Abstract
Background
Transfemoral Transcatheter Aortic Valve Replacement (TAVR) was superior to surgical aortic valve replacement (SAVR) in the Placement of Aortic Transcatheter Valves (PARTNER) 2A trial (P2). The generalizability of the trial results to the broader population of patients with intermediate surgical risk remains unknown.
Objective
To compare the outcomes of SAVR and TAVR among patients with intermediate surgical risk treated in the VA Healthcare System.
Methods
We retrospectively analyzed the clinical characteristics and outcomes on all SAVR (1987–2014) and TAVR procedures (2015–2017) performed at the Minneapolis VA Healthcare System. Patients were divided into three groups based on their estimated 30-day mortality risk. The primary outcome was a composite of death or stroke at 30-days.
Results
A total of 1,049 patients underwent SAVR with (n=468, 45%) or without CABG (n=581, 55%) and 110 underwent TAVR during the study period. Intermediate-risk patients represented 29.4% and 40% of patients undergoing SAVR and TAVR, respectively. The predicted 30-day mortality risk of intermediate-risk patients was 5.5% for the SAVR group and 5.2% for the TAVR group (p=0.54). The observed combined rate of stroke or death at 30-days for intermediate-risk patients treated with SAVR and TAVR was 11% and 2.2%, respectively (p=0.05). The results for SAVR and TAVR at the VA were comparable to the P2 trial and STS database (all p=NS). The results did not change when the analysis was restricted to a more contemporary (2005–2014) surgical cohort or isolated SAVR. The number needed to treat to prevent one death/stroke with TAVR was 10.
Conclusions
Adoption of TAVR as the preferred treatment modality in intermediate-risk patients may result in significant improvements in morbidity and mortality.
Introduction
Transcatheter aortic valve replacement (TAVR) is currently approved as an alternative to surgical aortic valve replacement (SAVR) in high-risk or inoperable patients with severe, symptomatic aortic stenosis (AS) (1,2). Recent data from the Placement of Aortic Transcatheter Valves (PARTNER) 2A trial (P2), and a propensity-matched Sapien 3 registry, showed that transfemoral TAVR is superior to SAVR in intermediate-risk patients (3,4). Based on these two trials, the Food and Drug Administration (FDA) has approved Sapien XT and Sapien 3 (S3) for intermediate-risk patients in August 2016.
The implications of expanding indications of TAVR to intermediate-risk patients could be considerable to the U.S. healthcare system. First, census projections estimate 88.5 million Americans will be over the age of 65 by 2050 (5). The most common form of AS is age-related or degenerative form (6). Therefore, the incidence of severe AS that requires treatment is likely to increase (7). Second, of the 141,905 isolated SAVR procedures reported in the Society of Thoracic Surgeons (STS) database in the last decade approximately 20,000 (14%) were performed in intermediate-risk patients (8). Although the number of TAVR programs in the US is proliferating, from 228 in 2012 to 348 in 2014, they still represent a small fraction of the more than 1,000 existing surgical programs that report outcomes to the STS database (9). Therefore, with the expansion of indications of TAVR a surge in the number of new TAVR programs may be expected. Finally, for existing TAVR programs expanding the procedure indications to intermediate risk patients will require additional resources, which will require advance planning and budgeting.
The main objective of this investigation is to compare the short-term outcomes of SAVR and TAVR among patients with intermediate surgical risk treated in the Veterans Affairs Health Care System. A secondary objective is to validate the results of pivotal trials of SAVR and TAVR with balloon-expandable valves in intermediate risk patients (P2 trial and S3 registry) in a real world cohort of patients.
Methods
Setting and Design
The Minneapolis VA Healthcare System (MVAHCS) is a tertiary, 250-bed hospital within the VA Midwest Heath Care Network (Veterans Integrated Service Network VISN 23), which spans 394,134 square miles, and includes 9 Hospitals and 62 community based outpatient clinics. The network serves more than 440,000 enrolled Veterans residing in the states of Iowa, Minnesota, Nebraska, North Dakota, South Dakota, and portions of Illinois, Kansas, Missouri and Wyoming (supplementary appendix, Figure 1). The MVAHCS is the only program to offer SAVR and TAVR within VISN 23 and one of the only 8 TAVR programs at VA Medical Centers nationwide in 2016.
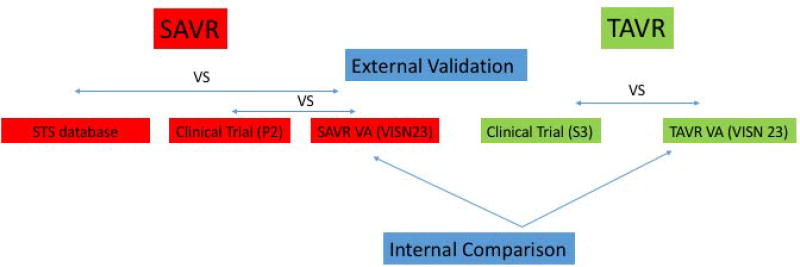
In this retrospective analysis, baseline characteristics and outcomes of intermediate-risk patients undergoing TAVR and SAVR at the MVAHCS were compared after ensuring that both programs had comparable outcomes relative to STS database (for SAVR) and pivotal TAVR trials with balloon-expandable valves (for TAVR and SAVR) (Figure 1).
Figure 1.
Schematic diagram of the study design. Outcomes of intermediate-risk patients undergoing TAVR and SAVR at the Minneapolis VA Healthcare System were compared after ensuring both programs had comparable outcomes relative to STS database and pivotal TAVR trials (PARTNER 2A and Sapien 3 registry)
Patient Selection
SAVR
We included all patients undergoing SAVR, with or without concomitant coronary artery bypass (CABG) surgery, at the MVAHCS between 1987 and 2014 (data for 2015–16 was not yet available). A subgroup of consecutive intermediate risk patients (i.e. estimated risk of perioperative mortality of 4%–8%) treated with SAVR in the last 10 years (2005–2014) was selected to serve as a more contemporary comparison with the TAVR cohort. This was done to account for advances in cardiac surgery that have occurred in the last decade, which have resulted in improved outcomes (8). We excluded patients that were unlikely to become TAVR candidates due to non-aortic pathology that required surgical correction. These included 1) patients undergoing complex operations (AVR plus great vessel surgery, double valve replacement or other complex surgical procedures) and 2) significant (>50%) left main coronary artery disease.
TAVR
We included all patients undergoing TAVR at our institution, irrespective of access and valve type, from 4/2015 to 1/2017. A subgroup of intermediate risk patients (estimated surgical perioperative mortality of 4%–8%) was selected for comparison with the the SAVR group and S3 registry.
Outcome
The primary outcome of the study was the incidence of death or stroke 30-days after aortic valve replacement, either surgical or transcatheter. Secondary outcomes were 30-day post-procedure mortality and hospital length of stay.
External Comparison Groups
First, two surgical control groups were selected to ensure that VA surgical outcomes were comparable to those obtained in the private sector or academic institutions (Groups 1 and 2 below). Second, a third comparison group of intermediate risk patients treated with S3 was used to ensure that VA TAVR outcomes were comparable to those obtained in the landmark clinical trial. This group (S3 registry) was selected because it provided the basis for FDA approval of TAVR for intermediate-risk patients. S3 is the most common valve type used in the US and in our program (9, 14).
Group 1 (SAVR STS)
This group consisted of 19,769 intermediate risk patients who underwent isolated SAVR from 2002 to 2010 at STS participating sites. Data was extracted from reference 8.
Group 2 (SAVR P2 trial)
This group consisted of 1,021patients treated with SAVR from December 2011 through November 2013 at any of the 57 participating PARTNER 2 (P2) sites in the US and Canada (3).
Group 3 (TAVR S3 registry)
This group consisted of 1,077 intermediate-risk patients treated with the balloon-expandable S3 valve as part of a large registry (4).
Estimation of Perioperative Mortality
The risk of perioperative mortality was estimated by the Veterans Administration (VA) risk score. Since 1987, data from all patients undergoing cardiac surgery VA medical centers have been collected as part of a quality improvement program (10). This ongoing, prospective database includes demographic information, preoperative clinical variables, surgical details, and postoperative outcomes including 30-day mortality and major complications. The database also includes a validated risk score, which measures patient risk at the time of cardiac surgery on a scale that ranges from 0% to 100%, with the higher numbers indicating greater risk (11,12). This score is calculated by multivariable logistic regression analysis where the variables with p less than 0.2 in univariable analysis are included in the multivariable logistic regression model. Twice each year, statistical analyses are done to assess the performance of each cardiac surgical center and to recalibrate the regression models that predict operative mortality. We have previously shown that the STS and VA risk score have good calibration and discrimination in this population (13). For this analysis, patients in whom the VA risk score predicted a mortality risk < 4% at 30-days were considered low risk, 4–8% intermediate risk, and >8% high risk.
Statistical Analysis
Continuous variables are presented as mean ± SD, or as median with interquartile range when appropriate. Categorical variables are reported as frequencies and percentages. Continuous variables were compared using the unpaired Student t test or Mann-Whitney U test as appropriate. Discrete variables were compared with the chi-square test or Fisher exact test as appropriate. A 2-sided p value < 0.05 was considered to be statistically significant. Medcalc® version 17.2 was used for analysis.
This study was approved by the Institutional Review Board of the Minneapolis VA Medical Center. Individual consent requirement was waived.
Sponsor
The study was supported by Edwards Lifesciences through an investigator-initiated grant to the Minnesota Veterans Research Foundation. The sponsor had no role in the design of the study or writing of the manuscript.
Results
A total of 1,049 patients underwent SAVR with (n=468, 45%) or without (n=581, 55%) CABG surgery between 1987 and 2014. Intermediate-risk patients (n=309) represented 29% of the patients undergoing SAVR at the MVAHCS. Baseline characteristics of this group are presented in Table 1. Their mean age was 72 (±8) years, 42 (13.6%) were reoperations, and the median (95% confidence interval) estimated mortality risk was 5.5% (95% CI: 5.4–5.7). The great majority of SAVR (n=296, 95.8%) were elective procedures performed in patients with normal left ventricular ejection fraction (Table 1).
Table 1.
Baseline characteristics of patients undergoing surgical aortic valve replacement (SAVR) between 1987 and 2014 at the Minneapolis VA Healthcare System (MVAHCS)
| Low-risk (n=565, 54%) |
Intermediate-risk (n=309, 29%) |
High-risk (n=175, 16%) |
P value |
|
|---|---|---|---|---|
| Age (±SD)- years | 66 (±9) | 72 (±8) | 75 (±6) | <0.01 |
| Men (%) | 561 (99.3%) | 307 (99%) | 172 (99%) | 0.98 |
| NYHA class III–IV | 298 (52.7%) | 211 (68.3%) | 152 (86.9%) | <0.01 |
| Previous MI | 91 (16%) | 101 (32.7%) | 83 (47.4%) | <0.01 |
| Hypertension | 342 (76.7%) | 174 (82.1%) | 108 (85.7%) | 0.02 |
| PAD | 199 (35.2%) | 137 (44.3%) | 92 (52.6%) | <0.01 |
| Chronic renal failure (eGFR <60 ml/m/m2) | 85 (15%) | 106 (34.3%) | 97 (55.4%) | <0.01 |
| Prior heart surgery | 21 (3.7%) | 42 (13.6%) | 63 (36%) | <0.01 |
| COPD | 149 (26.4%) | 114 (36.9%) | 85 (48.6%) | <0.01 |
| Current smoker | 85 (15.1%) | 32 (10.4%) | 15 (8.6%) | <0.01 |
| Diabetes | 134 (23.7%) | 81 (26.2%) | 65 (37.1%) | <0.01 |
| Functional Status – Partially dependent | 14 (2.5%) | 34 (11%) | 44 (25%) | <0.01 |
| Serum creatinine (mg/dl) | 1.09 (±0.2) | 1.24 (±0.4) | 1.60 (±1.1) | <0.01 |
| Hemoglobin g/dl (±SD) | 13 (±1.5) | 13 (±1.5) | 12.5 (±1.7) | <0.01 |
| Albumin g/dl | 4.04 (±0.4) | 3.9 (±0.4) | 3.87 (±0.5) | <0.01 |
| Surgical Variables | ||||
| Isolated AVR | 380 (67.3%) | 135 (43.7%) | 66 (37.7%) | <0.01 |
| AVR + CABG | 185 (32.7%) | 174 (56.3%) | 109 (62.3%) | <0.01 |
| 1-vessel CABG | 91 (16.1%) | 79 (25.6%) | 52 (29.7%) | <0.01 |
| 2-vessel CABG | 54 (9.6%) | 57 (18.4%) | 29 16.6%) | <0.01 |
| ≥3-vessel CABG | 40 (7.1%) | 38 (12.3%) | 28 (16%) | <0.01 |
| Elective surgery | 549 (97.2%) | 296 (95.8%) | 158 (90.3%) | <0.01 |
| EF > 55% | 339 (76%) | 141 (66.8%) | 57 (45.2%) | <0.01 |
| EF 35–54% | 81 (18.2%) | 59 (28%) | 47 (37.3%) | <0.01 |
| EF < 35% | 25 (5.6%) | 11 (5.2%) | 22 (17.5%) | <0.01 |
| CBP time (mins) | 151 (±36) | 167 (±42) | 178 (±47) | <0.01 |
| Total ischemic time- minutes (±SD) | 118 (±31) | 129 (±37) | 130 (±35) | <0.01 |
| Estimated mortality mean (95% CI) | 2.3% (2.2–2.4) | 5.5% (5.4–5.7) | 14 % (13–15) | <0.01 |
NYHA: New York Heart Association, MI: myocardial infarction, PAD: peripheral arterial disease, COPD: chronic obstructive pulmonary disease, BMI: body mass index, AVR: aortic valve replacement, CABG: coronary artery bypass surgery, CBP: cardiopulmonary bypass
A total of 110 patients underwent TAVR at the MVAHCS during the study period. Intermediate-risk patients (n=44) represented 40% of the patients undergoing TAVR at the MVAHCS. Baseline characteristic and outcomes of this group are presented in Table 2. Their mean age of patients undergoing TAVR was 80 (±7) years, 34% were reoperations and the median estimated mortality risk was 5.2% (95% CI: 5–8). The mean EF was 49 % (±12). The majority of TAVR procedures were done using transfemoral access (85%) and balloon-expandable valves (84.5%). Among intermediate risk patients, 40% of TAVR procedures were done under monitored anesthesia care. The median (interquartile range IQR) fluoroscopy time and contrast volume were 22 minutes (15–32) and 115 cc (74–190), respectively.
Table 2.
Clinical characteristics and outcomes of patients treated with transcatheter aortic valve replacement (TAVR) at the Minneapolis VA Healthcare System (MVAHCS)
| MVAHCS TAVR Overall (n=110) |
MVAHCS TAVR patients IR group (n=44) |
|
|---|---|---|
| Age –years (±SD) | 79 (9) | 80 (7) |
| STS score, median (95% CI) | 5 (4–6) | 5.2 (5–8) |
| Men (%) | 108 (98%) | 42 (95.5%) |
| NYHA class III–IV | 68 (61%) | 29 (65%) |
| Previous MI | 17(16%) | 8 (18%) |
| Previous PCI | 28 (26%) | 13 (30%) |
| Previous CABG | 31 (28%) | 15 (34%) |
| HTN | 70 (63%) | 27 (61%) |
| Peripheral arterial disease | 22 (20%) | 8 (18%) |
| Dialysis | 4 (3.6%) | 2 (5%) |
| COPD | 55 (50%) | 20 (45%) |
| Current smoker | 11 (10%) | 5 (11%) |
| Diabetes | 42 (38%) | 15 (34%) |
| Serum creatinine (mg/dl), mean (±SD) | 1.3 (0.9) | 1.1 (0.4) |
| Hemoglobin g/dl (±SD) | 12 (2) | 12 (1.9) |
| Albumin g/dl, mean (±SD) | 3.4 (0.4) | 3.3 (0.5) |
| Weight-Kg, mean (±SD) | 88 (21) | 88 (15) |
| EF %, mean (±SD) | 50 (11) | 49 (12) |
| Procedural Indication | ||
| Pure AS | 96 (88%) | 41 (93%) |
| AS/AI | 4 (3.7%) | 1 (2.3%) |
| Valve in Valve | 12 (11%) | 2 (5%) |
| Transfemoral access | 94 (85%) | 39 (89%) |
| Balloon-expandable valve | 93 (84.5%) | 38 (86%) |
| Device Size | ||
| 23 mm | 18 (16%) | 8 (18%) |
| 26 mm | 41 (37%) | 17 (38%) |
| 29 mm | 46 (41%) | 17 (38%) |
| 31 mm | 5 (4.5%) | 2 (4.5%) |
| Fluoroscopy time -minutes, median (95% CI) | 22 (19–27) | 21 (17–28) |
| Contrast volume –ml, median (95% CI) | 115 (93–138) | 115 (88–144) |
| Monitored anesthesia care (MAC) | 31 (28%) | 17 (40%) |
| Length of stay -days, median (95% CI) | 4 (3–4) | 4 (2–4) |
| Estimated Mortality | 5% | 5.2 % |
| Observed Mortality | 1.9% | 0% |
| Observed/Expected(O/E) mortality ratio | 0.38 | 0.01 (*) |
| TIA/stroke | 3% | 2.3% |
| Disabling stroke | 0% | 0% |
| Death or stroke | 4.9% | 2.2% |
| MI | 0% | 0% |
| Major vascular complications | 1 (0.9%) | 0% |
| New Pacemaker | 18 (16%) | 5 (11%) |
| Second valve | 1 (1.9%) | 0 % |
| Moderate/Severe PVL (% total) | 4/0 (3.6%) | 3/0 (6.8%) |
| Mean gradient- mmHg, median (IQR) | 10 (8–13) | 9 (7–11) |
STS: Society of Thoracic Surgeons, NYHA: New York Heart Association, MI: myocardial infarction, PCI: percutaneous coronary intervention, CABG: coronary artery bypass surgery, HTN: hypertension, COPD: chronic obstructive pulmonary disease, PAD: peripheral arterial disease, TIA, transient ischemic attack, CI: confidence interval
Given observed mortality of zero, 0.1 was added to numerator and denominator to allow for this calculation.
Outcomes
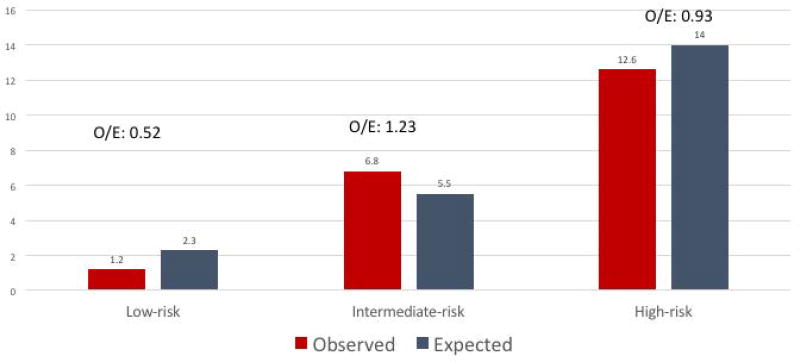
The observed in-hospital mortality (n=21, 6.8%) for intermediate-risk patients treated with SAVR was slightly higher than expected (5.5%) (Figure 2 and Table 3) with an observed/expected (O/E) mortality ratio of 1.23. In contrast, low- and high-risk patients treated with SAVR had an O/E mortality ratio of 0.52 and 0.9, respectively. Serious complications occurred in 24% of intermediate-risk patients undergoing SAVR and included stroke (n=13, 4.2%), myocardial infarction (n=7, 2.3%), reoperation for bleeding (n=21, 6.8%), renal failure needing hemodialysis (n=4, 1.3%), cardiac arrest (n=12, 4.1%), mechanical ventilation > 48 hours (n=41, 13.3%), and tracheostomy (n=3, 1.4%). The mean length of stay was 15 days (±18), with a median (IQR) of 10 days (8). The results did not change when the analysis was restricted to a more contemporary (2005–2014) surgical cohort or isolated SAVR (supplementary appendix, Figure 2).
Figure 2.
Observed (O) versus Expected (E) mortality for low, intermediate and high-risk patients treated with SAVR at MVAHCS
Table 3.
Outcomes of SAVR According to Pre-Operative Risk Assessment
| Low-risk (n=565) |
Intermediate- risk (n=309) |
High-risk (n=175) |
P value |
|
|---|---|---|---|---|
| Death at 30 days | 7 (1.2%) | 21 (6.8%) | 22 (12.6%) | <0.01 |
| Estimated mortality | 2.3% | 5.5% | 14 % | <0.01 |
| Observed/Expected (O/E) mortality | 0.52 | 1.23 | 0.9 | NA |
| Stroke | 16 (2.8%) | 13 (4.2%) | 15 (8.6%) | 0.01 |
| Myocardial infarction | 4 (0.7%) | 7 (2.3%) | 7 (4%) | 0.02 |
| Reoperation for bleeding | 20 (3.5%) | 21 (6.8%) | 11 (6.3%) | 0.07 |
| Renal failure | 5 (0.9%) | 4 (1.3%) | 6 (3.4%) | 0.07 |
| Cardiac arrest | 8 (1.3%) | 12 (4.1%) | 14 (4.7%) | <0.01 |
| Mechanical ventilation > 48 hours | 26 (4.6%) | 41 (13.3%) | 41 (23.4%) | <0.01 |
| Tracheostomy | 0 (0%) | 3 (1.4%) | 3 (2.4%) | <0.01 |
| Any complication | 8.6% | 24% | 33% | <0.01 |
| Length of stay – mean (±SD) | 9 (6) | 15 (18) | 19 (15) | <0.01 |
TAVR versus SAVR real-world comparison
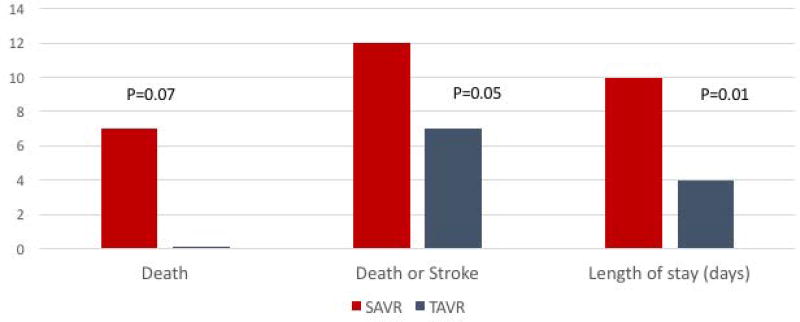
The outcomes of intermediate-risk patients treated with SAVR and TAVR at the MVAHCS are presented in Table 4 and Figure 3. Despite older age (80±7 versus 74±9, p<0.001) and a higher incidence of previous heart surgery (34% versus 13%, p=0.001) in the TAVR cohort, peri-procedural mortality (0% versus 7%, p=0.07) and the composite of death or stroke at 30-days was significantly lower with TAVR relative to SAVR (2.2% versus 12%, p= 0.05). Similarly, length of stay was shorter with TAVR (4 versus 10 days, p=0.01). The number needed to treat to prevent one surgical death or one surgical death plus stroke with TAVR was 14 and 10, respectively. These results were similar when the analysis was restricted to isolated SAVR procedures (supplementary appendix Table 1). The incidence of new permanent pacemaker (PPM) after SAVR was 4.9% and for TAVR was 11% (risk difference 6.1%, 95% CI= −2.82 to 19.52, p=0.14). The incidence of moderate or severe paravalvular regurgitation (PVL) was 3% for SAVR and 6.8% for TAVR (risk difference 3.80%, 95% CI= −3.19 to 15.83, p=0.26).
Table 4.
Comparison of baseline characteristics and outcomes of intermediate-risk patients treated with SAVR (2004–2010) and TAVR (2015–2017) at the Minneapolis VA Healthcare System (MVAHCS)
| MVAHCS TAVR Intermediate- risk (n=44) |
MVAHCS SAVR Intermediate-risk (n=142) |
P value |
|
|---|---|---|---|
| Age- years (±SD) | 80 (7) | 74 (9) | <0.01 |
| STS score, median | 5.2 | 5.5 | 0.9 |
| Men (%) | 42 (95.5%) | 141 (99%) | 0.09 |
| NYHA class III–IV | 29 (65%) | 114 (80%) | 0.04 |
| Previous MI | 8 (18%) | 51(36%) | 0.02 |
| Previous CABG | 15 (34%) | 18 (13%) | <0.01 |
| COPD | 20 (45%) | 67 (47%) | 0.81 |
| PAD | 8 (18%) | 75 (52%) | <0.01 |
| Creatinine (mg/dl)(±SD) | 1.1 (0.4) | 1.2 (0.6) | 0.30 |
| Hemoglobin g/dl (±SD) | 12 (1.9) | 13 (1.5) | <0.01 |
| Albumin g/dl(±SD) | 3.3 (0.5) | 4.1 (0.4) | <0.01 |
| Outcomes | |||
| Mortality | 0% | 10 (7%) | 0.07 |
| Stroke | 1 (2.2%) | 7 (4.9%) | 0.43 |
| Death or stroke | 1 (2.2%) | 17 (12%) | 0.05 |
| Length of stay –median in days (IQR) | 4 (3) | 11 (7) | <0.01 |
| New PPM | 11% | 4.9% | 0.14 |
| Moderate or Severe PVL | 6.8% | 3% | 0.26 |
STS: Society of Thoracic Surgeons, NYHA: New York Heart Association, COPD: chronic obstructive pulmonary disease, PAD: peripheral arterial disease, IQR: interquartile range
Figure 3.
Real-world outcomes of intermediate-risk patients treated with SAVR and TAVR at MVAHCS
External Validation
Comparison of surgical groups
A comparison of surgical groups in key baseline characteristics and outcomes is presented in the supplementary appendix. Despite some differences in baseline characteristics among groups, the predicted mortality rate at 30-days was similar (P2 trial:5.8%, VA:5.5%, STS: 5.5%, p=0.84). The observed combined rate of stroke or death at 30-days for intermediate risk patients treated with SAVR at the MVAHCS (11%) was similar to STS (8%, p=0.06) and P2 trial (10%, p=0.62). Other outcomes of interest are presented in the supplementary appendix Table 2.
Comparison of TAVR groups
A comparison of TAVR groups in key baseline characteristics and outcomes is presented in the supplementary appendix Table 3. Despite some differences in baseline characteristics, both cohorts had similar 30-day predicted mortality. The observed combined rate of stroke or death at 30-days was significantly lower than predicted for both groups treated with TAVR (MVAHCS: 2.2% vs. S3 registry: 2%, p=0.92)
Discussion
Using a contemporary cohort of patients with severe AS and intermediate surgical risk we found that TAVR offers a quantifiable improvement in outcome over SAVR, both in a clinical trial and real-world setting. Our results support an expanded role of TAVR, as the preferred treatment modality of patients with severe AS and intermediate surgical risk. To provide some perspective, the low number needed to treat (NNT) to prevent one death/stroke seen in our study (NNT:10) and S3 registry (NNT:16) are in line with the benefits of primary angioplasty over thrombolytic therapy for the treatment of acute myocardial infarction (NNT:17 for death/reinfarction/stroke) (15), which is a therapy widely accepted as the standard of care by practice guideline recommendations from professional societies (16).
Other important conclusions of our analysis are the following: First, intermediate-risk patients represent about a third of patients undergoing SAVR at VA Medical Centers, which is significantly higher than the STS registry (14%) (8). Second, intermediate risk-patients treated with SAVR outside a clinical trial in the US have higher (10–20%) than expected mortality (3–4,8), which highlights some of the limitations of existing risk prediction models. Finally, in addition to lower mortality and stroke, TAVR is also associated with a significantly shorter hospital length of stay and a higher proportion of patients being discharged home (4). All of these findings strongly suggest that TAVR may soon become the predominant treatment modality for intermediate-risk patients and could help hospital and healthcare systems with planning and resource allocation.
Reardon et al. recently reported results of the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial (17). A total of 1,276 intermediate-risk patients were randomized to TAVR, with a self-expanding valve, or SAVR. SURTAVI was designed as a noninferiority trial with a primary end-point of death or disabling stroke at 24 months. No difference was seen in the incidence of the primary outcome with TAVR (12.6%) relative to SAVR (14%, p >0.99) (17). At 30 days, the composite of death or disabling stroke was 2.8% for TAVR and 3.9% for SAVR (p=ns). Of note, SURTAVI enrolled lower-risk patients (mean STS: 4.5%, 15% had STS < 3) relative to P2 (STS: 5.8%) and had one of the lowest surgical mortality rates (1.7%) ever reported in randomized surgical studies (3–4,17). Thus, TAVR had similar outcomes to SAVR even when compared to a “best case scenario” surgical comparison group. However, these excellent surgical results may not be generalizable to the real world. The 30-day surgical mortality for intermediate-risk patients in the STS database is 5.8%, which emphasizes the potential improvements in outcome with TAVR (8). Further, given the minimally invasive nature of TAVR, patients seem to have developed a strong preference for this minimally invasive procedure. In the SURTAVI trial 71 patients out of 867 assigned to surgery (8%) withdrew their consent after randomization (17).
Limitations
Our study has several limitations. First, a comparison between different trials and registries carries inherent risks due to different enrollment criteria and end-point definitions. However, mortality and stroke are objective end-points unlikely to change with different study designs. Second, although TAVR is cost efficient in high-risk and inoperable patients the economics of TAVR in a lower-risk population have not yet been evaluated. Commercially available TAVR valves in the U.S. are at least 5 times more expensive than surgical valves and the cost of the valve represents approximately 40% of the cost of the procedure-related expense currently (18). Third, the long-term (> 5 years) durability of TAVR valves remains unknown (19). Although TAVR valves have yielded lower gradients and larger valve areas relative to surgical valves there is paucity of long-term data. Ongoing studies in low-risk patients should help clarify this important issue. Finally, paravalvular regurgitation and pacemaker rates were numerically higher in the TAVR group but these differences didn’t reach statistical significance. It is accepted that TAVR is associated with higher rates of paravalvular regurgitation and permanent pacemaker implantation than SAVR (2–4,17). In a previous study we showed that 6% of SAVR patients require a pacemaker implantation in the perioperative period (20), which is in line with the P2 trial. The incidence of moderate-severe paravalvular regurgitation (1.3%) appears to be decreasing with technological improvements such as a sealing skirt at the lower portion of the stent frame (4). In contrast, and probably related to the presence of the “skirt”, the incidence of PPM is increasing (12%) (9).
Conclusions
The results of the pivotal trials of TAVR with balloon-expandable valves in intermediate-risk patients are generalizable to the broader population of patients with AS treated in the VA Health Care System. Intermediate-risk patients represent a larger proportion of patients treated with SAVR at VA Medical Centers relative to the STS database and have higher than predicted mortality with SAVR. Adoption of TAVR as the preferred treatment modality in intermediate risk patients is likely to result in significant improvements in morbidity and mortality for patients.
Supplementary Material
Acknowledgments
Dr. Garcia is a consultant for Surmodics, Medtronic, Boston Scientific and Osprey Medical. Research support from Edwards Lifesciences, MN Veterans Research Foundation and the VA Office of Research and Development.
Dr. Yannopoulos is the PI and co-PI for the following NIH (NHLBI) grants: R01 HL123227, 1R01HL126092 -01, R01HL1223231, R43HL123194-01 1R43HL110517-01A1, R43HL115937-01. Dr Yannopoulos also received funds for the Minnesota Resuscitation Consortium from the Medtronic Foundation.
Footnotes
Disclosures:
The other authors have no conflicts to report related to this manuscript.
References
- 1.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 4.Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218–25. doi: 10.1016/S0140-6736(16)30073-3. [DOI] [PubMed] [Google Scholar]
- 5.Ortman JMO, Guarneri CE. United States Population Projections: 2000 to 2050. [Accessed April 20, 2017]; Available at: http://www.census.gov/population/projections/files/analytical-document09.pdf.
- 6.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118(15):e523–661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 8.Thourani VH, Suri RM, Gunter RL, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg. 2015;99:55–61. doi: 10.1016/j.athoracsur.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 9.Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, et al. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69(10):1215–30. doi: 10.1016/j.jacc.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Grover FL, Johnson RR, Shroyer AL, Marshall G, Hammermeister KE. The Veterans Affairs Continuous Improvement in Cardiac Surgery Study. Ann Thorac Surg. 1994;58(6):1845–51. doi: 10.1016/0003-4975(94)91725-6. [DOI] [PubMed] [Google Scholar]
- 11.Grover FL, Shroyer AL, Hammermeister KE. Calculating risk and outcome: the Veterans Affairs database. Ann Thorac Surg. 1996;62(5 Suppl):S6–11. doi: 10.1016/0003-4975(96)00821-1. discussion S31-2. [DOI] [PubMed] [Google Scholar]
- 12.Hammermeister KE, Johnson R, Marshall G, Grover FL. Continuous assessment and improvement in quality of care. A model from the Department of Veterans Affairs Cardiac Surgery. Ann Surg. 1994;219(3):281–90. doi: 10.1097/00000658-199403000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basraon J, Chandrashekhar YS, John R, Agnihotri A, Kelly R, Ward H, et al. Comparison of risk scores to estimate perioperative mortality in aortic valve replacement surgery. Ann Thorac Surg. 2011;92(2):535–40. doi: 10.1016/j.athoracsur.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Gurevich S, John R, Kelly RF, Raveendran G, Helmer G, Yannopoulos D, et al. Avoiding the Learning Curve for Transcatheter Aortic Valve Replacement. Cardiol Res Pract. 2017;2017:7524925. doi: 10.1155/2017/7524925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 16.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 17.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376(14):1321–31. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds MR, Magnuson EA, Lei Y, Wang K, Vilain K, Li H, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A) J Am Coll Cardiol. 2012;60(25):2683–92. doi: 10.1016/j.jacc.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Webb JG, Dvir D. Is Transcatheter Aortic Valve Replacement a Durable Therapeutic Strategy? JACC Cardiovasc Interv. 2015;8(8):1092–4. doi: 10.1016/j.jcin.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Raza SS, Li JM, John R, Chen LY, Tholakanahalli VN, Mbai M, et al. Long-term mortality and pacing outcomes of patients with permanent pacemaker implantation after cardiac surgery. Pacing Clin Electrophysiol. 2011;34(3):331–8. doi: 10.1111/j.1540-8159.2010.02972.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.