Abstract
Sickle cell disease (SCD) is a congenital hemoglobinopathy characterized by frequent acute care/emergency room visits and hospital admissions. Evidence from population-based studies demonstrating the role of hydroxycarbamide (HC) in reducing hospital readmission rates is limited. Our objective was to describe the use of HC and its association with acute care utilization and readmission rates using a large, nationally-representative U.S. health insurance claims database over a 6-year period between 2009 and 2014. We identified 20721 SCD-related inpatient and acute care encounters. HC exposure was present among 4263 (21%) of SCD-related admission events within 6 months prior to the admission. HC use was more common among children ages 10–17 years and young adults ages 18–29 years. HC was associated with lower 30-day all-cause readmission rates in adults treated with average daily doses ≥1g (odds ratio [OR], 0.72, 95% CI 0.52–0.99) and doses of 0.5–1g (OR, 0.73, 95% CI 0.57–0.93), compared to HC treatment with average daily doses of <0.5g; adherence to HC with proportion of days covered of ≥0.80 was also associated with significantly lower 30-day all-cause readmission risks (OR, 0.59, 95% CI 0.41–0.84). Optimal therapeutic dosing and adherence to HC treatment significantly reduces 30-day readmissions among patients with SCD.
Keywords: Sickle cell disease, Hydroxycarbamide, Epidemiology, Readmission, Acute Care, Adherence
Introduction
Sickle cell disease (SCD) is an inherited autosomal recessive disorder of hemoglobin that primarily affects African Americans in the United States (U.S.) (Piel, et al 2017). SCD is characterized by hemolytic anemia, recurring acute painful crises and acute chest syndrome (ACS) that require immediate treatment in the emergency room (ER) and/or inpatient settings. Patients with SCD have high admission rates and ER visits caused by complications related to the disease. Thirty-day and 14-day readmission rates are as high as 33% and 22%, respectively, among SCD patients with the highest rates among 18- to 30-year-olds (Brousseau, et al 2010). These frequent acute care encounters incur enormous financial burdens on health systems. It is estimated that annual costs for management of SCD in the U.S. exceed $1.1 billion with the majority of the costs (81%) attributable to inpatient admissions (Kauf, et al 2009). Therefore, reducing hospital readmission rates is essential to ensuring quality care and mitigating the rising health care costs for individuals with SCD.
Addressing high rates of hospital readmission is a priority for continuous SCD care improvement. The 30-day readmission rate is increasingly recognized as a quality metric for SCD care. The National Association of Children’s Hospital and Related Institutions (NACHRI) established the 30-day readmission rate as a benchmark for quality SCD care (Institute of Medicine (U.S.). Committee on Quality of Health Care in America. 2001). In addition, this quality measure has been adopted by the Centers for Medicare and Medicaid Services (CMS) for adjusting reimbursement for inpatient services (Brock and Jencks 2008).
Hydroxycarbamide (HC) is one of two medications approved by the U.S. Food and Drug Administration (FDA) for SCD; the second medication introduced in 2017, Endari® (L-glutamine oral powder), was the first approval for this rare blood disorder in nearly 20 years. HC has the potential of reducing complications of vaso-occlusive crises by reactivating fetal γ-globin, improving nitric oxide (NO) metabolism, and reducing adhesion of sickled red blood cells to the vascular endothelial walls (Ware 2010). The laboratory and clinical efficacy of HC has been demonstrated from numerous randomized clinical trials and smaller observational studies in both pediatric and adult populations (Charache, et al 1992, Charache, et al 1995, De Montalembert, et al 1997, Ferster, et al 1996, Jayabose, et al 1996, Kinney, et al 1999, Scott, et al 1996). Follow-up from the subjects enrolled in these trials showed salutary long-term benefits in morbidity, mortality, laboratory tests and preservation of organ function (McGann and Ware 2011, McGann and Ware 2015). Several studies conducted in Italy, Belgium and India have shown significant clinical benefits attributable to HC (Colombatti, et al 2018, Jain, et al 2012, Le, et al 2015, Nottage, et al 2013). However, these studies were limited with respect to a relatively small simple size, single institution design and shorter follow-up periods in randomized clinical trials (RCTs). Moreover, evidence is lacking demonstrating clinical effectiveness of HC in real-world settings from the U.S. (Lanzkron, et al 2006), despite being cost-effective in the Multicenter Study of Hydroxyurea (MSH) (Moore, et al 2000). There is growing evidence for the efficacy and safety of HC; yet, this treatment remains under-utilized (Mulaku, et al 2013, Ware, et al 2016). One study of 312 U.S. Medicaid enrollees with SCD demonstrated that adherence (medication possession ratio ≥0.80) was associated with reduced risks of SCD-related hospitalization (HR=0.65), all-cause and SCD-related ER visits (HR=0.72 and 0.58), vaso-occlusive events (HR=0.66), and reduced costs (Candrilli, et al 2011). However, this study did not examine outcomes of 30-day hospital readmission following SCD-related hospitalizations or associations with cumulative HC doses on hospitalization.
The objectives of this study were to use a large, nationally-representative U.S. administrative claims database to document patterns of HC use in a population of commercially-insured SCD patients and investigate associations of cumulative therapeutic doses and adherence levels of HC in relation to 30-day readmission and acute-care utilization rates.
Methods
Data Source
We conducted a retrospective cohort study utilizing data using the Truven Health MarketScan® Commercial Claims and Encounters and Medicare Supplemental and Coordinated Benefits databases (https://truvenhealth.com/Markets/Life-Sciences/Products/Data-Tools/MarketScan-Databases) from 1 January 2009 and 31 December 2014. The MarketScan® Commercial Databases and Medicare Supplemental Databases include health insurance claims data in addition to enrolment data from large groups of participating U.S. employers and health plans across all 50 states and Washington D.C. (Hansen 2017). The databases were linked to prescription drug claims and encounter data to provide individual-level inpatient, outpatient, and pharmacy services for employees, their spouses and dependents. All data were de-identified and Health Insurance Portability and Accountability Act (HIPAA) of 1996 compliant. This study was approved by the Institution Review Board of the University of Illinois at Chicago.
Patient Selection
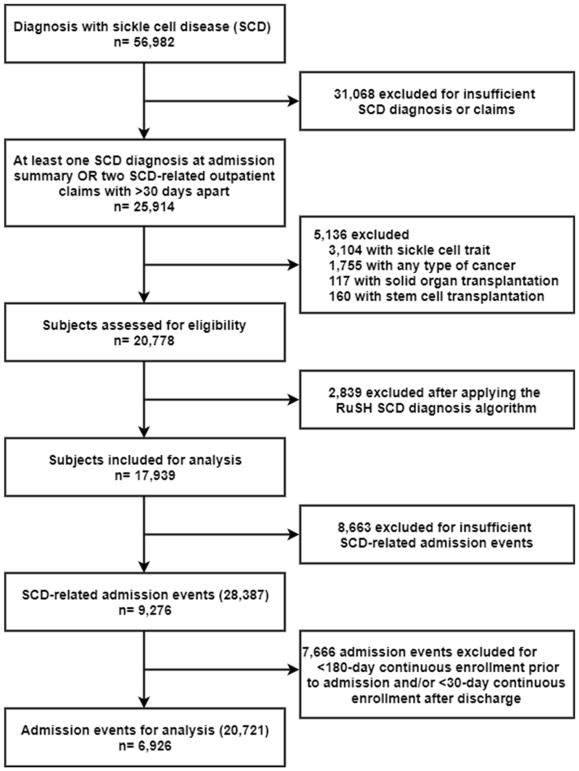
All subjects with one or more medical claims from an inpatient discharge summary or ≥2 separate medical claims from outpatient services that were at least 30 days apart indicative of SCD using International Classification of Diseases, Ninth Revision, Clinical Modification; (ICD-9-CM) code (See Supplemental Table 1) were initially selected for study inclusion. To ensure the homogeneity of cohort, patients with any claims for sickle cell trait during the follow-up period were excluded, as well as any type of cancer, or received a hematopoietic stem cell or solid organ transplantation. We applied an additional SCD-identifying algorithm (RuSH) to further specify SCD cases (Paulukonis, et al 2014). All SCD-related admission events were defined as the occurrence of a diagnosis for any SCD-related condition in discharge summaries evidenced by ICD-9CM codes (See Supplemental Table 2). Only admission events of patients who were continuously enrolled in the health plan for at least 6 months (180 days) before the admission date and at least 1 month (30 days) following discharge were selected. The discharge date was designated as the index date. Pharmacy dispensing claims for HC as well as other medications including nonsteroidal anti-inflammatory drugs (NSAIDs) and opiate analgesics were identified using relevant national drug codes on the outpatient prescription claims records. Under the participating health plans, a fixed copayment of 5 to 8 U.S. dollars is associated with each outpatient HC prescription. Selection of patients for this analysis is summarized in Figure 1.
Figure 1.
Patient flow diagram
Exposures
Using outpatient prescription drug records, we collected data on HC dispensed during the 6 months period preceding the admission events. We included only oral HC in the outpatient settings. To limit the effects of confounding by indication, we excluded all newly initiated HC occurring within the 14 days prior to the admission dates of the SCD-related admissions.
Proportion of days covered (PDC), one of the most commonly used and reproducible methods for determining medication adherence with administrative claims (Andrade, et al 2006), was calculated for each SCD-related admission event to determine HC adherence in the 6-month baseline period (Karve, et al 2008). For every admission event, the subject’s exposure status to HC was examined daily in a time-dependent manner. Prescription refill dates and corresponding days’ supply from pharmacy dispensing records were used to calculate PDC estimates. For example, if a patient with SCD refilled 2 HC prescriptions of 30 days’ supply and 1 prescription of 90 days’ supply over a 180 days’ period, his adherence to diabetes medication measured by PDC would be 0.83 ((30+30+90)/180). If the patient had early refills during the baseline period or left-over from time before the baseline period, the number of days covered by HC were adjusted to account for overlap. SCD patients who had PDC level above or equal to 0.80 were considered to be highly adherent HC users, whereas SCD patients with between 0.50 and 0.80 PDC were considered to be intermediate adherent users.
We defined HC exposure patterns as: 1) ever HC exposed compared to never HC exposed (main analyses limited to the whole cohort); 2) higher-dose HC exposure (≥1g average daily dose) and intermediate-dose HC exposure (<1g and ≥0.5g daily dose) compared to lower-dose HC exposure (>0mg and <0.5g daily dose, reference group); 3) recent HC exposure (presence of HC dispensing records during the 3 months period prior to admission date) compared to not recently HC exposed; and 4) highly adherent HC users (PDC≥0.80) and intermediate adherent HC users (0.80>PDC≥0.50) compared to non-adherent HC users (PDC<0.50, reference group).
Outcome Measures
The primary outcomes were SCD-related readmissions, all-cause readmissions, and presence of all-cause acute care encounters, defined as hospital admissions and/or emergency room visits. We evaluated the occurrence of these outcomes at both 7 and 30 days following discharge date. SCD-related readmissions were identified using the same criteria as primary SCD-related admission events. Secondary outcomes included length of stay for the index admission event in addition to death at discharge.
Covariates
We measured multiple a priori covariates considered to be possible confounders for the relation between HC and readmission and acute care/emergency department visits. Patient demographics, such as age and sex, were measured at admission date. Presence of concomitant ACS, pneumonia, asthma were measured using ICD-9CM discharge diagnoses associated with the index admission (See Supplemental Table 3). The presence of hospital admission and/or ER visits over the baseline period was determined by claims of inpatient and outpatient services. Erythrocyte transfusion events were identified using relevant ICD-9-CM diagnosis codes in addition to common procedure coding system codes documented among the administrative health insurance claims. Information on opioid medication doses were determined using pharmacy dispensing records and were converted to cumulative oral morphine equivalent (OME) using methods described previously (Han, et al 2016, Han, et al 2017).
Statistical Analysis
Differences in demographic and acute care utilization characteristics between HC exposure types were assessed using descriptive statistics. To compare groups, we used Student’s t-test and Kruskal-Wallis test for continuous variables and chi-square test for categorical variables.
Multivariable regression models were used to assess the association between HC exposure with acute care utilization (readmission events and ER visits) in 7-day and 30-day timeframes. Generalized estimating equation models assuming a first-order autoregressive covariance structure were used to account for correlation of multiple SCD-related admission events contributed per individual patient. Multivariable models were adjusted for patient demographic and clinical attributes (age, gender, presence of admission and ER visit in the 6-month period preceding admission, past NSAIDs and opiate analgesics exposure, measured by cumulative oral morphine equivalent), hospitalization characteristics (year and season of admission, weekend discharge, acute chest syndrome and pneumonia diagnosis code, co-occurring asthma diagnosis code, erythrocyte transfusion, and length of stay). Models were fit using terms on ever-exposure versus never-exposure using the whole analytical cohort. The impacts of other types of HC use patterns based on cumulative HC exposure, status of recent exposure and HC adherence on readmission and acute care services utilization rates were limited to the adult patients in the cohort. Odds ratios (OR) and 95% confidence intervals (CI) were estimated to determine the association between type of HC exposure and acute care utilization following discharge of the corresponding SCD-related hospitalization events. All analyses were performed using SAS, version 9.4 (SAS Institute SAS institute, Inc., Cary, North Carolina); two-tailed P values <0.05 were considered statistically significant.
Results
Overall, 20721 hospitalizations involving 6926 patients with SCD met the inclusion criteria (see Table 1), with 3388 (48.9%) contributing 1 SCD-related hospitalization, 1329 (19.2%) contributing 2 hospitalizations, and 2209 (31.9%) contributing 3 or more hospitalizations. Hospitalizations occurred in all age groups (median [interquartile range], 24 [16–39] years), with approximately one third occurring among younger adults ages 18–29 years. Hospitalization was slightly more common among female SCD patients (57.0%). ACS and pneumonia were associated with 2504 (21.8%) of the admission events with more admission events identified with a discharge diagnosis code for pneumonia 3574 (17.3%) as compared to ACS 1774 (8.6%). A total of 4553 (22.0%) hospitalizations had 1 or more erythrocyte transfusion.
Table 1.
Hydroxycarbamide use of hospitalized patients with sickle cell disease-related conditions
| Characteristics | Overall, No. (Column %) | hydroxycarbamide exposed in the past 6 months before hospitalization | hydroxycarbamide unexposed in the past 6 months before hospitalization | p-value |
|---|---|---|---|---|
| Hospitalization, No. (%) | 20,721 | 4263 | 16458 | |
| Age, in years | ||||
| 0–17 | 5831 (28.1) | 1203 (28.2) | 4628 (28.1) | <.0001 |
| 18–29 | 6761 (32.6) | 1733 (40.7) | 5028 (30.6) | |
| 30–39 | 3125 (15.1) | 603 (14.1) | 2522 (15.3) | |
| 40–49 | 2299 (11.1) | 442 (10.4) | 1857 (11.3) | |
| 50+ | 2705 (13.1) | 282 (6.6) | 2423 (14.7) | |
| Sex | ||||
| Male | 8916 (43.0) | 1894 (44.4) | 7022 (42.7) | 0.0383 |
| Female | 11805 (57.0) | 2369 (55.6) | 9436 (57.3) | |
| ACS diagnosis code | ||||
| ACS | 1774 (8.6) | 336 (7.9) | 1438 (8.7) | 0.0752 |
| Pneumonia | 3574 (17.3) | 582 (13.7) | 2992 (18.2) | <.0001 |
| ACS or pneumonia | 4513 (21.8) | 781 (18.3) | 3732 (22.7) | <.0001 |
| Asthma diagnosis code | 2504 (12.1) | 486 (11.4) | 2018 (12.3) | 0.1242 |
| Year of admission | ||||
| 2009 | 2088 (10.1) | 363 (8.5) | 1725 (10.5) | <.0001 |
| 2010 | 3781 (18.3) | 691 (16.2) | 3090 (18.8) | |
| 2011 | 3979 (19.2) | 768 (18.0) | 3211 (19.5) | |
| 2012 | 4055 (19.6) | 938 (22.0) | 3117 (18.9) | |
| 2013 | 3407 (16.4) | 775 (18.2) | 2632 (16.0) | |
| 2014 | 3411 (16.5) | 728 (17.1) | 2683 (16.3) | |
| Season of admission | ||||
| Winter (Dec–Feb) | 4517 (21.8) | 928 (21.8) | 3589 (21.8) | 0.2177 |
| Spring (Mar–May) | 4661 (22.5) | 985 (23.1) | 3676 (22.3) | |
| Summer (Jun–Aug) | 5373 (25.9) | 1055 (24.7) | 4318 (26.2) | |
| Fall (Sep–Nov) | 6170 (29.8) | 1295 (30.4) | 4875 (29.6) | |
| Discharge day of the week** | ||||
| Weekend | 5002 (24.1) | 1087 (25.5) | 3915 (23.8) | 0.0200 |
| Weekday | 15719 (75.9) | 3176 (74.5) | 12543 (76.2) | |
| Erythrocyte transfusion during admission | 4553 (22.0) | 995 (23.3) | 3558 (21.6) | 0.0155 |
| Medication exposure | ||||
| Opioids | 9203 (44.4) | 3291 (77.2) | 5912 (35.9) | <.0001 |
| NSAIDs | 3756 (18.1) | 1422 (33.4) | 2334 (14.2) | <.0001 |
| Health services use in the past 6 months | ||||
| Hospitalization | ||||
| Mean, SD | 1.56 (2.28) | 2.06 (2.50) | 1.42 (2.20) | <.0001*** |
| Median, IQR | 1 (0–2) | 1 (0–3) | 1 (0–2) | <.0001**** |
| ER visits | ||||
| Mean, SD | 3.03 (6.80) | 3.32 (6.16) | 2.95 (6.96) | 0.0008*** |
| Median | 1 (0–3) | 2 (0–4) | 1 (0–3) | <.0001**** |
ACS, acute chest syndrome; NSAIDs, Nonsteroidal anti-inflammatory drugs; ER, emergency room;
High-dose hydroxycarbamide exposure defined by the median of the cumulative hydroxycarbamide dose among patients ever-exposed. Set at 90g across age groups. Any patient with > or equal to 90g cumulative dose over the 180 days preceding the index admission event is considered as high-dose hydroxycarbamide users.
Weekend discharge status defined as Saturday and Sunday, while the rest of the week were weekdays (Mon-Fri).
Students t-test for comparing means across exposed groups
Wilcoxon Rank sum test for comparing medians across exposed groups
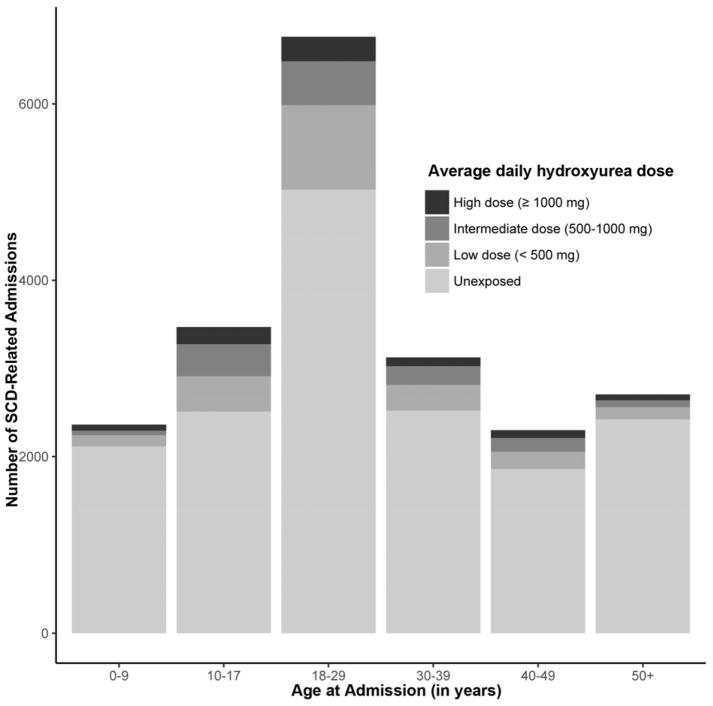
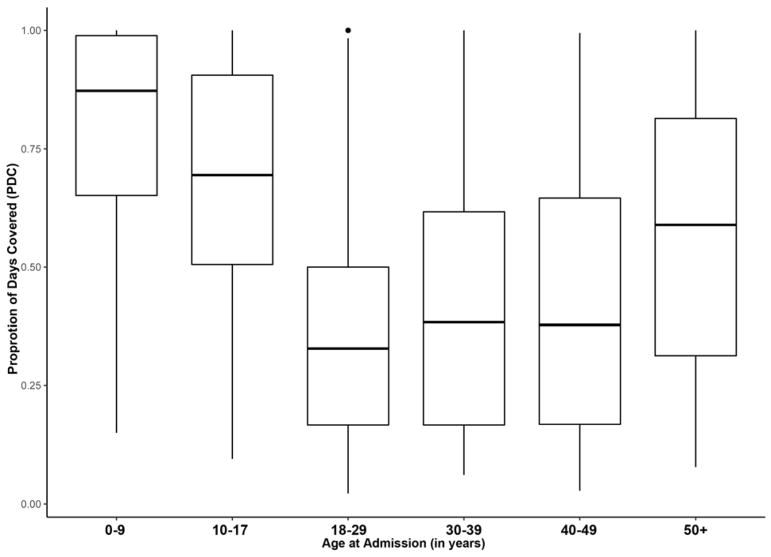
As shown in Figure 2, the proportion rates of hospitalizations with HC exposure were highest in the 10–17 years old, followed by the 18–29 years old. Figure 3 shows adherence rates to HC therapy by age groups. Among SCD-related admissions associated with HC exposure, estimated PDC was higher in the youngest pediatric patients (0–9 years) at 0.78 (0.87) [mean (median)], while lowest adherence was observed in younger adult patients (18–29 years) at 0.36 (0.33) [mean (median)].
Figure 2.
Number of SCD-related admissions associated with hydroxycarbamide therapy as measured by average daily dose in different age groups
Figure 3.
Adherence to hydroxycarbamide therapy as measured by proportion of days covered in different age groups
Patterns of HC use
Patients in 4263 (20.6%) hospitalizations had HC exposure 6 months prior to admission (Table 2). Among these primary admission events, 793 (18.6%) had higher dose HC exposure defined by an average daily dose of ≥1g, 1354 (31.8%) had intermediate dose HC exposure defined by an average daily dose of 0.5–1g; and 2116 (49.6%) had lower dose HC exposure (an average daily dose of <0.5g).
Table 2.
Outcomes by hydroxycarbamide exposure for hospitalization related to SCD
| Overall, No. (Column %) | HC exposed | HC not-exposed | p-value | High-dose HC *(≥1g/d) | Intermediate-dose HC (500mg-1g/d) | Low-dose HC Group (<500mg/d) | p-value | High adherent (PDC≥0.80) | Intermediate adherent (0.80>PDC≥0.50) | Non-adherent (PDC<0.50) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospitalizations, No. (%) | 20,721 | 4263 (20.6) | 16458 (79.4) | 793 (18.6) | 1354 (31.8) | 2116 (49.6) | 819 (19.2) | 1192 (28.0) | 2252 (52.8) | |||
| Primary outcomes | ||||||||||||
| SCD-related readmissions | ||||||||||||
| 7 day | 1318 (6.4) | 296 (6.9) | 1022 (6.2) | 0.0802 | 45 (5.7) | 81 (6.0) | 170 (8.0) | 0.0202 | 37 (4.5) | 66 (5.5) | 193 (8.6) | <.0001 |
| 30 day | 4076 (19.7) | 1034 (24.3) | 3042 (18.5) | <.0001 | 159 (20.1) | 295 (21.8) | 580 (27.4) | <.0001 | 140 (17.1) | 215 (18.0) | 679 (30.2) | <.0001 |
| All-cause readmission | ||||||||||||
| 7 day | 1436 (6.9) | 313 (7.3) | 1123 (6.8) | 0.2345 | 47 (5.9) | 86 (6.4) | 180 (8.5) | 0.0142 | 38 (4.6) | 69 (5.8) | 206 (9.1) | <.0001 |
| 30 day | 4527 (21.9) | 1103 (25.9) | 3424 (20.8) | <.0001 | 169 (21.3) | 315 (23.3) | 619 (29.3) | <.0001 | 152 (18.6) | 233 (19.5) | 718 (31.9) | <.0001 |
| All-cause acute care encounter | ||||||||||||
| 7 day | 2673 (12.9) | 555 (13.0) | 2118 (12.9) | 0.7947 | 72 (9.1) | 156 (11.5) | 327 (15.5) | <.0001 | 66 (8.1) | 125 (10.5) | 364 (16.2) | <.0001 |
| 30 day | 6762 (32.6) | 1583 (37.1) | 5179 (31.5) | <.0001 | 240 (30.3) | 455 (33.6) | 888 (42.0) | <.0001 | 216 (26.4) | 360 (30.2) | 1007 (44.7) | <.0001 |
| Secondary outcomes | ||||||||||||
| Length of stay (in days) | ||||||||||||
| median | 5 | 5 | 5 | <.0001 | 5 | 5 | 5 | 0.1217*** | 5 | 5 | 5 | <.0001*** |
| IQR | 3–7 | 3–8 | 3–7 | 3–8 | 3–7 | 3–8 | 3–7 | 3–7 | 4–8 | |||
| mean (SD) | 6.08 (5.65) | 6.19 (4.96) | 6.05 (5.81) | 0.1228 | 6.13 (5.55) | 6.13 (5.13) | 6.25 (4.61) | 0.7476 | 6.42 (4.74) | 6.21 (5.99) | 6.41 (4.74) | <.0001 |
| Died at discharge | 40 (0.2) ** | 9 (0.2) | 31 (0.2) | 0.7629 | 2 (0.3) | 4 (0.3) | 2 (0.1) | 0.2376**** | 3 (0.4) | 2 (0.2) | 4 (0.2) | 0.5592**** |
IQR, interquartile range
High-dose hydroxycarbamide exposure defined by the median of the cumulative hydroxycarbamide dose among the ever-exposed population. Set at 90g across age groups. Any patient with >90g cumulative dose over the 180 days preceding the index admission event is considered as high-dose hydroxycarbamide users.
A total of 1023 cases with missing discharge status
Kruskal-Wallis Test was used to assess the mean rank for the length of stay across exposed groups
A total of 221 cases with missing discharge status
HC-exposed admissions were more common among younger male patients and in more recent years in the study cohort. HC-exposed admission events were less likely to have acute chest syndrome (ACS) diagnosis at discharge. During the 6 months preceding admission, a greater proportion of HC exposed admissions had ≥1 emergency visits and ≥1 all-cause admissions. Further, HC exposed groups had a significantly higher proportion of erythrocyte transfusion during admission as well as history of both opiate analgesics and NSAIDs.
HC adherence measured by PDC among admission events in adult patients (age ≥18 years) in relation to outcomes are reported in Table 2. A total of 299 (9.8%) of the SCD-related admissions had high HC adherence (PDC≥0.80), 762 (24.9%) had intermediate adherence to HC (0.50≤PDC<0.80) and 1999 (65.3%) had low adherence (PDC <0.50). A total of 2225 hospitalizations (72.7%) had recent HC exposure, defined as presence of HC dispensing recording during the three months preceding admission, while 835 (27.3%) had discontinued HC for more than 3 months at the time of SCD-related index hospitalizations.
Association of HC exposure with readmission
As shown in Table 2, the unadjusted results demonstrated that HC unexposed admission events were associated with lower 30-day SCD-related (18.5% vs 24.3%, p<0.0001), all-cause readmissions (20.8% vs 25.9%, p <0.001) and all-cause acute-care utilizations (31.5% vs 37.1%, p <0.001) at 30 days after hospitalization. No statistically significant differences were observed between exposed and unexposed groups at 7-day post-discharge. The mean length of stay was the same between the two groups (6.05 vs 6.19 days, p=0.1228) and rates of inpatient mortality were similar at 0.2%.
In subgroup analyses assessing cumulative HC dose with our endpoints, increasing doses were correlated with significantly lower readmission and acute care encounter rates at both 7 (p<0.05) and 30 days (p<0.0001) after hospitalization. No differences in length of stay or death status at discharge were observed.
We also observed significantly reduced readmission and acute care utilization among HC users by medication adherence status. Patients who were more adherent to HC had significantly lower 7-day (p<0.0001) and 30-day (p<0.0001) readmission risks. High adherence to HC therapy (PDC≥0.80) was associated with a 13.3% reduction (crude risk difference) in 30-day all-cause readmission, a 13.1% SCD-related 30-day readmission and an 18.3% 30-day acute care encounter (Table 2).
In multivariable-adjusted models, high (PDC ≥ 0.80) and intermediate HC (0.50 ≤ PDC <0.80) adherence were associated with lower 30-day all-cause readmission (OR, 0.62; 95% CI 0.47–0.82, p=0.0007; and OR, 0.62; 95% CI 0.50–0.75, p<0.0001, respectively) compared to the HC users with low PDC (Table 3). In contrast, we did not observe any associations between HC exposure and readmission risks both in the whole analytical cohort and the adult subgroup when comparing HC users to never users. (See Supplemental Table 4 and 5).
Table 3.
Adjusted models of readmission or acute care utilization following hospitalized for SCD-related conditions
| Hydroxycarbamide exposure types | 7-day SCD-related readmission | 30-day SCD-related readmission | 7-day all-cause readmission | 30- day all-cause readmission | 7-day all-cause acute care | 30-day all-cause acute care | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |||||||
|
| ||||||||||||||||||
| Whole cohort (pediatric and adult patients) | ||||||||||||||||||
|
| ||||||||||||||||||
| High adherent user (PDC≥0.80) | 0.54 | 0.36–0.82 | 0.0034 | 0.60 | 0.45–0.79 | 0.0003 | 0.52 | 0.34–0.78 | 0.0019 | 0.62 | 0.47–0.82 | 0.0007 | 0.51 | 0.37–0.70 | <.0001 | 0.54 | 0.43–0.68 | <.0001 |
|
| ||||||||||||||||||
| Intermediate adherent users (0.80>PDC≥0.50) | 0.67 | 0.47–0.95 | 0.0228 | 0.58 | 0.48–0.71 | <.0001 | 0.65 | 0.46–0.92 | 0.0148 | 0.61 | 0.50–0.75 | <.0001 | 0.66 | 0.51–0.85 | 0.0014 | 0.60 | 0.50–0.71 | <.0001 |
|
| ||||||||||||||||||
| Nonadherent user (PDC<0.50) | ||||||||||||||||||
| Reference | ||||||||||||||||||
|
| ||||||||||||||||||
| All adult (18+) | ||||||||||||||||||
|
| ||||||||||||||||||
| Ever-HC exposure* | 0.96 | 0.76–1.21 | 0.7062 | 1.13 | 0.95–1.35 | 0.1782 | 0.94 | 0.75–1.18 | 0.6074 | 1.07 | 0.90–1.27 | 0.4362 | 0.88 | 0.74–1.05 | 0.147 | 1.07 | 0.93–1.23 | 0.3304 |
|
| ||||||||||||||||||
| Never-exposed | ||||||||||||||||||
| Reference | ||||||||||||||||||
|
| ||||||||||||||||||
| Adult (18+) and with any hydroxycarbamide exposure | ||||||||||||||||||
|
| ||||||||||||||||||
| High-dose ( >1g daily) | 0.72 | 0.47–1.11 | 0.1348 | 0.75 | 0.54–1.05 | 0.09 | 0.69 | 0.45–1.04 | 0.0767 | 0.72 | 0.52–0.99 | 0.0451 | 0.54 | 0.38–0.77 | 0.0006 | 0.65 | 0.49–0.86 | 0.0027 |
|
| ||||||||||||||||||
| Intermediate-dose (0.5–1g daily) | 0.87 | 0.56–1.36 | 0.5407 | 0.73 | 0.57–0.94 | 0.0157 | 0.86 | 0.56–1.30 | 0.4718 | 0.73 | 0.57–0.93 | 0.0122 | 0.83 | 0.61–1.13 | 0.2476 | 0.69 | 0.56–0.85 | 0.0005 |
|
| ||||||||||||||||||
| Low-dose (0–90g, 0–500mg daily) | ||||||||||||||||||
| Reference | ||||||||||||||||||
|
| ||||||||||||||||||
| Adult (18+) and with any hydroxycarbamide exposure | ||||||||||||||||||
|
| ||||||||||||||||||
| Recent exposure | 1.22 | 0.83–1.79 | 0.3105 | 0.99 | 0.82–1.19 | 0.896 | 1.25 | 0.86–1.84 | 0.2435 | 1.00 | 0.83–1.21 | 0.9846 | 1.28 | 0.98–1.67 | 0.0726 | 1.14 | 0.96–1.35 | 0.1345 |
|
| ||||||||||||||||||
| No Exposure in last 3-months | ||||||||||||||||||
| Reference | ||||||||||||||||||
|
| ||||||||||||||||||
| High adherent user (PDC≥0.80) | 0.66 | 0.36–1.20 | 0.0825 | 0.59 | 0.41–0.84 | 0.0002 | 0.60 | 0.33–1.10 | 0.0661 | 0.57 | 0.40–0.81 | 0.0005 | 0.56 | 0.36–0.89 | 0.0218 | 0.55 | 0.39–0.77 | <.0001 |
|
| ||||||||||||||||||
| Intermediate adherent users (0.80>PDC≥0.50) | 0.71 | 0.48–1.05 | 0.1713 | 0.64 | 0.51–0.81 | 0.0040 | 0.70 | 0.48–1.02 | 0.0981 | 0.67 | 0.54–0.84 | 0.0020 | 0.71 | 0.53–0.95 | 0.0141 | 0.62 | 0.50–0.76 | 0.0006 |
|
| ||||||||||||||||||
| Nonadherent user (PDC<0.50) | ||||||||||||||||||
| Reference | ||||||||||||||||||
Abbreviations: SCD, sickle cell disease; OR, odds ratio; CI, confidence interval; HC, hydroxycarbamide
The ever-exposure term in this model can be interpreted as estimating the allocation bias between exposed and unexposed SCD patients
The High-dose vs. Low-dose hydroxycarbamide exposure term in this model stratified SCD patients into ≥90g and <90g cumulative dose in the 6 months’ period before the index admission event.
Analysis limited to adults, the outcome is SCD-related 30-day admission. Adjusted for age, gender, length of stay, cumulative opioids exposure (measured by oral morphine equivalent), NSAIDs exposure, transfusion during hospitalization, all-cause hospitalization in the previous 6 months, any ER visits in the previous 6 months, asthma diagnosis code, acute chest syndrome code, year of admission, season of admission, and weekday of discharge.
Statistically significant at the p < .05 level.
HC was not approved by the U.S. FDA for pediatric patients ages 2 years and older with SCD with recurrent moderate to severe painful crises until December 21, 2017 (U.S. Food & Drug Administration 2017). In light of the differences in HC pharmacotherapy guidelines and SCD-related comorbidities in children and adults with SCD over the study period, we conducted subgroup analyses restricted to either pediatric (0–17 years of age at admission) and adult (18+ years at admission) SCD patients. In subgroup analyses limited to adult patients, we found that ≥1 g and 0.5–1g daily HC dose were associated with significant reduction in 30-day all-cause readmission risks as compared to 0–500mg daily HC dose (OR, 0.72; 95% CI 0.52–0.99, p=0.0451; and OR, 0.73 95% CI 0.57–0.93, p=0.0122, respectively) (Table 3).
In subgroup analyses limited to pediatric SCD patients, multivariable GEE regression models (data not shown) did not demonstrate any statistically significant relationship between adherence and the risk of inpatient readmission, or acute care encounter, or SCD-related readmission events in the 7- and 30-day period after discharge.
Discussion
To our knowledge, this is the first large, population-based study assessing HC utilization patterns in relation to hospital readmissions and ER visits in a commercially-insured, nationally representative sample of SCD patients. We demonstrated substantial benefits in reducing 30-day SCD-related, all-cause readmission and acute care use attributable to adherent HC treatment and higher therapeutic dosage (average daily dose ≥ 1g).
Readmission events in pediatric SCD patients are mainly attributed to vaso-occlusive crises (Bou-Maroun, et al 2018, Ellison and Bauchner 2007, Panepinto, et al 2005). Other factors including older age, inpatient use of steroids, admission for pain without other SCD-related complications (Sobota, et al 2012), absence of outpatient follow-up, concomitant asthma, oxygen within 24 hours of discharge and disease severity have been identified as risk factors for readmission in pediatric SCD patients (Frei-Jones, et al 2009). Risk factors for readmission in adult patients with SCD differ in nature and include premature discharge, withdrawal syndromes, and recurrence of acute painful episodes have been identified as causes for hospital readmission (Ballas and Lusardi 2005). Another recent study identified absence of a listed primary care provider and the number of past hospitalizations for vaso-occlusive pain as significant risk factors for 30-day readmission (Brodsky, et al 2017).
HC is a potent medication with proven efficacy and safety in clinical trials and observational studies. In a study of North Carolina Medicaid enrollees, adherence to HC therapy was demonstrated to reduce risks for inpatient and emergency room encounters in addition to a reduction in health care costs (Candrilli, et al 2011). A cost-effectiveness study also showed statistically significant differences in healthcare expenditures attributable to HC therapy when compared to placebo (Moore, et al 2000). While the potential benefits of HC treatment continue to be demonstrated, the adoption of this therapy in real-world practice settings can be slow and partly hampered by long-term safety concerns from both providers and families. In a study using a state administrative database, up to 70% of appropriate HC candidates were not taking the medication (Lanzkron, et al 2006). A study has shown that worse Health Related Quality of Life (HRQoL) and worse fatigue were associated with poor adherence among adolescents and young adults (Badawy, et al 2017a). Adherence to medication therapies like HC in SCD patients is a complex, multifactorial behavior with negative beliefs, recall barriers and access barriers among the factors causing poor adherence to HC therapy (Badawy, et al 2017b). In one study of 75 pediatric SCD cases, the adherence rate was 49% using pharmacy refill records while caregiver reported adherence ranged from 82%-85% using visual analog, modified Morisky scores or medical provider estimates (Thornburg, et al 2010). These inconsistent findings based on measure of medication adherence are reflective of possible inaccuracies when relying on self-reported adherence measures alone and in pediatric SCD patients where HC is dosed according to weight.
We observed lower adherence in adults compared to younger patients with SCD, highlighting the importance of care transition between pediatric and adult care providers. HC is demonstrated to be safe when children with SCD continue therapy through puberty and adulthood, with sustained hematological benefits and minimal safety concerns (Hankins, et al 2014). Our findings support efforts to create a smoother transition to adult SCD care for adolescents on HC therapy.
The observed lower readmission risks in the HC unexposed patients likely reflect the unexposed group being composed of subjects with a mild or less severe form of SCD not indicated for HC therapy, representing potential confounding by disease severity or indication when making comparisons between SCD patients exposed to HC and those never receiving HC (Psaty, et al 1999). Our results demonstrated that greater adoption of HC therapy has the potential to reduce SCD-related and all-cause readmissions and ER visits. Reducing acute care utilization without compromising SCD treatment outcomes would have substantial benefits to patients, their families, payers and society as a whole. Therefore, innovative strategies including adherence monitoring incorporating technological advances, education to the providers and caregivers, and psychological and social support programs should be considered to improve adherence and optimal dosing of HC therapy.
Our study had several strengths worth noting. We utilized administrative pharmacy dispensing records to collect information on HC utilization patterns and adherence in combination with records of acute care encounters, methods that are well-established and reliably describe medication use in patients with pharmacy coverage and enrolled on U.S. health plans (Andrade, et al 2006, Boudreau, et al 2004a, Boudreau, et al 2004b). Characterizing hospital encounters as unique index enabled us to quantify the impacts of HC cumulative dose and adherence on readmission risks at a hospitalization event-level in a clinically meaningful way. In addition, by limiting the analysis to SCD-related admissions, we determined our results to be robust to differences in non-SCD-related hospitalization events. Thus, we attempted to maximize the internal and external validity of our findings. Several limitations should also be considered when interpreting our results. First, we used ICD-9-CM codes to identify medical conditions, an inherent limitation to when using administrative health plan claims databases. We adopted a validated (RuSH) algorithm (Paulukonis, et al 2014) and a series of selection criteria to specify these conditions. Second, use of pharmacy dispensing records to evaluate medication adherence to HC therapy, while reproducible and not subject to recall bias, assumes that patients took the medication as directed. Also, we were unable to assess the reason for nonadherence to HC therapy. Poor adherence and early discontinuation to HC therapy could have been motivated by lack of treatment response. To address this, we employed more conservative measure of adherence (PDC) compared to other studies. Third, our study cohort consisted of commercially insured patients with SCD in the U.S. with continuous health plan coverage which may differ from SCD patients that lack insurance or are publicly insured. Still, our study population is nationally-representative, including approximately 15% of overall SCD patients in the U.S. Finally, as in any observational study, while we measured many important clinical factors, potential bias from unmeasured confounding such as weight and body mass index (BMI) cannot be ruled out. Further studies are needed to confirm our findings and should include outcomes of mortality, end-organ damage and laboratory test changes to comprehensively evaluate the impact of poor adherence to HC therapy in SCD.
Conclusion
We demonstrated reduced 30-day SCD-related and all-cause readmission among adherent HC users. Our findings suggest that efforts to improve adherence and optimal dosing of HC in patients with SCD would be well motivated.
Supplementary Material
Acknowledgments
Dr. Zhou was supported by the University of Illinois at Chicago-AbbVie Fellowship in Health Economics and Outcomes Research. Dr. Calip was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Numbers UL1TR002003 and KL2TR000048. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Authorship Contributions
J.Z., J.H. and G.S.C. designed and performed the research study. J.Z. and G.S.C. analyzed the data. All authors (J.Z., J.H., E.A.N., V.R.G., S.L.S. and G.S.C.) contributed to the drafting, revision and final approval of the manuscript.
References
- Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. discussion 575–567. [DOI] [PubMed] [Google Scholar]
- Badawy SM, Thompson AA, Lai JS, Penedo FJ, Rychlik K, Liem RI. Health-related quality of life and adherence to hydroxyurea in adolescents and young adults with sickle cell disease. Pediatric blood & cancer. 2017a:64. doi: 10.1002/pbc.26369. [DOI] [PubMed] [Google Scholar]
- Badawy SM, Thompson AA, Penedo FJ, Lai JS, Rychlik K, Liem RI. Barriers to hydroxyurea adherence and health-related quality of life in adolescents and young adults with sickle cell disease. Eur J Haematol. 2017b;98:608–614. doi: 10.1111/ejh.12878. [DOI] [PubMed] [Google Scholar]
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25. doi: 10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
- Bou-Maroun LM, Meta F, Hanba CJ, Campbell AD, Yanik GA. An analysis of inpatient pediatric sickle cell disease: Incidence, costs, and outcomes. Pediatric blood & cancer. 2018:65. doi: 10.1002/pbc.26758. [DOI] [PubMed] [Google Scholar]
- Boudreau DM, Daling JR, Malone KE, Gardner JS, Blough DK, Heckbert SR. A validation study of patient interview data and pharmacy records for antihypertensive, statin, and antidepressant medication use among older women. Am J Epidemiol. 2004a;159:308–317. doi: 10.1093/aje/kwh038. [DOI] [PubMed] [Google Scholar]
- Boudreau DM, Doescher MP, Jackson JE, Fishman PA, Saver BG. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004b;38:1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- Brock J, Jencks S. CMS targets readmission through payment, audits;’coaching’model reduces rates. Report on Medicare Compliance. 2008;17:1–2. [Google Scholar]
- Brodsky MA, Rodeghier M, Sanger M, Byrd J, McClain B, Covert B, Roberts DO, Wilkerson K, DeBaun MR, Kassim AA. Risk Factors for 30-Day Readmission in Adults with Sickle Cell Disease. Am J Med. 2017;130:601.e609–601 e615. doi: 10.1016/j.amjmed.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- Candrilli SD, O’Brien SH, Ware RE, Nahata MC, Seiber EE, Balkrishnan R. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;86:273–277. doi: 10.1002/ajh.21968. [DOI] [PubMed] [Google Scholar]
- Charache S, Dover GJ, Moore RD, Eckert S, Ballas SK, Koshy M, Milner P, Orringer EP, Phillips GJ, Platt OS. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia [see comments] Blood. 1992;79:2555–2565. [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR Anemia IotMSoHiSC. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. New England Journal of Medicine. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- Colombatti R, Palazzi G, Masera N, Notarangelo LD, Bonetti E, Samperi P, Barone A, Perrotta S, Facchini E, Miano M, Del Vecchio GC, Guerzoni ME, Corti P, Menzato F, Cesaro S, Casale M, Rigano P, Forni GL, Russo G, Sainati L Italian Multicenter Study of Hydroxyurea in Sickle Cell Anemia, I. Hydroxyurea prescription, availability and use for children with sickle cell disease in Italy: Results of a National Multicenter survey. Pediatric blood & cancer. 2018:65. doi: 10.1002/pbc.26774. [DOI] [PubMed] [Google Scholar]
- De Montalembert M, Belloy M, Bernaudin F, Gouraud F, Capdeville R, Mardini R, Philippe N, Jais J, Bardakdjian J, Ducrocq R. Three-year follow-up of hydroxyurea treatment in severely ill children with sickle cell disease. Journal of pediatric hematology/oncology. 1997;19:313–318. doi: 10.1097/00043426-199707000-00009. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Bauchner H. Socioeconomic status and length of hospital stay in children with vaso-occlusive crises of sickle cell disease. J Natl Med Assoc. 2007;99:192–196. [PMC free article] [PubMed] [Google Scholar]
- Ferster A, Vermylen C, Cornu G, Buyse M, Corazza F, Devalck C, Fondu P, Toppet M, Sariban E. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88:1960–1964. [PubMed] [Google Scholar]
- Frei-Jones MJ, Field JJ, DeBaun MR. Risk Factors for Hospital Readmission within 30-Days: A New Quality Measure for Children with Sickle Cell Disease. Pediatr Blood Cancer. 2009;52:481–485. doi: 10.1002/pbc.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Saraf SL, Zhang X, Gowhari M, Molokie RE, Hassan J, Alhandalous C, Jain S, Younge J, Abbasi T, Machado RF, Gordeuk VR. Patterns of opioid use in sickle cell disease. Am J Hematol. 2016;91:1102–1106. doi: 10.1002/ajh.24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Zhou J, Saraf SL, Gordeuk VR, Calip GS. Characterization of opioid use in sickle cell disease. Pharmacoepidemiol Drug Saf. 2017 doi: 10.1002/pds.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins JS, Aygun B, Nottage K, Thornburg C, Smeltzer MP, Ware RE, Wang WC. From infancy to adolescence: fifteen years of continuous treatment with hydroxyurea in sickle cell anemia. Medicine (Baltimore) 2014;93:e215. doi: 10.1097/MD.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. White Paper. [Accessed on October 18, 2017];The Truven Health MarketScan® Databases for life sciences researchers. 2017 Available at: https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf.
- Institute of Medicine (U.S.). Committee on Quality of Health Care in America. Crossing the quality chasm : a new health system for the 21st century. National Academy Press; Washington, D.C: 2001. [Google Scholar]
- Jain DL, Sarathi V, Desai S, Bhatnagar M, Lodha A. Low fixed-dose hydroxyurea in severely affected Indian children with sickle cell disease. Hemoglobin. 2012;36:323–332. doi: 10.3109/03630269.2012.697948. [DOI] [PubMed] [Google Scholar]
- Jayabose S, Tugal O, Sandoval C, Patel P, Puder D, Lin T, Visintainer P. Clinical and hematologic effects of hydroxyurea in children with sickle cell anemia. The Journal of pediatrics. 1996;129:559–565. doi: 10.1016/s0022-3476(96)70121-x. [DOI] [PubMed] [Google Scholar]
- Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Med Care. 2008;46:1125–1133. doi: 10.1097/MLR.0b013e31817924d2. [DOI] [PubMed] [Google Scholar]
- Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84:323–327. doi: 10.1002/ajh.21408. [DOI] [PubMed] [Google Scholar]
- Kinney TR, Helms RW, O’Branski EE, Ohene-Frempong K, Wang W, Daeschner C, Vichinsky E, Redding-Lallinger R, Gee B, Platt OS. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Blood. 1999;94:1550–1554. [PubMed] [Google Scholar]
- Lanzkron S, Haywood C, Segal JB, Dover GJ. Hospitalization rates and costs of care of patients with sickle - cell anemia in the state of Maryland in the era of hydroxyurea. Am J Hematol. 2006;81:927–932. doi: 10.1002/ajh.20703. [DOI] [PubMed] [Google Scholar]
- Le PQ, Gulbis B, Dedeken L, Dupont S, Vanderfaeillie A, Heijmans C, Huybrechts S, Devalck C, Efira A, Dresse MF, Rozen L, Benghiat FS, Ferster A. Survival among children and adults with sickle cell disease in Belgium: Benefit from hydroxyurea treatment. Pediatric blood & cancer. 2015;62:1956–1961. doi: 10.1002/pbc.25608. [DOI] [PubMed] [Google Scholar]
- McGann PT, Ware RE. Hydroxyurea for sickle cell anemia: What have we learned and what questions still remain? Current opinion in hematology. 2011;18:158–165. doi: 10.1097/MOH.0b013e32834521dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert Opinion on Drug Safety. 2015;14:1749–1758. doi: 10.1517/14740338.2015.1088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RD, Charache S, Terrin ML, Barton FB, Ballas SK. Cost-effectiveness of hydroxyurea in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Am J Hematol. 2000;64:26–31. doi: 10.1002/(sici)1096-8652(200005)64:1<26::aid-ajh5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Mulaku M, Opiyo N, Karumbi J, Kitonyi G, Thoithi G, English M. Evidence review of hydroxyurea for the prevention of sickle cell complications in low-income countries. Arch Dis Child. 2013;98:908–914. doi: 10.1136/archdischild-2012-302387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottage KA, Hankins JS, Smeltzer M, Mzayek F, Wang WC, Aygun B, Gurney JG. Hydroxyurea use and hospitalization trends in a comprehensive pediatric sickle cell program. PLoS One. 2013;8:e72077. doi: 10.1371/journal.pone.0072077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto JA, Brousseau DC, Hillery CA, Scott JP. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer. 2005;44:182–186. doi: 10.1002/pbc.20180. [DOI] [PubMed] [Google Scholar]
- Paulukonis ST, Harris WT, Coates TD, Neumayr L, Treadwell M, Vichinsky E, Feuchtbaum LB. Population based surveillance in sickle cell disease: methods, findings and implications from the California registry and surveillance system in hemoglobinopathies project (RuSH) Pediatric blood & cancer. 2014;61:2271–2276. doi: 10.1002/pbc.25208. [DOI] [PubMed] [Google Scholar]
- Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. New England Journal of Medicine. 2017;376:1561–1573. doi: 10.1056/NEJMra1510865. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, Pahor M, Furberg CD. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47:749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Scott JP, Hillery CA, Brown ER, Misiewicz V, Labotka RJ. Hydroxyurea therapy in children severely affected with sickle cell disease. The Journal of pediatrics. 1996;128:820–828. doi: 10.1016/s0022-3476(96)70335-9. [DOI] [PubMed] [Google Scholar]
- Sobota A, Graham DA, Neufeld EJ, Heeney MM. Thirty-day readmission rates following hospitalization for pediatric sickle cell crisis at freestanding children’s hospitals: Risk factors and hospital variation. Pediatr Blood Cancer. 2012;58:61–65. doi: 10.1002/pbc.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg CD, Calatroni A, Telen M, Kemper AR. Adherence to hydroxyurea therapy in children with sickle cell anemia. The Journal of pediatrics. 2010;156:415–419. doi: 10.1016/j.jpeds.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration. [Accessed: 26 March 2018];FDA approves hydroxyurea for treatment of pediatric patients with sickle cell anemia. 2017 2018 Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm590096.htm. [Google Scholar]
- Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, Odame I, Fuh B, George A, Owen W, Luchtman-Jones L, Rogers ZR, Hilliard L, Gauger C, Piccone C, Lee MT, Kwiatkowski JL, Jackson S, Miller ST, Roberts C, Heeney MM, Kalfa TA, Nelson S, Imran H, Nottage K, Alvarez O, Rhodes M, Thompson AA, Rothman JA, Helton KJ, Roberts D, Coleman J, Bonner MJ, Kutlar A, Patel N, Wood J, Piller L, Wei P, Luden J, Mortier NA, Stuber SE, Luban NL, Cohen AR, Pressel S, Adams RJ. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387:661–670. doi: 10.1016/S0140-6736(15)01041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.