Abstract
Objective
Triple-negative breast cancer (TNBC) is a heterogeneous disease with poor prognosis. Circulating tumor cells (CTCs) are a promising predictor for breast cancer prognoses but their reliability regarding progression-free survival (PFS) is controversial. We aim to verify their predictive value in TNBC.
Methods
In present prospective cohort study, we used the Pep@MNPs method to enumerate CTCs in baseline blood samples from 75 patients with TNBC (taken at inclusion in this study) and analyzed correlations between CTC numbers and outcomes and other clinical parameters.
Results
Median PFS was 6.0 (range: 1.0–25.0) months for the entire cohort, in whom we found no correlations between baseline CTC status and initial tumor stage (P=0.167), tumor grade (P=0.783) or histological type (P=0.084). However, among those getting first-line treatment, baseline CTC status was positively correlated with ratio of peripheral natural killer (NK) cells (P=0.032), presence of lung metastasis (P=0.034) and number of visceral metastatic site (P=0.037). Baseline CTC status was predictive for PFS in first-line TNBC (P=0.033), but not for the cohort as a whole (P=0.118). This prognostic limitation of CTC could be ameliorated by combining CTC and NK cell enumeration (P=0.049).
Conclusions
Baseline CTC status was predictive of lung metastasis, peripheral NK cell ratio and PFS in TNBC patients undergoing first-line treatment. We have developed a combined CTC-NK enumeration strategy that allows us to predict PFS in TNBC without any preconditions.
Keywords: Breast cancer, nanotechnology, circulating tumor cell, immunology
Introduction
Triple negative breast cancer (TNBC) accounts for nearly 15% of all breast cancers and is defined by the absence of estrogen receptors (ER), progesterone receptors (PgR) and human epidermal growth factor receptor 2 (HER2) expressions (1). These receptors are involved in promoting breast cancer cell growth and also served as targets of specific therapeutic drugs. Until now, only a few molecule-directed therapies, including poly(ADP-ribose) polymerase (PARP) inhibitors, were available for TNBC patients (2,3). Chemotherapy and radiotherapy are still standard treatments for patients with early-stage or advanced-stage TNBC, but their long-term results are not optimistic (4).
As currently-used cancer-related biomarkers do not precisely reflect tumor response in advanced-stage TNBC (5), clinical decisions mainly rely on radiological evaluation of tumor burden at regular intervals, which cannot be intensively used during a therapeutic course (4). A better real-time evaluating system for TNBC is urgently required.
In this setting, circulating tumor cells (CTCs) are considered to be potential real-time, minimally invasive “liquid biopsies”. CTCs have been shown capable of providing information on dynamic changes in tumor burden and even vital genetic information about the targeted tumor from a blood draw (6). Although numerous studies have proven CTCs to be an independent prognostic factor in all stages of breast cancer, until now, the only U.S. Food & Drug Administration-approved method of detecting CTC is the CellSearch assay ( 7). However, many epithelial cell adhesion molecule (EpCAM)-based CTC-isolation technologies, including CellSearch, have prognostic limitations for certain breast cancer subtypes, especially TNBC (8,9). Previous reports showed that baseline CTC enumeration can successfully predict overall survival (OS) of TNBC patients, but its predictive value for progression-free survival (PFS) is still controversial. In 2012, Munzone E et al. first evaluated the prognostic value of CTCs in different immunohistochemically (IHC)-defined breast cancer subtypes. They found that baseline CTC counts detected by CellSearch were borderline-significantly associated with OS but not with PFS, in TNBC patients who were receiving new courses of systemic therapy (8). Thereafter, three other studies also confirmed that baseline CTC counts were not predictive for both PFS and OS in TNBC patients (10-12). Particularly in 2015, a phase II clinical trial (TBCRC001) that used two parallel CTC-detecting technologies [CellSearch and immunomagnetic enrichment and flow cytometry (IE/FC)] again showed that the CTC status (as detected by both methods at baseline) was not predictive for time-to-progression in 102 women with TNBC (13). In contrast, results from another phase II trial (TBCRC019) showed that baseline CTC count higher than 5 CTCs per 7.5 mL whole blood is a negative prognostic factor for PFS in TNBC patients (14). Similar results were also reported by another study (15). However, these two studies drew their conclusions based on rather small patient cohorts (n=52 and n=16, respectively). We therefore conducted a prospective investigation using our previously reported nanotechnology-based CTC isolation system (Pep@MNPs) (16), to verify the prognostic efficacy of EpCAM-based CTC enumeration in TNBC patients.
For the first time, we also took peripheral lymphocyte enumeration into consideration to optimize the prognostic power of EpCAM-based CTCs enumeration in TNBC patients.
Materials and methods
Patients and samples
For this study, we enrolled 75 consecutive TNBC patients who received treatment at the Peking University Cancer Hospital & Institute from March 2014 to December 2016. The study was approved by the Medical Ethical Committee of Peking University Cancer Hospital & Institute (Approval No. 2013KT29). Our eligibility criteria were: 1) recurrent and metastatic breast cancer; 2) absence of ER, PgR or HER2 expression; 3) about to begin a new line of therapy; 4) expected survival time of at least 3 months; 5) measurable lesions; and 6) patient’s written informed consent.
We defined ER and PgR positivity by IHC-tested Allred scores ranging from 3 to 8, using antibodies against ER (Immunotech, Marseilles, France) and PgR (Novocastra Laboratories, Newcastle, UK). IHC staining against HER2 (Abcam PLC, Cambridge, UK) was categorized in intensity as 0, 1+, 2+ or 3+; 0–1+ was considered as HER2–, 3+ as HER2+, and HER2 amplification of 2+ samples was confirmed by fluorescence in situ hybridization (FISH).
We collected peripheral blood samples from the 75 patients, using their blood draws at baseline (time of inclusion) for CTC and lymphocyte enumeration. Of the 75 patients, 10 had subsequent blood draws at regular tumor evaluation intervals. Clinicopathological and complete ongoing follow-up information was recorded for all the patients at the time of blood collection. For each patient, one tube containing 8.0 mL peripheral blood was collected in CellSave® (Immunivest Corporation, Wilmington DE, USA) collection tubes for the Pep@MNPs assays. Samples were maintained at room temperature and processed within 96 h; only 2.0 mL blood was used for Pep@MNPs enumeration. Details of the Pep@MNPs technique have been described previously (16).
Lymphocytes phenotype by flow cytometry
Phenotypes were ascertained using whole blood. Peripheral lymphocytes were stained using the appropriate antibodies: CD3-PC5/CD4-FITC/CD8-PE (IM1650, Beckman Coulter, California, USA), CD3-FITC/(CD16+/CD56+)-PE (A07735, Beckman Coulter), CD (14+16)-FITC/CD85k (ILT3)-PE/CD33-PC5 (A23413, Beckman Coulter), CD4-FITC (A007750, Beckman Coulter, CD8-FITC (A07756, Beckman Coulter), CD19-PC5 (A07771; Beckman Coulter), CD25-PE (A07774, Beckman Coulter), CD314/NKG2D PE-Cyanine7 (25-5878-41, eBioscience, San Diego, CA, US), and CD159a-PC7 (B10246, Beckman Coulter). Tests were performed on FC500 flow cytometer (Beckman Coulter).
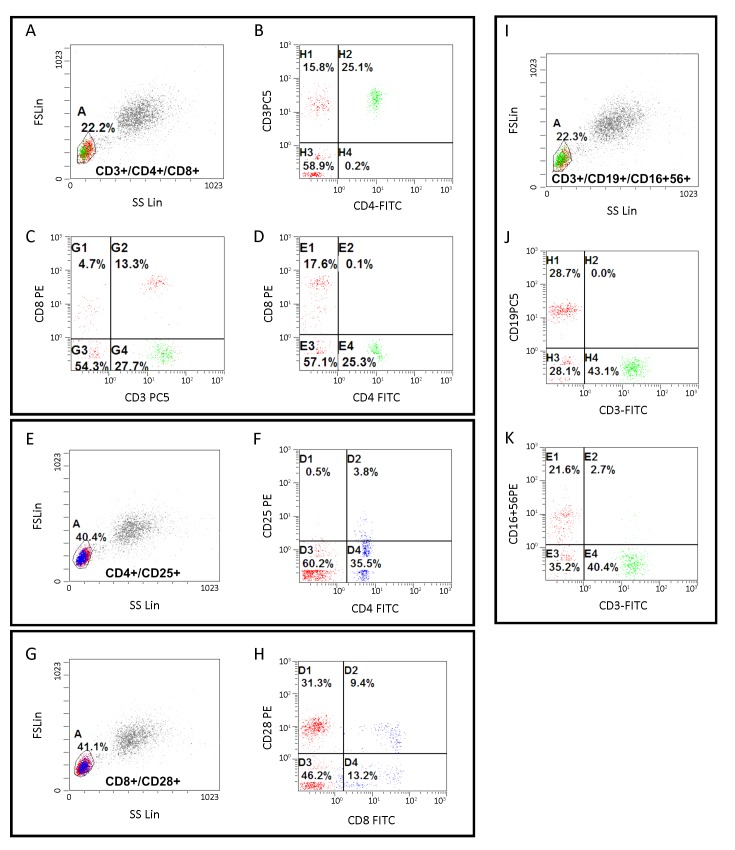
In short, 100 μL of fresh whole blood from TNBC patients were incubated with the appropriate antibodies on a rocking platform for 30 min. Erythrocytes were lysed with OptiLyse® C (A11895, Beckman Coulter) as directed by the manufacture’s protocol. Before and after reading patient samples, fluorescence intensities of the FACS Canto were standardized over time with PMT 7-Color Setup Beads (335775, BD Biosciences) to compensate for fluorescence intensity variability. The gating strategy consisted of eliminating doublets based on FCS-A and FCS-H parameters, followed by removal of dead cells. Levels of different peripheral lymphocyte subtypes were expressed as percentages of total lymphocytes (%). Phenotyping and grading of each lymphocyte subtype were set up according to previously reported standards (17-20). Detailed information is shown in Supplementary Table S1 and Supplementary Figure S1.
S1.
Discription, phenotype and reference range of peripheral lymphocyte subsets
| Peripheral lymphocyte subtypes | Molecular phenotype | Reference range (%) | ||
| Low | Median | High | ||
| Total T-cells | CD3+ | <62.72 | 62.72–78.30 | >78.30 |
| Helper T-cells | CD3+ & CD4 + | <31.47 | 31.47–43.80 | >43.80 |
| Cytotoxic T-cell | CD3+ & CD8 + | <19.22 | 19.22–34.80 | >34.80 |
| CD4+/CD8+ ratio | CD4+/CD8+ | <0.98 | 0.98–1.94 | >1.94 |
| Natural killer cell | CD3– & CD56 + & CD16 + | <9.15 | 9.15–21.51 | >21.51 |
| Natural killer T-cell | CD3+ & CD56 + & CD16 + | <1.05 | 1.05–6.05 | >6.05 |
| B cells | CD19+ | <7.48 | 7.48–15.55 | >15.55 |
| Regulatory T-cells | CD4+ & CD25 + | <1.36 | 1.36–5.48 | >5.48 |
| Activated naïve cytotoxic T-cells | CD8+ & CD28 + | <7.74 | 7.74–16.33 | >16.33 |
| Suppressor T-cells | CD8+ & CD28 – | <9.16 | 9.16–23.28 | >23.28 |
S1.
Cell staining and gating strategy for cytometry. (A) Using typical forward and side scatter characteristics, a gate was set on lymphocytes expressed CD3 and/or CD4 and/or CD8; (B) Within the CD3+/CD4+/CD8+ gate, helper T-cells were determined by gating on CD3+ & CD4 + (H2 phase); (C) Cytotoxic T-cells were determined by gating on CD3+ & CD8 + (G2 phase); (D) CD4/CD8 ratio was determined by gating on CD4+ & CD8 + (E4 phase/E1 phase); (E) A new gate was set on lymphocytes expressed CD4 and/or CD25; (F) Within the CD4+/CD25+ gate, regulatory T-cells were determined by gating on CD4+ & CD25 + (D2 phase); (G) Another gate was set on lymphocytes expressed CD8 and/or CD28; (H) Within the CD8+/CD28+ gate, activated naïve cytotoxic T-cells were determined by gating on CD8+ & CD28 + (D2 phase), and suppressor T-cells were determined by gating on CD8+ & CD28 – (D4 phase); (I) Lymphocytes expressed CD3 and/or CD19 and/or both CD16 and CD56 were isolated using preset gate as showed in Figure S1A; (J) Within the CD3+/CD19+/CD16+56+ gate, B cells were determined by gating on CD3– & CD19 + (H1 phase); (K) Natural killer cells were determined by gating on CD3– & CD16 +56+ (E1 phase); and natural killer T-cells were determined by gating on CD3– & CD16 + & CD56 + (E2 phase).
CTC isolation and enumeration by Pep@MNPs
We counted CTCs using the Pep@MNPs method as previously described (16). In brief, an EpCAM recognition peptide is attached with iron oxide magnetic nanoparticles (MNPs) via biotin-avidin interaction. For ease of detection, 5.0 μL pre-vortexed Pep@MNPs (10 mg Fe/mL) was added to 2.0 mL peripheral blood samples, and incubated with gentle shaking at 37 °C for 30 min. The Pep@MNPs-CTC complexes were subsequently isolated and washed with PBS at least 3 times under a magnetic field; the captured CTCs were then stained by multiple color immunocytochemistry: 4’,6-diamidino-2-phenylindole (DAPI) for cell nuclei, CD45-phycoerythrin for leukocytes (negative selection) and CK19-fluorescein isothiocyanate for cytokeratin (CK; positive selection, which was used to distinguish epithelial cells from leukocytes). Further identification and counting of CTCs were performed using ZeissVert A1 fluorescent microscope (Carl Zeiss Microscopy GmbH, München, Germany).
Definition of CTC-natural killer cell (CTC-NK) threshold
To generate the combined CTC and peripheral NK cell enumeration (CTC-NK), we used a binary logistic regression analysis which includes CTC and NK. In the CTC-NK regression equation, the response variable was whether patients with PFS≤6.0 months (poor prognosis), or with PFS>6 months (favorable prognosis). The predictive probabilities of each CTC-NK combinations were calculated and subsequently subjected to receiver operating characteristic curve (ROC) analysis for area under the curve (AUC). The true class for ROC analysis was patient with poor prognosis or with favorable prognosis. Thereafter, the AUCs for CTC, NK and CTC-NK were compared. Finally, the CTC-NK combination with maximum Youden-index was set as the threshold of CTC-NK. Youden-index was calculated by the following formula: sensitivity + specificity – 1.
Statistical analysis
The main objective was to test whether PFS was associated with CTC status at baseline in TNBC patients; and secondarily to find any correlations between CTC count, metastatic status, or peripheral lymphocyte ratios. PFS was calculated from the date of inclusion until the date of tumor progression (per clinical or imaging diagnosis) or cancer related death.
The positive rates of NK-associated activating receptor between health control and TNBC were compared by independent t-test (two-tailed). Data were expressed as
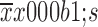 . Categorical variables (such as histological type, metastatic status and family history) were compared by Chi-square or Fisher’s exact test. Hierarchical variables (such as tumor grade or tumor stage) were compared by the Cochran-Armitage trend test. Correlations between CTC status and different peripheral lymphocyte subtypes were evaluated with Spearman rank correlation test, with CTC detection as the outcome variable of interest. The predictive probabilities of CTC-NK combination were calculated by logistic regression. The AUC of CTC, NK and CTC-NK was calculated by ROC analysis. Kaplan-Meier survival curves were computed according to CTC status alone or combined CTC and NK cell status. The PFS of each group was compared using log-rank tests. For all analyses, P<0.05 indicates statistically significant. All analyses were performed using IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA).
. Categorical variables (such as histological type, metastatic status and family history) were compared by Chi-square or Fisher’s exact test. Hierarchical variables (such as tumor grade or tumor stage) were compared by the Cochran-Armitage trend test. Correlations between CTC status and different peripheral lymphocyte subtypes were evaluated with Spearman rank correlation test, with CTC detection as the outcome variable of interest. The predictive probabilities of CTC-NK combination were calculated by logistic regression. The AUC of CTC, NK and CTC-NK was calculated by ROC analysis. Kaplan-Meier survival curves were computed according to CTC status alone or combined CTC and NK cell status. The PFS of each group was compared using log-rank tests. For all analyses, P<0.05 indicates statistically significant. All analyses were performed using IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA).
Results
Patient characteristics
The median age at diagnosis of the 75 patients in this cohort was 49 (range: 30–74) years. Their median PFS was 6.0 (range: 1.0–25.0) months. At last follow-up (December 31, 2016), 46 patients (61.3%) were confirmed to have progressive disease; and 21 patients (28.0%) were confirmed dead. Among the 75 patients, 50 (66.7%) underwent first-line therapy, 19 (25.3%) underwent second-line therapy and beyond, and 6 (8.0%) were found to have stage IV disease at initial diagnosis. All patients had baseline CTC levels and peripheral lymphocytes counts taken before starting new lines of therapy. Detailed information for this cohort is shown in Supplementary Table S2.
S2.
Clinicopathological characteristics of total TNBC patients (N=75)
| Characteristics | n (%) |
| TNBC, triple-negative breast cancer. | |
| Age at diagnosis (year) | |
| ≤45 | 28 (37.3) |
| >45 | 47 (62.7) |
| Unknown | 0 |
| Histology of primary tumor | |
| Ductal | 70 (94.6) |
| Lobular | 1 (1.4) |
| Others | 3 (4.1) |
| Unknown | 1 |
| Initial tumor grade | |
| I | 1 (2.1) |
| II | 28 (58.3) |
| III | 19 (39.6) |
| Unknown | 27 |
| Initial TNM stage | |
| I | 6 (9.1) |
| II | 24 (36.4) |
| III | 28 (42.4) |
| IV | 8 (12.1) |
| Unknown | 9 |
| Family history | |
| Yes | 8 (10.7) |
| No | 67 (89.3) |
| Unknown | 0 |
| Sites of metastases at inclusion | |
| Chest | 20 (26.7) |
| Lymph node | 49 (65.3) |
| Bone | 27 (36.0) |
| Lung | 27 (36.0) |
| Liver | 21 (28.0) |
| Brain | 9 (12.0) |
| Unknown | 0 |
| Therapeutic status at inclusion | |
| First-line | 50 (66.7) |
| Second-line | 15 (20.0) |
| Third-line and beyond | 4 (5.3) |
| Stage IV at initial diagnosis | 6 (8.0) |
Clinical relevance of baseline CTC count in TNBC
At baseline, the median CTC count of this cohort was 2.0 CTC/2 (range: 0.0–126.0 CTC/2) mL. The cut-off for CTC status used in the present study was defined by our previous study (CTC>2.0:>2.0 CTC/2 mL; CTC≤2.0: ≤2.0 CTC/2 mL). The CTC detection rate was 73.3% for the cohort as a whole. No significant correlation was found between baseline CTC status and initial tumor stage (P=0.164), tumor grade (P=0.781), histological type (P=0.084) or number of visceral metastatic sites (P=0.575) (Table 1). However, in the first-line therapy subgroup, 76.0% of the patients were CTC>2.0. Baseline CTC status was found to positively correlate with lung metastases (P=0.034) and number of visceral metastatic sites (P=0.037) (Table 2).
1.
Baseline CTC comparison according to clinical characteristics in total TNBC (N=75)
| Characteristics | Number of patients | n (%) | P | |
| ≤2 CTC/2 mL | >2 CTC/2 mL | |||
| Age at diagnosis (year) | 0.878a | |||
| ≤45 | 28 | 16 (57.1) | 12 (42.9) | |
| >45 | 47 | 26 (55.3) | 21 (44.7) | |
| Unknown | 0 | |||
| Histological type | 0.084a | |||
| Ductal | 70 | 38 (54.3) | 32 (45.7) | |
| Lobular | 1 | 1 (100) | 0 (0) | |
| Others | 3 | 3 (100) | 0 (0) | |
| Unknown | 1 | |||
| Tumor grade | 0.781b | |||
| I | 1 | 1 (100) | 0 (0) | |
| II | 28 | 15 (53.6) | 13 (46.4) | |
| III | 19 | 12 (63.2) | 7 (36.8) | |
| Unknown | 27 | |||
| Tumor stage | 0.164b | |||
| I | 6 | 3 (50.0) | 3 (50.0) | |
| II | 24 | 11 (45.8) | 13 (54.2) | |
| III | 28 | 20 (71.4) | 8 (28.6) | |
| IV | 8 | 5 (62.5) | 3 (37.5) | |
| Unknown | 9 | |||
| Bone metastasis at relapse | 0.587a | |||
| Yes | 27 | 14 (51.9) | 13 (48.1) | |
| No | 48 | 28 (58.3) | 20 (41.7) | |
| Unknown | 0 | |||
| Liver metastasis at relapse | 0.246a | |||
| Yes | 21 | 14 (66.7) | 7 (33.3) | |
| No | 54 | 28 (51.9) | 26 (48.1) | |
| Unknown | 0 | |||
| Lung metastasis at relapse | 0.131a | |||
| Yes | 27 | 12 (44.4) | 15 (55.6) | |
| No | 48 | 30 (62.5) | 18 (37.5) | |
| Unknown | 0 | |||
| Brain metastasis at relapse | 0.699a | |||
| Yes | 9 | 4 (44.4) | 5 (55.6) | |
| No | 66 | 38 (57.6) | 28 (42.4) | |
| Unknown | 0 | |||
| Number of visceral metastatic sitesc | 0.575b | |||
| 0 | 32 | 20 (62.5) | 12 (37.5) | |
| 1 | 32 | 17 (53.1) | 15 (46.9) | |
| 2 | 8 | 2 (25.0) | 6 (75.0) | |
| 3 | 3 | 3 (100) | 0 (0) | |
| Table 1 (continued) | ||||
2.
Baseline CTC comparison according to clinical characteristics in first-line TNBC (N=50)
| Characteristics | Number of patients | n (%) | P | |
| ≤2 CTC/2 mL | >2 CTC/2 mL | |||
| Age at diagnosis (year) | 0.754a | |||
| ≤45 | 17 | 9 (52.9) | 8 (47.1) | |
| >45 | 33 | 19 (57.6) | 14 (42.4) | |
| Unknown | 0 | |||
| Histological type | 0.497a | |||
| Ductal | 48 | 26 (54.2) | 22 (45.8) | |
| Lobular | 0 | 0 (NA) | 0 (NA) | |
| Others | 2 | 2 (100) | 0 (0) | |
| Unknown | 0 | |||
| Tumor grade | 1.000b | |||
| I | 1 | 1 (100) | 0 (0) | |
| II | 20 | 9 (45.0) | 11 (55.0) | |
| III | 15 | 8 (53.3) | 7 (46.7) | |
| Unknown | 14 | |||
| Tumor stage | 0.247b | |||
| I | 3 | 2 (66.7) | 1 (33.3) | |
| II | 17 | 7 (41.2) | 10 (58.8) | |
| III | 23 | 15 (65.2) | 8 (34.8) | |
| IV | 1 | 1 (100) | 0 (0) | |
| Unknown | 6 | |||
| Bone metastasis at relapse | 0.709a | |||
| Yes | 15 | 9 (60.0) | 6 (40.0) | |
| No | 35 | 19 (54.3) | 16 (45.7) | |
| Unknown | 0 | |||
| Liver metastasis at relapse | 0.815a | |||
| Yes | 11 | 7 (63.6) | 4 (36.4) | |
| No | 39 | 21 (53.8) | 18 (46.2) | |
| Unknown | 0 | |||
| Lung metastasis at relapse | 0.034a | |||
| Yes | 17 | 6 (35.3) | 11 (64.7) | |
| Table 2 (continued) | ||||
Positive correlation between baseline CTCs and functional peripheral NK cells
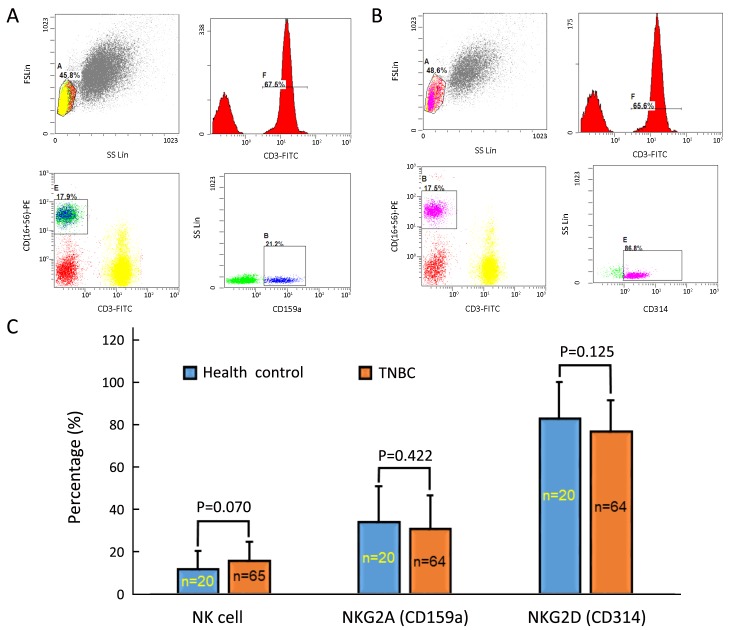
As chemotherapy can cause hematological toxicity (such as lymphocytopenia), we explored associations between CTC status and peripheral lymphocyte ratios only among patients who had not yet undergone any treatment. As the data show, baseline CTC status was not significantly associated with most lymphocyte subtypes. However, CTC status and peripheral NK ratios were positively correlated (P=0.032; Table 3). We also longitudinally monitored both CTC counts and peripheral NK cell ratios at regular intervals after chemotherapy in some patients, and found similar tendencies in variations of CTC counts and peripheral NK cell ratios in six patients. Although peripheral NK cell ratios and CTC status were positive correlated, the functional integrity of NK cells in TNBC patients still needed to be evaluated. In doing so, we compared positive rates of NK-associated activating receptor (NKG2D, also known as CD314) and inhibitory receptor (NKG2A, also known as CD159a) between healthy controls and the TNBC patients. The positive rate was defined as the percentage of NKG2A+ or NKG2D+ peripheral NK cells to active peripheral NK cells (CD3–/CD56+/CD16+), based on the gating strategies shown in Figure 1A, B . As the data indicate (Figure 1C), healthy controls and TNBC patients did not significantly differ in total NK cell ratios (11.7%±8.9% vs. 15.8%±8.8%, P=0.070), NKG2A+ NK cell ratios (34.1%±16.8% vs. 30.8%±15.9%, P=0.422) or NKG2D+ NK cell ratios (83.1%±17.4% vs. 76.9%±14.9%, P=0.125).
3.
Correlation between baseline CTC status and peripheral lymphocyte subtypes distribution in first-line TNBC
| Peripheral lymphocyte subtypes (Phenotype) | Correlation coefficient | P |
| CTC, circulating tumor cell; TNBC, triple-negative breast cancer. Baseline CTC status consists of CTC positivity (>2 CTC/2 mL) and CTC negativity (≤2 CTC/2 mL). | ||
| Total T-cells (CD3+) | –0.234 | 0.121 |
| Helper T-cells (CD3+ & CD4 +) | –0.130 | 0.391 |
| Cytotoxic T-cell (CD3+ & CD8 +) | –0.111 | 0.455 |
| CD4+/CD8+ratio (CD4+/CD8+) | –0.102 | 0.528 |
| Natural killer cell (CD3– & CD56 + & CD16 +) | 0.321 | 0.032 |
| Natural killer T-cell
(CD3+ & CD56 + & CD16 +) |
0.280 | 0.855 |
| B cells (CD3– & CD19 +) | –0.008 | 0.957 |
| Regulatory T-cells (CD4+ & CD25 +) | –0.220 | 0.143 |
| Activated naïve cytotoxic T-cells
(CD8+ & CD28 +) |
–0.214 | 0.157 |
| Suppressor T-cells (CD8+ & CD28 –) | 0.276 | 0.064 |
1.
Evaluating the functional integrity of natural killer (NK) cell in triple-negative breast cancer (TNBC) patients. (A) Gating strategy for NKG2A (CD159a); (B) Gating strategy for NKG2D (CD314); (C) Comparing the proportion of total NK cell, NKG2A positive NK cell and NKG2D positive NK cell between health control and TNBC.
Prognostic value of baseline CTC for PFS
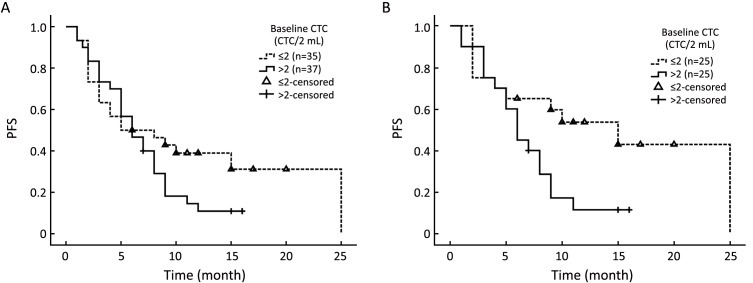
In consideration of the conflicting reports on the prognostic value of EpCAM+ CTC in TNBC with respect to PFS, we carried out a revalidation experiment using our self-developed CTC isolation system (Pep@MNPs). We divided the cohort into CTC>2.0 and CTC≤2.0 subgroups and compared their PFS. Regardless of lines of therapy, median PFS did not significantly differ between the CTC>2.0 group (5.0 months) and the CTC≤2.0 group (6.0 months; P=0.118;Figure 2A). However, among patients who were undergoing first-line therapy, significantly shorter median PFS was observed for the CTC>2.0 group (6.0 months) than the CTC≤2.0 group (15.0 months; P=0.033;Figure 2B).
2.
Kaplan-Meier estimates of progression-free survival (PFS) in months for triple-negative breast cancer (TNBC) patients. (A) PFS for patients with circulating tumor cell (CTC) positivity (>2CTC/2 mL of whole blood) compared to those with CTC negativity (≤2CTC/2 mL of whole blood) at baseline in total TNBC patients (P=0.118); (B) PFS for patients with CTC positivity compared to those with CTC negativity at baseline in patients who received first line therapy (P=0.033).
Enhanced prognostic power of CTC by adding NK cell enumeration
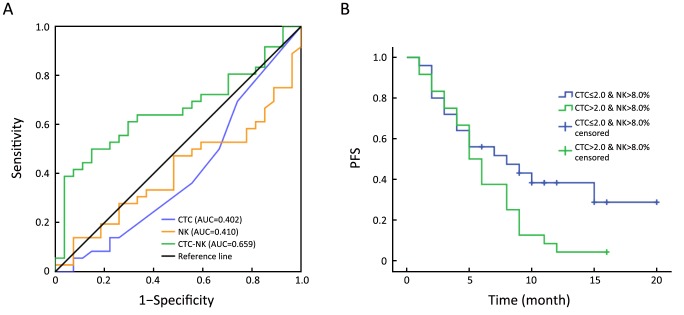
As baseline CTC status and peripheral NK cell ratios were positively correlated, and immune factors reportedly have prognostic value for breast cancer, we sought better predictions for PFS of TNBC by considering CTC and NK cell status together. In doing so, we used binary logistic regression to calculate the predictive probabilities of each CTC-NK combinations. Then the CTC number, NK proportion and predictive probabilities of CTC-NK were subjected to ROC curve analysis for their AUC. Data showed that CTC-NK has better AUC (0.659, P=0.031, 95% CI, 0.524–0.795) than CTC (0.402, P=0.185, 95% CI, 0.257–0.546) or NK (0.410, P=0.227, 95% CI, 0.270–0.551) alone (Figure 3A). Maximum Youden-index of CTC-NK was 0.352 and its correspondent combination was CTC>2.0 & NK>8.0% (data not showed). We further set this combination of CTC-NK as threshold to stratify TNBC patients, regardless of lines of therapy. Kaplan-Meier survival analysis showed that patients with CTC>2.0 & NK>8.0% at baseline have shorter median PFS than those with CTC≤2.0 & NK>8.0% (5.0vs. 8.0 months; P=0.049; Figure 3B).
3.
Kaplan-Meier estimates of progression-free survival (PFS) (in months) for patients with triple-negative breast cancer (TNBC), regardless of lines of therapy, using combined baseline circulating tumor cell-natural killer cell (CTC-NK) levels. (A) Comparison of area under the curve (AUC) among CTC, NK and CTC-NK using receiver operating characteristic (ROC) curve analyses; (B) Kaplan-Meier survival analysis for patients with CTC>2.0 & NK>8.0% and CTC≤2.0 & NK>8.0% (P=0.049).
Discussion
The prognostic value of EpCAM-dependent CTCs in metastatic breast cancer has been clearly demonstrated. However, breast cancer is a heterogeneous disease. According to previous reports, 3%–10% of breast cancers do not express EpCAM (21-23). Several clinical features, including molecular subtype, reportedly affect CTC detection rate. For instance, CTC were found more in luminal HER2– patients than in those with HER2+ cancer or TNBC (24). At least one explanation for this phenomenon is low EpCAM expression and more mesenchymal phenotypes of HER2+ and TNBC tumors. The CellSearch platform indicates that CTC detection rates in TNBC patients range from 33.3% to 81.0% (8,10,11,13,15,25,26); as CTCs in the present study were detected in 55 out of our 75 patients (73.3%), the efficacy of our CTC isolation system (Pep@MNPs) for TNBC seems to be satisfactory. We therefore sought to determine the association between baseline CTC status and other clinical parameters of TNBC, especially PFS. Using the threshold defined in our previous study (2.0 CTC/2 mL whole blood) (16), we found baseline CTC status was positively correlated with lung metastasis in first-line patients (P=0.034) and number of metastatic sites (P=0.037). However, this correlation became insignificant in the cohort as a whole, regardless of lines of therapy (P=0.131). We believe it partly due to the loss of epithelial markers, including EpCAM, as tumors progressed through multiple lines of therapy (27).
Notably, we also found a positive correlation between baseline CTC status and peripheral NK cells ratio in first-line TNBC patients. We also longitudinally monitored variations of both NK cell ratios and CTC numbers over times in several patients. Despite the effect of antitumor therapy on peripheral lymphocyte numbers (17,20), we observed synchronized variation curves for CTC numbers and peripheral NK cell ratios in most patients.
The bloodstream is a harsh environment for tumor cells, in which CTCs might face many fierce attacks, including elimination by immune cells (28). After CTCs are shed into circulation, they can be recognized and eliminated by NK cells, neutrophils, macrophages or cytotoxic T-cells (29-32), which supports the positive correlation between CTC status and peripheral NK cells ratio found in our study. However, in breast cancer patients, some peripheral lymphocytes, such as T helper cells and monocytic dendritic cells, are reportedly more vulnerable and likely to undergo apoptosis, especially in CTC positive patients (as defined by CellSearch) (33,34). To assure functional integrity of peripheral NK cells in TNBC patients, we evaluated the classic NK-associated inhibitory receptor, NKG2A, and activating receptor, NKG2D, and found that neither NKG2A (P=0.422) nor NKG2D (P=0.125) was abnormally expressed in TNBC patients compared with healthy controls; this indicates that peripheral NK cells normally responded to EpCAM+ CTCs in TNBC.
The main purpose of present study was to determine whether baseline CTC counts were related to PFS in TNBC patients, regardless of lines of therapy. In this study cohort as a whole, the CTC≤2.0 and CTC>2.0 groups did not significantly differ in PFS (using 2.0 CTC/2 mL as the cutoff; P=0.118). However, as discussed above, the total TNBC cohort contained multiline-therapy patients whose cancers might lose EpCAM expression during the course of tumor progression. This might explain the impaired prognostic power of EpCAM-dependent CTC enumeration in TNBC patients, regardless of lines of therapy. This speculation is supported by our data showing that baseline CTC status has predictive values regarding PFS (P=0.033) in TNBC patients undergoing first-line treatment. Some recent pilot studies that combined CTC enumeration with other clinical parameters, including tumor stage, presence of visceral metastasis, performance status and gene expression, were capable to estimate survival or tumor response (35,36). The presence of certain lymphocytes (such as tumor-infiltrating lymphocytes) has been reported as independent prognostic factors for OS and PFS (8,20,37-39) in breast cancer. In view of these findings, we wondered if we could optimize the prognostic performance of baseline CTC in TNBC by adding peripheral lymphocyte enumeration. The presence of active peripheral NK cells (CD3–/CD56+/CD16+) can clear CTCs or maintain them at certain levels (37,40). However, some CTCs could also develop escape or resistance mechanisms against NK cell-mediated elimination (41-43). These better evolved CTCs are the so-called “NK-resistant CTCs”. This terminology was first used in prostate cancer (44). The check-and-balance relationship between CTCs and NK cells may have an important implication for the prognosis of TNBC patients, and the presence of NK-resistant CTCs may portend a worse outcome in TNBC patients. According to this speculation, the CTC>2.0 TNBC patients with a certain level of peripheral NK cells should have shorter PFS than other patients. As expected, when we used the CTC-NK combined enumeration thresholds of >2.0 CTC/2 mL and NK>8.0%, we could stratify TNBC patients in terms of PFS, regardless of their lines of therapy (P=0.049). The ROC curve analysis also showed that CTC-NK had better AUC as compared to CTC or NK proportion alone (0.659vs. 0.402 and 0.659 vs. 0.410). These findings confirm the advantage of CTC-NK enumeration in predicting FPS of TNBC patients.
Conclusions
In this study, we reported that: 1) the EpCAM-based CTC assay is more sensitive to lung metastasis in TNBC patients; 2) baseline CTC status is positively correlated with functional peripheral NK cell ratio in TNBC; 3) baseline CTC counts can predict PFS only in first-line TNBC patients but not TNBC patients as a whole, without respect to lines of therapy; 4) but baseline CTC combined with NK enumeration (CTC-NK) can predict PFS of TNBC patients regardless of their lines of therapy. Thus CTC-NK combined enumeration could be a useful prognostic tool for patients with TNBC. Future studies are needed to explore the biological nature and clinical significance of NK-resistant CTCs in breast cancer.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81502269 and No. 21273051), and a grant from the Chinese Academy of Sciences (No. XDA09030306).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 3.O’Shaughnessy J, Schwartzberg L, Danso MA, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32:3840–7. doi: 10.1200/JCO.2014.55.2984. [DOI] [PubMed] [Google Scholar]
- 4.Telli M. Evolving treatment strategies for triple-negative breast cancer. J Natl Compr Canc Netw. 2015;13:652–4. doi: 10.6004/jnccn.2015.0194. [DOI] [PubMed] [Google Scholar]
- 5.Kalimutho M, Parsons K, Mittal D, et al. Targeted therapies for triple-negative breast cancer: Combating a stubborn disease. Trends Pharmacol Sci. 2015;36:822–46. doi: 10.1016/j.tips.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Yu T, Di G. Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chin J Cancer Res. 2017;29:237–52. doi: 10.21147/j.issn.1000-9604.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andree KC, van Dalum G, Terstappen LW. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol. 2016;10:395–407. doi: 10.1016/j.molonc.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munzone E, Botteri E, Sandri MT, et al. Prognostic value of circulating tumor cells according to immunohistochemically defined molecular subtypes in advanced breast cancer. Clin Breast Cancer. 2012;12:340–6. doi: 10.1016/j.clbc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Banys-Paluchowski M, Schneck H, Blassl C, et al. Prognostic relevance of circulating tumor cells in molecular subtypes of breast cancer. Geburtshilfe Frauenheilkd. 2015;75:232–7. doi: 10.1055/s-0035-1545788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang ZF, Cristofanilli M, Shao ZM, et al. Circulating tumor cells predict progression-free and overall survival in Chinese patients with metastatic breast cancer, HER2-positive or triple-negative (CBCSG004): a multicenter, double-blind, prospective trial. Ann Oncol. 2013;24:2766–72. doi: 10.1093/annonc/mdt246. [DOI] [PubMed] [Google Scholar]
- 11.Karhade M, Hall C, Mishra P, et al. Circulating tumor cells in non-metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2014;147:325–33. doi: 10.1007/s10549-014-3103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madic J, Kiialainen A, Bidard FC, et al. Circulating tumor DNA and circulating tumor cells in metastatic triple negative breast cancer patients. Int J Cancer. 2015;136:2158–65. doi: 10.1002/ijc.29265. [DOI] [PubMed] [Google Scholar]
- 13.Magbanua MJ, Carey LA, DeLuca A, et al. Circulating tumor cell analysis in metastatic triple-negative breast cancers. Clin Cancer Res. 2015;21:1098–105. doi: 10.1158/1078-0432.CCR-14-1948. [DOI] [PubMed] [Google Scholar]
- 14.Paoletti C, Li Y, Muñiz MC, et al. Significance of circulating tumor cells in metastatic triple-negative breast cancer patients within a randomized, phase II trial: TBCRC 019. Clin Cancer Res. 2015;21:2771–9. doi: 10.1158/1078-0432.CCR-14-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peeters DJ, van Dam PJ, Van den Eynden GG, et al. Detection and prognostic significance of circulating tumour cells in patients with metastatic breast cancer according to immunohistochemical subtypes. Br J Cancer. 2014;110:375–83. doi: 10.1038/bjc.2013.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu XR, Shao B, Peng JX, et al. Identification of high independent prognostic value of nanotechnology based circulating tumor cell enumeration in first-line chemotherapy for metastatic breast cancer patients. Breast. 2017;32:119–25. doi: 10.1016/j.breast.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Mellios T, Ko HL, Beuth J. Impact of adjuvant chemo- and radiotherapy on the cellular immune system of breast cancer patients. In Vivo. 2010;24:227–30. [PubMed] [Google Scholar]
- 18.Goedert JJ, Pfeiffer R, Zhu M, et al. Peripheral blood immunologic phenotype of population-based breast cancer cases and matched controls. Eur J Clin Invest. 2012;42:572–4. doi: 10.1111/j.1365-2362.2011.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber I, Landenberger N, Staebler A, et al. Relationship between circulating tumor cells and peripheral T-cells in patients with primary breast cancer. Anticancer Res. 2013;33:2233–8. [PubMed] [Google Scholar]
- 20.Jia Y, Xu L, Lin Q, et al. Levels of lymphocyte subsets in peripheral blood prior treatment are associated with aggressive breast cancer phenotypes or subtypes. Med Oncol. 2014;31:981. doi: 10.1007/s12032-014-0981-9. [DOI] [PubMed] [Google Scholar]
- 21.Rao CG, Chianese D, Doyle GV, et al. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol. 2005;27:49–57. [PubMed] [Google Scholar]
- 22.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–14. doi: 10.1158/0008-5472.Can-07-5644. [DOI] [PubMed] [Google Scholar]
- 23.Spizzo G, Fong D, Wurm M, et al. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol. 2011;64:415–20. doi: 10.1136/jcp.2011.090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punnoose EA, Atwal SK, Spoerke JM, et al. Molecular biomarker analyses using circulating tumor cells. PLos One. 2010;5:e12517. doi: 10.1371/journal.pone.0012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu YJ, Wang P, Wang X, et al. The significant prognostic value of circulating tumor cells in triple-negative breast cancer: a meta-analysis. Oncotarget. 2016;7:37361–9. doi: 10.18632/oncotarget.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall C, Karhade M, Laubacher B, et al. Circulating tumor cells after neoadjuvant chemotherapy in stage I-III triple-negative breast cancer. Ann Surg Oncol. 2015;22(Suppl 3):S552–8. doi: 10.1245/s10434-015-4600-6. [DOI] [PubMed] [Google Scholar]
- 27.Friedlander TW, Premasekharan G, Paris PL. Looking back, to the future of circulating tumor cells. Pharmacol Ther. 2014;142:271–80. doi: 10.1016/j.pharmthera.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Palucka K, Coussens LM, O’Shaughnessy J. Dendritic cells, inflammation, and breast cancer. Cancer J. 2013;19:511–6. doi: 10.1097/PPO.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Placke T, Örgel M, Schaller M, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72:440–8. doi: 10.1158/0008-5472.CAN-11-1872. [DOI] [PubMed] [Google Scholar]
- 30.Granot Z, Henke E, Comen EA, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–14. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gül N, Babes L, Siegmund K, et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J Clin Invest. 2014;124:812–23. doi: 10.1172/JCI66776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013;73:2381–8. doi: 10.1158/0008-5472.CAN-12-3932. [DOI] [PubMed] [Google Scholar]
- 33.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013;91:411–29. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green TL, Santos MF, Ejaeidi AA, et al. Toll-like receptor (TLR) expression of immune system cells from metastatic breast cancer patients with circulating tumor cells. Exp Mol Pathol. 2014;97:44–8. doi: 10.1016/j.yexmp.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Giordano A, Egleston BL, Hajage D, et al. Establishment and validation of circulating tumor cell-based prognostic nomograms in first-line metastatic breast cancer patients. Clin Cancer Res. 2013;19:1596–602. doi: 10.1158/1078-0432.CCR-12-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paoletti C, Muñiz MC, Thomas DG, et al. Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer. Clin Cancer Res. 2015;21:2487–98. doi: 10.1158/1078-0432.CCR-14-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamessier E, Pradel LC, Thibult ML, et al. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J Immunol. 2013;190:2424–36. doi: 10.4049/jimmunol.1200140. [DOI] [PubMed] [Google Scholar]
- 38.Song QK, Ren J, Zhou XN, et al. The prognostic value of peripheral CD4+CD25+ T lymphocytes among early stage and triple negative breast cancer patients receiving dendritic cells-cytokine induced killer cells infusion. Oncotarget. 2015;6:41350–9. doi: 10.18632/oncotarget.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ascierto ML, Idowu MO, Zhao Y, et al. Molecular signatures mostly associated with NK cells are predictive of relapse free survival in breast cancer patients. J Transl Med. 2013;11:145. doi: 10.1186/1479-5876-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mamessier E, Bourgin C, Olive D. When breast cancer cells start to fend the educational process of NK cells off. Oncoimmunology. 2013;2:e26688. doi: 10.4161/onci.26688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mamessier E, Sylvain A, Thibult ML, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–22. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamessier E, Bertucci F, Sabatier R, et al. " Stealth” tumors: Breast cancer cells shun NK-cells anti-tumor immunity. Oncoimmunology. 2012;1:366–8. doi: 10.4161/onci.18528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajitani K, Tanaka Y, Arihiro K, et al. Mechanistic analysis of the antitumor efficacy of human natural killer cells against breast cancer cells. Breast Cancer Res Treat. 2012;134:139–55. doi: 10.1007/s10549-011-1944-x. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Chen Q, Yan J, et al. MiRNA-296-3p-ICAM-1 axis promotes metastasis of prostate cancer by possible enhancing survival of natural killer cell-resistant circulating tumour cells. Cell Death Dis. 2013;4:e928. doi: 10.1038/cddis.2013.458. [DOI] [PMC free article] [PubMed] [Google Scholar]