Abstract
Background
Intracranial aneurysms represent a significant health concern and are poorly understood despite decades of research. Our study focused on understanding temporal patterns of endothelial cell distribution in different spatial locations within the aneurysm early after creation in a rabbit model.
Methods
Elastase induced saccular aneurysms were created in rabbits and harvested at day 1 (n=3) and 2 (n=5), 4 (n=4), 8 (n=5), and 12 (n=6) weeks. Sham operated controls (n=3) were harvested on the same day. Aneurysm and control tissue samples were subjected to en face whole-mount CD31 staining for endothelial cells. Semiquantitative scoring was performed on the basis of endothelial coverage of the vessel wall (proximal, middle, and distal portions of the aneurysm dome). Mixed-effects models were used to assess the effect of time and aneurysm section on endothelial coverage.
Results
Aneurysmsal segments were near completely deendothelialized at 4 and 8 weeks but had reendothelialized by 12 weeks. Compared to control, aneurysms at all the time points showed decreased endothelialization, but the difference was only significant compared 4- and 8-week groups. Both time (P=.03) and aneurysm section (P=.07) were significantly associated with degree of endothelialization. Proximal locations showed increased endothelialization compared with distal locations (P=.03).
Conclusion
In experimental aneurysms of rabbits, endothelial cells regress during the first month after creation, followed by ascending reendothelialization that stays incomplete. These findings suggest that repopulation of endothelial cells comes from resident cells in the adjacent parent artery and that deranged hemodynamics may affect full reconstitution of endothelial cells long term.
Keywords: Intracranial aneurysms, CD31 staining, endothelial distribution, endothelial pattern, endothelial score, rabbit model
INTRODUCTION
Intracranial aneurysms (ICA), prevalent in 3% to 4% of the population, are a relatively common vascular disorder1,2. ICAs have varied wall structure, cell content, and clinical outcome. The mechanism in healing an aneurysm continues to be enigmatic, and despite treatment with endovascular devices, the recurrence rates are still high (20%–25%) 3,4,5. Studies have shown that endothelialization forms the critical component in arterial hemostasis and in achievement of complete occlusion of the aneurysm neck6,7. Moreover, incomplete endothelialization at the aneurysm neck has been accounted for the increase in recurrences following coil embolization8,9. Therefore, it is essential to understand the pattern of distribution of endothelial cells over time after the formation of aneurysm. This paper is an attempt to fill the gaps in knowledge about aneurysm healing and explain the reason behind incomplete endothelialization noted after treatment with endovascular devices.
Since the wall represents the primary pathologic factor indeed, the actual site of enlargement and rupture we believe that a comprehensive exploration of aneurysm wall can provide valuable insight that improves patient treatment. Several studies have shown CD31 as a reliable marker for identification of endothelial cells, and hence scoring based on CD31 expression is a better way to understand the distribution of endothelial cells10,11.
The elastase-induced aneurysm model of the rabbit has been used as an established clinical model for studying aneurysms and has a hemodynamic status comparable to human aneurysms12–15. Several studies using the rabbit model have demonstrated the shear stress patterns and device testing of the aneurysms14–16. However, no study has highlighted the distribution pattern of endothelial cells over time after aneurysm formation. In our study, we attempted to characterize the pattern of deendothelialization and reendothelialization of vessel wall over time at different spatial locations in the aneurysm in our rabbit model.
METHODS
Aneurysm Creation
The Mayo Clinic Institutional Animal Care and Use Committee approved all protocols of the study. Elastase-induced saccular aneurysms were created in 26 New Zealand white rabbits and aneurysm tissues were harvested at day 1(n=3) and 2 (n=5), 4 (n=4), 8 (n=5), and 12 (n=6) weeks. In the control group (n=3, day 0), similar procedures of aneurysm creation was followed but instead of the elastase, saline incubation was done in RCCA. Detailed procedures for aneurysm creation have been described elsewhere17,18,19. In brief, rabbits (3–4 kg) were induced with intramuscular injection of ketamine and xylazine, (35 and 5 mg/kg, respectively) and maintained with 1–3% isofluorane. Using sterile technique, the right common carotid artery (RCCA) was exposed and ligated distally. A 5F sheath (Cordis Endovascular, Miami Lakes, Fla) was advanced retrograde in the RCCA to a point approximately 3 cm cephalad to the origin of RCCA. A roadmap image was obtained by injection of contrast through the sheath retrograde in the RCCA, to identify the junction between the RCCA and the subclavian and brachiocephalic arteries (Advantx; General Electric, Milwaukee, Wis). Through the indwelling sheath, a 3F Fogarty balloon (Baxter Healthcare Corporation, Irvine, Calif) was advanced to the origin of the RCCA at its junction with the right subclavian artery. The balloon was inflated with just enough iodinated contrast material to achieve flow arrest in the RCCA. Porcine elastase (5.23 U/mgP, 40.1 mgP/mL, approximately 200 U/mL; Worthington Biochemical, Lakewood, NJ) mixed with iodinated contrast material was incubated in the dead space of the RCCA, above the inflated balloon, through a microcatheter (Tracker 10; Target Therapeutics, Fremont, Calif). Balloon position and shape were documented with the use of fluoroscopic spot imaging, which was saved and printed. After incubation of the elastase solution, the balloon and sheath were removed, and the RCCA was ligated below the sheath entry site. The harvested aneurysm tissue was dissected along the midline into dorsal and ventral pieces. The dorsal piece was processed for whole-mount en face immunostaining with CD31 for endothelial cells.
Whole-mount en face immunostaining
The freshly harvested dorsal piece of aneurysm wall was pinned to a dish coated with Sylgard (Dow Corning Corp) to expose its lumen side and was completely immersed in 10% neutral buffered formalin to fix for 2 hours at room temperature (RT). The sample was washed with TBS for 15 minutes in 3 washes. It was then blocked with 5% donkey serum in TBST for 1 hour at RT, followed by incubation with primary antibody (CD31, 1:20–30 in 0.3% Triton X-100 [Sigma-Aldrich Co, LLC] in TBS) for 1 hour at RT, overnight at 4°C. The samples were washed again with TBS and incubated with secondary antibody (Cy3 conjugated donkey antimouse IgG, 1:200 in TBS) for 2.5 hours at RT and counterstained with Sytox green (ThermoFisher Scientific) (1:1000). Finally, the sample was scoped with confocal laser microscope.
Each sample was divided into 3 portions for score: distal (apical one-third far away from the parent artery), proximal (basal one-third close to the aneurysm neck), and middle (between proximal and distal areas). Five fields from each portion were randomly selected for evaluation to achieve 5 scores for each portion.
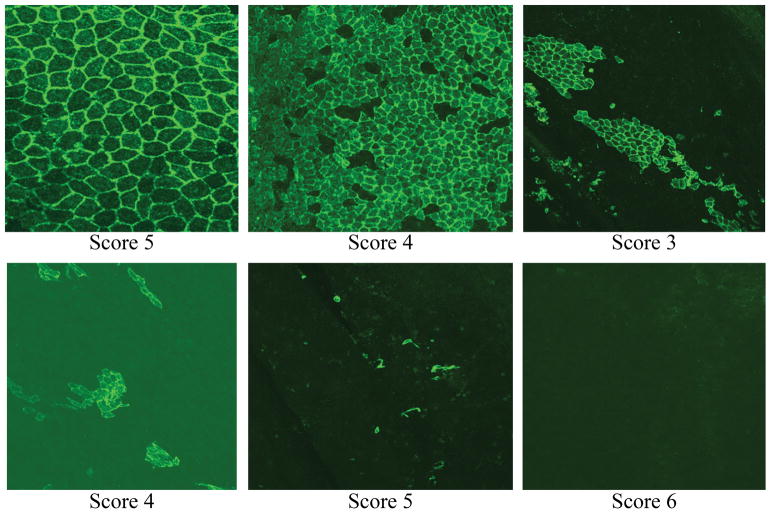
Scoring of endothelial cells are performed as stated below (Figure 1)
Figure 1.
CD31 stained endothelial cells categorized into five scores based on the distribution of endothelial cells. Score of 5=highly confluent endothelial cells; score 0=no endothelial cells.
0 -- Complete lack of coverage of CD31 positive endothelial cells
1-- Scattered, sparse cells positive for CD31, covered the lumen side of the wall
2 -- Small, isolated, patchy, cell clusters are positive for CD31
3 -- The cell clusters which are positive for CD31 are larger than that seen in 2
4 -- Majority of lumen side of the wall is covered with CD31 + cells, with the exception of some small gaps among cells
5 -- The lumen side of the wall is completely covered with CD31 + cells.
Statistical methods
Each rabbit had 5 fields scored from each aneurysm portion (proximal, middle, and distal). Scoring consistency between fields was tested using Krippendorf’sα. Field scores were averaged to create a composite score for each aneurysm location within the study rabbit. A mixed-effects model was used to test for association of score with time and location as shown Table 1. Denominator degrees of freedom were corrected using the Kenward-Rogers method. Model diagnostics were performed. Overall variable significance in the model and model-based means are presented. Because of the preliminary nature of the investigations, significance was set at α=.10. Analyses were performed with statistical software (SAS Institute Inc).
Table 1.
Estimate of Group Means From the Mixed-Effects Model.
| Effect | Value | Mean | 90% CI Upper | 90% CI Lower | P valuea |
|---|---|---|---|---|---|
| Location | Distal | 1.06 | 0.643 | 1.48 | 0.07 |
| Middle | 1.48 | 1.06 | 1.9 | ||
| Proximal | 1.66 | 1.24 | 2.08 | ||
| Time | Controls | 2.47 | 1.55 | 3.38 | 0.05 |
| 2 weeks | 1.81 | 1.11 | 2.52 | ||
| 4 weeks | 0.283 | −0.508 | 1.07 | ||
| 8 weeks | 0.813 | 0.105 | 1.52 | ||
| 12 weeks | 1.62 | 0.976 | 2.27 |
Represents variable in the overall mixed-effects model.
RESULTS
Aneurysms harvested at day 1 were excluded from the analysis due to the presence of fresh, unorganized thrombus within the dome, which prevented us from processing the samples for en face CD31 immunostaining.
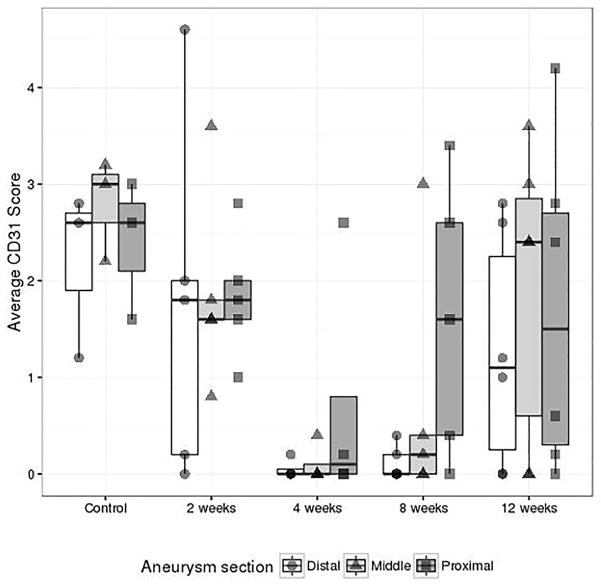
Sham operated control samples showed the highest level of endothelialization (Figure 2), but the difference was only significant compared to the 4- and 8-week groups (Table 2). Compared to the control group, the groups at 4 weeks and 8 weeks showed decreased endothelialization (−2.18 [90%CI −3.39, 0.974], p=0.006; −1.65[90%CI −2.81, – 0.497], p=0.023). Of the remaining comparisons, the 2-week and 12-week groups showed significantly greater endothelialization than the 4-week group (2 week – 4 week: 1.53[90%CI 0.468, 2.59], p=0.022; 2 week – 12 week −1.34[−2.36, −0.317], p=0.036); none of the other pairwise comparisons between different times of sacrifice were significantly different (Table 2). Thus our results show a trend of decrease in CD31 scores starting from day 0 to 4 weeks followed by increase in CD 31 scores at 8 and 12 weeks. However the scores at 12 weeks does not match the scores of control (day 0).
Figure 2.
Comparison of CD31 scores and time points of sacrifice at different aneurysm section locations. Each point represents the composite score for one rabbit. The center line on each box represents the median; the limits of the box, and the interquartile range. Endothelialization is greatest in the control group (mean 2.47) followed by de-endothelialization in week 2 (mean 1.81) and week 4 (mean 0.283). Re-endothelialization is noted again at week 8 in the proximal and middle portions of the aneurysms. Re-endothelialization is noted in all three regions at week 12 (mean 1.62).
Table 2.
Mixed-Model Pairwise Comparisons Between Levels of Each Variable
| Effect | Comparison | Estimate | 90% CI Upper | 90% CI Lower | p |
|---|---|---|---|---|---|
| Location | Middle vs Proximal | −0.183 | −0.627 | −0.262 | 0.493 |
| Middle vs Distal | 0.417 | −0.027 | 0.862 | 0.122 | |
| Proximal vs Distal | 0.6 | 0.156 | 1.04 | 0.028 | |
| Time | Week 2 vs Control | −0.653 | −1.81 | 0.503 | 0.340 |
| Week 4 vs Control | −2.18 | −3.39 | −0.974 | 0.006 | |
| Week 8 vs Control | −1.65 | −2.81 | −0.497 | 0.023 | |
| Week 12 vs Control | −0.844 | −1.96 | 0.275 | 0.207 | |
| Week 2 vs Week 4 | 1.53 | 0.468 | 2.59 | 0.022 | |
| Week 2 vs Week 8 | 1 | −0.001 | 2 | 0.100 | |
| Week 4 vs Week 8 | −0.53 | −1.59 | −0.532 | 0.398 | |
| Week 2 vs Week 12 | 0.191 | −0.768 | 1.15 | 0.734 | |
| Week 4 vs Week 12 | −1.34 | −2.36 | 0.317 | 0.036 | |
| Week 8 vs Week 12 | −0.809 | −1.77 | 0.15 | 0.161 |
The coefficient of reliability among the five raw CD31 positive scores at each animal × location combination was alpha=0.755. Descriptive statistics for the composite scores per rabbit were calculated and Figure 2 shows the box and whisker plot for composite CD31 scores at each time point and aneurysm section location.
Group mean estimates and confidence intervals (CI) from the linear mixed model are shown in Table 1. Both location and time of sacrifice were significant at the p=0.1 level (location p=0.078, time p=0.032). The interaction effect (time × location) was not significant and was dropped from the model. Both middle and proximal locations had higher endothelialization when compared to distal, though only proximal was significantly different (mean difference/[90%CI] 0.417[−0.027, 0.862], p=0.12; 0.600[0.16, 1.04], p=0.028 respectively). Middle locations were also decreased compared to proximal, but this difference was not significant (−0.183[−0.627, 0.262], p=0.49) (Table 2).
DISCUSSION
In our study, a moderate deendothelialization was noted in the sham operated control in the aneurysm wall. It was followed by further deendothelialization at 2 and 4 weeks and reendothelialization at 8 and 12 weeks. Endothelialization was not complete near the 12-week point, probably because of deranged hemodynamics that altered the blood flow within the aneurysm.
Aneurysm formation occurs because of weakening in the vessel wall due to stripping of the internal elastic lamina and tunica media, resulting in an outpouching of only tunica intima and adventitia. It was conventionally believed that endothelial dysfunction leading to denudation occurs during aneurysm formation, followed by reendothelialization and collagen deposition20. In our rabbit aneurysm model, we observed initial denudation of endothelial cells associated with advancement of sheath and balloon inflation during aneurysm creation. We were not able to clearly delineate the endothelial injury attributed to the elastase injection during aneurysm creation owing to the unorganized thrombus formation within the aneurysm 24 hours after creation. However, we see a pattern of early deendothelialization at aneurysm creation and further deendothelialization at 2 and 4 weeks could be attributed to the altered flow pattern within the aneurysm.. This finding is clinically relevant because deendothelialization and hypocellular vessel wall are important contributors in aneurysm rupture, as reported by Frosen et al21. We believe the wall is at increased risk of rupture around 4 weeks after aneurysm creation. Our rabbit model has histologic characteristics identical to human aneurysm22. This finding also sheds light on the reason for the rupture of small aneurysms in certain patients22–24
The current knowledge of endothelialization pertaining to aneurysm healing has been shown in relation to growth of cells on endovascular coils and flow diverter stents15,25. The source of endothelialization after endovascular treatment continues to be controversial. Dai et al26 have shown that initial endothelialization following coil embolization occurs as a result of continuation of cells from the parent artery. Liu et al 27 have shown that endothelial progenitor cells in circulation contribute to the endothelialization following coil embolization28–30. On the basis of our initial endothelialization noted till 2 weeks of aneurysm creation, we believe that endothelial cells remain contiguous with the parent artery. Following this we observed further deendothelialization at 4 weeks. We believe this further denudation of endothelial cells may be due toaltered flow pattern within the aneurysm. Overtime, the reendothelialization occurs, with the new flow pattern starting from the aneurysm neck, and reaches the dome around 12 weeks, as reported by findings in our study. This finding further confirms our theory of endothelial cell origin from the parent artery because reendothelialization begins in the neck close to the parent artery at 8 weeks, whereas no endothelial cells were noted at the dome in that timeframe. Moreover, endothelialization was noted to be greatest at baseline (day 0) of aneurysm formation and did not have a similar cell number around 12 weeks (Figure 2). This outcome might be due to the altered hemodynamics within the aneurysm leading to incomplete endothelialization, subsequently leading to recanalization of aneurysms.
Currently, no study delineates the pattern of distribution of endothelial cells in the aneurysm wall and no scoring system is available to quantify endothelialization across different time points. Our scoring system not only quantitates spatial distribution of endothelial cells in aneurysms but also can help predict the rate of healing of aneurysms after treatment with endovascular devices. This information will enable us to identify devices that promote better healing of aneurysms. Future studies are warranted to explore new options in stabilizing the endothelial cells during the first month of aneurysm formation that might lead to better outcomes in aneurysm treatment.
LIMITATIONS
Our study has limitations. The aneurysms produced in the elastase-induced rabbit model continue to be stable after 1 month and do not enlarge and rupture. Thus, the endothelialization in enlarging ruptured aneurysms cannot be correlated in our model. Even though vascular smooth muscle cells are also critical in aneurysm formation, owing to technical difficulty of scoring smooth muscle cells, we only analyzed endothelialation patterns. The presence of unorganized thrombus in the aneurysm sac 24 hours after aneurysm creation limiting us to understand the role played by elastase in the deendothelialization in our model. With regards to the role played by altered flow pattern within the aneurysm we can only postulate and not definitively state that deranged hemodynamics is responsible for the stripping of endothelial cells observed at 4 and 8 weeks following aneurysm creation.
CONCLUSION
In experimental aneurysms of rabbits, endothelial cells regress in all regions during the first month after creation, followed by ascending reendothelialization that remains incomplete. These findings suggest that repopulation of endothelial cells comes from resident cells in the adjacent parent artery and that deranged hemodynamics may affect the full reconstitution of endothelial cells long term.
Acknowledgments
FUNDING STATEMENT
This work was supported by grants from National Institute of Health awarded to Ramanathan Kadirvel (R01NS042646) and David F Kallmes (R21NS088256). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We would like to thank the Radiology research travel fund program, Mayo clinic for the support provided to present the findings of the study in SNIS conference.
Footnotes
These data were presented at the 14th Annual meeting of Society of Neurointerventional Surgery, Colorado Springs, Colorado, July 23–28, 2017.
COMPETING INTEREST STATEMENT
None.
AUTHOR CONTRIBUTORSHIP STATEMENT
PKP and DD contributed to tissue processing, slides staining, interpretation of data, and drafting of the manuscript.
YHD contributed to the aneurysm model creation.
TG contributed to statistics of the study.
DFK and KR contributed to the conception and design of the study and to revision of the article critically for important intellectual content.
References
- 1.Vlak MHM, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. The Lancet Neurology. 2011;10(7):626–36. doi: 10.1016/s1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 2.Wiebers DO. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. The Lancet. 2003;362(9378):103–10. doi: 10.1016/S0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 3.Crobeddu E, Lanzino G, Kallmes DF, et al. Review of 2 decades of aneurysm-recurrence literature, part 1: reducing recurrence after endovascular coiling. AJNR Am J Neuroradiol. 2013;34(2):266–70. doi: 10.3174/ajnr.A3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naggara ON, White PM, Guilbert F, et al. Endovascular treatment of intracranial unruptured aneurysms: systematic review and meta-analysis of the literature on safety and efficacy. Radiology. 2010;256(3):887–97. doi: 10.1148/radiol.10091982. [DOI] [PubMed] [Google Scholar]
- 5.Brinjikji W, Kallmes DF, Kadirvel R. Mechanisms of Healing in Coiled Intracranial Aneurysms: A Review of the Literature. AJNR American journal of neuroradiology. 2015;36(7):1216–22. doi: 10.3174/ajnr.A4175. [published Online First: 2014/11/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozawa T, Tamatani S, Koike T, et al. Histological evaluation of endothelial reactions after endovascular coil embolization for intracranial aneurysm. Clinical and experimental studies and review of the literature. Interv Neuroradiol. 2003;9(Suppl 1):69–82. doi: 10.1177/15910199030090S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara S, Mawad ME, Ogata K, et al. Histopathologic Findings in Human Cerebral Aneurysms Embolized with Platinum Coils: Report of Two Cases and Review of the Literature. American Journal of Neuroradiology. 2002;23(6):970–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond J, Darsaut T, Salazkin I, et al. Mechanisms of occlusion and recanalization in canine carotid bifurcation aneurysms embolized with platinum coils: an alternative concept. AJNR Am J Neuroradiol. 2008;29(4):745–52. doi: 10.3174/ajnr.A0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond J, Sauvageau E, Salazkin I, et al. Role of the Endothelial Lining in Persistence of Residual Lesions and Growth of Recurrences After Endovascular Treatment of Experimental Aneurysms. Stroke. 2002;33(3):850–55. doi: 10.1161/hs0302.104090. [DOI] [PubMed] [Google Scholar]
- 10.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54(4):385–95. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 11.Gill R, O’Donnell RJ, Horvai A. Utility of immunohistochemistry for endothelial markers in distinguishing epithelioid hemangioendothelioma from carcinoma metastatic to bone. Arch Pathol Lab Med. 2009;133(6):967–72. doi: 10.1043/1543-2165-133.6.967. [DOI] [PubMed] [Google Scholar]
- 12.Ding YH, Dai D, Lewis DA, et al. Angiographic and Histologic Analysis of Experimental Aneurysms Embolized with Platinum Coils, Matrix, and HydroCoil. American Journal of Neuroradiology. 2005;26(7):1757–63. [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng Z, Kallmes DF, Durka MJ, et al. Hemodynamics and anatomy of elastase-induced rabbit aneurysm models: similarity to human cerebral aneurysms? AJNR American journal of neuroradiology. 2011;32(3):595–601. doi: 10.3174/ajnr.A2324. [published Online First: 2011/01/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinjikji W, Ding YH, Kallmes DF, et al. From bench to bedside: utility of the rabbit elastase aneurysm model in preclinical studies of intracranial aneurysm treatment. J Neurointerv Surg. 2016;8(5):521–5. doi: 10.1136/neurintsurg-2015-011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai D, Ding YH, Danielson MA, et al. Histopathologic and Immunohistochemical Comparison of Human, Rabbit, and Swine Aneurysms Embolized with Platinum Coils. American Journal of Neuroradiology. 2005;26(10):2560–68. [PMC free article] [PubMed] [Google Scholar]
- 16.Kadirvel R, Ding YH, Dai D, et al. The influence of hemodynamic forces on biomarkers in the walls of elastase-induced aneurysms in rabbits. Neuroradiology. 2007;49(12):1041–53. doi: 10.1007/s00234-007-0295-0. [DOI] [PubMed] [Google Scholar]
- 17.Ding YH, Dai D, Lewis DA, et al. Can neck size in elastase-induced aneurysms be controlled? A retrospective study. AJNR American journal of neuroradiology. 2006;27(8):1681–4. [published Online First: 2006/09/15] [PMC free article] [PubMed] [Google Scholar]
- 18.Cloft HJ, Altes TA, Marx WF, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology. 1999;213(1):223–8. doi: 10.1148/radiology.213.1.r99oc15223. [published Online First: 1999/11/30] [DOI] [PubMed] [Google Scholar]
- 19.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR American journal of roentgenology. 2000;174(2):349–54. doi: 10.2214/ajr.174.2.1740349. [published Online First: 2000/02/05] [DOI] [PubMed] [Google Scholar]
- 20.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44(12):3613–22. doi: 10.1161/STROKEAHA.113.002390. [DOI] [PubMed] [Google Scholar]
- 21.Frosen J, Piippo A, Paetau A, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35(10):2287–93. doi: 10.1161/01.STR.0000140636.30204.da. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Dai D, Kolumam Parameswaran P, et al. Rabbit aneurysm models mimic histologic wall types identified in human intracranial aneurysms. Journal of neurointerventional surgery. 2017 doi: 10.1136/neurintsurg-2017-013264. [published Online First: 2017/08/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Study of Unruptured Intracranial Aneurysms I. Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339(24):1725–33. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 24.Weir B, Disney L, Karrison T. Sizes of ruptured and unruptured aneurysms in relation to their sites and the ages of patients. J Neurosurg. 2002;96(1):64–70. doi: 10.3171/jns.2002.96.1.0064. [DOI] [PubMed] [Google Scholar]
- 25.Dai D, Ding YH, Kelly M, et al. Histopathological findings following pipeline embolization in a human cerebral aneurysm at the basilar tip. Interv Neuroradiol. 2016;22(2):153–7. doi: 10.1177/1591019915622165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai D, Ding YH, Rezek I, et al. Characterizing patterns of endothelialization following coil embolization: a whole-mount, dual immunostaining approach. J Neurointerv Surg. 2016;8(4):402–6. doi: 10.1136/neurintsurg-2014-011513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Zhou Y, An Q, et al. Erythropoietin Stimulates Endothelial Progenitor Cells to Induce Endothelialization in an Aneurysm Neck After Coil Embolization by Modulating Vascular Endothelial Growth Factor. Stem Cells Transl Med. 2016;5(9):1182–9. doi: 10.5966/sctm.2015-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li ZF, Fang XG, Yang PF, et al. Endothelial progenitor cells contribute to neointima formation in rabbit elastase-induced aneurysm after flow diverter treatment. CNS Neurosci Ther. 2013;19(5):352–7. doi: 10.1111/cns.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aronson JP, Mitha AP, Hoh BL, et al. A novel tissue engineering approach using an endothelial progenitor cell-seeded biopolymer to treat intracranial saccular aneurysms. J Neurosurg. 2012;117(3):546–54. doi: 10.3171/2012.5.JNS091308. [DOI] [PubMed] [Google Scholar]
- 30.Marosfoi M, Langan ET, Strittmatter L, et al. In situ tissue engineering: endothelial growth patterns as a function of flow diverter design. Journal of neurointerventional surgery. 2017;9(10):994–98. doi: 10.1136/neurintsurg-2016-012669. [published Online First: 2016/11/01] [DOI] [PubMed] [Google Scholar]