To the Editor
Renal complications are one of the leading causes of mortality in sickle cell anemia (SCA). Sickle nephropathy (SN) encompasses a spectrum of renal pathologies including tubular defects [e.g., impaired urine concentrating ability (UCA)/hyposthenuria] and glomerular defects [albuminuria, focal segmental glomerulosclerosis (FSGS) and end-stage renal disease (ESRD)]. Sickling of RBCs in the hypoxic hyperosmotic renal medulla causes vaso-occlusion in the vasa-recta, ischemia, loss of osmotic gradient and papillary necrosis, resulting in hyposthenuria. However, the mechanism underlying glomerulopathy is unknown and assumed to be secondary to sickling-associated injury. Angiotensin converting enzyme-inhibitors (ACE-I) have been tested in small clinical trials for adults with SCA with albuminuria, based on their reno-protective properties in non-sickle nephropathies, and are included in the NHLBI guidelines for SCA.1
We recently showed that high circulating angiotensin-II (AT) in mice and humans with SCA increases mobilization of hematopoietic stem/progenitor cells.2 Angiotensin-II (AT), signals through tissue-bound AT receptors-1 (AT1R) and -2 (AT2R). While the detrimental role of increased AT signaling is known in non–SCA nephropathies, its role in SCA, and specifically SN, has not been studied.
We investigated whether high AT was secondary to increased renin in SCA. Renin catalyzes generation of AT from its precursor angiotensinogen (Supplementary Fig. S1a). Hypoxia increases renin expression, thereby increasing AT production, which is the basis of reno-vascular hypertension. We postulated that in SCA, hypoxia from vaso-occlusions in vasa-recta could increase renin and consequently AT production. However, renin expression was not increased in kidneys of young sickle mice, despite high urinary AT levels (AT, a small octapeptide, readily filters through the glomerulus, and urine AT level reflects circulating and renal-generated AT) (Supplemental Fig. S1b–d). Moreover, high AT was present in young children with SCA, in the absence of hypertension (Supplemental Fig. S1e–f). Hence, renin signaling does not appear to be the cause of high AT levels; its levels rise secondarily in older animals.
We next transplanted normal/wild-type mice (WT) mice with bone marrow from SS mice (SS→WT) to attain fully chimeric sickle mice (WT→WT chimeras were transplant controls). SS chimeras, but not WT chimeras, had hyperangiotensinemia (Supplemental Fig. S1g), demonstrating that this effect was not secondary to transplant-induced vasculopathy. This data suggests that perhaps sickle hematopoiesis mediates hyperangiotensinemia, although the mechanism needs to be explored.
We investigated the role of high AT in SN. The features of SN seen in humans were closely mimicked in both SCA mouse models (Berkeley sickle [SS] mice and knock-in-SS mice, both exclusively carrying human sickle hemoglobin), including glomerular hyper-filtration, albuminuria, hyposthenuria, and progressively worsening FSGS. Indeed, the renal pathology in older SS mice was similar to that in a SCA patient with macro-albuminuria (Supplemental Fig. S2a–f). These SCA mouse models were used for mechanistic studies.
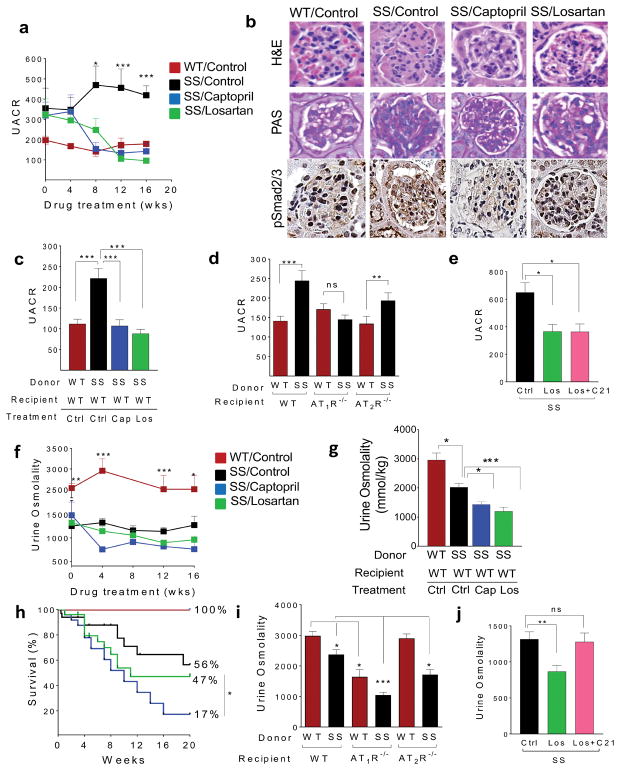
We blocked AT signaling with captopril (ACE-I), or losartan (AT1R blocker) in SS mice. Both captopril and losartan abrogated albuminuria within 8-weeks, an effect sustained when treatment was continued for another 8-weeks (Fig. 1a). This amelioration in glomerular defect was accompanied with improved renal histology (glomerulosclerosis, and mesangial proliferation) compared to the untreated SS controls (Fig. 1b, Supplemental Fig S3a–d). This effect of AT inhibition was also observed in WT mice transplanted with sickle hematopoiesis (SS/WT chimera); the albuminuria that developed in the SS/WT chimeric mice post-transplant was normalized with AT blockade (Fig. 1c). Like with other non-sickle nephropathies,3 SS mice had increased renal (glomerular) active TGFβ1 and Smad-2/3 (Supplemental Fig S4a–d), that were corrected by blocking AT-signaling with captopril/losartan (Fig.1b).
Figure 1. Hyperangiotensinemia in SCA promotes glomerulopathy (via AT1R signaling), but plays an important role in sustaining urine concentrating ability (via both AT1R and AT2R signaling) in the setting of SCA-associated hyposthenuria.
a) Temporal progression of albuminuria, shown as urine albumin/creatinine ratio (Y-axis), in non-transplanted WT mice or SS mice (that were untreated, or treated with losartan or captopril). The weeks of drug treatment shown on the X-axis. SS mice were started on drug treatment at 8–12 weeks of age. UACR was determined on 24hr-urine samples by the urine albumin to creatinine ratio and data are expressed in μg albumin/mg creatinine. (b) H &E staining (60X magnification), PAS staining (60X magnification) and immunohistochemistry for phosphorylated Smad-2/3 (100X magnification) in the kidneys of WT mice, untreated -SS control mice, or SS mice treated with captopril or losartan for 12 weeks. A single representative glomerulus is shown. Improvement in glomerular pathology including congestion, FSGS and mesangial proliferation is seen in SS mice on captopril or losartan compared to the untreated SS mice. Increased pSmad2/3 expression (brown staining) is seen in untreated SS compared to SS mice on captopril/losartan. (c) C57Bl/6 mice (WT; recipient) were transplanted with SS or WT donor bone marrow at 8–12 weeks of age following lethal irradiation (1275cGy) in a donor: recipient ratio of 1:7. Only SS/WT chimeras determined to be fully chimeric for SS by HPLC for hemoglobin S, 3 months after transplantation, were further analyzed for experiments. Untreated WT/WT chimeras (WT/WT-Ctrl; red bar), untreated SS chimeric mice (SS/WT-Ctrl; black bar), SS chimeric mice placed on captopril 3 months after transplant (SS/WT-Cap; blue bar) and SS chimeric mice placed on losartan 3 months after transplant (SS/WT-Los; green bar) were compared. Urine albumin and creatinine was analyzed 12 months after establishment of chimerism. UACR was determined on 24-hr urine samples by the urine albumin to creatinine ratio and data are expressed in μg albumin/mg creatinine is shown (n= 6–8 mice/group. (d) UACR levels in WT, AT1R−/− and AT2R−/− recipient mice transplanted with WT (red bars) or SS (black bars) donor bone marrow. Mice fully chimeric for WT or SS bone marrow at three months were analyzed. UACR was determined on 24-hr urine samples by the urine albumin to creatinine ratio and data are expressed in μg albumin/mg creatinine is shown (n=6–20 mice per group). (e) Urine albumin in SS mice (untreated or placed on only AT1R antagonist Losartan (Los) or placed on a combination of AT2R agonist C21, and Los; n = 10–20 mice/group. (f) Temporal progression of UCA, measured by urine osmolality (Y-axis) in non-transplanted WT mice and SS mice (that were untreated, or treated with captopril or losartan) with weeks of drug treatment shown on the X-axis. Mice were started on drug treatment at 8–12 weeks of age. Urine osmolality could only be measured in captopril-treated mice if they were given additionally water without captopril, due to high mortality from severe dehydration. The urine osmolality data are expressed in mmol/kg and was measured in 24-hr urine collections (g) C57Bl/6 mice (WT; recipient) were transplanted with SS or WT donor bone marrow at 8–12 weeks of age following lethal irradiation (1275cGy) in a donor: recipient ratio of 1:7. Only SS/WT chimeras determined to be fully chimeric for SS by HPLC for hemoglobin S, 3 months after transplantation, were further analyzed for experiments. Untreated WT/WT chimeras (WT/WT-Ctrl; red bar), untreated SS chimeric mice (SS/WT-Ctrl; black bar), SS chimeric mice placed on captopril 3 months after transplant (SS/WT-Cap; blue bar) and SS chimeric mice placed on losartan 3 months after transplant (SS/WT-Los; green bar) were compared. Urine osmolality analyzed 12 months after establishment of donor chimerism. The urine osmolality data are expressed in mmol/kg and was measured in 24-hr urine (n= 6–20 mice/group). (h) Kaplan-Meier survival curve in non-transplanted WT mice and SS mice (that were untreated or treated with captopril or losartan) for 20 weeks (X-axis). The percentage of mice surviving at the end of the experiment is indicated against the survival curve of each group. (i) Urine osmolality in WT, AT1R−/− and AT2R−/− recipient mice transplanted with WT (red bars) or SS (black bars) donor bone marrow. Mice fully chimeric for WT or SS bone marrow at three months were analyzed for urine osmolality (n=5–9 mice per group), in a 24hr- urine collection sample. (j) Urine osmolality in SS mice (untreated or placed on only AT1R antagonist Losartan (Los) or placed on a combination of AT2R agonist C21 and Los; n = 10–20 mice/group. All data are plotted as mean ±SEM. Statistical analysis was done either using Mann Whitney U test, where two groups are compared or using ANOVA (Dunnet’s multiple comparisons test) while comparing between multiple groups. Statistical significance is denoted by *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
To confirm the pharmacological data, we transplanted sickle hematopoiesis into mice genetically deficient in AT1R, or AT2R, to create mice with SCA that lacked either AT1R (SS/AT1R-deficient) or AT2R (SS/AT2R-deficient), and compared them with sickle mice with intact AT1R+AT2R (SS/WT) (Fig. 1d). Notably, only the SS/AT1R-deficient mice failed to develop sickle glomerulopathy: absent albuminuria and histopathological findings of FSGS and lack of increased TGFβ1-Smad2/3 signaling ((Fig. 1d, Supplemental Fig S4e,f). We also generated knock-in-SS/AT1R deficient mice via interbreeding the knock-in-SS mice with AT1R-deficient mice. Knock-in-SS/AT1R-deficient mice also had no albuminuria, unlike the knock-in mice with intact AT1R (Supplemental Fig. S5a).
AT2R signaling has been found to be glomerular-protective in other chronic renal injury disease models.4 However, we found that AT signaling via AT2R did not cause sickle glomerulopathy since SS/AT2R-deficient mice developed albuminuria and FSGS similar to SS/WT chimeras (Fig. 1d, Supplemental Fig. S4e). Next, we augmented AT2R signaling with C21 (an AT2R agonist) in the context of AT1R blockade. SS mice that were administered C21 with losartan showed no improvement/worsening of albuminuria, as compared to losartan alone. Therefore, neither abrogation nor augmentation of AT2R signaling affected the sickle glomerulopathy (Fig. 1e). Taken together, our pharmacological data and genetic models show that increased AT signaling promotes sickle glomerulopathy solely via AT1R.
Next, we analyzed how AT affected UCA. As expected, SS mice have significantly impaired UCA from sickling-mediated injury to vasa-recta and resultant loss of osmotic gradient. AT/AT1R signaling is known to increase sodium absorption from proximal tubules, and increase aquaporin expression, to concentrate urine.5 Therefore, it was not surprising that the reduced UCA in SS mice (or SS/WT chimeras) worsened further with AT1R inhibition with losartan/captopril (Fig. 1f,g). When SS mice (or SS/WT chimeras) were placed on captopril, however, we observed unusually high mortality from severe dehydration (Fig. 1h). Urine osmolality (and urine albumin) in this treatment group could only be measured in the “fittest”/least dehydrated sickle mice when they were additionally given non-captopril containing water supplementation. The significant increase in mortality from dehydration with captopril (that reduces AT production, thus interrupting AT signaling via both receptors; Supplementary Fig. S1a), but not with losartan (that only blocks AT1R signaling), suggests that AT may be improving UCA through AT2R as well. The anti-inflammatory and antifibrotic effects of AT2R activation have been proposed to be protective in glomerular injury; however its role in maintaining tubular function, specifically UCA, has not been explored. AT2R stimulation causes natriuresis and blood pressure regulation, and its deficiency is associated with severe experimentally-induced acute tubular necrosis.4 We hypothesized that increased AT2R signaling probably compensates for loss of UCA with losartan, which may explain the captopril effect.
We therefore determined urine osmolality in SS/AT1R-deficient and SS/AT2R-deficient chimeric mice and compared them to SS/WT chimeras (Fig. 1i). As expected, SS/WT mice had significantly lower urine osmolality than WT/WT mice. Both SS/AT1R-deficient and WT/AT1R-deficient mice had significantly lower UCA than their respective control hematopoietic chimeras i.e. SS/WT and WT/WT, highlighting the important role of AT1R in UCA. Moreover, SS/AT1R-deficient chimeras had the lowest UCA, from the dual effect of sickling-mediated hyposthenuria and lack of AT1R→aquaporin signaling. This was not a transplant effect, or an effect restricted to the SS mouse model, as UCA was also reduced in knock-in-SS/AT1R deficient mice (Supplemental Fig. S5b).
Urine osmolality in WT/WT versus WT/AT2R-deficient chimeras, however, was similar, suggesting that in healthy mice, AT2R plays no role in maintaining UCA. However, SS/AT2R-deficient mice had significantly lower urine osmolality than SS/WT chimeras demonstrating that AT2R is important in maintaining UCA in SCA, where it is likely recruited because the normal urine concentrating mechanisms are severely compromised (Fig. 1i). Indeed, stimulation of AT2R signaling by giving SS mice C21, along with concomitant blockade of AT1R with losartan, improved their UCA to levels seen in control SS mice, showing that the effect of AT1R-deficiency on UCA could be compensated for, by increased AT2R signaling. (Fig. 1j). In a diabetic rat model, C21 treatment has been shown to be effective in reducing tubulointerstitial fibrosis.4 Our data of improved UCA in SCA with C21 highlights another important reno-protective role of increased AT2R signaling. Taken together, our data shows that in SCA, AT signaling via both AT1R and AT2R compensates for the sickling-mediated loss of UCA..
Notably, ACE-I have been evaluated for their effect on albuminuria in patients with SCA, although urine osmolality was not assessed in these trials.6 It is conceivable that ACE-I may worsen UCA in SCA patients, which is either compensated for with increased water consumption and worsened enuresis/nocturia, or it results in dehydration (and consequently predisposes SCA patients to increased vaso-occlusion). In a recent Phase-II losartan study published by our group, losartan tended to lower urine osmolality in SCA patients with macro-albuminuria, although the difference was not statistically significant. Future studies should carefully assess UCA while addressing improvement in sickle glomerulopathy with AT blockade.
In conclusion, we show that the hyperangiotensinemia in SCA plays a protective role in preserving UCA in face of SCA-associated hyposthenuria, via both AT1R and AT2R signaling. However, the chronically increased AT1R signaling promotes glomerular pathology. Our data also reveals a novel reno-protective role of AT-AT2R signaling in promoting UCA in SCA. While worsened AT1R-induced hyposthenuria can compromise the quality of life of patients with SCA from nocturia/enuresis, it can be compensated by increased fluid intake. Sickle glomerulopathy, on the other hand, is an organ pathology that is important to prevent, as it leads to progressive albuminuria, FSGS and ESRD. Our study suggests that targeted blockade of AT1R along with agonism of AT2R signaling could preserve UCA and prevent sickle glomerulopathy.
Materials and methods (are available as supplemental material).
Supplementary Material
Acknowledgments
This study was supported by research funding from NIH-NHLBI R34 HL 108752 (PM) and the Excellence in Hemoglobinopathy Research Award (EHRA), U01HL117709 (PM). PR was the Translational Research Scholar on the EHRA U01HL117709. We would like to thank Dr. Charles Quinn for his helpful review and comments on the manuscript.
Footnotes
Authorship Contribution
SR performed the experiments, analyzed, plotted and interpreted the data, PR and MSEMM plotted and interpreted the data, KHC and JAC performed and interpreted the angiotensin assays, SKS and KV analyzed histopathology, TI generated the AT2R−/− mice, BA and JAC had intellectual discussions on the project with PM, PM conceived the project and designed the experiments and interpreted the data, SR, PR, MSEMM and PM wrote the manuscript, all authors reviewed and edited the manuscript.
Disclosure of Conflicts of Interest
The authors have no conflicts of interest.
References
- 1.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 2.Chang KH, Nayak RC, Roy S, et al. Vasculopathy-associated hyperangiotensinemia mobilizes haematopoietic stem cells/progenitors through endothelial AT(2)R and cytoskeletal dysregulation. Nat Commun. 2015;6:5914. doi: 10.1038/ncomms6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan HY, Chung AC. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012;32(3):236–243. doi: 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Kaschina E, Namsolleck P, Unger T. AT2 receptors in cardiovascular and renal diseases. Pharmacol Res. 2017;125(Pt A):39–47. doi: 10.1016/j.phrs.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Stegbauer J, Gurley SB, Sparks MA, et al. AT1 receptors in the collecting duct directly modulate the concentration of urine. J Am Soc Nephrol. 2011;22(12):2237–2246. doi: 10.1681/ASN.2010101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasongko TH, Nagalla S, Ballas SK. Angiotensin-converting enzyme (ACE) inhibitors for proteinuria and microalbuminuria in people with sickle cell disease. Cochrane Database Syst Rev. 2015;(6):CD009191. doi: 10.1002/14651858.CD009191.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.