLong mounting media (Invitrogen). Randomly selected (minimum of 5 images per experimental condition) fluorescence photomicrographs were collected using a Zeiss Axiovert fluorescence microscope and Plan-APOCHROMAT, 63× oil objective (Zeiss) with a Hamamatsu ORCA-ER digital camera (Hamamatsu Photonics). Quantification of non-adjacent cellular circumference and confluent claudin-5 junctional staining was determined with the tracing tool in ImageJ (NIH, v1.51p) for 20 randomly selected cells in each condition. Due to limited patient-derived serum samples, confocal experiments were run on four sets of patient samples. Patients were selected based on availability of serum and closeness of fit to the pooled average TEER curve.
Assessment of Gene Expression
Real time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis was conducted on mRNA isolated from HDMECs or HPMECs using an RNeasy Mini kit (Qiagen), per the manufacturer’s protocol. Cells were treated with 10% PBS as a negative control and 10 ng/mL of TNF as a positive control. Samples were collected in duplicate after 4 hours of exposure to sera. The high-capacity cDNA reverse transcription kit (Applied Biosystems) was used to synthesize cDNA from RNA. All PCR reactions were assembled with TaqMan gene expression master mix and manufacturer validated TaqMan gene expression probes (Applied Biosystems #Hs02786624_g1, Hs00900055_m1, Hs00169867_m1, Hs01065279_m1, Hs01574665_m1, Hs00174131_m1 and Hs00174057_m1). Reactions were analyzed on a CFX96 real-time system using CFX Manager software (BioRad Laboratories). Gene expression levels were normalized to GAPDH. Cells were treated for four hours with 10% PBS treated cells as a negative control and 10 ng/mL of TNF (R&D Systems) as a positive control. Differential gene expression was normalized to PBS treated control group. Due to limited patient-derived serum samples, gene expression experiments were run on four sets of patient samples. Patients were selected based on availability of serum and closeness of surgical and clinical course to the cohort averages (Table 1).
Table 1.
Patient demographic and outcome data
| Patient Characteristics | Result |
|---|---|
| Total Number of Patients | 20 |
| Gender (M/F) | 10M/10F |
| Median Age (months, IQR) | 31 (4.5, 152) |
| Weight (kg) | 22.2 ± 19.8 |
| Ethnicity | |
| Caucasian | 6 (30%) |
| Hispanic | 9 (45%) |
| African American | 5 (25%) |
| Diagnosis | |
| ASD | 3 (15%) |
| VSD | 2 (10%) |
| CAVC | 2 (10%) |
| Common atrium | 1 (5%) |
| TOF | 6 (30%) |
| Subaortic membrane | 2 (10%) |
| Tricuspid atresia | 2 (10%) |
| DORV | 2 (10%) |
| STAT | |
| Category 1 | 8 (40%) |
| Category 2 | 11 (55%) |
| Category 4 | 1 (5%) |
| Median CPB Time (min, IQR) | 68 (54, 101) |
| Median CCT Time (min, IQR) | 33 (23, 38) |
| Median 24-hour fluid balance (mL/kg, IQR) | +27 (12, 63) |
| Median duration of respiratory support (hours, IQR) | 5 (2, 17) |
| CPB Prime Protocol Standard | |
| >10 kg: Heparin, methylprednisolone, and aminocaproic acid | 11 |
| <10 kg: Heparin, methylprednisolone, and aminocaproic acid, red blood cells, fresh frozen plasma | 9 |
Abbreviations: ASD; atrial septal defect, CAVC; common atrioventricular canal, DOR; double outlet right ventricle, STAT; Society of Thoracic Surgery Congenital Heart Surgery Mortality Score, TOF; tetralogy of Fallot, VSD; ventricular septal defect, M; male, F; female, CPB; cardiopulmonary bypass time, CCT; aortic cross-clamp time, SD; standard deviation, IQR; interquartile range, CPB; Cardiopulmonary Bypass
Statistical Methods
The association between the primary outcome of area under the TEER curves (AUC) and time and type of cells were analyzed using a linear mixed effects model using Stata 15.1 (StataCorp LLC, College Station, TX). The association was adjusted for age and bypass time. Age, bypass time and the interaction between time and type of cells were not statistically significant and were thus dropped from the model. The final model had time and type of cells as dependent variables.
Gene expression data is expressed as mean and standard deviation. Unpaired t tests were used to make statistical comparisons between treatment conditions. Comparisons between multiple treatment conditions were performed using one-way ANOVA with a Tukey test for post hoc analysis. Statistical analyses were performed using GraphPad Prism software (v7.01). In all experiments, p <0.01 was considered statistically significant.
RESULTS
Patients Characteristics
We analyzed sera from twenty pediatric patients (10 boys and 10 girls) who had undergone CPB for congenital heart disease repair or palliation (Table 1). Median (interquartile range) age was 31 months (4.5, 152), and 40% were <1 year of age. The median CPB time was 68 (54, 101) minutes and the median cross clamp time was 33 (23, 38) minutes. No patients required repeat operations within 30 days. The median 24-hour fluid balance was positive 27 (12, 63) mL/kg and patients received invasive mechanical ventilation for a median of 5 (2, 17) hours. All patients were discharged alive from the hospital. There were no late complications such as infections, post-pericardiotomy syndrome, or readmissions.
Barrier Function
All serum samples induced a transient 15% increase in TEER over 2 hours followed by a sustained decrease in barrier function compared to pre-treatment levels (Figure 1A and B). Unexpectedly, sera collected after patient exposure to the CPB circuit (both T2 and T3) consistently caused a lesser decline in TEER compared to sera collected just prior to surgery and CPB (T1) in both HDMECs and HPMECs. This difference was significant for serum collection time-points in both cell types, although HPMECs were more responsive to serum induced changes. Area under the curve analysis revealed 9.4, 10.3 and 10.4 for HDMEC and 10.8, 11.3 and 11.6 for HPMEC treated with T1, T2 and T3 respectively (Supplementary Figure 1). Increasing the concentration of patient serum did not change the magnitude or pattern of TEER changes in HDMEC (Supplementary Figure 2). We did not identify any patterns of TEER loss or gain in relationship to any clinical parameters for individual TEER curves.
Figure 1.
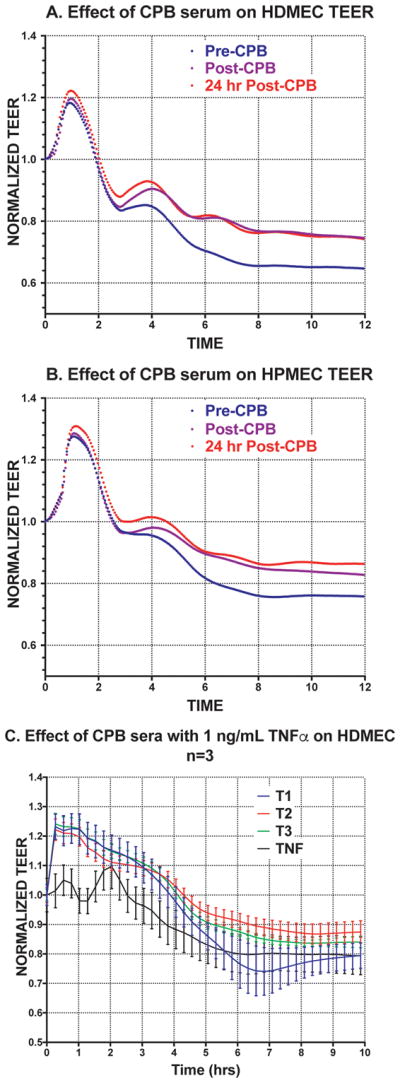
Effect of serum on HDMEC and HPMEC trans-endothelial electrical resistance. HDMEC (A) and HPMEC (B) monolayers display more leak when treated with pre-CPB (T1) sera compared to post-CPB sera (T2 and T3). Treatment of cells with sera from both T2 and T3 sera result in similar decreases in permeability compared to cells treated with T1 sera. Each tracing is the average of all 20 patient samples. Error bars omitted for clarity and data is analyzed as area under the curve (a measure of effect over time) through regression analsysis. To Invesitigate the presence of possible inhibitors, we screened three patient’s sera supplemented with 1 ng/mL of TNF (C) which resulted in a 10% increase in TEER for sera from all time points.
To detect the presence of inhibitors to compounds that may induce NF-κB mediated permeability, we screened patient sera on HDMEC. Given that we lacked sufficient sera to test all 20 patients, we assessed the effects of sera from three of the patients on the HDMEC TEER response induced by 1 ng/mL of TNF (Figure 1C). We chose a sub-maximal effect concentration of TNF for these experiments to increase chances that we could detect inhibition, additivity or synergy of effects with patient sera. When combined with patient sera, the addition of TNF resulted in approximately 10% less decrease in leak compared to the capillary leak induced TNF alone.
EC Junctional Structure
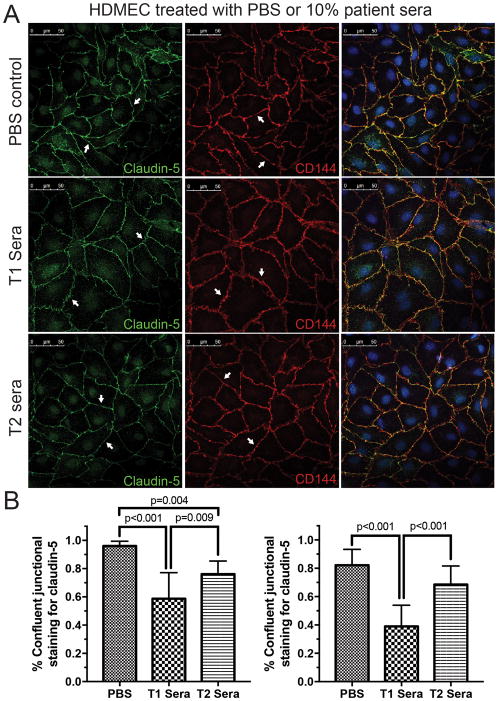
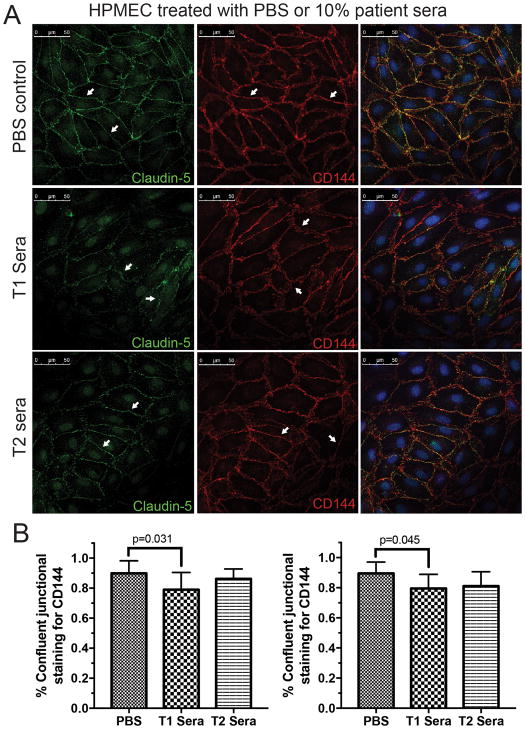
Confocal microscopy revealed differences in tight junction protein cladin-5 and adherens junction protein VE-cadherin staining in both HDMECs (Figure 2A, quantification 2B) and HPMECs (Figure 3A, quantification 3B). Only T2 sera was used for post-bypass comparison due to limited availability of patient samples and similarity of TEER curves between T2 and T3 sera. Staining for the tight-junctional protein claudin-5 was qualitatively darker in HDMEC and HPMEC treated with post-CPB sera (T2) compared to pre-CPB sera. Staining for the adherins-junction protein VE-cadherin demonstrated less disruption in HDMEC and HPMEC treated with post-CPB sera compared to pre-CPB sera. This observation is quantified by measuring linearly confluent staining divided by the total cell circumference. PBS treated cells demonstrated more continuous staining of claudin-5 (96 +/− 3.3% for HDMEC and 82 +/− 11% in HPMEC) than cells treated with T1 (58 +/− 18%, p<0.001 and 39 +/− 15%, p<0.001 respectively) and T2 (76 +/− 9%, p=0.004 and 69 +/− 13%, p=ns) sera. For VE-cadherin, the staining pattern was less disrupted with more continuous staining evident in all conditions (90 +/− 8%, 79 +/− 12%, p=0.031 and 86 +/− 7%, p=ns for HDMECs treated with PBS, T1 and T2 sera compared to 89 +/− 7%, 80 +/− 10%, p=0.045, and 81 +/− 10%, p=ns for HPMEC respectively). Overall, the staining pattern for HDMECs and HPMECs revealed similar patterns of junctional disruptions (Figure 2).
Figure 2.
Changes in the tight-junction protein claudin-5 and adherin-junction protein VE-cadherin in human dermal microvascular endothelial cells (HDMECs) after 6 hours of treatment with saline (PBS), pre- (T1) or post-CPB (T2) sera. (A) HDMECs display smooth and continuous junctional staining for both claudin-5 (Left panel) and VE-cadherin (CD144, Middle panel) in the untreated group (arrows). Contiguous junctional staining is disrupted and disjointed after 6 hours of stimulation with 10% pre-CPB sera (T1). The disruption of junctional staining is markedly less in cells treated with the immediately post-CPB serum (T2, arrows). Merged images indicate well defined junctional overlap (Right panels). Representative data from three experiments. (B) Quantification of circumferential disruption of junctional staining demonstrates significant differences in claudin-5 (left) and VE-cadherin (right) in HDMECs treated with PBS, T1 and T2 sera. Compiled data from three experiments, p values indicated.
Figure 3.
Changes in the tight-junction protein claudin-5 and adherin-junction protein VE-cadherin in human pulmonary microvascular endothelial cells (HPMECs) after 6 hours of treatment with saline (PBS), pre- (T1) or post-CPB (T2) sera. (A) HPMECs display smooth and continuous junctional staining for both claudin-5 (Left panel) and VE-cadherin (CD144, Middle panel) in the untreated group (arrows). Contiguous junctional staining is disrupted and disjointed after 6 hours of stimulation with 10% pre-CPB sera (T1). The disruption of junctional staining is markedly less in cells treated with the immediately post-CPB serum (T2, arrows). Merged images indicate well defined junctional overlap (Right panels). Representative data from three experiments. (B) Quantification of circumferential disruption of junctional staining demonstrates significant differences in claudin-5 (left) and VE-cadherin (right) in HPMECs treated with PBS, T1 and T2 sera. Compiled data from three experiments, p values indicated.
EC Gene Expression
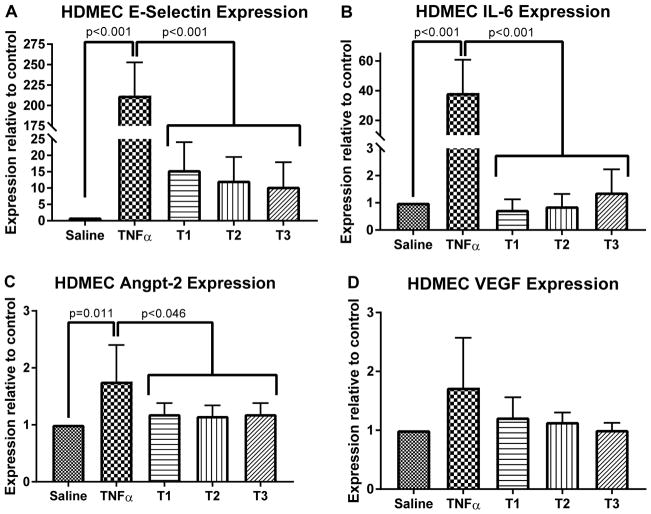
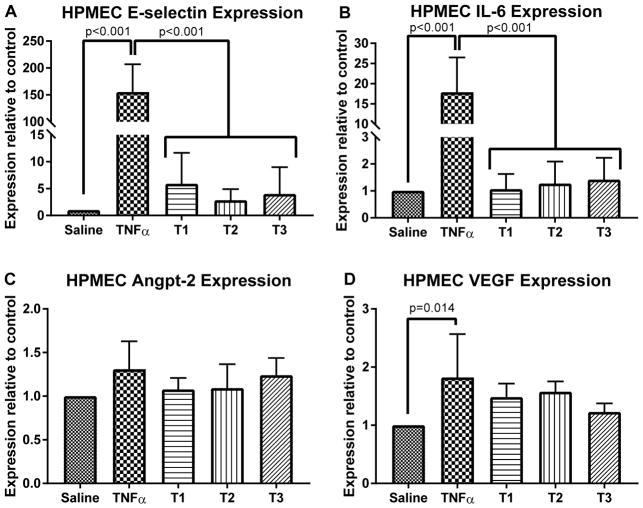
Transcriptional changes in HDMECs (Figure 4) and HPMECs (Figure 5) were analyzed after exposure to pre- and post-bypass serum. Overall, there were no significant differences in transcript levels for any of the measured inflammatory genes induced among the responses to T1 vs. T2 vs. T3 sera. Treatment of EC with 10 ng/mL TNF (positive control) resulted in significant increases in transcript levels of E-selectin and IL-6 as well as anpt-2 in HDMEC and VEGF in HPMEC compared to cells treated with PBS (negative control).
Figure 4.
Changes in mRNA levels for pro-inflammatory transcripts in human dermal microvascular endothelial cells (HDMECs) treated with 10% patient serum for four hours. Serum from pre-CPB (T1), post-CPB (T2) and 18 hours after CPB (T3) were assessed with saline treated (negative control) and TNF treated (positive control). E-selectin, IL-6 (both markers of NF-κB mediated endothelial activation) and angpt-2 expression were significantly different between control groups and patient samples (A, B, C) while vascular-endothelial growth factor (VEGF) expression was not significantly changed (D) Most importantly, no significant differences were observed between T1, T2 or T3 sera. Compilation of three different patient sera experiments run in duplicate, p values indicated.
Figure 5.
Changes in mRNA levels for pro-inflammatory transcripts in human pulmonary microvascular endothelial cells (HPMECs) treated with 10% patient serum for four hours. Serum from pre-CPB (T1), post-CPB (T2) and 18 hours after CPB (T3) were assessed with saline treated (negative control) and TNF treated (positive control). E-selectin and IL-6 expression were significantly different between control groups and patient samples (A, B) while angiopoietin-2 and vascular-endothelial growth factor (VEGF) expression did not differ between control and patient samples conditions (C, D). Most importantly, no significant differences were observed between T1, T2 or T3 sera. Compilation of three different patient sera experiments run in duplicate, p values indicated.
DISCUSSION
The results of this study suggest that sera from pediatric patients after CPB period do not contain stable mediators that induce barrier disruption or inflammatory gene expression on cultured human microvascular ECs compared to pre-procedure control samples from the same patients. While there was evidence of barrier disruption across all timepoints, post-CPB sera (T2 and T3) induced less barrier disruption in cultured human microvascular endothelial cells than sera obtained prior to bypass (T1). These results are unlikely to be due to dosage or dilution effects as increased sera concentrations, up to 50% volume to volume, did not change the pattern of TEER responses in HDMEC. Another possibility is the effects of medications administered in the peri-CPB setting such as heparin or protamine. The TEER results are supported by observations that EC junction structure appeared less disrupted by post-CPB sera. Disruption of EC junction in culture by stimulation with individual cytokines is known to depend upon new gene transcription and previous studies have shown increase in individual cytokine levels post-CPB(24). However, our analysis of marker inflammatory genes in HDMECs and HPMECs did not reveal significant transcriptional differences induced by pre- or post-CPBN sera. These data suggest that the clinical manifestations of capillary leak may not be induced on ECs by long lived soluble mediators released in the post-CPB setting.
We chose to use HDMECs and HPMECs in these experiments because, unlike more widely studied human umbilical vein endothelial cells (HUVECs), HDMECs and HPMECs replicate the capillary vascular segment. Specifically, HDMECs and HPMECs form claudin-5 dependent tight junctions and more accurately reflect the physiology of the capillary vascular segment(16). HUVEC junctions are characterized primarily by adherens junctions that depend on VE-cadherin and are sensitive to extracellular calcium levels. HUVECs cannot provide information on capillary endothelial permeability involving tight junction breakdown like HDMECs or HPMECs(20). Therefore, our study may be a more accurate in vitro investigation of the effects of CPB on capillary ECs. This distinction may explain the difference between our results and previous investigations that demonstrate an increase in permeability(7) and apoptosis(25) when HUVECs are treated with post-CPB samples. Our findings of minimal changes in capillary permeability are consistent with human observations that changes in lung mechanics after CPB may not be attributable to pulmonary capillary vascular permeability changes(9, 26).
A possible explanation for our findings would be the release of compounds that act as inhibitors to by pro-inflammatory cytokines such as TNF, as has been observed in septic patients(27). If such compounds were released by CPB, the effects of barrier destabilizing compounds in serum (like TNF) may not be detected by TEER changes. To determine if the post-CPB sera used in our study contained inhibitors of inflammation that could mask an effect, we combined these sera with TNF. We observe characteristic breakdown of capillary tight junctions with TNF stimulation alone and the effects of patient sera augmented with 1 ng/mL TNF appear roughly additive of the two treatments with no evidence that pre- or post-CPB sera either neutralize or synergize with TNF actions. The observed changes seemed to reflect the persistent addition of the initial increase in barrier that all the CPB patient sera induce rather than effective neutralization. Interestingly, the smallest reduction in the cytokine response was caused by pre-bypass sera.
Our study focused on the functional effects of patient sera rather than the individual cytokines or mediators present pre- or post-CPB. Our results suggest investigating other factors that may instigate or propagate capillary leak. Such considerations include patient factors (i.e. cooling-rewarming or total fluid administered) short lived factors in the vascular compartment (i.e. proteases or coagulation proteins). Alternatively, patient sera could act on other cell types to produce contact-dependent or paracrine signals that cause EC responses and therefore not detected when using pure EC cultures. Although previous studies have shown increase in cytokine levels post-CPB(28) our analysis of HDMEC and HPMEC did not reveal transcriptional differences induced by pre- or post-CPBN sera. These data suggest that systemically detected cytokines may not be produced by capillary ECs stimulated by long lived systemic signaling mediators post-CPB or that such cytokines may originate predominately from other cell types.
Two additional factors complicate the interpretation of our results. There is no generally accepted definition or diagnostic criteria for capillary leak(29) and our cohort generally did very well with minimal requirements for ventilatory and inotropic support. Our findings may not be generalizable to sicker patients and serum from such patients may produce different TEER changes. However, these patients represent a common post-cardiac population that is often thought to be affected by capillary leak. A set of standardized and generally accepted clinical criteria to diagnose capillary leak and score its severity would allow more precise comparisons for future investigations into capillary leak.
Our study, which challenges a widely held assumption about the causes of capillary leak following CPB, has several additional limitations. While the simplest interpretation of these data is that CPB does not induce release of soluble mediators that cause capillary leak, sera differ from whole blood in significant ways, such as absence of blood cells (platelets, leukocytes), presence of active coagulation cascade components or the presence of short-lived or protease-sensitive vasoactive mediators. Nevertheless, our findings seem to go against the commonly held beliefs about the systemic dissemination of mediators that induce capillary leak and may help to guide future research on capillary leak. Furthermore, we analyzed a relatively small sample size of 20. We chose this number because we leveraged samples from a previous study that had observed an effect. Furthermore, samples from newly enrolled patients are unlikely to be comparable with the original cohort because there have been changes in the practice of CPB in our institution since the original study was completed. We also have limited data related to immune-modulators administered peri-operatively, such as specific dosing of corticosteroids or anti-inflammatories. We recognize this may limit the interpretation of capillary leak in this population. Each sample had experienced a freeze-thaw cycle; however, our screen was aimed to detect the net effects of stable long-lived mediators of capillary leak. Last, we lacked sufficient sample volume to assay the sera for specific cytokines.
CONCLUSION
We demonstrate that sera collected from patients after CPB had less destabilizing effects on human capillary endothelial barrier function and junctions compared to pre-CBP samples and may not induce pro-inflammatory gene expression. A standardized definition of capillary leak would help to guide future studies that may consider alternative explanations for post-CPB capillary leak.
Supplementary Material
(A) Area under the curve (AUC) analysis for TEER for each timepoint on HDMEC and HPMEC (n = 20). The AUC is significantly lower in HDMEC and HPMEC treated with pre-CPB sera, indicating a more detrimental effect over time.
Effect of patient serum titration on trans-endothelial electrical resistance of HDMECs acclimated to growth media containing only human sera. HDMEC monolayers that have been transitions to human-serum based media treated with 30% (A) or 50% (B) patient sera. Consistent with HDMEC treated with 10% sera, more leak is observed when treated with pre-CPB (T1) sera compared to post-CPB sera (T2 and T3). Treatment of cells with sera from both T2 and T3 sera result in similar decreases in permeability compared to cells treated with T1 sera. Mixed effects regression analysis of the area under the curve reveal no significant differences between 10, 30 and 50% sera. Each tracing is the average of 4 patient samples. Error bars omitted for clarity.
Acknowledgments
This work was supported by NIH and T32HD068201 as well as funds from the Department of Pediatrics, Yale School of Medicine.
We would like to acknowledge the patients and families who are the inspiration for this work, PICU and cardiac operating room physician and nursing staff. Dr. Pierce received support from the NIH (T32HD068201) and the Department of Pediatrics with Dr. Kandil.
Abbreviations
- HDMEC
human dermal microvascular cell
- HPMEC
human pulmonary microvascular cell
- Angpt-2
angiopoietin-2
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- MUF
modified ultra-filtration
- PICU
pediatric intensive care unit
- TEER
trans-endothelial electrical resistance
- ECIS
electrical cell-substrate impedance sensing
- HUVEC
human umbilical vein endothelial cell
- PBS
phosphate buffered saline
Footnotes
No reprints will be requested.
Copyright form disclosure: Drs. Pierce and Zahr received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at Yale University and Yale New Haven Children’s Hospital
References
- 1.Verrier ED, Morgan EN. Endothelial Response to Cardiopulmonary Bypass Surgery. Ann Thorac Surg. 1998;66(Suppliment):S17–S19. doi: 10.1016/s0003-4975(98)00965-5. [DOI] [PubMed] [Google Scholar]
- 2.Krispinsky L, Lamb F, Stark R, et al. Endothelial Dysfunction in Children after Cardiopulmonary Bypass. FASEB Journal. 2016;30(1 Supplement):948.911. [Google Scholar]
- 3.Tassani P, Barankay A, Haas F, et al. Cardiac surgery with deep hypothermic circulatory arrest produces less systemic inflammatory response than low-flow cardiopulmonary bypass in newborns. The Journal of Thoracic and Cardiovascular Surgery. 2002;123(4):648–654. doi: 10.1067/mtc.2002.121285. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg L, Forsell C, Jogi P, et al. Effects of dexamethasone on clinical course, C-reactive protein, S100B protein and von Willebrand factor antigen after paediatric cardiac surgery. British Journal of Anaesthesia. 2003;90(6):728–732. doi: 10.1093/bja/aeg125. [DOI] [PubMed] [Google Scholar]
- 5.Madhok AB, Ojamaa K, Haridas V, et al. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27(4):408–413. doi: 10.1007/s00246-006-0934-y. [DOI] [PubMed] [Google Scholar]
- 6.Giuliano JS, Jr, Lahni PM, Bigham MT, et al. Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive Care Med. 2008;34(10):1851–1857. doi: 10.1007/s00134-008-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koning NJ, Overmars MA, van den Brom CE, et al. Endothelial hyperpermeability after cardiac surgery with cardiopulmonary bypass as assessed using an in vitro bioassay for endothelial barrier function. Br J Anaesth. 2016;116(2):223–232. doi: 10.1093/bja/aev411. [DOI] [PubMed] [Google Scholar]
- 8.Clajus C, Lukasz A, David S, et al. Angiopoietin-2 is a potential mediator of endothelial barrier dysfunction following cardiopulmonary bypass. Cytokine. 2012;60(2):352–359. doi: 10.1016/j.cyto.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tassani P, Schad H, Schreiber C, et al. Extravasation of albumin after cardiopulmonary bypass in newborns. J Cardiothorac Vasc Anesth. 2007;21(2):174–178. doi: 10.1053/j.jvca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Boldt J, Osmer C, Linker L, et al. Circulating adhesion moleculres in pediatric cardiac surgery. Pediatric Anesthesia. 1995;81:1129–1135. doi: 10.1097/00000539-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Komai H, Haworth SG. Thrombomodulin and Angiotensin-Converting Enzyme Activity During Pediatric Open Heart Operations. Ann Thorac Surg. 1996;62:533–538. [PubMed] [Google Scholar]
- 12.Komai H, Haworth SG. Effect of Cardiopulmonary Bypass on the Circulating Level of Soluble GMP-140. Ann Thorac Surg. 58:478–482. doi: 10.1016/0003-4975(94)92233-0. [DOI] [PubMed] [Google Scholar]
- 13.Burns SA, DeGuzman BJ, Newburger JW, et al. P-Selectin expression in myocardium of children undergoing cardiopulmonary bypass. Journal of Thoracic and Cardiovascular Surgery. 110(4):924–933. doi: 10.1016/s0022-5223(05)80159-x. [DOI] [PubMed] [Google Scholar]
- 14.Dagan O, Prince T, Mishali D, et al. Plasma soluble L-selectin following cardiopulmonary bypass (CPB) in children: is it a marker of the postoperative course? Med Sci Monitor. 2002;8(7):CR467–472. [PubMed] [Google Scholar]
- 15.Pierce RW, Giuliano JS, Pober JS. Endothelial cell function and dysfunction in critically ill children. Pediatriacs. 2017;140(1):e20170355. doi: 10.1542/peds.2017-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pober JS, Sessa WC. Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol. 2014;7(1):a016345. doi: 10.1101/cshperspect.a016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehne M, Sasse M, Karch A, et al. Systemic inflammatory response syndrome after pediatric congenital heart surgery: Incidence, risk factors, and clinical outcome. J Card Surg. 2017;32(2):116–125. doi: 10.1111/jocs.12879. [DOI] [PubMed] [Google Scholar]
- 18.Redl H, Dinges HP, Buurman WA, et al. Expression of endothelial leukocyte adhesion molecule-1 in spetic but not traumatic/hypovolemic shock in the baboon. American Journal of Pathology. 1991;139(2):461–466. [PMC free article] [PubMed] [Google Scholar]
- 19.Kluger MS, Clark PR, Tellides G, et al. Claudin-5 controls intercellular barriers of human dermal microvascular but not human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(3):489–500. doi: 10.1161/ATVBAHA.112.300893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abou Zahr R, Faustino EV, Carpenter T, et al. Vitamin D Status After Cardiopulmonary Bypass in Children With Congenital Heart Disease. J Intensive Care Med. 2016 doi: 10.1177/0885066616652077. [DOI] [PubMed] [Google Scholar]
- 21.Comhair SA, Xu W, Mavrakis L, et al. Human primary lung endothelial cells in culture. Am J Respir Cell Mol Biol. 2012;46(6):723–730. doi: 10.1165/rcmb.2011-0416TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark PR, Manes TD, Pober JS, et al. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol. 2007;127(4):762–774. doi: 10.1038/sj.jid.5700670. [DOI] [PubMed] [Google Scholar]
- 23.Clark PR, Kim RK, Pober JS, et al. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-kappaB-dependent phases. PLoS One. 2015;10(3):e0120075. doi: 10.1371/journal.pone.0120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komarova YA, Kruse K, Mehta D, et al. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ Res. 2017;120(1):179–206. doi: 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aebert H, Kirchner S, Keyser A, et al. Endothelial apoptosis is induced by serum of patients after cardiopulmonary bypass. European Journal of Cardio-Thoracic Surgery. 2000;18:589–593. doi: 10.1016/s1010-7940(00)00565-0. [DOI] [PubMed] [Google Scholar]
- 26.MacNaughton PD, Braude S, Hunter DN, et al. Changes in lung function and pulmonary capillary permability after cardiopulmonary bypass. Critical Care Medicine. 1992;20(9):1289–1294. doi: 10.1097/00003246-199209000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Spooner C, Markowitz N, Saravolatz L. The role of tumor necrosis factor in sepsis. Clinical Immunology and Immunopathology. 1992;62(1):S11–S17. doi: 10.1016/0090-1229(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 28.Misop M, Babin-Ebel J. Interindividual variations in cytokine levels following cardiopulmonary bypass. Heart Vessels. 1997;12:119–127. doi: 10.1007/BF02767129. [DOI] [PubMed] [Google Scholar]
- 29.Faustino E, Luckett P, Pierce R. A Survey of Pediatric Critical Care Providers on the Presence, Severity, and Assessment of Capillary Leak in Critically Ill Children. Journal of Pediatric Intensive Care. 2016 doi: 10.1055/s-0036-1593388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Area under the curve (AUC) analysis for TEER for each timepoint on HDMEC and HPMEC (n = 20). The AUC is significantly lower in HDMEC and HPMEC treated with pre-CPB sera, indicating a more detrimental effect over time.
Effect of patient serum titration on trans-endothelial electrical resistance of HDMECs acclimated to growth media containing only human sera. HDMEC monolayers that have been transitions to human-serum based media treated with 30% (A) or 50% (B) patient sera. Consistent with HDMEC treated with 10% sera, more leak is observed when treated with pre-CPB (T1) sera compared to post-CPB sera (T2 and T3). Treatment of cells with sera from both T2 and T3 sera result in similar decreases in permeability compared to cells treated with T1 sera. Mixed effects regression analysis of the area under the curve reveal no significant differences between 10, 30 and 50% sera. Each tracing is the average of 4 patient samples. Error bars omitted for clarity.