Abstract
In humans and rodent animal models, the brain oxytocin system is paramount for facilitating social bonds, from the formation and consequences of early-life parent-infant bonds to adult pair bond relationships. In social species, oxytocin also mediates the positive effects of healthy social bonds on the partners’ well-being. However, new evidence suggests that the negative consequences of early neglect or partner loss may be mediated by disruptions in the oxytocin system as well. With a focus on oxytocin and its receptor, we review studies from humans and animal models, i.e. mainly from the biparental, socially monogamous prairie vole (Microtus orchogaster), on the beneficial effects of positive social relationships both between offspring and parents and in adult partners. The abundance of social bonds and benevolent social relationships, in general, are associated with protective effects against psycho- and physiopathology not only in the developing infant, but also during adulthood. Furthermore, we discuss the negative effects on well-being, emotionality and behaviour, when these bonds are diminished in quality or are disrupted, for example through parental neglect of the young or the loss of the partner in adulthood. Strikingly, in prairie voles, oxytocinergic signalling plays an important developmental role in the ability to form bonds later in life in the face of early-life neglect, while disruption of oxytocin signalling following partner loss results in the emergence of depressive-like behaviour and physiology. This review demonstrates the translational value of animal models for investigating the oxytocinergic mechanisms that underlie the detrimental effects of developmental parental neglect and pair bond disruption, encouraging future translationally relevant studies on this topic that is so central to our daily lives.
1 Introduction
Social relationships are vital for the well-being of humans. The first and most crucial social relationship in life is developed at birth between the offspring and the parents. The formation of relationships persists throughout life, where new ties are established between individuals and family, friends, co-workers, and partners. Here, the neuropeptide oxytocin (OT) plays an important role in the formation of bonds of many kinds, including those between parents and offspring and between partners. OT is a ring-structured neuropeptide consisting of nine amino acids that is mainly synthesised in the hypothalamic paraventricular (PVN) and supraoptic nucleus (SON) (Burbach, Young, & Russell, 2006; Neumann & Landgraf, 2012). When released within the brain, OT facilitates the formation of pair bonds and friendships as well as the positive effects that result thereof. By contrast, the consequences of disruption or the loss of a bond severely impair the OT system. The subsequent dysfunction of the OT system then results in detrimental physiological as well as psychological effects. This review will briefly summarise the significance of positive social relationships and the negative effects of disrupting the parent-infant relationship or breaking the bond in humans with a focus on the OT system. Moreover, we review the existing literature on studies concerning impaired maternal care and models of neglect. In the case of the neural mechanisms of impairments of paternal care and partner loss, the focus is on studies using the biparental and socially monogamous prairie vole (Microtus orchogaster). We present links between the maladaptive behaviour as well as physical and psychological effects following impaired parental care and partner loss and the dysregulation of the OT system.
2 Positive impact of social relationships in humans
Humans, being naturally social, show a fundamental need to connect with others and gain acceptance within a social group (Ryan & Deci, 2000). The presence of rewarding social relationships is regarded as an important factor in the regulation of health and well-being, exerting a beneficial influence on both physical and emotional health not only in early life but also during adulthood (Holt-Lunstad, Robles, & Sbarra, 2017; House, Landis, & Umberson, 1988; Uchino, 2006; Yang et al., 2016). For example, studies show a positive correlation between the abundance of social relationships and longevity. A multidimensional assessment of the integration in social structures revealed a 91 % increase in the likelihood of survival in more socially integrated individuals (Holt-Lunstad, Smith, & Layton, 2010). Interestingly, not only the quantity but also the perception of possessing ample social connectedness is linked to a lower risk for the development of neurotic symptoms and mental health (Cruwys, Haslam, Dingle, Haslam, & Jetten, 2014; Henderson, 1981; Saeri, Cruwys, Barlow, Stronge, & Sibley, 2017). Studies investigating the effect of relationships between friends, co-workers, and roommates showed a negative correlation between the quality of relationships with susceptibility towards depression and distress (Beach, Martin, Blum, & Roman, 1993; Kenny, Dooley, & Fitzgerald, 2013; Lepore, 1992). Furthermore, the prognosis of personality disorders is positively correlated with the quantity of rewarding interpersonal relationships (Skodol et al., 2007). Qualitatively, family relationships and marriage exert the biggest influence on the human well-being (Glenn & Weaver, 1981; Uchino, Cacioppo, & Kiecolt-Glaser, 1996), partly by buffering against negative events or experiences in life, as do other positive social relationships. Such buffering effects are associated with a higher resilience against stress and the curtailment of the risk for the development of psychiatric or physiological illnesses following adverse events (Cassel, 1976; Cobb, 1976; Cohen & Wills, 1985; Hostinar, Sullivan, & Gunnar, 2014). Social buffering attenuates the cortisol response by dampening the reactivity of the HPA axis and has been shown to elevate the levels of peripheral OT (Grewen, Girdler, Amico, & Light, 2005). Additionally, intranasal administration of OT potentiates the calming effects of social support; these effects are most likely based on a reduction of the stress-induced cortisol levels (Ditzen & Heinrichs, 2014; Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Heinrichs, von Dawans, & Domes, 2009; Olff et al., 2013; Quirin, Kuhl, & Düsing, 2011). In addition to the calming effects of intranasal OT, several other studies investigate its pro-social properties, e.g. in social interactions, enhancement of trust, emotion recognition, and social cognition, by potentially increasing the sensitivity to social cues (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008; Ebstein et al., 2009; Guastella et al., 2010; Kirsch et al., 2005; Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005; Meyer-Lindenberg, 2008; Opar, 2008). Interestingly, the latter not only increases the perception of positive, but also on negative cues, hinting at context-dependent regulation of the perception of social cues by OT (Olff et al., 2013). However, while human studies on intranasal OT reveal interesting and important effects on social behaviour the results should be viewed in the light of their statistical power to ensure the exclusion of false-positives (Walum, Waldman, & Young, 2016).
2.1 The bond between parents and their infants
The first bond in life develops immediately after parturition. This bond between the parents and their child is of utmost importance. Not only is it ensuring the survival of the progeny, but it is also decisive for the child’s cognitive, social and emotional development, thereby influencing physiological and psychological health even later in life (Ammerman, 1991; Fernald & Gunnar, 2009; Insel & Young, 2001; Rilling & Young, 2014). The developing infant is highly responsive to changes in the environment and received maternal care due to the high plasticity of the brain that is still present (Ammerman, 1991; Fernald & Gunnar, 2009; Kolb & Gibb, 2011). For example, sufficient care and sensitivity for the offspring’s needs can prime them for coping with stressful events in adulthood by shaping their reactivity towards stress (Kundakovic & Champagne, 2015; Rutter, 1979; J. Smith & Prior, 1995). Therefore, quality child care, expressed through affection, emotional warmth, empathy, and closeness, lowers the susceptibility for the development of psychiatric disorders such as depression and positively influences the child’s functionality and health later in life (Campbell et al., 2014; Y. L. Liu, 2003; Rilling & Young, 2014). Furthermore, children that receive undisrupted parental care (i.e. parents not separated) show a more secure attachment style in adulthood and report fewer marital problems (Amato & Rogers, 1997; Braithwaite, Doxey, Dowdle, & Fincham, 2016).
2.2 Bonding to a partner in humans
Only up to 5 % of mammalian species live in a monogamous relationship (Cockburn, 2006; Kleiman, 1977). Among primate species, this form of sociality is more abundant with approximately 29 % being socially monogamous (Lukas & Clutton-Brock, 2013). Interestingly, it is speculated that the bond between partners in adulthood originates evolutionarily from the mother-infant bond since both types of bonds rely on overlapping brain areas and neurotransmitter systems (Numan & Young, 2016; Young, 2009; Young & Alexander, 2012).
Marriage has beneficial effects for general well-being in humans as it promotes better physical health and a lower susceptibility to develop long-term illnesses or work disability (Murphy, Glaser, & Grundy, 1997; Waite & Lehrer, 2003). Like the general abundance of social relationships, successful marriages also increase longevity which can be attributed to its beneficial effects on general health (Waite & Gallagher, 2002; Waite & Lehrer, 2003). The formation of a partnership and cohabitation increase the general satisfaction with life. Interestingly, there are no differences in satisfaction between unmarried cohabitating couples and married couples (Zimmermann & Easterlin, 2006).
The formation of relationships and partnerships is governed by social interactions. One important mediator of social behaviours is OT. It has been proposed that OT enhances the perception and salience of social cues, e.g. social recognition, social memory, social perception, and trust (Bartz, Zaki, Bolger, & Ochsner, 2011; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011; Young, 2015). Intranasal administration of OT increases the perception of the partners’ attractiveness compared to the perceived attractiveness of familiar and unfamiliar women and, at the same time, increases the activity of the nucleus accumbens (NAcc) when viewing images of the partner (Scheele et al., 2013), an effect that has been explored in detail in prairie voles (Johnson & Young, 2015).
3 Negative effects of adverse relationships in humans
However, as always in life - the pendulum swings both ways. Antagonistic to the beneficial effects of social relationships lies their maleficial nature when they are troubled or terminated, e.g., through breakup or death. This accounts for detrimental consequences of negative social relationships in work life or between close friends as well as in partnerships and marriages (Curtis, 1995; Steptoe, 1991). In general, stressful relationships or even the loss of a partner are associated with psychiatric conditions and health irregularities. Conflict within the family or between two partners is regarded as being the most influential on health and everyday functioning and has been linked to elevated plasma OT concentrations in women (Taylor, Saphire-Bernstein, & Seeman, 2010). Interestingly, another study (Holt-Lunstad, Birmingham, & Light, 2015) did not report such correlation. This study demonstrated that higher plasma and salivary OT levels are associated with higher relationship quality, not distress. This contradictory finding might be due to differences in the context in which the samples were collected, or also due to differences in controlling for potential covariates, e.g. stress and depression levels (Holt-Lunstad et al., 2015).
3.1 Impaired parent-infant bond impacts on the offspring’s well-being
Impaired parental investment in the offspring is often the result of the separation of the caregivers, either through termination of their relationship or even death of one parent. When single mothers raise their children without the regular presence of the fathers, both quality and quantity of the contact between the fathers and their children are reduced (Amato, 1987). The resulting negative effects of impaired parental care on the offspring’s psychological and physiological health can even last into adulthood. For example, early life adversity is linked to physiological dysregulation, socio-behavioural maladaptation during adulthood, increased susceptibility for the development of psychopathology like depression and post-traumatic stress disorder (PTSD) as well as substance abuse later in life (Agorastos et al., 2014; Felitti et al., 1998; Friedman, Montez, Sheehan, Guenewald, & Seeman, 2015; Heim & Binder, 2012; Heim et al., 2002; Nusslock & Miller, 2016).
In general, single parent-raised children show a deterioration of their physical and mental health in comparison to children raised by both parents (Scharte, Bolte, & Group, 2013). Also, single parent-raised adults, or those raised by families with family conflict, either inter-parental or parent-child, report lower life satisfaction with a worse marital quality, a higher risk for the development of depression, internalising or externalising problems, and lower socio-economic status indicated by lower education and income (Buss et al., 2017; Cherlin, Chase-Lansdale, & McRae, 1998; Cummings, George, McCoy, & Davies, 2012; Fergusson, McLeod, & John Horwood, 2014). Furthermore, lower levels of education, a higher marital divorce rate and lower quality of relationships with their parents are also observed in grandchildren of divorced parents, thereby hinting at long-lasting concomitants of marital dissolution (Amato & Cheadle, 2005; Buss et al., 2017). The impact on child development of divorce and the factors that precede divorce itself, like inter-parental conflict can have impact on the child’s well-being not only later in life but are also present before the dissolution of the marital bond (Amato & Anthony, 2014). Adding to the effect of divorce on the child’s well-being are factors that mediate the outcomes of aforesaid event, such as positive parenting by the single mother, child IQ, pre-divorce family income, mental health status of the mother following divorce, and risk factors such as genetic predispositions or (prenatal) epigenetic changes (Mitchell, Schneper, & Notterman, 2016; C. Monk, Spicer, & Champagne, 2012; Nestler, Pena, Kundakovic, Mitchell, & Akbarian, 2016; Provencal & Binder, 2015; Wang, Davis, Wootton, Mottershaw, & Haworth, 2017; Weaver & Schofield, 2015; Weder et al., 2014; Yang et al., 2016).
Regarding brain neuroendocrinology, negative early life experiences have the power to modulate the OT system, even lasting into adulthood. A history of maltreatment during childhood negatively influences the cerebrospinal fluid OT concentrations in adult women and substantially decreases OT levels in the plasma of adult men (Heim et al., 2009; Opacka-Juffry & Mohiyeddini, 2012). Moreover, parental separation influences the sensitivity to intra-nasally administered OT; the decrease of plasma cortisol following OT administration is attenuated in men that experienced parental separation compared to control individuals (Meinlschmidt & Heim, 2007).
3.2 Physiological and psychological consequences of separation from the partner
In contrast to the beneficial effects on physical and psychological health arising from social kinships, social isolation or even the loss of the bonded partner are associated with a deterioration of both physical (e.g. immune system disruption and cardiovascular diseases) and mental health (Barger, 2013; Cacioppo & Hawkley, 2003; Carey et al., 2014; Gerra et al., 2003; Goforth et al., 2009; Holt-Lunstad & Smith, 2016; Shear & Shair, 2005; Uchino, 2006). Interestingly, even though divorce or separation from a partner exerts a negative influence on the health and survival of individuals, never having been married is even more closely linked to the development of poor health conditions (Kaplan & Kronick, 2006). With respect to mental health, never having been married has similar effects as being married, with divorce/separation inducing the most negative influence (Afifi, Cox, & Enns, 2006). The unexpected parting of the spouse is oftentimes being referred to as the most traumatic experience in one’s life (Keyes et al., 2014). The loss of the partner or bereavement causes acute grief lasting up to 6 months and is not regarded as being harmful to the individual’s health in the long-term (Shear et al., 2011). However, in roughly 10–20 % of bereaved people, the manifestation of complicated grief is observed (Prigerson et al., 2009; Shear, 2015). In contrast to acute grief, complicated grief persists for a prolonged period, marked by the disability to show normal functioning in everyday life, social relationships and work (Boelen & Prigerson, 2007; T. H. Monk, Houck, & Shear, 2006; Simon et al., 2007). Complicated grief can also be accompanied by an increase in the consumption of alcohol and nicotine, an elevated risk for the development of cardiovascular diseases, sleep disturbances, psychosomatic and psychiatric disorders, as well as an elevated suicidal risk (Buckley et al., 2012; Latham & Prigerson, 2004; Shear, 2015). The observed psychiatric outcomes following the breakup of a romantic relationship or even the death of a loved one range from a heightened risk for the 1st onset of a major depressive disorder, to PTSD, and anxiety disorders (Keyes et al., 2014; Monroe, Rohde, Seeley, & Lewinsohn, 1999; Zisook, Chentsova-Dutton, & Shuchter, 1998). Interestingly, these disorders have been described as being modulated by the OT system (Dodhia et al., 2014; Heim et al., 2009; Labuschagne et al., 2010; Ozsoy, Esel, & Kula, 2009; Parker et al., 2010) (for a review on the relationship between OT and stress-related disorders, see (Sippel et al., 2017)). One brain area that has been associated with changes during complicated grief in humans is the NAcc. When questioned for the self-reported yearning for the deceased, higher NAcc activity correlates with the amount of longing, independent of the time that has passed since the death (O’Connor et al., 2008). This might indirectly link the psychological and physiological maladaptation observed in complicated grief to animal models, as the NAcc has also been linked to dysregulations of the OT-ergic signalling following the disruption of a pair bond in animal models (see following section).
4 Prairie voles as a model organism to understand the neuroendocrine basis of social relationships
The ability to examine the underlying mechanisms of social relationships or of the consequences of their disruptions experimentally is limited in humans. However, supportive social relationships are advantageous for the development and well-being in social animals, too. Therefore, research has focused on animal models that exhibit the formation of a pair bond and/or biparental care to explore the underlying neurobiological mechanisms contributing to the adverse effects of bond disruption or neglect. In that context, the behavioural and neuroendocrine factors associated with social bonding in male and female prairie voles have been investigated extensively in the past decades. The first study to describe the prairie vole as a monogamous species characterised the mating system in laboratory settings (Thomas & Birney, 1979). Follow-up studies examined the social structures of wild prairie voles using multiple-capture live-trap data (Getz, Carter, & Gavish, 1981). Strikingly, previously caught male-female pairs were repeatedly re-caught together, independent of the breeding season, thereby indicating the existence of pair bonds and social monogamy in prairie voles. Because prairie voles are highly affiliative, form enduring social bonds between both sexes, and provide biparental care towards the offspring, they are a valuable model organism for examining social monogamy and to investigate the neuronal pathways involved in the formation of a partner preference (Johnson & Young, 2015; Lieberwirth & Wang, 2016; McGraw & Young, 2010; Young & Wang, 2004).
4.1 Molecular underpinnings of the formation of a pair bond in prairie voles
Monogamous behaviour in prairie voles, including the formation of a pair bond, is associated with the brain neuropeptides OT and arginine vasopressin (AVP), both being also involved in the development of mother-infant bonds and the onset of maternal behaviour (Bosch & Neumann, 2012; Numan & Young, 2016; Young, 2003), as well as in reproductive behaviour (Donaldson & Young, 2008). For a detailed review of the OT/AVP neural network and its implications in social behaviour, see (Johnson & Young, 2017).
In male prairie voles, AVP-ergic signalling in the ventral pallidum (VP) facilitates the formation of a partner preference (Barrett et al., 2013; Donaldson, Spiegel, & Young, 2010; Lim & Young, 2004; Young & Wang, 2004). In addition to AVP, the brain OT system is significantly involved in the formation of pair bonds. Centrally administered OT or endogenous OT signalling facilitates the formation of pair bonds in female (Williams, Insel, Harbaugh, & Carter, 1994) and male prairie voles (Cho, DeVries, Williams, & Carter, 1999; Johnson et al., 2016). Furthermore, the expression of OT receptors (OTR) within the caudate putamen (CP) and the NAcc is higher in the monogamous prairie vole compared to the non-monogamous montane vole (Microtus montanus) (Young & Wang, 2004). In prairie voles, blockade of OTR by infusion of a receptor-specific antagonist locally into the prefrontal cortex (PFC) or the NAcc impairs partner preference formation (Johnson et al., 2016; Young, Lim, Gingrich, & Insel, 2001). In confirmation, silencing the expression of OTR within the NAcc by RNAi knockdown inhibits social attachment (Keebaugh, Barrett, Laprairie, Jenkins, & Young, 2015), whereas selective overexpression of OTR accelerates partner preference formation in female prairie voles (Keebaugh & Young, 2011; Ross et al., 2009). Furthermore, male prairie voles with a genetic polymorphism in the OTR gene that results in lower OTR density in the NAcc show an impairment in social bond formation (King, Walum, Inoue, Eyrich, & Young, 2016). Also, OT is thought to interact with dopamine to link the neural encoding of the partner with the reward system (Young & Wang, 2004). More recent electrophysiological and optogenetic studies have demonstrated that dynamic communication between the PFC and the NAcc is critical for partner preference formation, and OT signalling may influence the strength of the communication and the latency to display huddling behaviour (Amadei et al., 2017).
4.2 Importance of positive early life social experience
The first and undoubtedly one of the most important social relationships in life is established between the newborn and the mother. The quality and quantity of care provided by the mother is important for the current physical well-being of the offspring (Bosch & Neumann, 2012). Furthermore, parental nurturing is of significance for the offspring’s gene expression on an epigenetic basis and, consequently, for their social- and non-social behaviour in adulthood as well as their response to fearful stimuli, as has been extensively studied in rats (Caldji, Diorio, Anisman, & Meaney, 2004; Parent, Del Corpo, Cameron, & Meaney, 2013; Perkeybile, Griffin, & Bales, 2013). For example, increased levels of maternal care can be induced by early handling of the offspring and the subsequent reunion with their mother (D. Liu et al., 1997; Pryce, Bettschen, & Feldon, 2001). Such treatment results in increased nursing behaviour by handled females towards their pups in adulthood compared to mothers that were raised without early handling (Fleming, O’Day, & Kraemer, 1999; Francis & Meaney, 1999; D. Liu et al., 1997; Pryce et al., 2001). Maternal care decisively impacts the development of the OT system of the offspring thereby modulating their own behavioural phenotype in adulthood, including maternal behaviour (Champagne, 2011; Champagne & Meaney, 2007). For example, high levels of pup-directed licking and grooming are associated with higher OTR levels in the hypothalamus and the medial preoptic area (MPOA) of the offspring at maturity, which in turn leads to high licking and grooming behaviour in the pups as they become mothers (Champagne, Diorio, Sharma, & Meaney, 2001; Francis, Champagne, & Meaney, 2000; Francis, Diorio, Liu, & Meaney, 1999).
More recently, studies aimed to investigate specifically the role of biparental care, mirroring the human parenting strategy. Biparental care is thought to be present in species where there is a high probability that the father is caring for his own offspring or the offspring of a close relative (Lukas & Clutton-Brock, 2013; Thomas & Birney, 1979). Interestingly, monogamy does not exclusively coincide with biparental care as in only 59 % of the investigated monogamous mammals both parents contribute care towards the offspring (Lukas & Clutton-Brock, 2013). In many cases though, the offspring profit from being raised by both parents as the quantity of parental care is higher and, hence, this has beneficial effects on the offspring’s well-being (Ahern & Young, 2009). Therefore, this aides the development of the offspring, either directly by increased licking and grooming or indirectly by defending the territory and the nest (Oliveras & Novak, 1986).
4.3 Impaired parent-offspring interactions have long-lasting effects on the young
The early life period is very sensitive and susceptible not only to positive but also to negative influences from outside as the brain still has a high degree of plasticity (Kolb & Gibb, 2011). Therefore, various kinds of disturbances within this sensitive time effectively influence the development of the brain and consequently behaviour.
The experience of impaired parental care can induce a multitude of behavioural changes in the offspring. Among those is an increase in the response to fearful stimuli, a dysregulation of the stress reactivity, as well as impairments in social interactions (Chen & Baram, 2016; Sanchez, 2006; Sanchez, Ladd, & Plotsky, 2001). However, differences between the sexes as well as the period during which adverse life conditions are encountered can be decisive for its impact on health later in life (Curley & Champagne, 2016; Lehmann, Pryce, Bettschen, & Feldon, 1999).
For example, in rats, impairments in, or lower levels of, maternal care are associated with lower maternal nurturing behaviour towards pups in the F1 generation (Champagne & Meaney, 2001; Francis, Caldji, Champagne, Plotsky, & Meaney, 1999; Lomanowska, Boivin, Hertzman, & Fleming, 2017). This effect persists even into the F2 generation (Francis, Diorio, et al., 1999). Such differences in the natural variation of maternal care are mimicked under laboratory conditions by maternal separation and/or artificial rearing (Bosch & Neumann, 2012; Champagne & Meaney, 2007; Lovic, Gonzalez, & Fleming, 2001). Aversive rearing conditions induce alterations of the stress response (Murgatroyd et al., 2009; Wigger & Neumann, 1999), modulate anxiety-like behaviour (Ishikawa, Nishimura, & Ishikawa, 2015), cause cognitive and social impairments in adulthood (Aisa, Tordera, Lasheras, Del Rio, & Ramirez, 2007; Haller, Harold, Sandi, & Neumann, 2014), and even induce lower pup-directed maternal care in adulthood of artificially reared pups (Gonzalez & Fleming, 2002; Gonzalez, Lovic, Ward, Wainwright, & Fleming, 2001; Lovic et al., 2001) (but also see (Zhang et al., 2014)). Strikingly, deficits in maternal nurturing behaviour are linked to a dysregulation of the brain OT signalling (Jin et al., 2007; Takayanagi et al., 2005); the offspring’s OTR density in the MPOA is decreased when receiving less maternal care (Champagne et al., 2001).
Similar to those findings, in prairie voles, the disruption of the postnatal rearing period or post-weaning isolation are linked to changes in gene expression, neural pathways, and social behaviour (Ahern, Hammock, & Young, 2011; Ahern & Young, 2009; Barrett, Arambula, & Young, 2015; Pan, Liu, Young, Zhang, & Wang, 2009; Tabbaa, Lei, Liu, & Wang, 2017). Moreover, prairie voles are biparental (Thomas & Birney, 1979; Young & Wang, 2004), which paved the way for studies investigating the effects of single mother- versus biparental-rearing on the offspring. The absence of the father results in being less licked and groomed and increases the time in which the pups are left unattended, especially since deserted mothers do not compensate for the loss of the father (Ahern et al., 2011; Ahern & Young, 2009; McGuire, Parker, & Bemis, 2007; Tabbaa et al., 2017). Furthermore, paternally deprived pups mature slower in comparison to biparentally reared ones (Ahern et al., 2011; Ahern & Young, 2009). Consequently, paternal deprivation causes long-lasting behavioural changes; the single mother-reared prairie voles show impaired alloparental and socio-sexual behaviour, marked by the manifestation of delayed partner preference formation (Ahern et al., 2011; Ahern & Young, 2009). Interestingly, investigation of the brain OT system reveals sex-specific differences following single mother-rearing. Female, but not male, offspring reared by the mother alone have higher OT mRNA expression in the PVN compared to biparentally-reared conspecifics (Ahern & Young, 2009) (but also see (Tabbaa et al., 2017)). However, in the socially monogamous and biparental mandarin voles (Lasiopodomys mandarinus) single mother-rearing and parental separation induce lower social interaction and higher anxiety-related behaviours in adulthood (Jia, Tai, An, Zhang, & Broders, 2009).
In addition, female prairie voles that were socially isolated for 3 hours per day during the neonatal period exhibit impairments in the formation of a partner preference in adulthood (Barrett et al., 2015). This study further hints at the interplay between the susceptibility to malignant early life experiences and the natural variation of OTR density in the NAcc (Barrett et al., 2015). Juvenile females that naturally express a high NAcc OTR density seem to be resilient against early life adversity as they spend more time in contact with their partner at adulthood compared to females that have a low OTR density. These findings suggest that in those pups the OT release stimulated by licking and grooming following the reunion with the parents has important developmental consequences such as the ability to form relationships in adulthood (Barrett et al., 2015; Rilling & Young, 2014). Interestingly, the natural variation in OTR density in the NAcc that determines susceptibility or resilience to early-life neglect is not determined by epigenetic factors induced by experience as is the case in rats, but is genetically determined by polymorphisms in the OTR gene (King et al., 2016). Importantly, the induction of endogenous OT release by pharmacological intervention during the neonatal period buffers against the negative effects of early isolation on partner preference formation (Barrett et al., 2015).
4.4 Positive impact of persisting social relationships in adult prairie voles
Comparable to the beneficial properties of social support in humans (see Section 2), prairie voles also show lower anxiety-related and depressive-like behaviour following a stressor in the presence of the partner, termed social buffering (Smith & Wang, 2014). Briefly, female prairie voles (demonstrator) that are subjected to immobilisation stress show increased stress-related behaviours such as route tracing and repetitive auto grooming but only when recovering alone (Smith & Wang, 2014). In contrast, when the stressed females are returned to their male partners (observer) these stress-related behaviours are absent. In addition, the males display increased female-directed social behaviour interpreted as consoling behaviour. Interestingly, the soothing effect of the male partner’s presence on the stressed female is mediated by the brain OT system. Females that recover with their partner have a substantial increase of OT release within the PVN compared to females that recover alone. Importantly, in the latter, OT microinjection into the PVN could normalise their stress-related behaviours to a similar level as in females with social buffering (Smith & Wang, 2014).
When switching perspectives, the consoling behaviour together with the parallel processes in the brain of the unstressed observer is also fascinating (Burkett et al., 2016). After the stressed (mild foot shock) demonstrator is placed back in the home cage, the observing prairie vole displays increased grooming towards the stressed pair bonded partner, but not towards a stranger. Furthermore, the stress-induced heightened levels of anxiety-related behaviour are diminished when receiving consolation from the bonded partner. This consoling behaviour is mediated by OT-ergic signalling in the anterior cingulate cortex (ACC), a brain region linked to empathy in humans (Lamm, Decety, & Singer, 2011). Validating the previous findings, subsequent infusion of an OTR antagonist locally into the ACC blocks stress-induced consolation behaviour in the observing partner (Burkett et al., 2016).
4.5 Physiological and psychological consequences of pair bond disruption in adult prairie voles
In the past years, studies characterised the consequences of social isolation in general and the loss of a bonded partner more specifically on behavioural, physiological, and molecular levels. Social isolation from same-sex individuals evokes gender-specific responses (Bosch et al., 2016; Grippo, Lamb, Carter, & Porges, 2007; Grippo, Wu, Hassan, & Carter, 2008). In female voles, separation from other females causes depressive-like symptoms (Grippo et al., 2011; Grippo, Cushing, & Carter, 2007; Grippo, Gerena, et al., 2007), whereas such an emotional consequence is absent in males separated from other males (Bosch et al., 2016; Bosch, Nair, Ahern, Neumann, & Young, 2009). In contrast to same-sex isolation, the sudden disruption of opposite-sex pair bonds provokes an increase in behaviours associated with psychological maladies (Bosch et al., 2016; Bosch et al., 2009; Bosch & Young, 2017; McNeal et al., 2014; Sun, Smith, Lei, Liu, & Wang, 2014; Tabbaa, Paedae, Liu, & Wang, 2016). Both separated male and female prairie voles display increased passive stress-coping behaviour, reminiscent of depressive-like behaviour (Cryan & Slattery, 2007), as well as increased anxiety-related behaviour (Bosch et al., 2009; McNeal et al., 2014; Sun et al., 2014). These maladaptations are even further pronounced when the separated prairie vole is exposed to chronic mild stress (McNeal et al., 2017) probably due to the absence of the consoling behaviour of the partner (Burkett et al., 2016). On a physiological level, the cardiovascular system becomes dysregulated (McNeal et al., 2014); losing the partner induces an upregulation of the heart rate and downregulation of its variability. In addition, the sympathetic drive increases, whereas the parasympathetic innervation of the heart is downregulated (McNeal et al., 2014). Furthermore, basal plasma adrenocorticotropic hormone and corticosterone levels are upregulated and the adrenal weight is increased hinting to a chronically activated stress axis (Bosch et al., 2009; McNeal et al., 2014; Sun et al., 2014). In line with that, blocking the corticotropin-releasing factor (CRF) receptors centrally normalises the emotionality of the separated males (Bosch et al., 2009). Interestingly, CRF mRNA expression in the bed nucleus of the stria terminalis is increased in male prairie voles separated from the female partner but not when isolated from a male cage mate (Bosch et al., 2009). Surprisingly, CRF mRNA is also upregulated in the PVN of males that are still with the female partner; this might indicate that the CRF system is not active but primed for the event of separation (Bosch & Young, 2017). It is hypothesised that this constellation guarantees that the CRF system is able to swiftly respond to the separation, thereby causing all of the above-mentioned negative changes and, hence, ensuring the maintenance of the pair bond possibly by motivating the partners to actively seek the presence of each other in order to reduce the separation-induced stress reaction.
4.6 The impact of partner loss on the dysregulation of the brain OT system
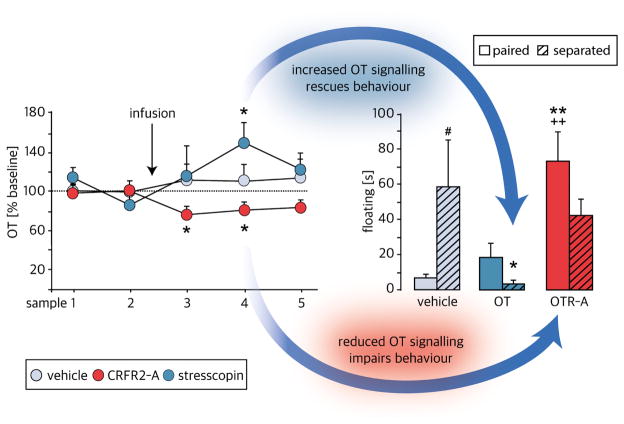
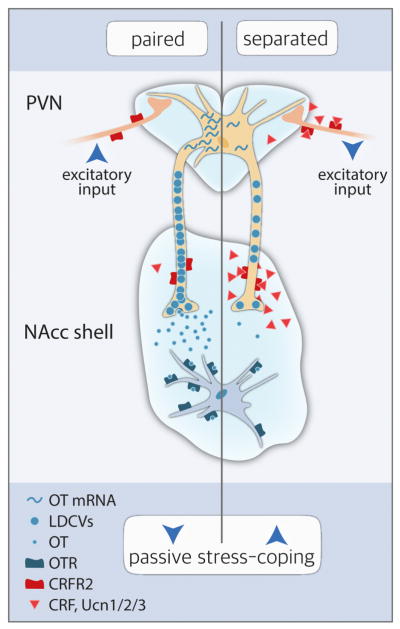
The formation of a pair bond relies on the activity of the brain OT system in the NAcc (see Section 4.1). However, separation of both partners induces impairments of the OT signalling on multiple levels, partly through an activation of CRF receptor type 2 (CRFR2) (Bosch et al., 2016). Upon short-term separation from the female bonded partner for five days, the expression of OT mRNA in the PVN, but not SON, is significantly reduced (Bosch et al., 2016). Consistent with this finding, the immunoreactivity for OT positive cells in the PVN is increased following a long-term separation of four weeks, which is interpreted to reflect a decrease of release and receptor activity in respective areas (Sun et al., 2014). Moreover, the OT neurones from the PVN represent the main source for OT released within the NAcc with approximately 90 % of the innervations of OT fibres arising from the PVN (Bosch et al., 2016). This highlights the PVN’s significance for OT signalling within the NAcc. Importantly, these OT fibres express CRFR2 (Bosch et al., 2016). When activating CRFR2 via acute central infusion of stresscopin (i.e. urocortin 3) the local release of OT within the NAcc decreases (Fig. 1). On the other hand, reduced CRFR2 signalling via acute central blockade with astressin-2b increases OT release locally in the NAcc (Bosch et al., 2016). Importantly, chronic infusion of OT into the NAcc reverses passive stress-coping in males separated from their partner (Fig. 1). Furthermore, and as proof of concept, chronic inhibition of OTR via local infusion of an antagonist (Bosch et al., 2016) or downregulation of OTR expression by 60 % via shRNA (Keebaugh et al., 2015) induces passive stress-coping in non-separated males (Bosch et al., 2016). Moreover, electrophysiological measurements of the excitability of OT neurons in the PVN following the central infusion of stresscopin revealed that activation of CRFR2 is decreasing the frequencies of sEPSCs and, thereby, presynaptically regulating the excitability of PVN OT neurons by downregulating glutamatergic transmission (Bosch et al., 2016). Hence, short-term separation leads to increased CRFR2 activation thereby significantly impairing OT signalling within the NAcc (Fig. 2). In addition, the OTR density within the NAcc is decreased after short-term separation (Bosch et al., 2016). Thus, these findings not only show that breaking the pair bond has strong emotional and physical effects on the prairie voles but, furthermore, also indicate that healthy OT signalling is important for the maintenance of the pair bond.
Figure 1. Local OT release within the NAcc shell of male prairie voles is affected by central CRFR2 manipulations.
Blockade of CRFR2 by astressin-2B (CRFR2 antagonist) decreases the release of OT, whereas activation of CRFR2 by stresscopin (CRFR2 agonist) increases OT release. In continuation, such CRFR2-evoked (de-) activation of OT signalling in the NAcc shell has similar effects on passive-stress coping as local OTR manipulation in the NAcc shell. In detail, synthetic OT rescues the increased passive stress-coping after separation whereas the OTR antagonist increases passive stress-coping in non-separated males.
Figure 2. Scheme of the influence of CRFR2 on OT signalling from the PVN to the NAcc shell under paired conditions as well as following the loss of the partner.
Separation from the pair bonded partner induces the release of CRFR ligands, which bind to CRFR2 on glutamatergic neurons, thereby inhibiting their activity. The resulting decreased excitability of OT neurons in the PVN causes decreased OT mRNA expression in neurones projecting to the NAcc shell. Subsequently, reduced OT mRNA expression may cause reduced OT-carrying large dense-core vesicles (LDCVs), thereby leading to impaired OT release within the NAcc shell. Furthermore, activation of CRFR2 on the terminals of OT neurons in the NAcc causes a decrease of OT release within the NAcc. In addition, OTR density within the NAcc shell is decreased following separation from the partner. Hence, loss of the partner results in impaired OT signalling from the PVN to the NAcc shell on multiple levels due to heightened brain CRFR2 activation.
5 Conclusions
In this review, we discussed the importance of social relationships for the individuals’ well-being partly by examining the negative outcomes when subjects are separated from a loved one. Intact social relationships are accompanied by increased brain OT signalling, which helps to buffer against adverse life experiences and to promote lower susceptibility to the development of psychiatric conditions and health deterioration. However, broken social relationships, e.g. neglect early in life or through the loss of a partner, can induce negative long-lasting effects on both physiological and psychological health. This is mediated at least partly by an impaired OT signalling. Treatments targeting the brain OT system might be a powerful tool to counteract the maladaptation following such adverse events, nevertheless trials that shed light on the long-term effects of chronic treatment with OT have not yet been conducted in humans as seen in animal models (Bales et al., 2013; Bales et al., 2014). However, animal models have demonstrated that stimulating the OT system has the potential to reverse the deleterious effects of maternal separation and/or paternal deprivation as well as of the outcomes following pair bond disruption. Furthermore, prairie voles are a well-suited model organism for the investigation of the deleterious effects of social neglect (Bosch & Young, 2017; Tabbaa et al., 2016). Therefore, forthcoming studies investigating the role of OT in social relationships as well as the effects on OT when being disrupted are valuable for translational implications to understand and - at most - to reverse the resulting adverse effects. Future studies involving manipulation of the OT system either directly via intranasal OT or indirectly by pharmacologically evoking OT release via drugs targeting systems such as the melanocortin system (Modi et al., 2015; Young & Barrett, 2015) may have important translational applications for psychiatric and physiological consequences of neglect and social loss.
Highlights.
The brain oxytocin system facilitates social bonds
Disruption of bonds negatively impacts on oxytocin system
We review the resulting physiological and emotional consequences
We reveal the translational value of animal models for investigating oxytocinergic mechanisms
Acknowledgments
The authors would like to acknowledge support from the Deutsche Forschungsgemeinschaft Grant DFG GRK 2174/1 to OJB, NIH Grants R01MH096983, and 1P50MH100023 to LJY and P51-OD011132-55 to YNPRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afifi TO, Cox BJ, Enns MW. Mental health profiles among married, never-married, and separated/divorced mothers in a nationally representative sample. Soc Psychiatry Psychiatr Epidemiol. 2006;41(2):122–129. doi: 10.1007/s00127-005-0005-3. [DOI] [PubMed] [Google Scholar]
- Agorastos A, Pittman JO, Angkaw AC, Nievergelt CM, Hansen CJ, Aversa LH, Parisi SA, Barkauskas DA, Baker DG The Marine Resiliency Study Team. The cumulative effect of different childhood trauma types on self-reported symptoms of adult male depression and PTSD, substance abuse and health-related quality of life in a large active-duty military cohort. J Psychiatr Res. 2014;58:46–54. doi: 10.1016/j.jpsychires.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Ahern TH, Hammock EA, Young LJ. Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster) Dev Psychobiol. 2011;53(2):118–131. doi: 10.1002/dev.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32(3):256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Jun Kwon Y, Shpiner AC, Saravanan V, Mays WD, … Liu RC. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature. 2017;546(7657):297–301. doi: 10.1038/nature22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato PR. Family Processes in One-Parent, Stepparent, and Intact Families: The Child’s Point of View. Journal of Marriage and the Family. 1987;49(2):327–337. doi: 10.2307/352303. [DOI] [Google Scholar]
- Amato PR, Anthony CJ. Estimating the Effects of Parental Divorce and Death With Fixed Effects Models. Journal of Marriage and Family. 2014;76(2):370–386. doi: 10.1111/jomf.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato PR, Cheadle J. The long reach of divorce: Divorce and child well-being across three generations. Journal of Marriage and Family. 2005;67(1):191–206. doi: 10.1111/j.0022-2445.2005.00014.x. [DOI] [Google Scholar]
- Amato PR, Rogers SJ. A longitudinal study of marital problems and subsequent divorce. Journal of Marriage and the Family. 1997;59(3):612–624. doi: 10.2307/353949. [DOI] [Google Scholar]
- Ammerman RT. The role of the child in physical abuse: a reappraisal. Violence Vict. 1991;6(2):87–101. [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, … Mendoza SP. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74(3):180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, … Mendoza SP. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger SD. Social integration, social support and mortality in the US National Health Interview Survey. Psychosomatic Medicine. 2013;75(5):510–517. doi: 10.1097/PSY.0b013e318292ad99. [DOI] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry. 2015;5:e606. doi: 10.1038/tp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav. 2013;63(3):518–526. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Beach SRH, Martin JK, Blum TC, Roman PM. Effects of marital and co-worker relationships on negative affect: Testing the central role of marriage. The American Journal of Family Therapy. 1993;21(4):313–323. doi: 10.1080/01926189308251002. [DOI] [Google Scholar]
- Boelen PA, Prigerson HG. The influence of symptoms of prolonged grief disorder, depression, and anxiety on quality of life among bereaved adults: a prospective study. Eur Arch Psychiatry Clin Neurosci. 2007;257(8):444–452. doi: 10.1007/s00406-007-0744-0. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, … Young LJ. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology. 2016;64:66–78. doi: 10.1016/j.psyneuen.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34(6):1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61(3):293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Young LJ. Oxytocin and Social Relationships: From Attachment to Bond Disruption. Curr Top Behav Neurosci. 2017 doi: 10.1007/7854_2017_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SR, Doxey RA, Dowdle KK, Fincham FD. The Unique Influences of Parental Divorce and Parental Conflict on Emerging Adults in Romantic Relationships. Journal of Adult Development. 2016;23(4):214–225. doi: 10.1007/s10804-016-9237-6. [DOI] [Google Scholar]
- Buckley T, Sunari D, Marshall A, Bartrop R, McKinley S, Tofler G. Physiological correlates of bereavement and the impact of bereavement interventions. Dialogues Clin Neurosci. 2012;14(2):129–139. doi: 10.31887/DCNS.2012.14.2/tbuckley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach JPH, Young LJ, Russell JA. Oxytocin: Synthesis, Secretion, and Reproductive Functions. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3. Vol. 2. St. Louis: Gulf Professional Publishing; 2006. pp. 3055–3128. [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351(6271):375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, … Wadhwa PD. Intergenerational Transmission of Maternal Childhood Maltreatment Exposure: Implications for Fetal Brain Development. J Am Acad Child Adolesc Psychiatry. 2017;56(5):373–382. doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46(3 Suppl):S39–52. [PubMed] [Google Scholar]
- Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29(7):1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y. Early childhood investments substantially boost adult health. Science. 2014;343(6178):1478–1485. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey IM, Shah SM, DeWilde S, Harris T, Victor CR, Cook DG. Increased risk of acute cardiovascular events after partner bereavement: a matched cohort study. JAMA Intern Med. 2014;174(4):598–605. doi: 10.1001/jamainternmed.2013.14558. [DOI] [PubMed] [Google Scholar]
- Cassel J. The contribution of the social environment to host resistance: the Fourth Wade Hampton Frost Lecture. Am J Epidemiol. 1976;104(2):107–123. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Maternal imprints and the origins of variation. Horm Behav. 2011;60(1):4–11. doi: 10.1016/j.yhbeh.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121(6):1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Chen Y, Baram TZ. Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacology. 2016;41(1):197–206. doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherlin AJ, Chase-Lansdale PL, McRae C. Effects of parental divorce on mental health throughout the life course. American Sociological Review. 1998;63(2):239–249. doi: 10.2307/2657325. [DOI] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cobb S. Social Support as a Moderator of Life Stress. Psychosomatic Medicine. 1976;38(5):300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- Cockburn A. Prevalence of different modes of parental care in birds. Proc Biol Sci. 2006;273(1592):1375–1383. doi: 10.1098/rspb.2005.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- Cruwys T, Haslam SA, Dingle GA, Haslam C, Jetten J. Depression and Social Identity: An Integrative Review. Pers Soc Psychol Rev. 2014;18(3):215–238. doi: 10.1177/1088868314523839. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Curr Opin Psychiatry. 2007;20(1):1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Cummings EM, George MR, McCoy KP, Davies PT. Interparental conflict in kindergarten and adolescent adjustment: prospective investigation of emotional security as an explanatory mechanism. Child Dev. 2012;83(5):1703–1715. doi: 10.1111/j.1467-8624.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Champagne FA. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front Neuroendocrinol. 2016;40:52–66. doi: 10.1016/j.yfrne.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R. Stress, personality and illness: The move from generality to specificity in current research trends. Irish Journal of Psychology. 1995;16(4):299–321. doi: 10.1080/03033910.1995.10558067. [DOI] [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci. 2014;32(1):149–162. doi: 10.3233/RNN-139008. [DOI] [PubMed] [Google Scholar]
- Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology. 2014;39(9):2061–2069. doi: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124(1):159–163. doi: 10.1037/a0018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I, … Yirmiya N. Arginine vasopressin and oxytocin modulate human social behavior. Ann N Y Acad Sci. 2009;1167:87–102. doi: 10.1111/j.1749-6632.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, McLeod GF, John Horwood L. Parental separation/divorce in childhood and partnership outcomes at age 30. J Child Psychol Psychiatry. 2014;55(4):352–360. doi: 10.1111/jcpp.12107. [DOI] [PubMed] [Google Scholar]
- Fernald LC, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Soc Sci Med. 2009;68(12):2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, O’Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23(5):673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999;46(9):1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12(12):1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr Opin Neurobiol. 1999;9(1):128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Montez JK, Sheehan CM, Guenewald TL, Seeman TE. Childhood Adversities and Adult Cardiometabolic Health: Does the Quantity, Timing, and Type of Adversity Matter? J Aging Health. 2015;27(8):1311–1338. doi: 10.1177/0898264315580122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Monti D, Panerai AE, Sacerdote P, Anderlini R, Avanzini P, … Franceschi C. Long-term immune-endocrine effects of bereavement: relationships with anxiety levels and mood. Psychiatry Res. 2003;121(2):145–158. doi: 10.1016/s0165-1781(03)00255-5. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS, Gavish L. The Mating System of the Prairie Vole, Microtus-Ochrogaster - Field and Laboratory Evidence for Pair-Bonding. Behavioral Ecology and Sociobiology. 1981;8(3):189–194. doi: 10.1007/Bf00299829. [DOI] [Google Scholar]
- Glenn ND, Weaver CN. The Contribution of Marital Happiness to Global Happiness. Journal of Marriage and the Family. 1981;43(1):161–168. doi: 10.2307/351426. [DOI] [Google Scholar]
- Goforth HW, Lowery J, Cutson TM, McMillan ES, Kenedi C, Cohen MA. Impact of bereavement on progression of AIDS and HIV infection: a review. Psychosomatics. 2009;50(5):433–439. doi: 10.1176/appi.psy.50.5.433. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Fleming AS. Artificial rearing causes changes in maternal behavior and c-fos expression in juvenile female rats. Behav Neurosci. 2002;116(6):999–1013. doi: 10.1037//0735-7044.116.6.999. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Dev Psychobiol. 2001;38(1):11–32. doi: 10.1002/1098-2302(2001)38:1<11::aid-dev2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Carter CS, McNeal N, Chandler DL, Larocca MA, Bates SL, Porges SW. 24-hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: implications for the study of depression and cardiovascular disease. Psychosomatic Medicine. 2011;73(1):59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69(2):149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32(8–10):966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007;62(10):1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25(6):E17–26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Haller J, Harold G, Sandi C, Neumann ID. Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. J Neuroendocrinol. 2014;26(10):724–738. doi: 10.1111/jne.12182. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15(3):117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14(10):954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Henderson S. Social relationships, adversity and neurosis: an analysis of prospective observations. Br J Psychiatry. 1981;138:391–398. doi: 10.1192/bjp.138.5.391. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WC, Light KC. Relationship quality and oxytocin: Influence of stable and modifiable aspects of relationships. Journal of Social and Personal Relationships. 2015;32(4):472–490. doi: 10.1177/0265407514536294. [DOI] [Google Scholar]
- Holt-Lunstad J, Robles TF, Sbarra DA. Advancing social connection as a public health priority in the United States. Am Psychol. 2017;72(6):517–530. doi: 10.1037/amp0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB. Loneliness and social isolation as risk factors for CVD: implications for evidence-based patient care and scientific inquiry. Heart. 2016;102(13):987–989. doi: 10.1136/heartjnl-2015-309242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2(2):129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Nishimura R, Ishikawa A. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur J Neurosci. 2015;41(4):442–453. doi: 10.1111/ejn.12825. [DOI] [PubMed] [Google Scholar]
- Jia R, Tai F, An S, Zhang X, Broders H. Effects of neonatal paternal deprivation or early deprivation on anxiety and social behaviors of the adults in mandarin voles. Behav Processes. 2009;82(3):271–278. doi: 10.1016/j.beproc.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, … Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446(7131):41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, Young LJ. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav. 2016;79:8–17. doi: 10.1016/j.yhbeh.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Curr Opin Behav Sci. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev. 2017;76(Pt A):87–98. doi: 10.1016/j.neubiorev.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RM, Kronick RG. Marital status and longevity in the United States population. J Epidemiol Community Health. 2006;60(9):760–765. doi: 10.1136/jech.2005.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015;10(5):561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60(5):498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny R, Dooley B, Fitzgerald A. Interpersonal relationships and emotional distress in adolescence. J Adolesc. 2013;36(2):351–360. doi: 10.1016/j.adolescence.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Pratt C, Galea S, McLaughlin KA, Koenen KC, Shear MK. The burden of loss: unexpected death of a loved one and psychiatric disorders across the life course in a national study. Am J Psychiatry. 2014;171(8):864–871. doi: 10.1176/appi.ajp.2014.13081132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, Young LJ. Variation in the Oxytocin Receptor Gene Predicts Brain Region-Specific Expression and Social Attachment. Biol Psychiatry. 2016;80(2):160–169. doi: 10.1016/j.biopsych.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, … Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q Rev Biol. 1977;52(1):39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20(4):265–276. [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology. 2015;40(1):141–153. doi: 10.1038/npp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, … Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Latham AE, Prigerson HG. Suicidality and bereavement: complicated grief as psychiatric disorder presenting greatest risk for suicidality. Suicide Life Threat Behav. 2004;34(4):350–362. doi: 10.1521/suli.34.4.350.53737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 1999;64(4):705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Lepore SJ. Social conflict, social support, and psychological distress: evidence of cross-domain buffering effects. J Pers Soc Psychol. 1992;63(5):857–867. doi: 10.1037//0022-3514.63.5.857. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. The neurobiology of pair bond formation, bond disruption, and social buffering. Curr Opin Neurobiol. 2016;40:8–13. doi: 10.1016/j.conb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125(1):35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis DD, Freedman A, … Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu YL. Parent-child interaction and children’s depression: the relationships between Parent-Child interaction and children’s depressive symptoms in Taiwan. Journal of Adolescence. 2003;26(4):447–457. doi: 10.1016/S0140-1971(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Boivin M, Hertzman C, Fleming AS. Parenting begets parenting: A neurobiological perspective on early adversity and the transmission of parenting styles across generations. Neuroscience. 2017;342:120–139. doi: 10.1016/j.neuroscience.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Lovic V, Gonzalez A, Fleming AS. Maternally separated rats show deficits in maternal care in adulthood. Dev Psychobiol. 2001;39(1):19–33. doi: 10.1002/dev.1024. [DOI] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341(6145):526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci. 2010;33(2):103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B, Parker E, Bemis WE. Sex differences, effects of male presence and coordination of nest visits in prairie voles (Microtus ochrogaster) during the immediate postnatal period. American Midland Naturalist. 2007;157(1):187–201. doi: 10.1674/0003-0031(2007)157[187:Sdeomp]2.0.Co;2. [DOI] [Google Scholar]
- McNeal N, Appleton KM, Johnson AK, Scotti ML, Wardwell J, Murphy R, … Grippo AJ. The protective effects of social bonding on behavioral and pituitary-adrenal axis reactivity to chronic mild stress in prairie voles. Stress. 2017;20(2):175–182. doi: 10.1080/10253890.2017.1295444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti MA, Wardwell J, Chandler DL, Bates SL, Larocca M, … Grippo AJ. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton Neurosci. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol Psychiatry. 2007;61(9):1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. Impact of prosocial neuropeptides on human brain function. Prog Brain Res. 2008;170:463–470. doi: 10.1016/S0079-6123(08)00436-6. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Schneper LM, Notterman DA. DNA methylation, early life environment, and health outcomes. Pediatr Res. 2016;79(1–2):212–219. doi: 10.1038/pr.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, Landgraf R, Young LJ. Melanocortin Receptor Agonists Facilitate Oxytocin-Dependent Partner Preference Formation in the Prairie Vole. Neuropsychopharmacology. 2015;40(8):1856–1865. doi: 10.1038/npp.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Dev Psychopathol. 2012;24(4):1361–1376. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Houck PR, Shear MK. The daily life of complicated grief patients--what gets missed,what gets added? Death Stud. 2006;30(1):77–85. doi: 10.1080/07481180500348860. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Rohde P, Seeley JR, Lewinsohn PM. Life events and depression in adolescence: relationship loss as a prospective risk factor for first onset of major depressive disorder. J Abnorm Psychol. 1999;108(4):606–614. doi: 10.1037//0021-843x.108.4.606. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, … Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12(12):1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Murphy M, Glaser K, Grundy E. Marital status and long-term illness in Great Britain. Journal of Marriage and the Family. 1997;59(1):156–164. doi: 10.2307/353669. [DOI] [Google Scholar]
- Nestler EJ, Pena CJ, Kundakovic M, Mitchell A, Akbarian S. Epigenetic Basis of Mental Illness. Neuroscientist. 2016;22(5):447–463. doi: 10.1177/1073858415608147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Miller GE. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol Psychiatry. 2016;80(1):23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain’s reward center. Neuroimage. 2008;42(2):969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, … van Zuiden M. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38(9):1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Oliveras D, Novak M. A Comparison of Paternal Behavior in the Meadow Vole Microtus-Pennsylvanicus, the Pine Vole Microtus-Pinetorum and the Prairie Vole Microtus-Ochrogaster. Animal Behaviour. 1986;34(2):519–526. doi: 10.1016/S0003-3472(86)80120-8. [DOI] [Google Scholar]
- Opacka-Juffry J, Mohiyeddini C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress. 2012;15(1):1–10. doi: 10.3109/10253890.2011.560309. [DOI] [PubMed] [Google Scholar]
- Opar A. Search for potential autism treatments turns to ‘trust hormone’. Nat Med. 2008;14(4):353. doi: 10.1038/nm0408-353. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Esel E, Kula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009;169(3):249–252. doi: 10.1016/j.psychres.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454(1):67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent CI, Del Corpo A, Cameron NM, Meaney MJ. Maternal care associates with play dominance rank among adult female rats. Dev Psychobiol. 2013;55(7):745–756. doi: 10.1002/dev.21070. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010;178(2):359–362. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Griffin LL, Bales KL. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster) Front Behav Neurosci. 2013;7:21. doi: 10.3389/fnbeh.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson HG, Horowitz MJ, Jacobs SC, Parkes CM, Aslan M, Goodkin K, … Maciejewski PK. Prolonged grief disorder: Psychometric validation of criteria proposed for DSM-V and ICD-11. PLoS Med. 2009;6(8):e1000121. doi: 10.1371/journal.pmed.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencal N, Binder EB. The effects of early life stress on the epigenome: From the womb to adulthood and even before. Exp Neurol. 2015;268:10–20. doi: 10.1016/j.expneurol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol. 2001;38(4):239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36(6):898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29(5):1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Protective factors in children’s responses to stress and disadvantage. Ann Acad Med Singapore. 1979;8(3):324–338. [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Saeri AK, Cruwys T, Barlow FK, Stronge S, Sibley CG. Social connectedness improves public mental health: Investigating bidirectional relationships in the New Zealand attitudes and values survey. Aust N Z J Psychiatry. 2017 doi: 10.1177/0004867417723990. 4867417723990. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Scharte M, Bolte G, Group GMES. Increased health risks of children with single mothers: the impact of socio-economic and environmental factors. Eur J Public Health. 2013;23(3):469–475. doi: 10.1093/eurpub/cks062. [DOI] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, … Hurlemann R. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A. 2013;110(50):20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153–160. doi: 10.1056/NEJMcp1315618. [DOI] [PubMed] [Google Scholar]
- Shear MK, Shair H. Attachment, loss, and complicated grief. Dev Psychobiol. 2005;47(3):253–267. doi: 10.1002/dev.20091. [DOI] [PubMed] [Google Scholar]
- Shear MK, Simon N, Wall M, Zisook S, Neimeyer R, Duan N, … Keshaviah A. Complicated grief and related bereavement issues for DSM-5. Depress Anxiety. 2011;28(2):103–117. doi: 10.1002/da.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Shear KM, Thompson EH, Zalta AK, Perlman C, Reynolds CF, … Silowash R. The prevalence and correlates of psychiatric comorbidity in individuals with complicated grief. Compr Psychiatry. 2007;48(5):395–399. doi: 10.1016/j.comppsych.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Sippel LM, Allington CE, Pietrzak RH, Harpaz-Rotem I, Mayes LC, Olff M. Oxytocin and Stress-related Disorders: Neurobiological Mechanisms and Treatment Opportunities. Chronic Stress (Thousand Oaks) 2017;1:247054701668799. doi: 10.1177/2470547016687996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodol AE, Bender DS, Pagano ME, Shea MT, Yen S, Sanislow CA, … Gunderson JG. Positive childhood experiences: resilience and recovery from personality disorder in early adulthood. J Clin Psychiatry. 2007;68(7):1102–1108. doi: 10.4088/jcp.v68n0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76(4):281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Prior M. Temperament and stress resilience in school-age children: a within-families study. J Am Acad Child Adolesc Psychiatry. 1995;34(2):168–179. doi: 10.1097/00004583-199502000-00012. [DOI] [PubMed] [Google Scholar]
- Steptoe A. Invited review. The links between stress and illness. J Psychosom Res. 1991;35(6):633–644. doi: 10.1016/0022-3999(91)90113-3. [DOI] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z. Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav Brain Res. 2014;265:22–31. doi: 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbaa M, Lei K, Liu Y, Wang Z. Paternal deprivation affects social behaviors and neurochemical systems in the offspring of socially monogamous prairie voles. Neuroscience. 2017;343:284–297. doi: 10.1016/j.neuroscience.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbaa M, Paedae B, Liu Y, Wang Z. Neuropeptide Regulation of Social Attachment: The Prairie Vole Model. Compr Physiol. 2016;7(1):81–104. doi: 10.1002/cphy.c150055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, … Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102(44):16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci. 2010;21(1):3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Birney EC. Parental Care and Mating System of the Prairie Vole, Microtus Ochrogaster. Behavioral Ecology and Sociobiology. 1979;5(2):171–186. doi: 10.1007/Bf00293304. [DOI] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29(4):377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119(3):488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Waite LJ, Gallagher M. The case for marriage: Why married people are happier, healthier and better off financially. Broadway Books; 2002. [Google Scholar]
- Waite LJ, Lehrer EL. The Benefits from Marriage and Religion in the United States: A Comparative Analysis. Popul Dev Rev. 2003;29(2):255–276. doi: 10.1111/j.1728-4457.2003.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Waldman ID, Young LJ. Statistical and Methodological Considerations for the Interpretation of Intranasal Oxytocin Studies. Biol Psychiatry. 2016;79(3):251–257. doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RAH, Davis OSP, Wootton RE, Mottershaw A, Haworth CMA. Social support and mental health in late adolescence are correlated for genetic, as well as environmental, reasons. Sci Rep. 2017;7(1):13088. doi: 10.1038/s41598-017-13449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JM, Schofield TJ. Mediation and moderation of divorce effects on children’s behavior problems. J Fam Psychol. 2015;29(1):39–48. doi: 10.1037/fam0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder N, Zhang H, Jensen K, Yang BZ, Simen A, Jackowski A, … Kaufman J. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J Am Acad Child Adolesc Psychiatry. 2014;53(4):417–424. e415. doi: 10.1016/j.jaac.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66(2):293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6(3):247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. Social relationships and physiological determinants of longevity across the human life span. Proc Natl Acad Sci U S A. 2016;113(3):578–583. doi: 10.1073/pnas.1511085112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ. The Neural Basis of Pair Bonding in a Monogamous Species: A Model for Understanding the Biological Basis of Human Behavior. In: Wachter KW, Bulatao RA, editors. Offspring: Human Fertility Behavior in Biodemographic Perspective. Washington (DC): 2003. pp. 91–103. [Google Scholar]
- Young LJ. Being Human: Love: Neuroscience reveals all. Nature. 2009;457(7226):148–148. doi: 10.1038/457148a. [DOI] [PubMed] [Google Scholar]
- Young LJ. Oxytocin, social cognition and psychiatry. Neuropsychopharmacology. 2015;40(1):243–244. doi: 10.1038/npp.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Alexander B. The Chemistry Between Us: Love, Sex, and the Science of Attraction. Penguin Publishing Group; 2012. [Google Scholar]
- Young LJ, Barrett CE. Neuroscience. Can oxytocin treat autism? Science. 2015;347(6224):825–826. doi: 10.1126/science.aaa8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang B, Jin J, An S, Zeng Q, Duan Y, … Cao X. Early deprivation reduced anxiety and enhanced memory in adult male rats. Brain Res Bull. 2014;108:44–50. doi: 10.1016/j.brainresbull.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Zimmermann AC, Easterlin RA. Happily Ever After? Cohabitation, Marriage, Divorce, and Happiness in Germany. Population and Development Review. 2006;32(3):511–528. doi: 10.1111/j.1728-4457.2006.00135.x. [DOI] [Google Scholar]
- Zisook S, Chentsova-Dutton Y, Shuchter SR. PTSD following bereavement. Ann Clin Psychiatry. 1998;10(4):157–163. doi: 10.1023/a:1022342028750. [DOI] [PubMed] [Google Scholar]