Abstract
Introduction
Integrated HIV-non-communicable disease (NCD) services have the potential to avert death and disability, but require data on program costs to assess the impact of integrated services on affordability.
Methods
We estimated the incremental costs of NCD screening as part of home-based HIV testing and counseling (HTC) and referral to care in KwaZulu-Natal, South Africa. All adults in the households were offered integrated HIV-NCD screening (for HIV, diabetes, hypertension, hypercholesterolemia, obesity, depression, tobacco and alcohol use), counseling, and linkage to care. We conducted comprehensive program micro-costing including ingredient-based and activity-based costing, staff interviews, and time assessment studies. Sensitivity analyses varied cost inputs and screening efficiency.
Results
Integrating all-inclusive NCD screening as part of home-based HTC in a high HIV prevalence setting increased program costs by $3.95 (42%) per person screened (from $9.36 to $13.31 per person). Integrated NCD screening, excluding point-of-care cholesterol testing, increased program costs by $2.24 (24%). Further, NCD screening integrated into HTC services reduced the number of persons tested by 15–20% per day.
Conclusions
Integrated HIV-NCD screening has the potential to efficiently utilize resources compared with stand-alone services. While all-inclusive NCD screening could increase the incremental cost per person screened for integrated HIV-NCD services over 40%, a less costly lipid assay or targeted screening would result in a modest increase in costs with the potential to avert NCD death and disability. Our analysis highlights the need for implementation science studies to estimate the cost-effectiveness of integrated HIV-NCD screening and linkage per disability-adjusted life year and death averted.
Keywords: NCDs, integrated screening, integrated care, HIV, cost analysis
Introduction
The dual burden of HIV and non-communicable diseases (NCDs) in sub-Saharan Africa (SSA) is rapidly growing.1 The largest number of people living with HIV (PLWH) reside in South Africa, where nearly half of the estimated seven million HIV-infected persons are on antiretroviral treatment (ART).2 Expanded ART coverage has increased life expectancy, increasingly shifting the burden of premature death and disability to non-AIDS causes including NCDs.3 In response to these epidemiologic trends, the South African Ministry of Health recognizes the need to identify high value strategies to deliver both NCD and HIV services for scale-up.4
In an effort to reach the UNAIDs 90-90-90 target, community-based strategies (occurring outside of healthcare facilities) have been utilized to test more people and link persons testing HIV-positive to care.5 Community-based HIV testing and counseling (HTC) approaches achieved higher rates of HIV testing than facility-based HTC, as well as high linkage to care and treatment when combined with enhanced linkage to care strategies.6 As community programs expand, there is growing support for the integration of NCD screening and linkage to care to identify higher risk persons, improve efficiency by broadening services, leverage existing chronic care systems and destigmatize HIV.7 One concern is that expanding services offered with HIV testing may reduce the number of PLWH who access ART due to a greater burden of services for staff.
Integrated delivery offers the potential to decrease costs by distributing fixed costs over several health interventions and increasing efficiency of scope. Services may also improve effectiveness through synergy and improve quality of delivery.8 Integrated tuberculosis and HIV programs have shown evidence of cost-effectiveness and improved linkage to HIV care and tuberculosis prevention.9,10 The effect of integrated NCD/HIV screening on expenditures and health outcomes to existing HIV programs, however, has not been established.7 Existing studies assessing costs associated with NCD integration to community-based HTC in SSA are limited, and the few studies conducted were of campaign and mobile van strategies.11–14 The aim of this analysis was to conduct a cost analysis to determine the per-person incremental costs associated with integrating NCD screening and counseling to a home-based HIV counseling and testing program in KwaZulu-Natal, South Africa.
Methods
We conducted a cross-sectional costing analysis of an integrated HIV-NCD home-based testing and counseling (HTC) program in KwaZulu-Natal, South Africa from the payer perspective. The integrated platform was implemented in a subset of HIV-infected and non-infected adults ≥ 18 years old who enrolled in the Linkages Study, a prospective, cohort study of community-based HTC delivery in 2012–2013. These households were revisited (N = 570) in 2015 and any family member enrolled during the initial study visit were offered repeat home-based HTC with integrated NCD screening and counseling. Exclusion criteria included any household members not previously enrolled and members unable to given written consent. The NCD screening included point-of-care assessment of non-fasting blood glucose and total cholesterol, blood pressure, depression, and associated NCD risk factors including obesity, tobacco and alcohol use. The counselors interpreted NCD results and provided participants with counseling and result cards. Participants with abnormal results were encouraged to follow-up with their clinic and linkage to care was not assessed. The primary study is described in full in the burden of NCD analysis.15
Procedures
Integrated home-based HIV-NCD testing and counseling was conducted by lay counsellors trained by a study nurse in HIV testing, anthropometric measurement, and point of care NCD screening. Enrolled participants completed a comprehensive interviewer-led self-report health questionnaire including information on demographics, mental health, NCD risk factors, chronic conditions and health care utilization. Anthropometric data were collected using a Seca Stadiometer (United Kingdom) and an electronic scale to the nearest 0.1kg (0.22 pounds). Blood pressure and pulse were measured in accordance with the American Heart Association recommendations using the HBP-1300-E device from Omron (Japan). A push button lancet safety needle was used for HIV testing using the Determine HIV 1–2 rapid test by Alere Medical Co Ltd. (United States), random plasma glucose (RPG) and low-density lipoprotein (LDL) using the point-of-careET-202 Easy Touch (GC) device and test strips. HTC was delivered in accordance to the South African National HIV Counselling and Testing Policy Guidelines (2015). NCD counseling was based on National South African Guidelines and WHO Guidelines for Primary Care in Low-Resource Settings, adopted for outreach activities using motivational interviewing techniques.16 All survey and measurement data were captured using the Mobenzi Researcher mobile Android application (Mobenzi Researcher, Durban, South Africa). The study was approved by both Human Sciences Research Council Research Ethics Committee (REC: 1/26/05/11) and the University of Washington Institutional Review Board (48733).
Cost Analysis
Baseline costs (HIV intervention only) were defined as those incurred by participants receiving home-based HTC in 2013 as part of the primary micro-costing study.17,18 Incremental costs of the integrated HIV-NCD testing and counseling (NCD intervention costs) were collected on-site in March 2016. A combination of ingredient and activity-based costing were used. Research costs were separated from operational costs. The time assessment was completed retrospectively through semi-structured interviews with research staff and review of study logs to estimate time per activity and change in the number of participants screened per day. We categorized costs into personnel, transportation, equipment, supplies, buildings and overhead, start-up, recurring meetings, and mobile phone data usage (Table 1). We assumed 5-years of useful life for vehicles, training and equipment. Costs were discounted annually at 3%.19 We calculated costs for an operational costing model, removing research-related costs and assuming task-shifting from professional counselors to lay staff (community care workers).18 Tornado diagrams were used to present the results of one-way sensitivity analyses varying costs inputs and screening efficiency.20 Baseline costs (2013 USD) were inflated to 2015 USD (see supplement). Integrated home-based HIV-NCD testing and counseling costs were collected in 2015 ZAR and converted to 2015 USD using World Bank exchange rates. Analyses were conducted using Excel 2008 (Microsoft, Redmond, WA, USA).
Table 1.
Costs Sources of Integrated Home-based HIV-NCD Testing and Counseling
| Cost Item1 | Marginal Component | Source | ||
|---|---|---|---|---|
| Transportation | Additional fuel for fasting glucose and lipid check on return visits | Review of budgets, invoices | ||
|
| ||||
| Equipment | Point of care cholesterol and glucose meter, measuring tape, stadiometer, automated blood measure cuff | Review of budgets, invoices | ||
|
| ||||
| Supplies | Glucose and lipid strips, lancets, paperwork | Review of budgets, invoices | ||
|
| ||||
| Start-up | Clinician and ministry of health consultation for NCD component | Staff interviews | ||
|
| ||||
| Training | Nursing, community workers, mobilizers, data technician and administrative assistant training | Staff interviews | ||
|
| ||||
| Data capture | Additional development and data usage cost | Staff interviews | ||
|
| ||||
| Testing efficiency2 | Numbers persons assumed tested per day | Staff Interviews | ||
| Home-based HTC3 | HIV - + | |||
| Community health worker | 7 | 5 | ||
| Nurses | 4 | 3 | ||
| Home-based HIV-NCD | HIV - + | |||
| Community health worker | 6 | 4 | ||
| Nurses | 3 | 2 | ||
Assumed no incremental increase of total cost of personnel, buildings and overhead and recurring meetings
Assume staff of N=20 community health workers, N=4 nurses to act as supervisors per WHO HTC guidelines and 30% HIV prevalence
Based on prior time and motion and task shifting assumption1
Results
Cohort characteristics
Five hundred and seventy people received integrated HIV-NCD testing, counseling, and referral to care in January 2015. HIV prevalence was 33%. Seventy one percent of persons tested were overweight or obese, 33% had stage 1 or 2 hypertension, 4% had an elevated non-fasting glucose, 20% had elevated total cholesterol, and 12% had a PHQ9 score of >10 consistent with depression. About 80% of the cohort had one measured NCD risk factor and over half (56%) had two or more. Similar rates of NCDs and risk factors were found between HIV positive and negative persons.15
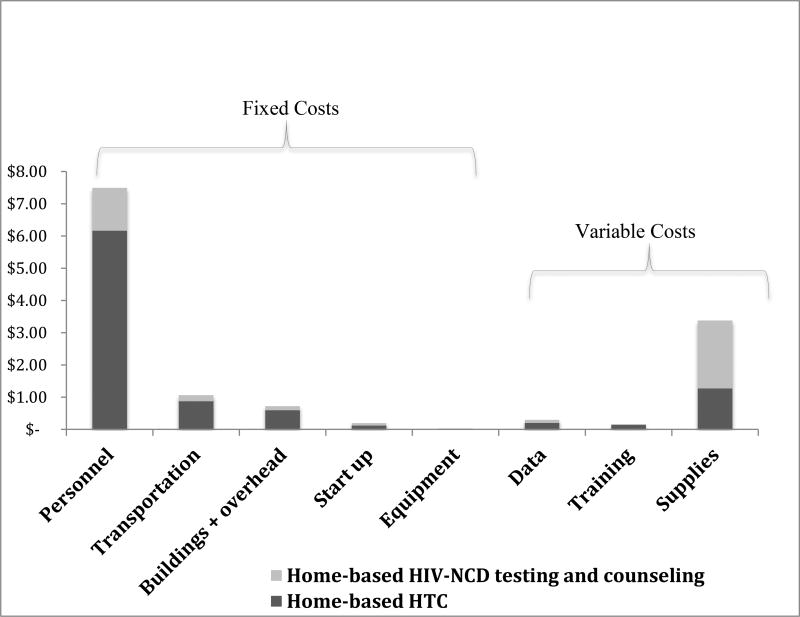
The cost per-person tested and counseling at home increased from $9.36 to $13.31, a 42% increase, with the addition of NCD screening and referral to care, to home-based HTC. Personnel cost was the largest input among all the cost categories, representing roughly 56% of the total cost of intervention, followed by supplies (25%), transportation (8%), and building and overhead (5%). Variable costs (supplies, yearly training, data capture) only comprised of 29% of total program costs. Table 2 presents the input costs by category for home-based HTC and home-based HIV-NCD testing and counseling.
Table 2.
Per-person costs of home-based HTC and home-based HIV-NCD testing and counseling in 2015 US
| Cost Input | Home-based HTC | Home-based HIV-NCD testing and counseling |
||
|---|---|---|---|---|
|
| ||||
| Per-person | % per-person | Per-person | % per-person | |
| Personnel | $6.16 | 66% | $7.49 | 56% |
|
| ||||
| Supplies | $1.27 | 14% | $3.37 | 25% |
|
| ||||
| Transportation | $0.87 | 9% | $1.06 | 8% |
|
| ||||
| Building and Overhead | $0.59 | 6% | $0.72 | 5% |
|
| ||||
| Data capture | $0.20 | 2% | $0.29 | 2% |
|
| ||||
| Start-up | $0.12 | 1% | $0.19 | 1% |
|
| ||||
| Training | $0.13 | 1% | $0.16 | 1% |
|
| ||||
| Equipment | $0.02 | 0% | $0.03 | 0% |
|
| ||||
| Total (annualized) | $9.36 | $13.31 | ||
The cost of supplies was the most pronounced marginal costs for integrated HIV-NCD testing and counseling (Figure 1), followed by personnel, transportation and building and overhead. Excluding lipid test strips, which cost $1.71 per strip, decreased marginal supply costs by over 80% and overall incremental cost of integrated screening to 24%.
Figure 1.
Fixed and variable marginal costs of NCD integration per-person to home-based HTC 2015 USD
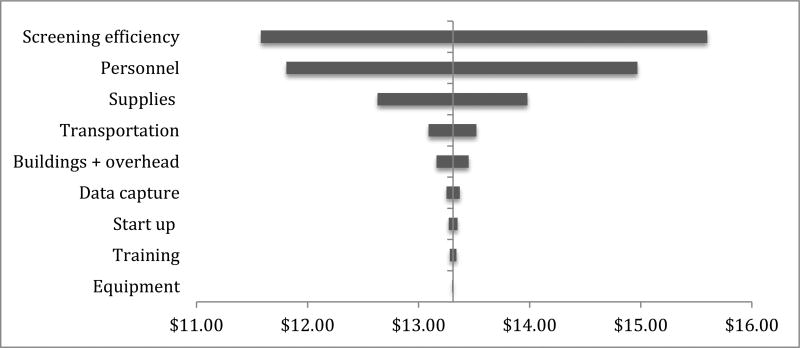
Total programmatic costs associated with personnel, building/overhead and start-up were largely unchanged from total costs of home-based HTC alone. Time assessments revealed an average 20% increase in testing and counseling time, estimating roughly one to two less persons reached per day with integrated screening as compared to HTC only. One-way sensitivity analysis of 20% change by each cost input and screening efficiency revealed that screening efficiency was a key driver of program costs.
Discussion
We estimated the incremental costs of combining NCD screening with a home-based HIV testing and counseling platform in KwaZulu-Natal, South Africa, and found that integration could increase the person screened costs by 20%-40% and decrease the number of people reached per day by 15%-20%. This study estimates the incremental costs of integrated HIV-NCD screening that is both comprehensive (diabetes, hypertension, cardiovascular risks and depression), and exclusively home-based. These cost data build on the sparse economic evidence assessing the value of innovative integrated models of care in low-middle income settings.7
The marginal per-person screening costs increase of 24% (excluding cholesterol screening) are consistent with previously published integrated screening platforms in Sub-Saharan Africa.11–14 We excluded lipid supplies when comparing prior studies since we would expect the cholesterol strip costs to decrease with increased guaranteed volume purchased and targeted screening. A mobile integrated screening program in Zambia included hypertension, diabetes and CVD risk factor screening among work employees. They found comparable incremental cost of screening (18%) with related incremental time component to NCD portion of care (20%) to our time estimation.13 The SEARCH collaboration, a community-based integrated campaign strategy in Uganda, estimated incremental NCD costs approximately 5%.11,12 Their relatively low marginal costs may be related to higher baseline overhead with campaign delivery and broader screening, including tuberculosis and malaria.
Fixed costs comprised the bulk of program, comprising 79% of overall costs (88% excluding cholesterol testing). The higher fixed costs in this study suggest efficiency gains for integration with increased utilization of services with home HTC (scale), as seen in integrated cervical cancer screening.8 A number of previous integrated HIV screening programs with cervical cancer found higher variable costs that rely more heavily on diagnostic costs, including lab and transport.21,22 We would expect improved efficiencies in scope with the implementation of single-device, multi-disease point-of-care testing.
The increase in time needed to perform NCD testing and counseling was the primary driver of costs in this analysis. Compared to previously cited integrated platforms, this study relied heavily on a time-intensive questionnaire, including depression, cardiovascular disease risk factors (including BMI, smoking) screen and counseling. This highlights the need for site-specific burden analyses to ‘fine-tune’ targeted counseling, appreciating the time trade-off between broad screening and diminishing return. Using the WHO/ISH risk stratification tool,23 we estimated only 3% of the Linkages study participants met criteria for cardiovascular disease risk. Although likely underestimating risk since the risk tool does not account for HIV as risk factor, this cohort was majority aged <50 with low rates of both smoking and diabetes. These results are comparable to an HIV cohort in another screening program in South Africa.24 As opposed to a ‘one size fits all’ approach, targeted, age-specific counseling towards diet and activity to address high burden of HIV, obesity and hypertension in this setting may optimize both technical efficiency and health outcomes. Additional longitudinal studies are needed to identify age-appropriate screening cut-offs and effective preventative strategies among younger, comorbid adults.
Our study had several limitations. The health benefits of integration cannot be assessed since this analysis did not include effectiveness outcomes. We anticipate there will be a rapid growth in implementation science studies estimating effectiveness of such non-clinic NCD preventative and management strategies that will shed light on the value of varying platforms. Further research is also needed to assess if enhanced linkages in home-based NCD screening has similar success in NCD linkages and retention to care as in home-based HTC.17,25 Additionally, high baseline HTC participation17 may have masked potential gains in participation through integration. Future work in settings with lower baseline participation should measure the impact of integrated screening on participation rates, where varying reach impacts costs per screen. The time estimates are based on semi-formal interviews with staff and review of daily logs, which is subject to recall bias. The comparative home HTC arm was conducted nearly a year prior; we adjusted for inflation but HTC costs may have varied over time. Since the study cohort had undergone home HTC within 2 years, generalizability is limited given the cohort’s familiarity with HTC that would impact time efficiency of the program. We did not assess the societal perspective, where savings could be associated with patient transportation and patients’ time, as found in an integrated HIV and cervical cancer program.26 Despite these limitations, these results estimate the incremental costs and high-impact variables to add a comprehensive NCD package to home-based HTC.
In conclusion, comprehensive home-based HIV-NCD testing and counseling results in a modest increase in costs with the potential to avert NCD death and disability. The additional time burden of NCD screening and testing was the major driver of costs, emphasizing the need for a targeted approach that bridges to an integrated public health model. Our analysis highlights the need for further costing analyses to characterize the technical efficiency of integration screening and implementation science studies to estimate the effectiveness of NCD screening on care linkages, cardiovascular risk reduction and cost-effectiveness of integrated models.
Supplementary Material
Figure 2.
Tornado diagram of one-way sensitivity analysis of cost inputs by 20% increase and decrease from base case scenario (See appendix Table S3 for inputs)
Acknowledgments
The authors would like to acknowledge the contribution of colleagues from the International Clinical Research Center at the University of Washington and the staff at Human Sciences Research Council. This work has been supported by the National Institutes of Health (NIH) 5 R01 AI083034, 3 R0 AI083034-02S2 and NIH Directors Award RC4 AI092552 and Centers for AIDS Research (CFAR)/NIH (P30 AI027757)
Footnotes
Competing interests
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript
Authors’ contributions
Conception of the work – AVH, HVR, CC, RVB
Data collection – IG, MS, AVH
Data analysis and interpretation – IG, MS, AVH, RVB
Drafting the article – IG, MS, RVB
Critical revision of the article – IG, MS, AVH, HVR, JMB, CC, RVB
Final approval of the version to be published – IG, RVB
Bibliography
Smith JA SM, Levin C, et al. Cost-effectiveness of communitybased strategies to strengthen the continuum of HIV care in rural South Africa: a health economic modelling analysis. Lancet HIV 2015;2:e159–e168.
References
- 1.Dalal SBJ, Volmink J, Adebamowo C, Bajunirwe F, Njelekela M, et al. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiology. 2011;40:885–901. doi: 10.1093/ije/dyr050. [DOI] [PubMed] [Google Scholar]
- 2.Nations U. [Accessed April 20, 2017];UNAIDS Gap Report. 2014 http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf.
- 3.Levitt NSSK, Bradshaw D. Chronic noncommunicable diseases and HIV-AIDS on a collisioncourse: relevance for health care delivery, particularly in low-resource settings–insight from South Africa. Am J Clin Nutr 2011. 2011;94:1690S–1696S. doi: 10.3945/ajcn.111.019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health. Mo. [Accessed April 20, 2017];Strategic Plan for the Prevention and Control of Non-Communicable Diseases 2013–17. 2013 http://www.hsrc.ac.za/uploads/pageContent/3893/NCDs%20STRAT%20PLAN%20%20CONTENT%208%20april%20proof.pdf.
- 5.WHO. Planning, Implementating, and Monitoring Home-Based HIV Testing and Counselling; A practical handbook for sub-Saharan Africa. WHO Library Cataloguing-in-Publication Data. 2012 [Google Scholar]
- 6.Suthar ABFN, Bachanas PJ, et al. Towards universal voluntary HIV testing and counselling: a systematic review and metaanalysis of community-based approaches. PLoS Med. 2013;10:e1001496. doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabkin M, Nishtar S. Scaling up chronic care systems: leveraging HIV programs to support noncommunicable disease services. Journal of acquired immune deficiency syndromes (1999) 2011;57(Suppl 2):S87–90. doi: 10.1097/QAI.0b013e31821db92a. [DOI] [PubMed] [Google Scholar]
- 8.Obure CD, Guinness L, Sweeney S, Initiative I, Vassall A. Does integration of HIV and SRH services achieve economies of scale and scope in practice? A cost function analysis of the Integra Initiative. Sexually transmitted infections. 2016;92(2):130–134. doi: 10.1136/sextrans-2015-052039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausler HPSE, Kumaranayake L, et al. Costs of measures to control tuberculosis/HIV in public primary care facilities in Cape Town, South Africa. Bull World Health Orga. 2006;84(7):528–536. doi: 10.2471/blt.04.018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drain PKHE, Noubary F, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014;14(3):239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamie G, Kwarisiima D, Clark TD, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One. 2012;7(8):e43400. doi: 10.1371/journal.pone.0043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang W, Chamie G, Mwai D, et al. Implementation and Operational Research: Cost and Efficiency of a Hybrid Mobile Multidisease Testing Approach With High HIV Testing Coverage in East Africa. Journal of acquired immune deficiency syndromes (1999) 2016;73(3):e39–e45. doi: 10.1097/QAI.0000000000001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Beer I, Chani K, Feeley FG, Rinke de Wit TF, Sweeney-Bindels E, Mulongeni P. Assessing the costs of mobile voluntary counseling and testing at the work place versus facility based voluntary counseling and testing in Namibia. Rural and remote health. 2015;15(4):3357. [PubMed] [Google Scholar]
- 14.Labhardt ND, Motlomelo M, Cerutti B, et al. Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial. PLoS Med. 2014;11(12):e1001768. doi: 10.1371/journal.pmed.1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Heerden A, Barnabas RV, Norris SA, Micklesfield LK, van Rooyen H, Celum C. High prevalence of HIV and non-communicable disease (NCD) risk factors in rural KwaZulu-Natal, South Africa. J Int AIDS Soc. 2017;20(2) doi: 10.1002/jia2.25012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Prevention and Control of Noncommunicable Diseases: Guidelines for primary health care in low resource settings. WHO Library Cataloguing-in-Publication Data; 2012. [PubMed] [Google Scholar]
- 17.van Rooyen HBR, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64:e1–e8. doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JASM, Levin C, et al. Cost-effectiveness of communitybased strategies to strengthen the continuum of HIV care in rural South Africa: a health economic modelling analysis. Lancet HIV. 2015;2:e159–e168. doi: 10.1016/S2352-3018(15)00016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. [Last accessed 17 June 2017];World Health Organization Statistical Information System: CHOICE (Choosing Interventions that are Cost Effective) www.who.int/choice/en/
- 20.Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force--6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Schnippel K, Michelow P, Chibwesha CJ, et al. Cost-effectiveness of using the Cervex-Brush (broom) compared to the elongated spatula for collection of conventional cervical cytology samples within a high-burden HIV setting: a model-based analysis. BMC health services research. 2015;15:499. doi: 10.1186/s12913-015-1163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lince-Deroche N, Phiri J, Michelow P, Smith JS, Firnhaber C. Costs and Cost Effectiveness of Three Approaches for Cervical Cancer Screening among HIV-Positive Women in Johannesburg, South Africa. PLoS One. 2015;10(11):e0141969. doi: 10.1371/journal.pone.0141969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Prevention of cardiovascular disease: Guidelines for assessment and management of total cardiovascular risk. Geneva: 2007. [Google Scholar]
- 24.Rabkin MMA, Chung C, Zhang Y, Wei Y, El-Sadr WM. Missed Opportunities to Address Cardiovascular Disease Risk Factors amongst Adults Attending an Urban HIV Clinic in South Africa. PLoS ONE. 2015;10(10):e0140298. doi: 10.1371/journal.pone.0140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77–85. doi: 10.1038/nature16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vodicka EL, Babigumira JB, Mann MR, et al. Costs of integrating cervical cancer screening at an HIV clinic in Kenya. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2017;136(2):220–228. doi: 10.1002/ijgo.12025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.