Abstract
A facile process to prepare zinc oxide (ZnO) nanoparticles from an aqueous zinc chloride (ZnCl2) solution and an aqueous hydroxide solution under a glycerol stabilizer at room temperature was developed. ZnCl2 aqueous solutions as concentrated as 65–80 wt% were used as the concentrated zinc source. The concentration of ZnCl2 solutions and the molar ratio of glycerol to Zn2+ had obvious effects on the sizes and shapes of the ZnO nanoparticles. The shape of ZnO nanoparticles changed from rods approximately 50–120 nm long and 30–70 nm in diameter to globular with diameters of approximately 20 nm with the increasing of the concentration of the ZnCl2 solution and the mole ratio of glycerol to Zn2+. Glycerol, as a stabilizer, played an important role in the formation of ZnO nanostructures at room temperature, even for a highly concentrated zinc source.
Keywords: Nano-ZnO, Glycerol, Zinc chloride
Background
Zinc oxide (ZnO) nanoparticles are one of the most important multifunctional semiconductor materials for applications in electronic and optoelectronic devices [1], solar cells [2], field-emission devices [3], sensors [4], and photocatalysts [5]. ZnO nanoparticles are also generally recognized as safe and biocompatible and have been used as drug carriers and medical filling materials [6], photoluminescence agents in biosensors [7], UV-absorbers in sun creams and coating materials [8], and antibacterial agents in many industrial products [9, 10].
Many methods have been developed to synthesize ZnO nanoparticles with different sizes and morphologies, including chemical vapor deposition [11], sol-gel methods [12], hydrothermal methods [13], laser ablation [14], microemulsion techniques [15], and others [16]. Due to their large specific surface areas as well as high surface energies, ZnO nanoparticles tend to agglomerate easily. Most approaches require the use of stabilizers and low concentrations of ZnO precursors [17–20]. Thus, most processes require a large amount of water or organic solvents. Furthermore, hard agglomerates regularly appear with the use of water in synthesis processes, which presents an obstacle to the application of ZnO nanoparticles [21]. The polyol approach was shown to be suitable for the preparation of metal oxide nanoparticles [22]. ZnO nanoparticles have been successfully synthesized in various polyol media such as ethylene glycol (EG) [23], diethylene glycol (DEG) [24], 1,3-propanediol (PD) [25], tetraethylene glycol (TEG) [23], and 1,4-butanediol (BD) [26]. The nucleation and growth of ZnO nanoparticles were carried out in a high boiling polyol. The polyol serves as both solvent and stabilizing agent to prevent the agglomeration of nanoparticles. Chieng and Loo fabricated ZnO nanoparticles of different sizes and shapes by refluxing 1 mol/L (M) zinc acetate (Zn(CH3COO)2) in EG, DEG, and TEG at 160 °C for 12 h. They found the average particle size of synthesized ZnO increased with increasing glycol chain length. The shape of the ZnO nanoparticles changed from spherical (EG), to spherical and rod (DEG), to ‘diamond’-like structures (TEG) [23]. Mezni et al. prepared ZnO nanoparticles with a mean diameter of 5 nm using 1,3-propanediol as a solvent and 1.2 mM Zn(CH3COO)2 as a precursor at 160 °C for 1 h. 1,3-propanediol also plays the roles of stabilizer and template [25].The drawbacks of the polyol process are the low concentrations of ZnO precursors and high temperatures of reaction systems.
ZnCl2 is a highly soluble salt in water; its solubility is 432 g/100 g water at 25 °C (highest concentration up to 81.2 wt%) [27]. Moreover, it is a ZnO precursor. If nano-sized ZnO could be obtained from a concentrated ZnCl2 aqueous solution, many problems might be solved, such as the formation of hard agglomerates and the use of an aqueous environment. In our previous study, ZnO nanoparticles could be prepared via in situ synthesis of ZnO in dissolved starch or cellulose by a highly concentrated ZnCl2 aqueous solution (such as 65 wt% of ZnCl2 aqueous solution) [28, 29]. However, in such processes, starch or cellulose would need to be dissolved by concentrated ZnCl2 aqueous solution at ~ 80 °C for approximately 2 h. To make the synthesis process more easily performed in one step, with a high concentration of ZnO precursors at room temperature and in a short reaction time, glycerol was used as a stabilizer in a highly concentrated ZnCl2 aqueous solution to generate ZnO nanoparticles in this study. Glycerol is an environmentally friendly polyol that has high solubility in water. However, it has been rarely employed in the synthesis of ZnO nanoparticles [22–26, 30]. Therefore, our study focuses on the correlations between ZnO nanoparticle size and morphology as well as the concentration of ZnCl2 solutions, mole ratios of glycerol/Zn2+, and types of hydroxides. Furthermore, at present, little attention has been paid to a simple route for fabricating ZnO nanoparticles in a water/glycerol system, where water acts as a solvent and glycerol acts as both stabilizer and template. The synthesis was easily performed in one step with high concentrations of ZnO precursors (ZnCl2 and NaOH aqueous solutions up to 80 and 50 wt% in water, respectively) and under mild reaction conditions such as room temperature and a short reaction time (10 min). Moreover, the size and morphology of ZnO nanoparticles could be controlled by the amount of glycerol and the concentration of ZnCl2 solutions.
Methods/Experimental
Materials
ZnCl2, NaOH, KOH, LiOH, ammonia water, and glycerol of analytical grade (Nanjing chemical reagent factory, China) were used without further purification.
Preparation of the ZnO Nanoparticle
First, glycerol was added to ZnCl2 aqueous solutions with a certain mole ratios of glycerol to Zn2+. Then an alkaline solution was added dropwise to the ZnCl2-glycerol solution at room temperature under continuous mechanical agitation to achieve a final pH value of 12, following which the reaction continued for 5 min, the conditions of preparation of the ZnO nanoparticles was seen Table 1. At the end of the reaction, a white emulsion was generated. The white emulsions were washed twice with water and ethanol, respectively, and centrifuged (6000 rpm, 10 min). After drying in an oven at 80 °C, the ZnO nanoparticles were obtained.
Table 1.
The conditions of preparation of the ZnO nanoparticles
| Mole ratio of glycerol to Zn2+ | Concentration of ZnCl2 solution (wt%) | Hydroxides | Concentration of hydroxide (wt%) |
|---|---|---|---|
| 0:1 | 65 | NaOH | 50 |
| 0.33:1 | 65 | NaOH | 50 |
| 1:1 | 65 | NaOH | 50 |
| 1.67:1 | 65 | NaOH | 50 |
| 3.33:1 | 65 | NaOH | 50 |
| 1:1 | 50 | NaOH | 50 |
| 1:1 | 65 | NaOH | 50 |
| 1:1 | 80 | NaOH | 50 |
| 3.33:1 | 65 | LiOH | 8 |
| 3.33:1 | 65 | NH4OH | 25 |
| 3.33:1 | 65 | NaOH | 50 |
| 3.33:1 | 65 | KOH | 60 |
Characterization of ZnO Nanoparticles
The X-ray diffraction patterns (XRD) were recorded using an X-ray diffractometer (Ultima IV, Japan). The morphology of the ZnO nanoparticles was investigated by a scanning electron microscope (SEM) (JSM-7600F; JEOL, Tokyo, Japan) and a transmission electron microscope (TEM) (JEM-2100, JEOL, Japan). An X-ray photoelectron spectroscopy (XPS) (AXIS Ultra DLD system, UK) was used to identify the chemical bonding states of the Zn and O. The UV spectrum of the ZnO nanoparticles was recorded with a UV-visible spectrophotometer (Lambda 950, Perkin Elmer, USA), and the maximum excitation wavelength was 325 nm.
Results and Discussion
The Influence of the Mole Ratio of Glycerol to Zn2+ on ZnO Nanoparticles Size and Morphology
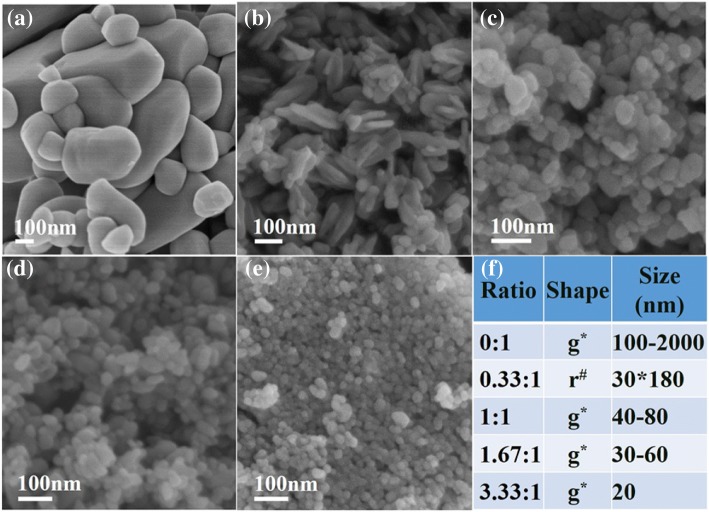
First, the role of glycerol in the synthesis of ZnO nanoparticles was studied. The influence of the mole ratio of glycerol to Zn2+ on the morphology of ZnO nanoparticles was investigated. Figure 1 demonstrates the effect of the mole ratio of glycerol to Zn2+ on the morphology of ZnO nanoparticles, as the reactants were 65 wt% ZnCl2 and 50 wt% NaOH in water. Apparently, the sizes of ZnO nanoparticles prepared without glycerol (Fig. 1a) were much larger than those prepared with glycerol stabilizer (Fig. 1b–e) under the same concentration of ZnCl2. The ZnO nanoparticles prepared without glycerol were shown to be heterogeneous (Fig. 1a). These results indicate that glycerol, as a stabilizer, played an important role in the formation of ZnO nanostructures. When the mole ratio of glycerol to Zn2+ was 0.33, the obtained ZnO consisted of a few globular particles and many ZnO rods, approximately 180 nm in length and 30 nm in diameter; the aspect ratio of the rod-shaped ZnO nanoparticles was approximately 6 (Fig. 1b). When the mole ratio of glycerol to Zn2+ increased to 1 and 1.67, the obtained ZnO was globular, with approximately 40–80 nm and 30–60 nm diameters, respectively (Fig. 1c and Fig. 1d). In addition, uniform and globular ZnO nanoparticles with a diameter of approximately 20 nm were obtained at the 3.33 mol ratio of glycerol to Zn2+ (Fig. 1e). It may be inferred that glycerol played an important role in the synthesis procedure described in this work.
Fig. 1.
SEM images of ZnO nanoparticles obtained from 65% ZnCl2 aqueous solution under the different mole ratios of glycerol to Zn2+ (a, 0:1; b, 0.33:1; c, 1:1; d, 1.67:1; e, 3.33:1) and (f) the statistics table about the morphology and size of ZnO nanoparticles (g*: globular, r#: rod)
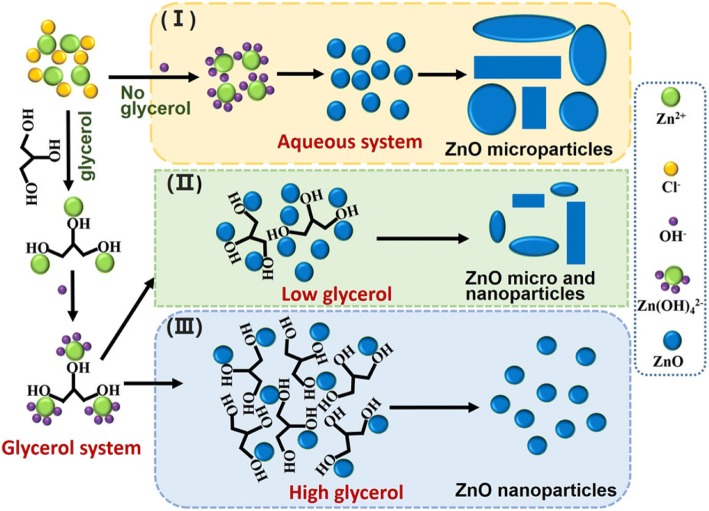
When the concentration of zinc ions and OH− increase without stabilizer, supersaturated ZnO nuclei would more easily aggregate, growing larger and heterogeneous ZnO nanoparticles (seen from Fig. 2(I)). Glycerol has three hydroxyl groups, with which the zinc ions could interact to form a zinc-glycerol complex. When NaOH solution is added, the zinc-glycerol complex reacts with OH− to form Zn(OH)42− around the hydroxyl groups of glycerol. Then, Zn(OH)42− dehydrates into ZnO near the glycerol. Meanwhile, the high NaOH concentration (50 wt%) causes a burst of initial homogeneous nucleation of ZnO crystals, and the supersaturated ZnO nuclei aggregate together near the glycerol, which acts as a stabilizer. As seen from Fig. 2(II), at a low glycerol content in the glycerol/water system, the blocking effect of glycerol was reduced, meaning that since less glycerol is preventing the growth and agglomeration of ZnO in the reaction system, the resulting ZnO nanoparticles were uneven and had a larger size. Meanwhile, with a high glycerol content, the combination of the hydroxyl groups of glycerol and zinc ions greatly reduced the concentration of unbonded zinc ions. The increase of the blocking effect of glycerol causes ZnO to be much more uniform and to exhibit the smallest size (seen from Fig. 2(III)). In fact, the morphologies of ZnO could be controlled by the mole ratio of glycerol to Zn2+. In brief, glycerol operates as an obstructer of agglomeration and a stabilizer of nano-sized ZnO under condition of an extremely concentrated zinc source and an alkaline solution.
Fig. 2.
Schematic presentation of glycerol role in the synthesis process of ZnO nanoparticles
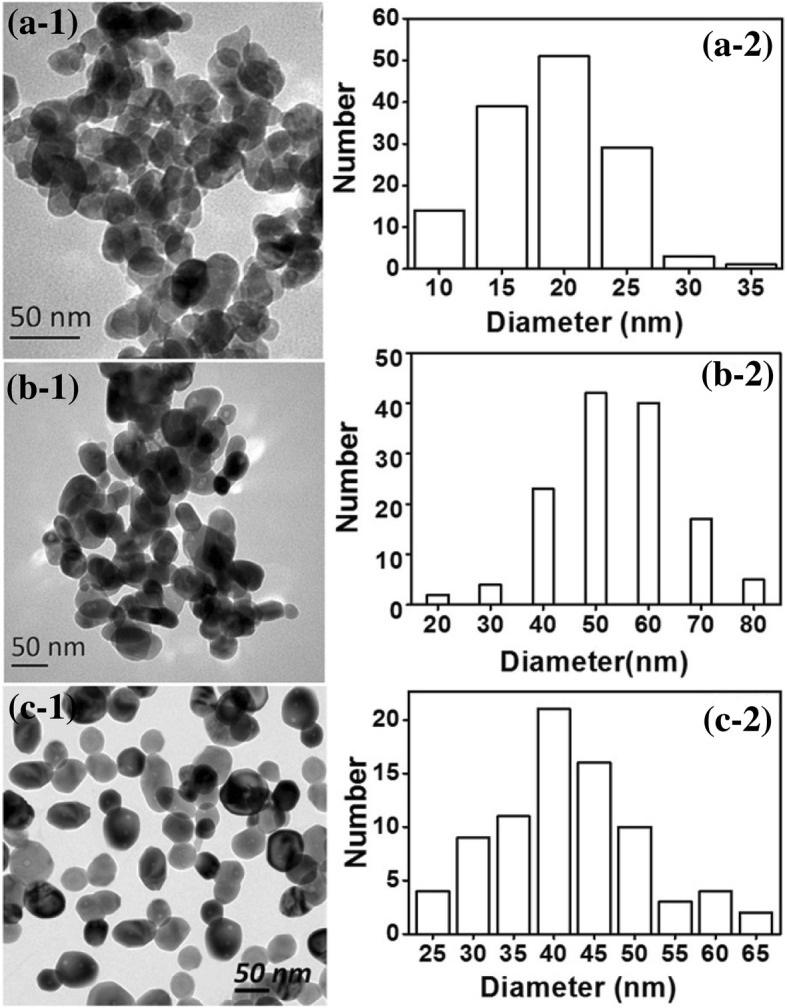
ZnO nanoparticles were characterized by TEM, XPS, XRD, and UV-visible spectrophotometry. Figure 3 illustrates that mostly ZnO rods (with some globular particles) were obtained from the 0.33 mol ratio of glycerol to Zn2+ at a concentration of 65 wt% ZnCl2. The ZnO rods had a wide range of lengths from 20 to 160 nm, namely, due to the uneven directional growth that appeared in the process. Globular ZnO particles with a diameter of approximately 40–50 nm were obtained when the mole ratio of glycerol to Zn2+ was 1 and the concentration of ZnCl2 was 65 wt% in aqueous solution. Furthermore, uniform and globular ZnO nanoparticles with a diameter of approximately 15–25 nm were obtained from the 3.33 of mole ratio of glycerol to Zn2+ when the concentration of ZnCl2 aqueous solution was 65 wt%. These results were consistent with the SEM results (Fig. 1). It was further confirmed that glycerol has an important effect on the preparation of ZnO nanoparticles. Moreover, ZnO nanoparticles could be generated in the presence of glycerol using a relatively highly concentrated ZnCl2 aqueous solution at room temperature.
Fig. 3.
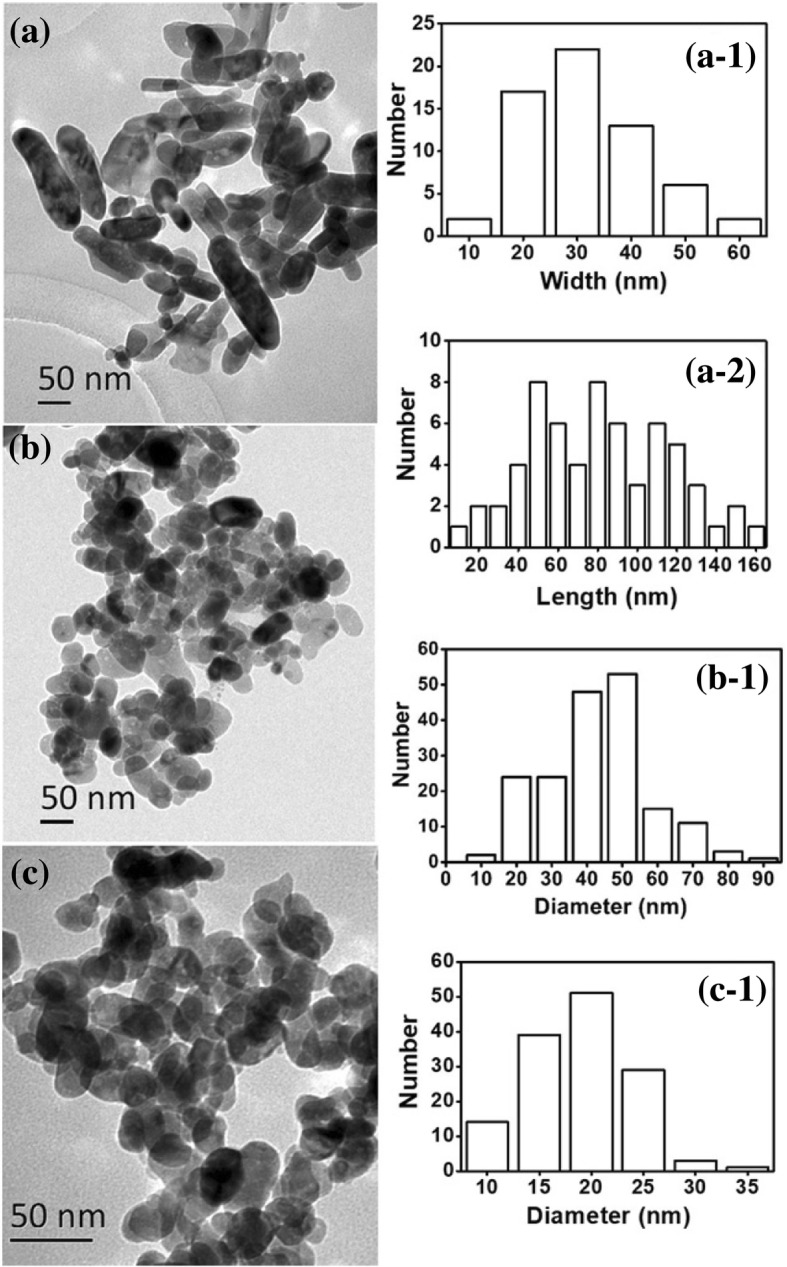
TEM images of ZnO nanoparticles obtained from the different mole ratios of glycerol to Zn2+ (a, 0.33:1; b, 1:1; c, 3.33:1). a-1, a-2 corresponding histogram of (a) sample; b-1, c-1 corresponding histogram of (b) and (c) sample respectively
The element composition and chemical bond of the ZnO samples were further analyzed by XPS technique, as shown in Fig. 4. Zn 3d, Zn 3p, Zn 3 s, Zn Auger, and Zn 2p peaks besides the C1s and O1s peaks were identified in Fig. 4a. The existence of C1s peak demonstrated little residual glycerol in the three ZnO nanoparticles. The Zn 2p spectrum shows a doublet (Fig. 4b), which can be identified as the Zn 2p3/2 and Zn 2p1/2 lines, respectively. The binding energy differences between the two lines are 23.0 eV (from mole ratio of glycerol to Zn2+ 0.33:1 and 3.33:1) or 23.1 eV (from mole ratio of glycerol to Zn2+ 1:1), which confirms that the Zn atoms are in a completely oxidized state in all samples. Figure 4c shows the O1s high-resolution XPS spectra of ZnO. For rod or globular ZnO particles, the peaks all exhibited at around 530.4 eV is attributed to the oxidized metal ions in the nanoparticles, namely, O-Zn in the ZnO lattice.
Fig. 4.
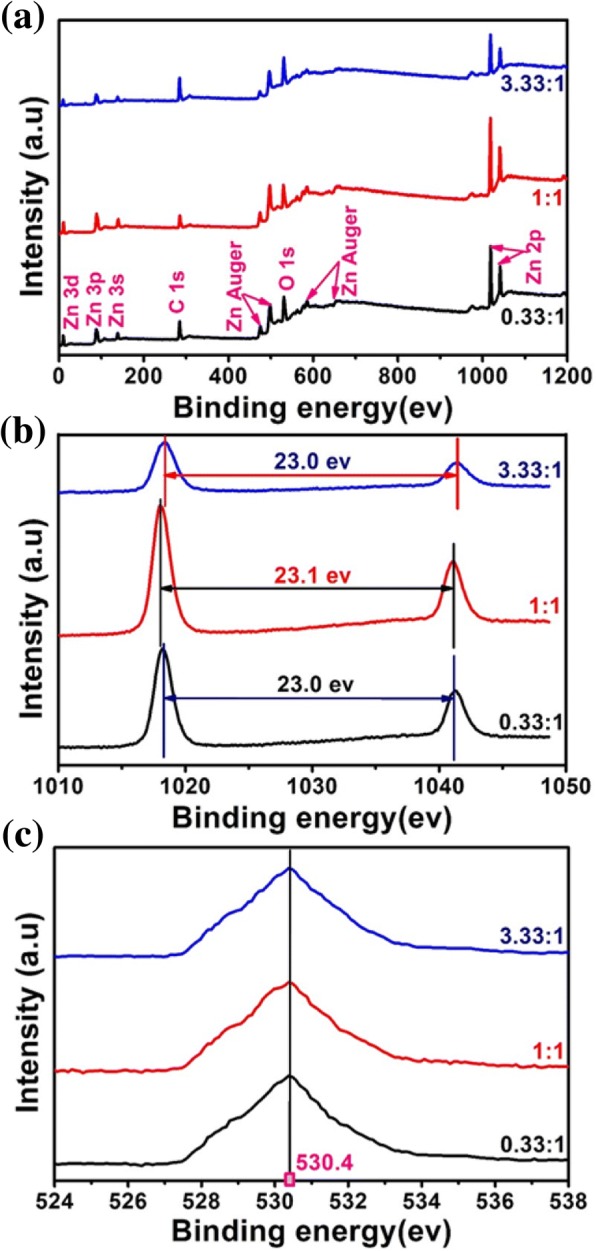
XPS spectra of ZnO nanoparticles obtained from the different mole ratios of glycerol to Zn2+ (a, wide; b, Zn-2p; c, O-1s)
Only diffraction peaks typical of the wurtzite ZnO crystal structure can be seen in Fig. 5a. The UV-vis absorption spectra of the ZnO nanoparticles are shown in Fig. 5b. The ZnO nanoparticles exhibited broad and strong absorption, with a maximum at approximately 380 nm. The figure shows that pure nano-sized ZnO could be prepared from a concentrated zinc source and a glycerol stabilizer and template. Furthermore, the ZnO nanoparticles possess UV blocking properties. Therefore, the ZnO nanoparticles prepared from our approach have a potential application in sun creams or coating materials.
Fig. 5.
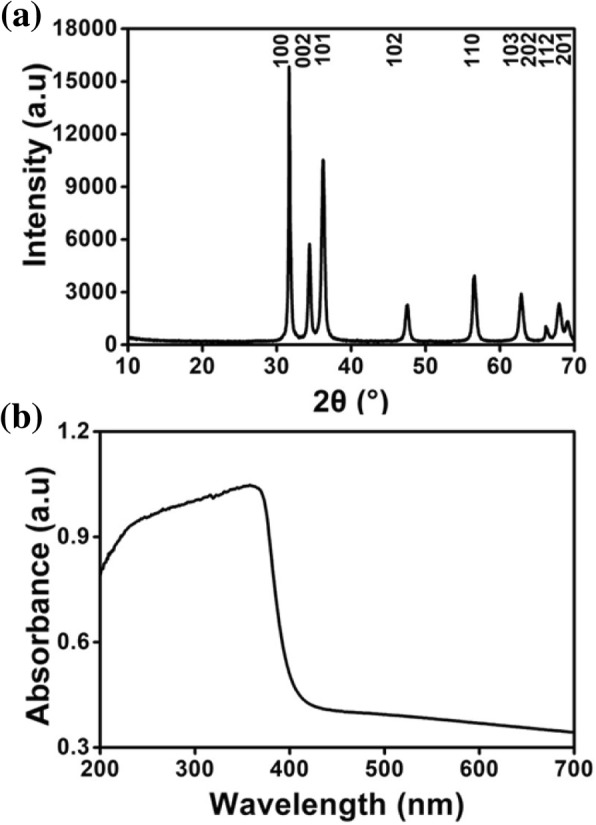
XRD pattern (a) and UV-vis absorption spectrum (b) of ZnO nanoparticles
The Influence of the Concentration of ZnCl2 Aqueous Solutions on ZnO Nanoparticle Size and Morphology
Globular ZnO nanoparticles could be prepared via 65 and 50 wt% ZnCl2 and NaOH aqueous solutions, respectively, at a 1:1 mol ratio of glycerol to Zn2+ (Fig. 1c). To investigate the influence of concentration of ZnCl2 on ZnO nanoparticle size and morphology, 50 wt% of NaOH solution and a 1:1 mol ratio of glycerol to Zn2+ were employed.
Figure 6 shows that the concentration of ZnCl2 in solution had an obvious effect on the shapes and sizes of ZnO nanoparticles. The shape of ZnO changed from rods to globular as the concentration of ZnCl2 increased under obstruction with glycerol as a stabilizer. In the presented approach, the shape of ZnO nanoparticles was changed, and the particle size decreased when the concentration of ZnCl2 aqueous solution was increased (in other words, the hydration ratio was decreased). The results obtained in this system present glycerol as a stabilizer because, otherwise, the homogeneous ZnO nanoparticle cannot be prepared from such a highly concentrated zinc source (see Fig. 1a). For a 50 wt% concentration of ZnCl2 (hydration ratio of 7.56), the obtained ZnO rods were approximately 130 nm in length and 30–70 nm in diameter (Fig. 6a). When the concentration of the ZnCl2 aqueous solution increased to 65 wt% (hydration ratio decreased to 4.07), the obtained ZnO was globular, with an approximately 40–80 nm diameter (Fig. 6b). In addition, as shown in Fig. 6c, uniform and globular ZnO nanoparticles with a diameter of approximately 40 nm were obtained from 80 wt% ZnCl2 aqueous solution (hydration ratio of 1.89). In fact, the morphologies of ZnO could be controlled by the concentration of ZnCl2 aqueous solution (or the hydration ratio). The results are consistent with those of Poul et al. [31]. However, in their polyol process, DEG served as a solvent, and a low concentration of ZnO precursors (lower than 0.3 M) at the DEG boiling point was used. Moreover, uneven and larger ZnO nanoparticles were fabricated in the absence of glycerol at a 65 wt% concentration of ZnCl2 (Fig. 1a). In this study, the ZnO nanoparticle was obtained using relatively highly concentrated (80 wt%, namely, 29.3 M) ZnCl2 at room temperature. In addition, in the presence of glycerol, the size of ZnO nanoparticles decreased as the concentration of ZnCl2 aqueous solution increased, which disagreed with the previous results. It may be that as the concentration of ZnCl2 was increasing (less water) in our approach, the interaction abilities of zinc ions and the hydroxyl oxygens of glycerol increased, meaning that the blocking effect of glycerol was enhanced, resulting in smaller ZnO nanoparticles.
Fig. 6.
SEM images of ZnO nanoparticles obtained from the different concentration of ZnCl2 aqueous solution under the 1:1 of mole ratio of glycerol to Zn2+ (a, 50 wt%; b, 65 wt%; c, 80 wt%)
The Influence of the Hydroxides on ZnO Nanoparticles Size and Morphology
The impact of the types of hydroxides on ZnO nanoparticles size and morphology were also investigated under obstruction with glycerol as stabilizer. The objective was to prepare ZnO nanoparticles with the highest concentration of substances in this study. Therefore, saturated solution of NaOH, KOH, LiOH, and NH4OH were prepared at room temperature: LiOH at 8 wt% (3.63 M), NaOH at 50 wt% (25 M), KOH at 60 wt% (26.74 M), and ammonia at 25 wt% (9.51 M). Meanwhile, to generate more even and smaller ZnO nanoparticles, a 3.33:1 mol ratio of glycerol to Zn2+ was employed.
Figure 7 shows SEM images of ZnO nanoparticles obtained from 65% ZnCl2 aqueous solution by reaction with various hydroxides. The results indicated that the hydroxides had an obvious influence on the size of ZnO nanoparticles. The ZnO nanoparticles obtained from NaOH, KOH, LiOH, and NH4OH were all granular, and the sizes of ZnO nanoparticles were approximately 20 nm from NaOH, 50 nm from KOH, 80–150 nm from LiOH, and 50–300 nm from NH4OH, respectively. It could be proposed that due to high concentrations of ZnCl2 solution (65 wt%) and hydroxides, the formation of ZnO was very fast during the early nucleation stage, generating many ZnO nuclei near glycerol. Meanwhile, the cations, such as Na+, K+, Li+, or NH4+, could provide a passivating layer around the ZnO surface, slowing the growth of ZnO nanoparticles and preventing particle agglomeration. The radii sequence of hydrated cationic is Li+ > Na+ > NH4+ > K+. Higher concentrations of K+ or NH4+ than Li+ or Na+ were required to provide the near-complete passivation on the ZnO surfaces. Additionally, the saturation concentrations of LiOH and ammonia water were 3.63 and 9.51 M, which are much lower than those of NaOH (25 M), and KOH (26.74 M). The amount of Li+ and NH4+ could not provide the near-complete passivation on the ZnO surfaces, inhibiting any further growth of ZnO nanoparticles. In addition, the concentrations of LiOH and ammonia water were low, meaning that more water reduced the interaction abilities of zinc ions and the hydroxyl oxygens of the glycerol, resulting simultaneously in the reduction of the blocking effect of glycerol. Therefore, ZnO nanoparticles obtained from LiOH and NH4OH were bigger.
Fig. 7.
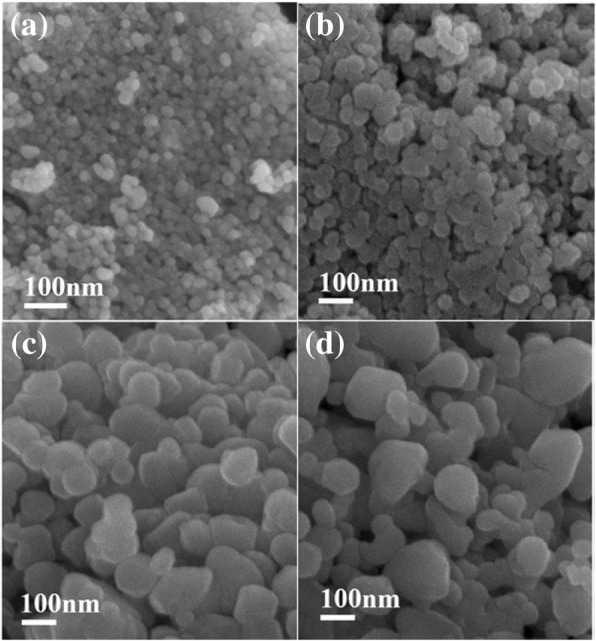
SEM images of ZnO nanoparticles obtained from 65% ZnCl2 aqueous solution by reactivity with various hydroxides (a, NaOH; b, KOH; c, LiOH; d, NH4OH)
Preparation of ZnO Nanoparticles Obstruction by Glycerol, Starch, or Cellulose Systems
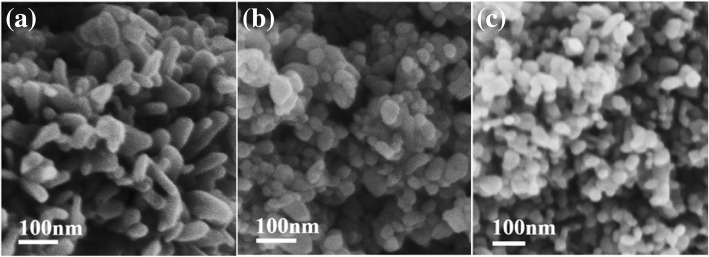
In our previous study, ZnO nanoparticles were prepared via in situ synthesis of ZnO in dissolved starch or cellulose by a highly concentrated ZnCl2 aqueous solution [28, 29]. The glycerol system is compared with the dissolved starch or cellulose systems in this paper. As seen from Fig. 8, the ZnO nanoparticles were globular from all three processes. The ZnO nanoparticles obtained from dissolved starch or cellulose systems were 50–60 nm [28] or 40–50 nm [29], respectively, while those obtained from the glycerol system were 15–25 nm. More uniform and smaller ZnO nanoparticles could be prepared from the glycerol system. Although there are many hydroxyls on the molecular chains of starch and cellulose, there are three hydroxyls on the glycerol chain, viscosities of the zinc-cellulose or the zinc-starch composite are higher than that of the zinc-glycerol. So the colloid mill made the zinc-glycerol or ZnO-glycerol composite more easily into smaller droplets than ZnO-cellulose or ZnO-starch, resulting in smaller ZnO nanoparticles generated from the glycerol system.
Fig. 8.
TEM images of ZnO nanoparticles obtained from 65% ZnCl2 aqueous solution obstruction by glycerol (a), starch (b), and cellulose (c) systems
In dissolved starch or cellulose systems, starch or cellulose were dissolved in a highly concentrated ZnCl2 aqueous solution, and then the ZnO-starch or cellulose nanocomposites were generated when the 40 wt% NaOH solution was added into ZnCl2 aqueous solution. The ZnO nanoparticles were obtained by calcining the dried ZnO-starch or cellulose nanocomposites. For the glycerol system, the glycerol was added into a concentrated ZnCl2 aqueous solution. The ZnO nanoparticles were easily obtained as the 50 wt% NaOH solution was added into glycerol-ZnCl2 aqueous solution. Therefore, the process using the glycerol system is easier and more cost-effective.
Conclusions
Approximately 20 nm ZnO nanoparticles were prepared by a simple process in which hydroxide aqueous solutions were added to a glycerol-zinc chloride solution at room temperature to adjust the pH value to 12 with an extremely concentrated zinc source. The morphologies of ZnO could be controlled by the mole ratio of glycerol to Zn2+, the type of hydroxide, and the concentration of ZnCl2 aqueous solution. The glycerol acted as a stabilizer during the synthesis process; its blocking effect enhanced as the concentration of ZnCl2 aqueous solution or the mole ratio of glycerol to Zn2+ increased. The shape of ZnO changed from rods to globular, and the particle size decreased as the concentration of ZnCl2 aqueous solution or the mole ratio of glycerol to Zn2+ increased. Under optimal conditions, globular ZnO with an approximately 40–80 nm diameter was obtained from a ZnCl2 aqueous solution with a concentration of 65 wt% and a 1:1 mol ratio of glycerol to Zn2+. Moreover, the hydroxides also had an obvious influence on the size of ZnO particles. The granular ZnO nanoparticles with diameters approximately 20 or 50 nm could be generated from NaOH or KOH solutions, respectively, with saturation concentrations at room temperature. This study thus proposed a facile and size-controllable process for the synthesis of ZnO nanoparticles.
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31570576) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- BD
1,4-butanediol
- DEG
Diethylene glycol
- EG
Ethylene glycol
- i-PrOH
Iso-propanol
- PD
1,3-propanediol
- SEM
Scanning electron microscope
- TEG
Tetraethylene glycol
- TEM
Transmission electron microscope
- XRD
X-ray diffraction patterns
- ZnO
Zinc oxide
Authors’ Contributions
ZW designed the research project; participated in TEM, SEM, and XRD analysis; and drafted the manuscript. HL and FT carried out the whole experimental progress. JM and XZ participated in the design and coordination of this research as well as the revision of the manuscript. All of the above authors have read and approved the final manuscript.
Authors’ Information
ZW is an associate professor and Master supervisor in the Jiangsu Provincial Key Lab of Pulp and Paper Science and Technology, Nanjing Forestry University. HL and FT are graduates in the Jiangsu Provincial Key Lab of Pulp and Paper Science and Technology, Nanjing Forestry University. JM and XZ are professors and Doctor supervisors in the Jiangsu Provincial Key Lab of Pulp and Paper Science and Technology, Nanjing Forestry University.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhiguo Wang, Email: wzg@njfu.edu.cn.
Hongwei Li, Email: 1490027384@qq.com.
Fanghua Tang, Email: 1194754488@qq.com.
Jinxia Ma, Phone: +86-25-85427118, Email: jxma@njfu.edu.cn.
Xiaofan Zhou, Email: zxiaofan@njfu.com.cn.
References
- 1.Chi PW, Wei DH. Dielectric enhancement with low dielectric loss in textured ZnO films inserted with NiFe. J Mater Chem C. 2017;5:1394–1401. doi: 10.1039/C6TC05164B. [DOI] [Google Scholar]
- 2.Law M, Greene LE, Johnson JC, Saykally R, Yang PD. Nanowire dye-sensitized solar cells. Nat Mater. 2005;4:455–459. doi: 10.1038/nmat1387. [DOI] [PubMed] [Google Scholar]
- 3.Umar A, Kim SH, Lee H, Lee N, Hahn YB. Optical and field emission properties of single-crystalline aligned ZnO nanorods grown on aluminium substrate. J Phys D Appl Phys. 2008;41(6):065412. doi: 10.1088/0022-3727/41/6/065412. [DOI] [Google Scholar]
- 4.Kathiravan D, Huang BR, Saravanan A. Multifunctional sustainable materials: the role of carbon existing protein in the enhanced gas and UV sensing performances of ZnO-based biofilms. J Mater Chem C. 2017;5:5239–5247. doi: 10.1039/C7TC01305A. [DOI] [Google Scholar]
- 5.Liu YT, Cai T, Wang LL, Zhang SQ, Zhang GZ, Xia XN. Hollow microsphere TiO2/ZnO p-n heterojuction with high photocatalytic performance for 2, 4-dinitropheno mineralization. Nano. 2017;12(06):1750076. doi: 10.1142/S179329201750076X. [DOI] [Google Scholar]
- 6.Mirhosseini M, Firouzabadi FB. Antibacterial activity of zinc oxide nanoparticle suspensions on food-borne pathogens. Int J Dairy Technol. 2013;66(2):291–295. doi: 10.1111/1471-0307.12015. [DOI] [Google Scholar]
- 7.Dorfman A, Kumar N, Hahm JI. Highly sensitive biomolecular fluorescence detection using nanoscale ZnO platforms. Langmuir. 2006;22(11):4890–4895. doi: 10.1021/la053270+. [DOI] [PubMed] [Google Scholar]
- 8.Gomez JL, Tigli O. Zinc oxide nanostructures: from growth to application. J Mater Sci. 2013;48(2):612–624. doi: 10.1007/s10853-012-6938-5. [DOI] [Google Scholar]
- 9.Chauhan I, Aggrawal S, Mohanty P. ZnO nanowire-immobilized paper matrices for visible light-induced antibacterial activity against Escherichia coli. Environ Sci: Nano. 2015;2:273–279. [Google Scholar]
- 10.Lizundia E, Urruchia A, Vilasa JL, Leon LM. Increased functional properties and thermal stability of flexible cellulose nanocrystal/ZnO films. Carbohydr Polym. 2016;136:250–258. doi: 10.1016/j.carbpol.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Park WI, Lee CH, Chae JH, Lee DH, Yi GC. Ultrafine ZnO nanowire electronic device arrays fabricated by selective metal-organic chemical vapor deposition. Small. 2009;5:181–184. doi: 10.1002/smll.200800617. [DOI] [PubMed] [Google Scholar]
- 12.Dejene FB, Ali AG, Swart HC, Botha RJ, Roro K, Coetsee L, Biggs MM. Optical properties of ZnO nanoparticles synthesized by varying the sodium hydroxide to zinc acetate molar ratios using the sol-gel process. Cent Eur J Phys. 2011;9:1321–1326. [Google Scholar]
- 13.Li Y, Wang LL, Liang J, Gao FX, Yi K, Dai P. Hierarchical heterostructure of ZnO@ TiO2 hollow spheres for highly efficient photocatalytic hydrogen evolution. Nanoscale Res Lett. 2017;12(1):531. doi: 10.1186/s11671-017-2304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amarilio-Burshtein I, Tamir S, Lifshitz Y. Growth modes of ZnO nanostructures from laser ablation. Appl Phys Lett. 2010;96:103104–103106. doi: 10.1063/1.3340948. [DOI] [Google Scholar]
- 15.Sarkar D, Tikku S, Thapar V, Srinivasa RS, Khilar KC. Formation of zinc oxide nanoparticles of different shapes in water-in-oil microemulsion. Colloids Surf A Physicochem Eng Asp. 2011;381:123–129. doi: 10.1016/j.colsurfa.2011.03.041. [DOI] [Google Scholar]
- 16.Lee S, Jeong S, Kim D, Hwang S, Jeon M, Moon J. ZnO nanoparticles with controlled shapes and sizes prepared using a simple polyol synthesis. Superlattice Microst. 2008;43:330–339. doi: 10.1016/j.spmi.2008.01.004. [DOI] [Google Scholar]
- 17.Kolodziejczak-Radzimska A, Jesionowski T. Zinc oxide-from synthesis to application: a review. Materials. 2013;7(4):2833–2881. doi: 10.3390/ma7042833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanje AS, Sharma SJ, Ningthoujam RS, Ahn JS, Pode RB. Low temperature dielectric studies of zinc oxide (ZnO) nanoparticles prepared by precipitation method. Adv Powder Technol. 2013;24(1):331–335. doi: 10.1016/j.apt.2012.08.005. [DOI] [Google Scholar]
- 19.Li H, Jiao S, Bai S, Li H, Gao S, Wang J, Yu Q, Guo F, Zhao L (2014) Precursor-controlled synthesis of different ZnO nanostructures by the hydrothermal method. Phys Status Solidi A 211:595–600
- 20.Espitia PJP, Soares NDF, Coimbra JSD, de Andrade NJ, Cruz RS, Medeiros EAA. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012;5:1447–1464. doi: 10.1007/s11947-012-0797-6. [DOI] [Google Scholar]
- 21.Hong R, Pan T, Qian J, Li H. Synthesis and surface modification of ZnO nanoparticles. Chem Eng J. 2006;119:71–81. doi: 10.1016/j.cej.2006.03.003. [DOI] [Google Scholar]
- 22.Feldmann C. Polyol-mediated synthesis of nanoscale functional materials. Solid State Sci. 2005;7:868–873. doi: 10.1016/j.solidstatesciences.2005.01.018. [DOI] [Google Scholar]
- 23.Chieng BW, Loo YY. Synthesis of ZnO nanoparticles by modified polyol method. Mater Lett. 2012;73:78–82. doi: 10.1016/j.matlet.2012.01.004. [DOI] [Google Scholar]
- 24.Trenque I, Mornet S, Duguet E, Gaudon M. New insights into crystallite size and cell parameters correlation for ZnO nanoparticles obtained from polyol-mediated synthesis. Inorg Chem. 2013;52:12811–12817. doi: 10.1021/ic402152f. [DOI] [PubMed] [Google Scholar]
- 25.Mezni A, Kouki F, Romdhane S, Warot-Fonrose B, Joulie S, Mlayah A, Smiri LS. Facile synthesis of ZnO nanocrystals in polyol. Mater Lett. 2012;86:153–156. doi: 10.1016/j.matlet.2012.07.054. [DOI] [Google Scholar]
- 26.Visinescu D, Scurtu M, Negrea R, Birjega R, Culita DC, Chifiriuc MC, Draghici C, Moreno JC, Musuc AM, Balint I. Additive-free 1,4-butanediol mediated synthesis: a suitable route to obtain nanostructured, mesoporous spherical zinc oxide materials with multifunctional properties. RSC Adv. 2015;5(121):99976–99989. doi: 10.1039/C5RA20224H. [DOI] [Google Scholar]
- 27.Chen G, et al. et al. Zinc and zinc alloys- cemented carbide. In: Chen G, et al.et al., editors. Encyclopedia of the Chemical Industry, Chemical Industry Press of China, Beijing. 1996. pp. 32–34. [Google Scholar]
- 28.Ma J, Zhu W, Tian Y, Wang Z (2016) Preparation of zinc oxide-starch nanocomposite and its application on coating. Nanoscale Res Lett 11(1):1–9 [DOI] [PMC free article] [PubMed]
- 29.Ma J, Sun Z, Wang Z, Zhou X. Preparation of ZnO-cellulose nanocomposites by different cellulose solution systems with a colloid mill. Cellulose. 2016;23:3703–3715. doi: 10.1007/s10570-016-1081-0. [DOI] [Google Scholar]
- 30.Bell NS, Tallant DR. Ripening and growth of zinc oxide nanorods from nanoparticles in 1,4 butanediol solvent. J Sol-Gel Sci Technol. 2009;51(2):158–168. doi: 10.1007/s10971-009-1967-5. [DOI] [Google Scholar]
- 31.Poul L, Ammar S, Jouini N, Fievet F, Villain F. Metastable solid solutions in the system ZnO-CoO: synthesis by hydrolysis in polyol medium and study of the morphological characteristics. Solid State Sci. 2001;3:31–42. doi: 10.1016/S1293-2558(00)01129-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.