Abstract
Background
Anti-sclerostin antibody is a promising new bone anabolic therapy. While anti-sclerostin antibody stimulates bone formation and repair in the appendicular and axial skeleton, its efficacy in the craniofacial skeleton is still poorly understood.
Methods
Using an established model of Down syndrome-dependent bone deficiency, 10 Ts65Dn mice and 10 wild-type mice were treated weekly via i.v. tail vein injection with vehicle or anti-sclerostin for 3 weeks and sacrificed 1 week after.
Results
Wild-type mice treated with the anti-sclerostin antibody had increased mandibular bone, trabecular thickness, and alveolar height compared to controls. Anti-sclerostin antibody increased Ts65Dn mandibular bone parameters such that they were statistically indistinguishable from those in vehicle-treated wild-type mandibles.
Conclusions
Treatment with anti-sclerostin antibody significantly increased mandibular bone mass and alveolar height in wild type mice and normalized mandibular bone mass and alveolar height in Ts65Dn mice. Anti-sclerostin antibody therapy represents a novel method for increasing mandibular bone formation.
Introduction
Advances in the study of bone remodeling have led to the development of a monoclonal antibody therapy designed to stimulate osteoblastic activity. This anti-Sclerostin antibody (Scl-Ab) has emerged as a reliable and robust osetoanabolic therapy for bone diseases with a deficiency in bone formation [1–3]. Scl-Ab binds and inhibits Sclerostin, a glycoprotein expressed by bone-embedded osteocytes [4, 5]. Sclerostin is a potent inhibitor of the osteogenic Wnt pathway[6]. Sclerostin was identified by studying the loss-of function mutation in patients with the rare genetic disorder Sclerosteosis [1, 4, 5, 7]. Sclerosteosis is characterized by extremely high bone mass throughout the skeleton, tall stature, and enlarged jaws [8–11].
Targeted inhibition of sclerostin using Scl-Ab has several therapeutic applications. By stimulating bone formation and suppressing bone resorption [8], Scl-Ab increases axial bone formation, density and strength. Scl-Ab also significantly improves bone repair in a number of animal models [5, 12–14]. Clinical studies with Scl-Ab have shown dramatic improvements in bone mineral density at the spine and hip in men and women with osteoporosis[15, 16]. Furthermore, Sclerostin is induced by inflammation and promotes inflammatory bone loss, which makes Scl-Abs an attractive therapeutic option for mandibular pathologies characterized by inflammation and reduced bone formation, such as periodontitis and mandibular osteoradionecrosis (ORN)[17]. However, there is limited data on the effect Scl-Ab on the craniofacial skeleton. Two previous studies have shown anabolic effects of Scl-Ab in maxillary alveolar bone in the setting of periodontitis, but there is no previous description of Scl-Ab effect on mandibular bone [2, 18].
Patients with Down Syndrome (DS) have low bone mass with reduced osteoblast activity and bone turnover [19]. Craniofacial analyses of humans with DS reveal several skeletal abnormalities including small atrophic mandibles with significantly reduced alveolar height [20]. Murine models of DS exhibit reduced bone volume and trabecular thickness in the axial skeleton, although, no studies have evaluated mandibular bone [21]. One of the most studied mouse models of Down syndrome is the Ts65Dn (Ts65) mouse [22, 23]. The Ts65 mouse strain is a DS model characterized by segmental trisomy for the region of mouse chromosome 16 that contains approximately 75% of the human chromosome 21-homologous genes [22]. The low bone mass phenotype of Ts65Dn mice is due to cell-intrinsic defects in osteoblast differentiation, which leads to a reduction in bone formation. In addition, osteoclast mediated bone resorption is also reduced, but not enough to overcome the low bone formation rate [21]. The Ts65Dn mouse is the most widely used and accepted model of trisomy 21 in mice. These animals display many of the cognitive and behavioral phenotype of Down syndrome patients as well as the skeletal, craniofacial and cardiovascular and megakaryocytopoiesis that characterizes people with Down syndrome. Other murine models do exist, however, Ts65Dn provides the strongest low bone mass phenotype of all the Down syndrome murine models [24, 25]. Since the analysis of the Ts65 skeletal phenotype focused on the axial skeleton, the extent to which this mouse model recapitulates the mandibular atrophy observed in human DS remains to be determined.
An osetoanabolic therapy that could increase mandibular alveolar bone and enhance mandibular bone repair could be highly beneficial for DS and other conditions. Mandibular bone is particularly susceptible to inflammatory bone loss, which has historically been a very difficult problem to treat[26–28]. Anti-resorptive bisphosphonates are the most common therapy used to treat bone loss in the axial skeleton. However, they provide little benefit to mandibular bone and, in some cases, can be harmful and lead to bisphosphonate-related osteonecrosis of the jaw (BRONJ) [29, 30]. A targeted osetoanabolic agent such as Scl-Ab could provide a novel therapeutic option for diseases with mandibular bone insufficiency, such as mandibular ORN and DS.
Fowler, et al. previously demonstrated that the low bone mass phenotype of Ts65 mice successfully responded to periodic parathyroid hormone (PTH) treatment [21]. Thus, the Ts65 DS mouse may be an ideal model to determine the effect of new osetoanabolic therapies on DS-related mandibular bone disease. Furthermore, PTH is currently contradicted for pediatric conditions and a novel anabolic agent could provide substantial benefit to pediatric patients. The present study investigates the osetoanabolic effects of Scl-Ab on mandibular bone of wild type mice and Ts65 DS mice with an established deficiency in osteoblast function.
Methods
Experimental Design
All animal handling and experimentation was performed in accordance with approved University of Arkansas for Medical Sciences (UAMS) institutional guidelines and protocols, and approved by the UAMS Institutional Animal Care and Use Committee (IACUC). Ts65 male mice and wild type euploid littermate controls were purchased from the Jackson Laboratory (Bar Harbor, ME). Upon arrival they were housed individually with food and water available ad libitum throughout the 12-hr light:dark cycle in accordance with the requirements of the US Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Only male mice were evaluated due to the decreased fertility of Ts65 male mice and the necessity of Ts65 female mice for colony maintenance. Animals were purchased at 6 weeks and aged as required.
Twenty 8-week old male mice were used in this study. However, one mouse in the Ts65 group died independently of treatment, leaving 19 (10 WT, 9 Ts65) mice for final analysis. Vehicle (0.9% saline) or Scl-Ab (monoclonal Scl-AbVI; Novartis, Switzerland) was administered intravenously at 100 mg/kg, weekly for 3 weeks. 5 WT and 4 Ts65 mice were treated with Scl-Ab. The dose was chosen based on efficacy in previous studies and on supplier recommendations. All mice were sacrificed 8 days after the last injection and right and left hemimandibles were harvested.
Radiologic Analysis
All right hemimandibles were first fixed in 10% neutralized buffered formalin overnight and then stored in 70% ethanol for microcomputed X-ray tomography (micro-CT). Micro-CT was performed using a Scanco μCT 50 high-resolution specimen scanner (Scanco Medical AG, Bassersdorf, Switzerland). After identification of the hemi-mandible on a scout view radiograph, images were acquired at an isotropic resolution of 10μm in all three dimensions. Additional scan settings include an integration time of 500ms, an X-ray beam potential of 55kVp and a beam intensity of 109μA. For each 180° of imaging, 1000 projections were acquired. After scanning, 3D microstructural image data was reconstructed using the Scanco cone-beam reconstruction algorithm. Structural and density calibration of the scanner is performed regularly using a calibration phantom provided by the manufacturer.
Volumetric measurements were carried out following the selection of a standardized region of interest (ROI), which was composed of the alveolar bone surrounding the roots of molars M1, M2, and M3. The length of the ROI extended from the most mesial aspect of the M1 root to the most distal aspect of the M3 root. The width of the ROI extended from the most buccal aspect of any root of the molars to the most palatal aspect of any root. The height of the ROI extended from the inferior most aspect of any root to the alveolar bone crest (ABC). The abovementioned ROI defined the border for volumetric analysis on 2-D parasagittal images by a single blinded investigator who drew the contour of the desired alveolar bone region so as to maximize the quantification of bone and minimize the inclusion of roots (Figure 1A). Bone volume per total volume (BV/TV), and trabecular thickness (TT), trabecular number, and trabecular spacing were then calculated from each specimen [31].
Figure 1.

Similar to the method described by Chen, et al., linear measurements of the distance between the cemento-enamel junction (CEJ) and the ABC were done to assess the length of exposed tooth and the height of the alveolar bone[2]. The greater the measured distance, the more exposed tooth and the shorter alveolar crest height. This linear measurement for each specimen was taken at the mesial surface of M1, in the plane where the distal root of M1 was the longest in sagittal sections of micro-CT scans (Figure 1B, C).
Histologic Analysis
All left hemimandibles were fixed in formalin overnight and then decalcified in 10% EDTA (pH 7.4) for 14 days. Paraffin embedding and sectioning was performed by the Gladstone Translational Pathology Core Laboratory. Sagittal sections (7 μm) of the left hemimandibles were stained with hematoxylin and eosin (H&E) and viewed for qualitative histologic analysis using camera assisted light microscopy (Nikon Labophot-2; Nikon, Tokyo, Japan). The cancellous alveolar bone between the anterior and middle molar roots was used as a standardized area for evaluation (Figure 2).
Figure 2.

Results
Anabolic effect of Scl-Ab on clinically relevant mandibular bone outcomes
To determine if Scl-Ab treatment can induce mandibular bone formation, we evaluated several measures of mandibular bone using micro-computed tomography of wild-type mice treated with vehicle or Scl-Ab for 3 weeks. Volumetric analysis of a defined mandibular region of interest (Figure 1A) demonstrated that wild-type mice treated with Scl-Ab had higher average mandibular bone volume (BV/TV) than vehicle-treated wild type mice (Figure 2, Table 1). Scl-Ab also increased the trabecular thickness (TT) of alveolar bone, a clinically relevant measure of bone quality that relates to tooth retention [32].
Table 1.
Micro-CT Results for Vehicle, Scl-AB, Ts65, and Ts65 + Scl-AB
| Micro-CT Results | Vehicle (N=5) | Scl-AB (N=5) | Ts65 (N=4) | Ts65 + Scl-AB (N=5) | P-value |
|---|---|---|---|---|---|
| Mean Tooth to Alveolar Crest Length (mm), (SD) | 0.416, (0.04) | 0.280, (0.03)a | 0.490, (0.05)b | 0.301, (0.06)c |
aP<0.001 vs Vehicle bP = 0.04 vs Vehicle cP = 0.002 vs Ts65 |
| Bone Volume/Total Volume (%), (SD) | 65%, (0.02) | 70%, (0.02)d | 60% (0.03)e | 67%, (0.04)f |
dP = 0.003 vs Vehicle eP = 0.05 vs Vehicle fP = 0.05 vs Ts65 |
| Trabecular Thickness (mm), (SD) | 0.154, (0.01) | 0.177, (0.01)g | 0.140, (0.01)h | 0.167, (0.02)i |
gP = 0.001 vs Vehicle hP = 0.01 vs Vehicle iP = 0.02 vs Ts65 |
| Trabecular Number (mm), (SD) | 4.297, (0.11) | 4.007, (0.2)j | 4.427, (0.06)k | 4.088, (0.3)l |
jP = 0.03 vs Vehicle kP = 0.06 vs Vehicle IP = 0.07 vs Ts65 |
| Trabecular Spacing (mm), (SD) | 0.079, (0.004) | 0.073, (0.005)m | 0.085, (0.007)n | 0.079, (0.005)o |
mP = 0.08 vs Vehicle n P = 0.2 vs Vehicle oP = 0.2 vs Ts65 |
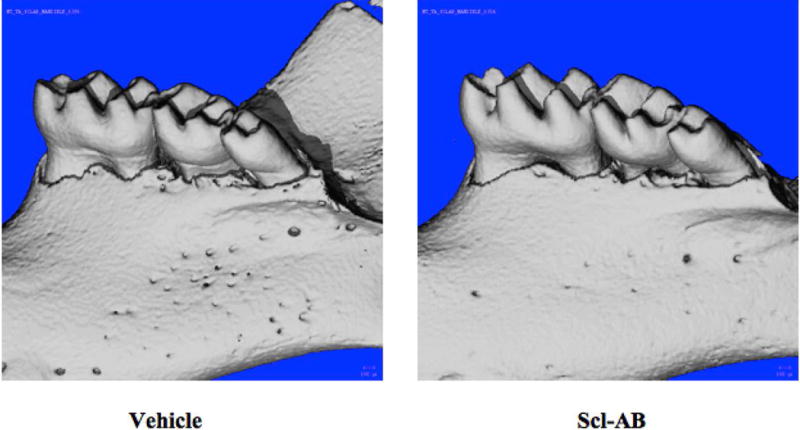
One of the most critical measures of overall mandibular bone health is the height of the alveolar bone crest (ABC). The height of the bone crest is a hallmark of mandibular bone health in several diseases including osteoradionecrosis, periodontitis and Down syndrome, all of which have reduced bone crest heights in humans [2, 33, 34]. To measure the height of the alveolar bone crest, standard landmarks were used to identify the plane for measurement of the linear distance between the mandibular cemento-enamel junction and alveolar bone crest (CEJ-ABC distance) for each specimen (Fig. 1B–C). The longer the linear distance, the shorter the bone crests. Relative to vehicle-treated wild-type mice, Scl-Ab treatment significantly decreased the average CEJ-ABC distance (Figure 1B–C, Figure 3, Table 1). Therefore, consistent with the anabolic effects of the Scl-Ab on the axial and appendicular skeleton, Scl-Ab also promotes the anabolism of mandibular bone in multiple clinically relevant outcomes. Reconstructions of micro-CT scans support the quantitative increase in alveolar bone in specimens treated with Scl-Ab compared to controls (Figure 4).
Figure 3.

Figure 4.
The skeletal phenotype in Down syndrome also affects mandibular bone
Although Ts65 mice have low bone mass in the appendicular and axial skeleton, whether or not the Ts65 mice also recapitulate the human DS craniofacial bone phenotype is unknown. Using the same micro-CT-based measures as above, we found that Ts65DN mice had reduced bone volume (BV/TV) and trabecular thickness, and an increased average CEJ-ABC distance compared to wild type mice (Figure 2–3, Table 1). Therefore, the low bone mass phenotype of Ts65 DS mice extends to the mandible and is consistent with the craniofacial phenotype observed in human DS [20].
Rescue of DS mandibular skeletal phenotype with Scl-Ab
Since the Scl-Ab stimulated anabolic bone formation in the mandible, we sought to determine if Scl-Ab could rescue the low bone mass phenotype of DS mandibular bone in the Ts65 mice. Remarkably, Scl-Ab treatment completely normalized each aspect of the Ts65 mandibular bone phenotype. The BV/TV, TT, and CEJ-ABC measures in Scl-Ab-treated Ts65 mice were statistically indistinguishable from the vehicle-treated wild-type controls (Figure 2–3, Table 1). Therefore, Scl-Ab exerts an anabolic effect on DS mandibular bone, rescuing it to wild-type levels according to several clinically relevant outcomes.
Histologic Analysis of alveolar bone
To determine if the newly formed bone was morphologically normal, the alveolar bone in this region of interest was evaluated histologically for all specimens. Similar to the results of Micro-CT analysis, Scl-Ab-treated specimens had qualitatively more alveolar bone in between root of M1 and M2; whereas Ts65 DS mice appeared to have reduced amounts of bone. This appeared to normalize with Scl-Ab therapy (Figure 5). We evaluated several other features of the alveolar bone, including collagen and canalicular organization, but observed no differences (data not shown). Thus, our histological evaluation revealed that the bone in all specimens was well organized and histologically normal in each outcome that we examined.
Figure 5.

Discussion
The current study is the first report on the effects of Scl-Ab on mandibular alveolar bone, on either wild type mice or in the context of Down syndrome. The osteoanabolic effects of Scl-Ab were significant in both groups. Diseases that compromise mandibular bone cause a loss of bone at the alveolar ridge, predisposing the mandible for tooth loss or poor integration of dental implants. The CEJ and ABC are established measures of alveolar ridge and are surrogates for understanding the health of the mandibular bone. Scl-Ab was not only able to increase the mandibular bone volume, but detailed micro-CT analysis demonstrated that Scl-Ab also increased ABC height. This is the first report of an osetoanabolic agent increasing wild type mandibular ABC height and bone volume. This suggests treatment with Scl-Ab has a wide potential clinical application for mandibular alveolar bone defect diseases such Down syndrome.
Sclerostin inhibits osteoblast activity by antagonizing Wnt signaling[1, 5]. Scl-Ab has exciting potential as an anabolic bone therapy [5] and is currently in phase III clinical trials for the treatment of osteoporosis. In phase II clinical trials, all dose levels of Scl-Ab significantly increased BMD in the lumbar spine with no increase in adverse events [15]. These trials focused on treatment for osteoporosis and evaluated bone mineral density (BMD) of the lumbar spine as the primary endpoint. Although Scl-Ab increases axial bone density, there is limited evidence on the effects of Scl-Ab on the craniofacial skeleton and none specifically on mandibular bone.
Sclerosteosis and Van Buchem disease are two rare autosomal recessive syndromes caused by loss of function mutations in the Sclerostin gene. Several craniofacial abnormalities have been described in these patients including mandibular overgrowth, facial palsy, loss of hearing, delayed tooth eruption, and malocclusion. Sclerosteosis patients characteristically have tall stature, with increased cortical density throughout the skeleton. These findings support the idea that Sclerostin is an important regulator of mandibular bone growth and thus a potential therapeutic target [35].
With improved medical management, individuals with Down syndrome have a longer life expectancy. Only recently has the low bone mass phenotype in DS been documented [21]. Individuals with DS suffer from a reduction in bone accrual during childhood and adolescence, which leads to low bone mass in young adulthood, and later osteoporosis and increased risk of fracture. The low bone mass phenotype of DS patients was replicated in Ts65 male mice. Fowler, et al. found that Ts65 mice had significantly decreased numbers of osteoblasts and osteoclasts on bone surfaces, resulting in decreased bone formation and bone resorption. The balance of osteoblast and osteoclast activity in this low bone turnover state favored bone resorption, which underlies the reduced bone mass in Ts65 mice. Because this low bone mass phenotype is accompanied by abnormally low bone resorption, Ts65 mice are uniquely candidates for osetoanabolic therapy but not anti-resorptive bone therapy [21]. In support of this idea, anabolic parathyroid hormone (PTH) treatment increased BMD in the axial skeleton of Ts65 mice [21], and we find that Scl-Ab increases bone formation in the mandible.
Mandibular bone has previously been shown to have unique metabolic properties and discrete responses to pharmacologic, mechanical and hormonal stimuli. Studies have demonstrated differences in both osteoclastogenic potential and osteoclast numbers of mandibular bone compared to long bone. Specifically, mandibular bone had fewer osteoclasts in basal conditions and following PTH+1,25D3 stimulation. Although detailed histomorphometry of the mandible was not performed in this study, the low osteoclast activity of mandibular bone would be expected to exacerbate the low bone resorption phenotype reported for the long bone of Ts65 mice [21]. These observations, together with those reported here, suggest that the beneficial effects of Scl-Ab on mandibular bone likely occur by stimulating bone formation, rather than by further suppressing bone resorption [36, 37].
The ability to stimulate new mandibular bone formation would also be highly beneficial for mandibular osteoradionecrosis (ORN). In mandibular ORN, both bone mass and bone turnover are reduced. Like DS, the low bone turnover in mandibular ORN is due to a reduction in osteoblast activity [38, 39]. This dysregulated bone remodeling predisposes the ORN mandible for necrosis and a limited ability to heal after dental extractions[28, 38]. ORN of the mandible continues to be one of the most devastating complications for patients undergoing treatment for head and neck cancer. Severe bone necrosis causes significant morbidity without any reliable treatment options. Current strategies for treating mandibular ORN focus on hyperbaric oxygen therapy to increase tissue perfusion or inhibiting osteoclast activity with bisphosphonates[28, 30]. Neither method provides a reliable benefit and patients often must undergo major mandibular resections and free tissue transfer reconstruction despite being cancer free. Furthermore, bisphosphonates may actually be harmful in the setting of mandibular inflammation leading to bisphosphonate related osteonecrosis of the jaw (BRONJ)[26, 29]. Thus, an osetoanabolic therapy, such as the Scl-Ab, would be ideal for mandibular ORN treatment, as well as for the treatment of DS. The encouraging results of this proof of principle study motivate additional research to identify a dose that would be optimal in a clinical context.
Conclusion
In conclusion, Scl-Ab treatment was able to create greater alveolar crest height and higher alveolar bone density in both wild type mice and in a murine model of Down syndrome. Although we did not perform dynamic histomorphmetry, prior studies suggest that this response is primarily due to increased bone formation. This novel finding may have several new valuable clinical applications for Scl-Ab in treating mandibular pathologies with reduced bone mass such as in human Down syndrome and mandibular ORN.
Acknowledgments
Funding:
This research was supported by NIH-NIAMS P30 AR066262-01 (TA), NIH-NIDCR R01 DE019284 (TA), DOD PRORP OR130191 (TA), and NIH-NCI F32 CA203402-01A1 (TWF).
References
- 1.Clarke BL. Anti-sclerostin antibodies: utility in treatment of osteoporosis. Maturitas. 2014;78(3):199–204. doi: 10.1016/j.maturitas.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, et al. Sclerostin antibody treatment causes greater alveolar crest height and bone mass in an ovariectomized rat model of localized periodontitis. Bone. 2015;76:141–8. doi: 10.1016/j.bone.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Kawai M, et al. Emerging therapeutic opportunities for skeletal restoration. Nat Rev Drug Discov. 2011;10(2):141–56. doi: 10.1038/nrd3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 5.Ke HZ, et al. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33(5):747–83. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 6.Babij P, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18(6):960–74. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 7.Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–89. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2016 doi: 10.1016/j.bone.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassler N, et al. Sclerostin deficiency is linked to altered bone composition. J Bone Miner Res. 2014;29(10):2144–51. doi: 10.1002/jbmr.2259. [DOI] [PubMed] [Google Scholar]
- 10.Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet. 2003;63(3):192–7. doi: 10.1034/j.1399-0004.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 11.Gardner JC, et al. Bone mineral density in sclerosteosis; affected individuals and gene carriers. J Clin Endocrinol Metab. 2005;90(12):6392–5. doi: 10.1210/jc.2005-1235. [DOI] [PubMed] [Google Scholar]
- 12.Li X, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24(4):578–88. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 13.Feng G, et al. Systemic administration of sclerostin monoclonal antibody accelerates fracture healing in the femoral osteotomy model of young rats. Int Immunopharmacol. 2015;24(1):7–13. doi: 10.1016/j.intimp.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Ominsky MS, et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011;26(5):1012–21. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- 15.McClung MR, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 16.Cosman F, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016;375(16):1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 17.Findlay DM, Atkins GJ. TWEAK and TNF regulation of sclerostin: a novel pathway for the regulation of bone remodelling. Adv Exp Med Biol. 2011;691:337–48. doi: 10.1007/978-1-4419-6612-4_34. [DOI] [PubMed] [Google Scholar]
- 18.Taut AD, et al. Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res. 2013;28(11):2347–56. doi: 10.1002/jbmr.1984. [DOI] [PubMed] [Google Scholar]
- 19.McKelvey KD, et al. Low bone turnover and low bone density in a cohort of adults with Down syndrome. Osteoporos Int. 2013;24(4):1333–8. doi: 10.1007/s00198-012-2109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suri S, Tompson BD, Cornfoot L. Cranial base, maxillary and mandibular morphology in Down syndrome. Angle Orthod. 2010;80(5):861–9. doi: 10.2319/111709-650.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler TW, et al. Low bone turnover and low BMD in Down syndrome: effect of intermittent PTH treatment. PLoS One. 2012;7(8):e42967. doi: 10.1371/journal.pone.0042967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonarakis SE, et al. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–38. doi: 10.1038/nrg1448. [DOI] [PubMed] [Google Scholar]
- 23.Gardiner K, et al. Mouse models of Down syndrome: how useful can they be? Comparison of the gene content of human chromosome 21 with orthologous mouse genomic regions. Gene. 2003;318:137–47. doi: 10.1016/s0378-1119(03)00769-8. [DOI] [PubMed] [Google Scholar]
- 24.Hawli Y, Nasrallah M, El-Hajj Fuleihan G. Endocrine and musculoskeletal abnormalities in patients with Down syndrome. Nat Rev Endocrinol. 2009;5(6):327–34. doi: 10.1038/nrendo.2009.80. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, et al. Genetic analysis of Down syndrome-associated heart defects in mice. Hum Genet. 2011;130(5):623–32. doi: 10.1007/s00439-011-0980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SO, et al. A retrospective study of osteomyelitis and osteonecrosis of the jaws and its etiologic implication of bisphosphonate in Asians. Clin Oral Investig. 2016 doi: 10.1007/s00784-016-1973-2. [DOI] [PubMed] [Google Scholar]
- 27.Armin BB, et al. Brachytherapy-mediated bone damage in a rat model investigating maxillary osteoradionecrosis. Arch Otolaryngol Head Neck Surg. 2012;138(2):167–71. doi: 10.1001/archoto.2011.1176. [DOI] [PubMed] [Google Scholar]
- 28.Bras J, de Jonge HK, van Merkesteyn JP. Osteoradionecrosis of the mandible: pathogenesis. Am J Otolaryngol. 1990;11(4):244–50. doi: 10.1016/0196-0709(90)90084-9. [DOI] [PubMed] [Google Scholar]
- 29.Koth VS, et al. Bisphosphonate-related osteonecrosis of the jaw: from the sine qua non condition of bone exposure to a non-exposed BRONJ entity. Dentomaxillofac Radiol. 2016:20160049. doi: 10.1259/dmfr.20160049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan AA, et al. Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management From the International Task Force on ONJ. J Clin Densitom. 2016 doi: 10.1016/j.jocd.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 32.Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip, and spine. Calcif Tissue Int. 1996;59(6):433–7. doi: 10.1007/BF00369206. [DOI] [PubMed] [Google Scholar]
- 33.Park CH, et al. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol. 2007;78(2):273–81. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang B, et al. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res. 2013;28(7):1631–40. doi: 10.1002/jbmr.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beighton P, et al. The syndromic status of sclerosteosis and van Buchem disease. Clin Genet. 1984;25(2):175–81. doi: 10.1111/j.1399-0004.1984.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg S, Grynpas MD, Glogauer M. Heterogeneity of osteoclast activity and bone turnover in different skeletal sites. Arch Oral Biol. 2016;71:134–143. doi: 10.1016/j.archoralbio.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Chaichanasakul T, et al. Diverse osteoclastogenesis of bone marrow from mandible versus long bone. J Periodontol. 2014;85(6):829–36. doi: 10.1902/jop.2013.130376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamplen M, et al. Standardized analysis of mandibular osteoradionecrosis in a rat model. Otolaryngol Head Neck Surg. 2011;145(3):404–10. doi: 10.1177/0194599811400576. [DOI] [PubMed] [Google Scholar]
- 39.Cohen M, et al. Animal model of radiogenic bone damage to study mandibular osteoradionecrosis. Am J Otolaryngol. 2011;32(4):291–300. doi: 10.1016/j.amjoto.2010.06.001. [DOI] [PubMed] [Google Scholar]


