Abstract
Background
Locomotor muscle fatigue (LMMF) and exercise-induced muscle damage (EIMD) are common conditions experienced during long-distance running due to the pooled effect of mechanical and metabolic strain on the locomotor muscles. However, little is known about the instant effects of combined LMMF and EIMD on pacing behaviour and performance during the decisive final stages of ‘real-world’ long-distance running events.
Methods
Twenty-two highly trained runners (11 females) completed two maximal self-paced 20-km treadmill time trials in a counterbalanced crossover design: (A) in a tapered condition and (B) with LMMF and EIMD. Indicators of muscle damage, muscle metabolic strain, and endocrinological stress were assessed to investigate the physiological effects, and a three-dimensional framework of perceived fatigability was applied to investigate the perceptual effects of running with LMMF and EIMD on performance fatigability.
Results
LMMF and EIMD caused restrictions in work capacity and medium increases in blood leucocyte and neutrophil count, interleukin-6, and cortisol concentrations, collectively constituting a physiological milieu likely not conducive to high performance. LMMF and EIMD further caused large increases in perceived physical strain and large decreases in valence as well as large increases and decreases in action crisis and flow state, respectively.
Conclusions
Under the constraint of amplified physical duress, findings are suggestive of heuristic and rational antecedents in the goal disengagement process. Dynamic changes in physiological and perceptual effects of LMMF and EIMD are hypothesised to underpin the observed alterations in pacing behaviour and performance fatigability during long-distance running. The applied three-dimensional framework provides a more comprehensive understanding of strain-perception-thinking-action coupling in centrally regulated and goal-directed exercise behaviour.
Electronic supplementary material
The online version of this article (10.1186/s40798-018-0143-2) contains supplementary material, which is available to authorized users.
Keywords: Locomotor muscle fatigue, Exercise-induced muscle damage, Pacing behaviour, Endurance performance, Long-distance running, Central regulation, Psychophysiology, Perceived fatigability, Performance fatigability
Keypoints
Little is known about the instant effects of combined locomotor muscle fatigue (LMMF) and exercise-induced muscle damage (EIMD) on pacing behaviour and performance fatigability during the decisive final stages of ‘real-world’ long-distance running events.
Running with LMMF and EIMD caused medium increases in cardiovascular, respiratory, and metabolic responses as well as a non-adaptive physiological distress response, collectively constituting a physiological milieu not conducive to high performance.
Running with LMMF and EIMD further caused large increases in perceived physical strain and decreases in valence as well as deteriorations in action crisis and flow state suggestive of heuristic and rational antecedents in the goal disengagement process.
Dynamic changes in physiological and perceptual effects are hypothesised to underpin the observed alterations in pacing behaviour and performance during long-distance running with LMMF and EIMD.
Background
Unaccustomed muscular exertion during training for, and competition in, prolonged endurance events can result in significant locomotor muscle fatigue (LMMF) and exercise-induced muscle damage (EIMD) [1]. Particularly, activities involving muscle-lengthening contractions such as running are more likely to induce muscle damage due to high muscular strain coinciding with low neuromuscular recruitment. Thus, the underlying cause of EIMD is suggested to be largely mechanical in nature [2]. However, EIMD also occurs in activities predominantly involving muscle shortening contractions such as swimming and cycling. Metabolic deficiencies are therefore also suggested to play a significant role in muscle damage when metabolic strain outweighs mechanical strain [3]. Accordingly, EIMD is a commonly experienced condition in long-distance running events characterised by a pooled effect of mechanical and metabolic strain on skeletal muscle injury [1, 4–6].
So far, research has focussed on the physiological consequences of EIMD day(s) after inducing it and therefore largely on delayed onset of muscular soreness (DOMS). For example, review articles discussed indicators of muscle damage, the repeated bout effect, and sex differences [7]; impact on neuromuscular function and excitation-contraction coupling, sarcomere insertion, and damage to contractile machinery and selective fibre types [8]; impaired metabolism [3]; and inflammatory and cytokine response [9, 10], as well as molecular and cellular mechanisms of damage and recovery [11].
More specifically, EIMD can negatively impact endurance performance by altering cardiorespiratory, metabolic, biomechanical, and thermoregulatory variables. This includes but is not limited to tachycardia [12, 13], augmented ventilatory response and oxygen consumption [14, 15], attenuated peak oxygen uptake and ventilatory threshold [16], increased intramuscular carbohydrate oxidation [17], increased blood lactate concentrations [18–20], compensatory modifications in running kinematics [21], decreased running economy [18, 22], and increased core temperature [23]. Critically, differential responses in these variables are more likely to occur once ventilatory threshold intensities are surpassed. In addition, the effect sizes of individual variables are only small to moderate, which might explain why experimental studies with usually small sample sizes of ≤ 10 participants do not consistently reach statistical significance, thereby erroneously suggesting conflicting findings or null effects.
Furthermore, these debilitating effects of increased physiological strain on endurance performance are accompanied by debilitating effects of increased perceived fatigability (defined as the changes in perceptions that regulate the integrity of the performer [24]). This includes and is currently limited to increased perception of effort and perceived exertion [12, 13, 15, 25] and exercise-induced pain [26, 27]—two of several determinants in the central regulation of pacing behaviour and endurance performance [28].
However, most of the hitherto mentioned studies investigated the impact of EIMD day(s) after inducing it and are therefore confounded by the pronounced systemic inflammatory response characterising DOMS. Accordingly, little is known about the instant effects of combined LMMF and EIMD on pacing behaviour and endurance performance as experienced during the decisive final stages of ‘real-world’ long-distance running events.
In contrast, two studies investigated the instant effects of LMMF and mild EIMD (average force loss of 19%) on high-intensity cycling exercise of ≈ 15 min in moderately trained participants. Marcora et al. [13] used a time-to-exhaustion protocol at 80% of peak power output and observed significant decreases in endurance performance as well as significant increases in tachycardia, tachypnoea, and perception of effort during the fatigue trial. de Morree and Marcora [29] used a 15-min time trial and observed large decreases in average power output (without differences in pacing behaviour), large decreases in vastus lateralis electromyogram activity over time, large reductions in blood lactate concentrations at the end, and large increases in perception of effort at the start of the intervention time trial. Both authors proposed a mediatory role of perception of effort in the relationship between LMMF and mild EIMD and endurance performance consistent with increased central motor command and reafferent signalling.1 However, the findings are of limited use in understanding the psychophysiological regulation of pacing behaviour and performance in high-performance long-distance runners, as they used cyclists rather than runners, moderately rather than highly trained athletes, and short rather than prolonged time trials, and further solely assessed effort perception rather than using a more comprehensive multi-dimensional approach.
Recently, a three-dimensional framework of centrally regulated and goal-directed exercise behaviour was proposed, which emphasises the dynamic and complex interplay of sensory, affective, and cognitive processes that underpin perceived fatigability [30]. This framework more completely accounted for perception-thinking-action coupling in response to psychological distress (i.e. falling behind a performance matched competitor) than the traditional Gestalt concept of perceived exertion [31]. Here, the proposed framework was applied under the constraint of physical distress investigating the psychophysiological determinants of pacing behaviour and endurance performance in highly trained long-distance runners during two self-paced maximal 20-km treadmill time trials: (A) running in a tapered condition versus (B) running with LMMF and EIMD produced by a standardised drop-jump protocol. We hypothesised that the physiological and perceptual effects of amplified physical strain are associated with dynamic changes in performance fatigability (defined as the observed decline in an objective measure of endurance performance over a discrete period of time [24]) and that strain-perception-thinking-action coupling of sensory, affective, and cognitive processes more completely accounts for the dynamic changes in observed pacing behaviour.
Methods
Participants
Twenty-two runners, 11 females, estimated to fit performance level 4 criteria [32], were recruited from local running clubs via email distribution of digital flyers. Main inclusion criteria were a half marathon personal best of less than 1 h:40 min and 1 h:25 min for female and male runners, respectively. The 15% absolute performance difference accurately reflects the average sex difference in the TOP-100 finishing times of an international half marathon race event (www.twooceansmarathon.org.za) hosted locally. Performance level categorisation of each participant was subsequently verified according to criteria by DePauw and colleagues [32, 33]. Sample size estimation using conventional methods (one-tailed alpha of < .05, β > .80, and large effect size) and G*Power 3 software (G*Power, Version 3.1.9.2, Kiel, Germany) indicated overall 20 participants would be needed. All participants provided prior written informed consent to the procedures used in this study, which were approved by the institutional ethics committee and carried out in accordance with the Declaration of Helsinki.
Study Design
Participants completed in total four visits including three maximal self-paced 20-km treadmill time trials over a simulated profiled course. A 10-km time trial took place after preliminary testing and acted as a submaximal familiarisation time trial (FTT). Before returning for their second visit, participants were instructed to log their training and diet for 48 h prior to the baseline time trial (BTT) and to prepare in a way that resembled their routine before an important race. They were then advised to repeat this ‘mini taper’ for the following experimental time trials. Participants then completed the intervention time trial (ITT) and control time trial (CTT) in a counterbalanced AB/BA crossover design. The ITT was preceded by a standardised drop-jump protocol (for details, see the “Drop-Jump Protocol” section below), while the CTT was preceded by a rest period of equal length. A washout period of 4 weeks took place before the crossover trial to (A) allow for complete recovery of muscle damage and (B) control for menstrual cycle in female runners (two were using hormonal contraception and two were amenorrhoeic), who were instructed to schedule experimental trials 5 to 9 days after the start of menses (early follicular phase).
Procedures
Preliminary Measurements
During the first laboratory attendance, each participant had their age, stature, and body mass recorded. Body fat percentage was estimated using the seven skinfold method [34].
Peak Treadmill Running Speed Test
A peak treadmill running speed (PTRS) test with peak oxygen consumption (VO2peak) was performed on a motor-driven treadmill (Viasys LE500 CE, Hoechberg-Wuerzburg, Germany). The treadmill gradient was set to 1% simulating the energetic costs of outdoor running [35]. After a 10-min self-paced warm-up, participants started the PTRS at 10 and 11 km h−1 for female and male runners, respectively, after which speed increased stepwise by 0.5 km h−1 every minute. PTRS was calculated as the last completed stage added to the product of the speed increment and the completed fraction of the incomplete stage. During the progressive exercise test, expired ventilation volume (VE), oxygen uptake (VO2), and carbon dioxide production (VCO2) were measured with an online breath-by-breath gas analyser and pneumotach (Cosmed Quark b2, Rome, Italy). Calibration took place before each trial according to the manufacturer’s instructions using a 3-l syringe (Hans Rudolph 5530, Inc., Kansas City, MO, USA) and a gas mixture of known composition (5.05% CO2; 15.97% N2). Peak oxygen uptake was determined as the highest recorded VO2 measurement averaged over 30 s. The first ventilatory threshold and respiratory compensation point were determined according to the methods described by Lucia et al. [36]
Maximum Isokinetic Power Output Test
Maximum isokinetic power output of the quadriceps and hamstring muscles of the dominant leg was measured using the Biodex 3 Isokinetic dynamometer (Biodex Medical Systems, Shirley, NY, USA). Participants were familiarised with the procedure before the FTT and BTT. Participants were seated with their arms crossed over their chest, the hip flexed at 90°, and a knee angle of 90° from full leg extension. The lateral condyle of the femur was aligned with the rotational axis of the dynamometer, and the ankle was secured to the lever arm of the dynamometer using a padded Velcro strap. Shoulder and waist straps were used to fixate joint positions during trials. Standardisation was enhanced by performing gravity correction. After a warm-up set of increasing intensity (50, 70, and 90%), participants performed six maximal isokinetic contractions at 120° s−1. Isokinetic measurements were chosen due to the repetitive cyclic nature of running specific muscle contractions, and the above movement velocity was selected as it more closely resembles running specific contraction speeds as well as provides a trade-off between peak and explosive power output usually measured at < 60 s−1 and > 180° s−1, respectively. Power output curves were displayed on a screen in front of the participant, and verbal encouragement was given during maximal contractions.
During the experimental trials, maximum isokinetic power output was assessed twice: (1) after the 10-min self-paced warm-up and (2) within 2 min after completing the drop-jump protocol and control period, respectively.
Running Economy Test
Assessment of running economy took place before each of the three maximal 20-km time trials. After 5 min of running at a constant speed of 10 and 11 km h−1 for female and male runners, respectively, participants ran for 5 min at a speed corresponding to ∆ 1/3 between the first ventilatory threshold and the respiratory compensation point determined during the PTRS. The average values of breath-by-breath VO2 and VCO2 during the final minutes were used to calculate the oxygen cost and energy cost of running. Updated non-protein respiratory quotient equations were used to estimate substrate use (g min−1) during the monitored period [37]. The mean energy content of the metabolised substrates was then calculated according to the methods described by Jeukendrup and Wallis [38], scaled to body mass (BM−1) and expressed per kilometre (km−1) [39].
Time Trial Procedure
Participants performed three maximal self-paced 20-km time trials over a simulated profiled course on the same treadmill. A customised course profile was written using h/p/cosmos para-graphics® software (Version 2.6.14, h/p/cosmos sports and medical GmbH, Nussdorf-Traunstein, Germany) simulating a 20-km long time trial with two uphill sections of 2 km length and a gradient of 7%, starting after 4 and 12 km, respectively. The uphill sections were immediately followed by two downhill sections of the same length and gradient. Participants were assisted with gradient-dependent alterations in running speed, but custom-made modifications of the treadmill enabled participants to self-select running speed at any time with a handheld remote control in 0.1 km h−1 increments. Only course profile, gradient, and distance covered were displayed to participants during each time trial. Participants were instructed to complete the time trial in the shortest possible time, but no verbal encouragement was given during the time trial itself.
A fan was placed 1.5 m in front of the participants, and the fan level was adjusted in accordance with the course profile to simulate speed-related alterations in peripheral cooling. During the uphill sections, fans were set on level 1, during the flat sections on level 2, and during the downhill sections on level 3, respectively, creating average wind speeds of 3.15, 3.80, and 4.25 ms−1. Participants were permitted to consume water ad libitum, and a commercially available carbohydrate drink (Enduren™ Endurance Energy Drink) was provided at 2-km intervals and upon request for an average rate of ≈ 60 g h−1. All experimental trials were conducted under stable climatic conditions (temperature 20.5 ± 0.7 °C; humidity 58.3 ± 5.1%) and commenced at 8 am to control for diurnal variations.
During each running economy test and time trial, speed, gradient, and distance covered were recorded and stored at 1-s intervals using h/p/cosmos para-graphics® software. Heart rate was recorded at 2-s intervals throughout each trial by telemetry (Suunto® T6, Suunto Oy, Vantaa, Finland). Performance and heart rate data were subsequently analysed with TrainingPeaks™ analysis software (WKO edition+, Version 3.0, Lafayette, CO, USA). All continuously captured data were averaged into 2-km bins before statistical analyses were performed.
Drop-Jump Protocol
During the intervention trial only, participants performed muscle-lengthening contraction exercise consisting of 100 drop-jumps from a step of 45 cm height [40] known to induce force losses consistent with mild EIMD and without confounding effects of significant muscle metabolite accumulation (blood lactate concentration [mmol/l]: CTT = 1.5 ± 0.6 vs ITT = 1.4 ± 0.6) and cardiovascular demands (heart rate [bpm]: CTT = 65 ± 10 vs ITT = 100 ± 11) [29]. Participants dropped to an approximate knee angle of 90° before jumping upward as high as possible. Drop-jumps were performed every 20 s for a total time of ≈ 33 min. Vertical displacement of the centre of mass (COM) was calculated by means of a floor-embedded force plate (AMTI, Watertown, MA, USA) and a customised programme written in MATLAB® (R2013a, The Mathworks Inc., Natick, MA, USA) determining vertical take-off velocity of COM [41]:
Equation 1: determining jump-height from vertical take-off velocity
where TOV = vertical velocity of COM at take-off, g = 9.81 ms−2.
Before and after the drop-jump protocol and rest period, perceived muscle discomfort and unpleasantness were assessed by means of 100 mm visual analogue scales (VAS) ranging from ‘no muscle discomfort/unpleasantness at all’ to ‘unbearable muscle discomfort/unpleasantness’. The extent to which participants experienced DOMS in the 84-h recovery period after experimental trials was assessed using a 7-point Likert-type scale [42].
Measurements
Measures of Perceived Fatigability
During the final minutes of the running economy test and at 2-km intervals during each time trial, participants provided ratings on the following four single-item scales presented in random order. All scales were anchored during the PTRS test.
The 15-point (6–20) Borg scale with the indicator terms ‘light’ and ‘strong’ was used to approximate perceived physical strain by phrasing ‘How strong are the physical sensations from your legs, lungs, and body?’ Participants were instructed to include only the subjective perception of physical sensations caused by the task and to focus on location, quality, and intensity of physical sensations before returning an overall perceived physical strain score.
The 15-point (0–14) Borg scale with the indicator terms ‘easy’ and ‘hard’ was used to approximate perceived mental strain by phrasing ‘How difficult is it to run at this pace?’ Participants were instructed to include only perceived task difficulty and mental effort invested into/required to continue with the task before returning a perceived mental strain score.
In line with recent recommendations [43], a detailed three-tiered justification process has been outlined for measurement selection in the assessment of dynamic changes in core affective state during prolonged endurance exercise [44]. Ultimately, the 11-point (− 5 ‘very bad’ to 0 ‘neutral’ to + 5 ‘very good’) Feeling Scale (FS) and 6-point (1 ‘low activation’ to 6 ‘high activation’) Felt Arousal Scale (FAS) were chosen to approximate dynamic changes in valence and felt activation, respectively [45, 46].
The Action Crisis Scale (ACRISS) and short Flow State Scale (FSS) were administered during the 30-min recovery period retrospectively measuring the extent to which participants experienced a shift from an implemental to a deliberative mindset. Both scales were administered for seven sections of the profiled TT course (three flat, two uphill, and two downhill) and rated on 5-point Likert-type scales.
The ACRISS comprises six items: conflict, setbacks, implemental disorientation, rumination, disengagement impulses, and procrastination [47]. The internal consistency estimate of reliability was good with mean Cronbach’s alpha of 0.83 ± 0.08 and 0.89 ± 0.04 during the control and intervention time trial, respectively.
The FSS comprises nine items: challenge-skill balance, action-awareness merging, clear goals, unambiguous feedback, concentration on task at hand, sense of control, loss of self-consciousness, transformation of time, and autotelic experience [48]. The internal consistency estimate of reliability was good with mean Cronbach’s alpha of 0.81 ± 0.06 and 0.81 ± 0.07 during the control and intervention time trial, respectively.2
Physiological Measures
Venous blood samples from a superficial antecubital vein were taken at rest, after the drop-jump protocol, after the running economy test, halfway through and at the end of experimental time trials as well as after the 30-min recovery period. Blood samples were placed into four different pre-chilled Vacutainers, respectively containing (A) serum clot activator for the analysis of cortisol, (B) potassium oxalate and sodium fluoride for the analysis of lactate concentrations, and (C) K2-ethylenediaminetetraacetic acid (EDTA) for the analysis of interleukin-6, differentiated white blood cell count, haemoglobin, and haematocrit. Where appropriate, samples were inverted five times, immediately centrifuged at 3000 rpm at 4 °C for 10 min, plasma/serum pipetted off, and kept on ice until stored at − 80 °C for subsequent analysis. Plasma lactate concentrations were determined using glucose oxidase method (YSI 2300 STAT PLUS, Ohio, USA). Serum cortisol was determined using an automated chemiluminescence system (Architect iSR, Abbott Diagnostics, IL, USA) with conventional reagent kits and calibrators. Interleukin-6 was analysed by means of enzyme-linked immunosorbent assay (ELISA) (eBioscience, Bender MedSystems, Vienna, Austria). Differentiated white blood cell count, haematocrit, and haemoglobin were determined by Lancet Laboratories using conventional methods. The degree of haemoconcentration was calculated, and all blood samples were subsequently corrected for plasma volume changes [49].
Measures of Performance Fatigability
Besides the assessment of global time trial performance between experimental conditions, this study aimed to analyse the decline in objective measures of endurance performance over time. The second-half to first-half split time quotient was calculated as a crude assessment of the degree of positive or negative pacing, and repeated measures of 2-km split time intervals were used to locate onset and extent of performance fatigability. For a clearer graphical presentation, dynamics in perceived fatigability were further indicated by the percentage increase in split times during ITT compared to CTT and accordingly assessed via one-way repeated measures ANOVA.
Statistical Analysis
Data were tested for assumptions, normality, equality of variances, equality of covariance matrices, and sphericity where appropriate. Independent samples t tests were used to analyse inter-individual differences in parameters between the sexes, and paired samples t tests were used for intra-individual comparisons in parameters between experimental trials. Between-group effect sizes were calculated using Cohen’s d (trivial < 0.20, small 0.21–0.60, medium 0.61–1.20, large 1.21–2.00, very large 2.01–4.00, and near perfect > 4.00). A non-parametric Mann-Whitney U test was performed when the assumption of equality of variance was violated. Confidence intervals and magnitude-based inferences for meaningful differences between time trial performances were derived from p values in accordance with Hopkins [50]. Two-way repeated measures ANOVAs were used to compare changes in parameters over time between experimental trials. Significant interaction effects were followed up with Bonferroni correction procedure. A Greenhouse-Geisser epsilon adjustment was made when sphericity was violated. Aligned rank transformation procedure (ARTool software 1.5.1, Washington, USA) was used to perform non-parametric factorial analysis on all perceptive data and when assumptions of parametric tests were violated [51]. All ANOVA effect sizes were calculated as partial eta squared (ηp2) and classified as small 0.02–0.13, medium 0.13–0.26, and large > 0.26. All data are presented as mean ± one standard deviation, and an alpha level of < .05 (two-tailed) was used to indicate statistical significance.
Results
Participant Characteristics
Anthropometric characteristics and performance parameters of female (n = 11) and male (n = 11) participants are listed in Table 1.
Table 1.
Sex comparison of anthropometric, training, and performance data
| Male (n = 11) | Female (n = 11) | p value | Effect size | |
|---|---|---|---|---|
| Descriptive data | ||||
| Age (years) | 29 ± 7 | 28 ± 10 | n.s. | – |
| Stature (cm) | 173 ± 7 | 163 ± 5 | .001 | Large |
| Body mass (kg)* | 64.7 ± 8.9 | 54.2 ± 5.2 | .016 | Large |
| Percentage body fat (%) | 5.9 ± 1.7 | 14.8 ± 2.1 | < .001 | Near perfect |
| Training data | ||||
| Weekly volume (km week−1) | 126 ± 61 | 61 ± 38 | .008 | Large |
| Other training (h week−1)* | 3.1 ± 4.9 | 5.8 ± 4.2 | .021 | Small |
| Training history (years) | 8.3 ± 4.4 | 4.2 ± 2.5 | .019 | Medium |
| Performance data | ||||
| PTRS (km h−1) | 20.1 ± 1.0 | 17.1 ± 0.7 | < .001 | Very large |
| Speed at VT-1 (km h−1) | 15.6 ± 0.9 | 12.8 ± 0.7 | < .001 | Very large |
| Speed at RCP (km h−1) | 17.6 ± 0.8 | 14.8 ± 0.9 | < .001 | Very large |
| abs VO2peak (ml min−1 kg−1) | 4.49 ± 0.53 | 3.15 ± 0.43 | < .001 | Very large |
| rel VO2peak (ml min−1 kg−1) | 69.8 ± 5.6 | 58.0 ± 4.6 | < .001 | Very large |
| VO2·VO2peak−1 @ VT-1 (%) | 78.8 ± 4.0 | 79.3 ± 2.7 | n.s. | – |
| VO2·VO2peak−1 @ RCP (%) | 89.5 ± 3.0 | 90.4 ± 2.9 | n.s. | – |
| CTT time (h:min:s) | 1:14:39 ± 0:04:31 | 1:31:43 ± 0:06:54 | < .001 | Very large |
* = the assumption of equality of variances was violated and thus non-parametric Mann-Whitney U test was performed. Abbreviations: PTRS peak treadmill running speed, abs absolute; rel = relative, VO2peak peak oxygen consumption, VT-1 first ventilatory threshold, RCP respiratory compensation point, CTT control time trial; data represented as mean ± SD
Despite the large to very large differences in anthropometric, training, and performance parameters between the sexes, there were neither significant differences in perceived fatigability and observed pacing behaviour during time trials nor in running performance after controlling for sex-dependent differences in body composition. Thus, psychophysiological responses to running in a tapered condition versus running with LMMF and EIMD are hereafter presented for female and male participants combined.
Psychophysiological Responses to Intervention and Running Economy Test
A summary of perceptual responses to the drop-jump protocol and its subsequent impact on the isokinetic power output of knee extensors, psychophysiological responses during the running economy test, and time trial performance is provided in Table 2.
Table 2.
Responses to drop-jump protocol and impact on power output, running economy, and time trial performance
| Control (n = 22) | Intervention (n = 22) | p value | Effect size | |
|---|---|---|---|---|
| Drop-jump protocol | ||||
| Perceptual responses | ||||
| Muscle discomfort pre* | 1.0 ± 0.9 | 0.7 ± 0.6 | n.s. | – |
| Muscle discomfort post* | 0.8 ± 0.8 | 2.4 ± 1.7 | < 0.001 | Large |
| Unpleasantness pre* | 0.8 ± 0.7 | 0.8 ± 0.8 | n.s. | – |
| Unpleasantness post* | 0.8 ± 0.7 | 2.3 ± 1.6 | < 0.001 | Large |
| Isokinetic power output test | ||||
| Knee extension pre* (W) | 152 ± 40 | 150 ± 45 | n.s. | – |
| Knee extension post* (W) | 150 ± 40 | 133 ± 45 | < 0.001 | Large |
| Running economy test | ||||
| Oxygen cost (ml kg−1 km−1) | 199 ± 11 | 205 ± 14 | 0.005 | Small |
| Energy cost (kcal kg−1 km−1) | 0.99 ± 0.05 | 1.03 ± 0.07 | 0.002 | Medium |
| Carbohydrate usage (g min−1) | 2.74 ± 0.78 | 3.17 ± 0.96 | 0.013 | Small |
| Heart rate (bpm) | 158 ± 11 | 164 ± 11 | 0.001 | Small |
| Perceptual responses | ||||
| Perceived physical strain | 12.3 ± 1.9 | 13.5 ± 1.9 | 0.001 | Medium |
| Perceived mental strain | 6.2 ± 1.5 | 7.7 ± 1.6 | < 0.001 | Medium |
| Valence | 2.9 ± 1.3 | 1.5 ± 1.6 | < 0.001 | Medium |
| Felt activation | 3.8 ± 1.0 | 4.0 ± 0.7 | n.s. | – |
| Time trial times | ||||
| TT time (h:min:s) | 1:23:11 ± 0:10:25 | 1:26:37 ± 0:11:36 | < 0.001 | Small |
| 2nd-half/1st-half split | 0.1 ± 1.1 | 1.4 ± 2.2 | 0.003 | Medium |
* = simple (main) group effects (using partial eta squared as effect size measure) of two-way repeated measures ANOVA after significant treatment * time interaction effect. TT = time trial. Data represented as mean ± SD
Time Trial Times
Times taken to complete the CTT and ITT were 1 h:23 min:11 s ± 10 min:25 s and 1 h:26 min:37 s ± 11 min:36 s, respectively. The average time difference was 3.96%. The mechanistic inference for this difference is most likely substantially negative (99.9% most likely substantially negative, 0.1% most unlikely trivial, and 0.0% most unlikely substantially positive).
Haematological Indicators of Muscle Damage and Physiological Strain
Graphical depictions of haematological markers of muscle damage, muscle metabolic strain, and endocrinological stress during experimental trials are summarised in Fig. 1.
Fig. 1.
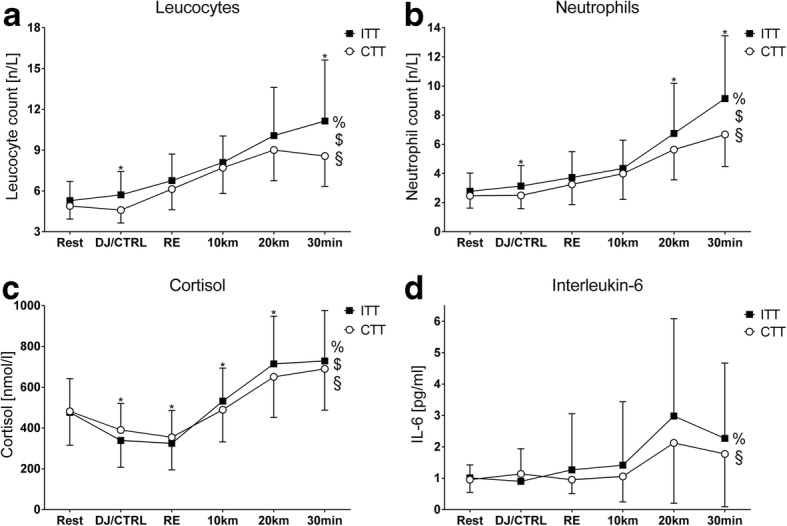
Differential responses in haematological markers of muscle damage, muscle metabolic strain, and endocrinological stress response. Panels show: a = blood leucocyte count; b = blood neutrophil count; c = blood cortisol concentration; d = blood interleukin-6 concentration. Note: blood interleukin-6 concentrations violated the assumption of normal distribution and thus non-parametric ANOVA after aligned rank transformation was conducted. Despite a significant interaction effect, simple main treatment effects did not reach significance. Abbreviations: # = main time effect; & = main group effect; % = time × group interaction effect; $ = simple (main) time effect for intervention trials; § = simple (main) time effect for control trials; * = simple (main) trial effect; ITT = intervention time trial; CTT = control time trial; DJ = drop-jump protocol; CTRL = control; RE = running economy
Sensory, Affective, and Cognitive Indicators of Perceived Fatigability
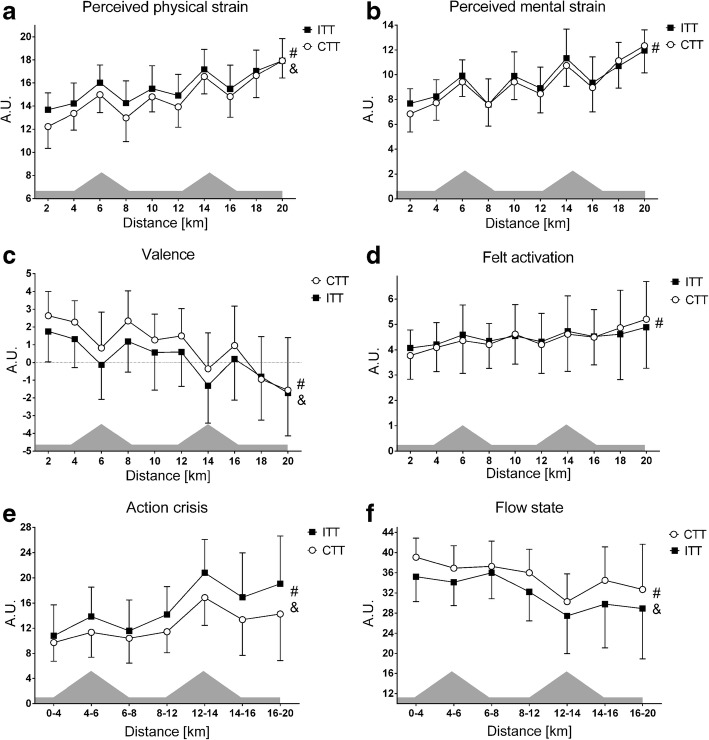
Graphical depictions of differential responses in sensory-discriminatory, affective-motivational, and cognitive-evaluative indicators of perceived fatigability during experimental trials are summarised in Fig. 2.
Fig. 2.
Differential responses in sensory, affective, and cognitive markers of perceived fatigability. Panels show: a = perceived physical strain; b = perceived mental strain; c = valence; d = felt activation; e = action crisis; f = flow state. Note the differences in the x-axes of action crisis and flow state due to different sampling times. The shaded topography represents the course profile of the 20-km treadmill time trial. Abbreviations: # = main time effect; & = main trial effect; ITT = intervention time trial; CTT = control time trial
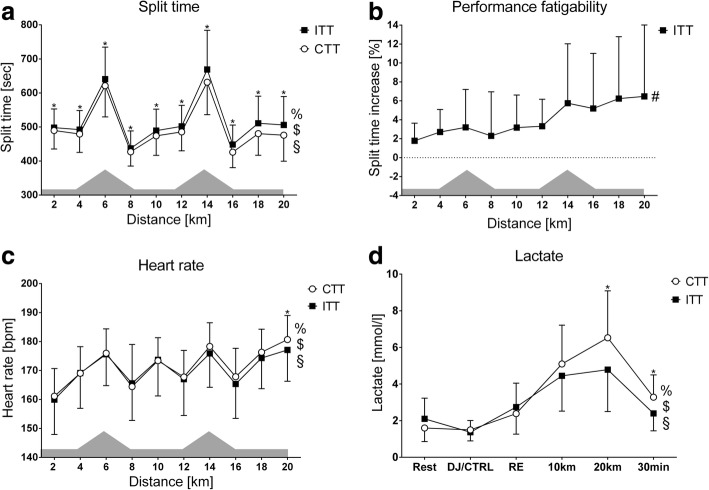
Indicators of Performance Fatigability
Graphical depictions of differential responses in markers of performance fatigability are summarised in Fig. 3.
Fig. 3.
Differential responses in markers of performance fatigability. Panels show: a = split time; b = performance fatigability; c = heart rate; d = blood lactate concentration. Note the differences in the x-axes of blood lactate concentrations due to different sampling times. Performance fatigability is indicated by the percentage increase in split time during the intervention time trial compared to control time trial and assessed via one-way repeated measures ANOVA. The shaded topography represents the course profile of the 20-km treadmill time trial. Abbreviations: # = main time effect; % = time × trial interaction effect; $ = simple (main) time effect for intervention trials; § = simple (main) time effect for control trials; * = simple (main) trial effect; ITT = intervention time trial; CTT = control time trial; DJ = drop-jump protocol; CTRL = control; RE = running economy
Performance, Perceptive, and Haematological Data
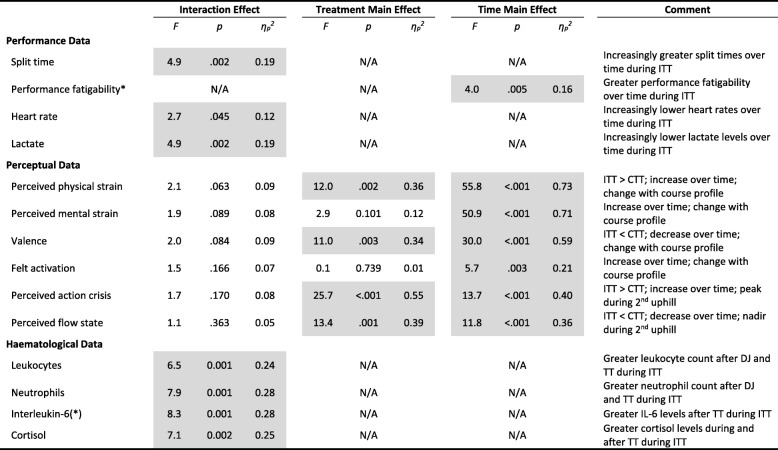
A summary of statistical findings in main performance, perceptive, and haematological variables is provided in Table 3.
Table 3.
Summary of treatment, time, and interaction effects in main performance, perceptive, and haematological variables
* = performance fatigability is indicated by the relative percentage increase in split times during ITT compared to CTT and assessed via one-way repeated measures ANOVA. (*) = blood interleukin-6 concentrations violated the assumption of normal distribution and thus non-parametric ANOVA after aligned rank transformation was conducted. For details, see Additional file 1. Note: shaded areas significant at p < .05; TT = time trial; ITT = intervention time trial; CTT = control time trial; DJ = drop-jump protocol
Due to space restrictions, the following results are provided in Additional file 1 only. Findings of deterioration in jump-height during the drop-jump protocol and the development of DOMS in the 84-h recovery period after experimental trials are provided in Additional file 1: SM1. The distributions of individual differences in knee extensor power output, time trial time, and time trial splits are provided in Additional file 1: SM2. Statistical details of two-way repeated measures ANOVA’s (trial × time) including follow-up tests and non-parametric ANOVA’s in perceptive variables are provided in Additional file 1: SM3 and results of three-way mixed repeated measures ANOVA’s (trial × time × sex) are provided in Additional file 1: SM4.
Discussion
According to Enoka and Duchateau [24], human fatigue is a psychophysiological symptom underpinned by interactions between (A) performance fatigability—the observed decline in an objective measure of endurance performance over a discrete period of time—and (B) perceived fatigability—changes in the perceptions that regulate the integrity of the performer based on the maintenance of homeostasis and the psychological state of the individual. The main findings of the current study indeed provide supportive evidence for physiological and perceptual mechanisms underlying observed pacing behaviour and performance fatigability.
Physiological Effects of LMMF and Mild EIMD on Performance Fatigability
First, the muscle-lengthening contraction protocol induced the desired muscle damage and reduction in power output generating the capacity of locomotor muscles (see Additional file 1: SM1) without confounding effects of significant muscle metabolite accumulation and cardiovascular stress. The large decrease of 11% in isokinetic strength is consistent with mild EIMD [10] (see Table 2), but only about half the decrease in isometric strength was observed in other studies [13, 29]. Although not directly comparable, the lesser strength loss is best explained by the high-performance calibre of our participants and the ‘repeated bout effect’ rendering runners more resistant to muscle damage induced by muscle-lengthening contractions than are moderately trained cyclists.
Second, running with LMMF and mild EIMD stimulated amplified responses in cardiovascular, respiratory, and metabolic variables during the running economy test. A small increase in heart rate, oxygen consumption, and carbohydrate usage (including concomitant trend towards decreased fat usage) as well as a medium increase in energy cost of running collectively point towards increased physiological demands (see Table 2). Thus, running with LMMF and mild EIMD at ∆ 1/3 between the first ventilatory threshold and respiratory compensation point (i.e. close to long-distance ‘race-pace’) increased absolute physiological strain and relative exercise intensity. Potential causes of augmented responses are manifold and, for example, could stem from inefficient alterations to running kinematics [18, 21, 22], increased afferent feedback from groups III and IV muscle afferent fibres resulting from structural disruption in the extrafusal muscle fibres and local microvasculature [52, 53], and increased compensatory efferent motor command to weakened locomotor muscles [13, 29]. Both, afferent feedback and efferent feedforward components therefore seem to impact and modulate physiological responses and collectively support the notion of an underlying reafference principle in the allostatic regulation of homeostasis [30].
Third, medium increases in blood leucocyte and neutrophil counts, interleukin-6, and cortisol concentrations were found during the ITT, respectively, indicating greater mobilisation and migration to damaged tissue (and thus indirectly EIMD), greater muscle metabolic strain [54, 55], and endocrinological stress (see Fig. 1 and Table 3). The absolute values in these variables closely resemble those observed after ‘real-world’ half marathon events [56]. Together, leucocytosis, neutrophilia, and pro-inflammatory immune response reflect the exacerbated physiological strain of running with LMMF and mild EIMD [57, 58]. Additionally, endocrinological stress response during the ITT was aggravated despite substantial performance decrement, thereby clearly indicating a non-adaptive distress response [59, 60]. Collectively, the previously described responses constitute a physiological milieu that is likely not conducive to high performance as evidenced by the one-tailed detrimental effects of LMMF and mild EIMD on exercise performance (see Additional file 1: SM2).
Accordingly, we suggest that muscle damage and the amplified physiological responses to running with LMMF and mild EIMD per se have a debilitating effect on endurance performance due to increased absolute physiological strain and relative exercise intensity conveyed by peripheral and central fatigue mechanisms [61, 62]. Time trial performance during ITT was constrained from the start indicating restricted work capacity and, potentially underpinned by accumulating EIMD, deteriorated further excessively from the second uphill onwards (see Fig. 3). Del Coso et al. [63] observed a similar differential pattern of pacing behaviour in marathon runners either maintaining (i.e. even split) or reducing (i.e. positive split) running speed and found significant correlations between increased performance fatigability and indirect haematological markers of muscle damage. However, running with LMMF and mild EIMD also elicited potentially negative effects on performance fatigability via deterioration in sensory, affective, and cognitive processes hypothesised to underpin perceived fatigability.
Perceptual Effects of LMMF and Mild EIMD on Performance Fatigability
First, a large increase in perceived muscle discomfort was found in response to the muscle-lengthening contractions protocol, although absolute scores are still relatively mild. In addition, medium increases in perceived physical and mental strain were observed in response to running with LMMF and mild EIMD during the running economy test (see Table 1). Perceived physical and mental strain showed a trend towards greater perceived strain scores during the start of the ITT, but eventually approached similar end-points (see Fig. 2). This is coherent with the findings from de Morree et al. [29] suggesting that both are primary regulatory variables of trajectory pacing behaviour [44].
A large strain construct × treatment interaction effect (F1,21 = 10.1; p = .005; = 0.32) was observed with a large main treatment effect for greater perceived physical strain only during the ITT (see Table 3). This points towards the accuracy of perceived physical strain in the psychophysiological integration of homeostatic disturbance and the ability of athletes to differentiate between perceived physical strain and perceived mental strain [64, 65]. Further empirical support was recently provided by Girard et al. [66], who observed associations between increased physiological disturbance and subsequent augmented exercise-related physical sensations and performance fatigability during mental effort-clamped ‘all-out’ sprints in hypoxia compared to normoxia. This suggests that under conditions of intensified muscular, respiratory, and/or thermal strain, perceived physical strain can dissociate from perceived mental strain and may play a mediatory role in performance regulation.
Second, a large decrease in valence in response to the muscle-lengthening protocol and a medium decrease in response to running with LMMF and mild EIMD during the running economy test (see Table 2) was associated with increases in perceived strain variables. Importantly, a large main treatment effect of attenuated valence during the ITT was associated with a large main treatment effect of augmented perceived physical strain (see Fig. 2). This attachment of valence to interoceptive stimuli facilitates the awareness of homeostatic disturbance and confirms the paramount importance of valence in interoception, awareness, and computation of conflicting motivational drives [67, 68]. Thus, valence is suggested to be the driving force by which attentional focus is shifted from goal-driven towards stimulus-driven processes. It thereby functions as an attractor in attention allocation and decision-making, and ultimately performance regulation [69, 70].
Third, the decrease of valence during the ITT was associated with large increases and decreases in action crisis and flow state, respectively (see Fig. 2). An action crisis has been defined as an intra-psychic conflict between further goal pursuit and goal disengagement resulting from negatively valenced events in goal striving [71]. In an action crisis, athletes deliberate once again the desirability and feasibility of the pursued and alternative goals, thereby undermining effective goal striving [72]. In contrast, flow has been described as a state of optimal experience, where athletes are totally immersed in goal striving and goal attainment [73]. Accordingly, their mirror-like dynamic responses suggest they form opposing ends of the mindset spectrum [74] and clearly identify a shift from an implemental mindset cognitively tuned towards the ‘how’ of behaviour to a deliberative mindset cognitively tuned towards the ‘why’ of behaviour.
Critically, increased cost-benefit thinking of further goal pursuit and goal disengagement has been shown to vary independently from the linearly increasing physiological strain experienced during a marathon, thereby independently predicting performance decrement [72, 75]. Within the sport domain, mediation analyses showed that an action crisis undermines performance by augmenting the endocrinological distress response [31, 72], while cross-lagged panel path analyses in the academic domain showed attenuation of goal desirability and perceived goal attainability [72, 76]. Thus, an action crisis can be understood as an antecedent in the goal disengagement process by draining physiological and self-regulatory resources, eventually leading to the dissolution of the pursued goal.
In support, during the second climb of the ITT, the large deterioration and peak in action crisis indeed coincided with an excessive increment in performance fatigability, thereby suggesting that runners were experiencing a decisional conflict between further goal pursuit and goal disengagement just before they (had to) made the decision to slow-down.3 Supportive evidence (although only associative) for the notion that runners re-evaluated and partly disengaged from their focal goal comes from the observations of increasingly lower heart rates and blood lactate concentrations towards the finish (see Fig. 3) as well as perceptual inflexions towards more sustainable rates of change in valence and action crisis after the second climb (see Fig. 2).
Conclusions
The current findings demonstrate that running with LMMF and mild EIMD caused medium-sized physiological and large-sized perceptual effects that were associated with discrete and poignant onset of unintended alteration in performance fatigability and observed pacing behaviour, thereby closely resembling defining characteristics of the ‘hitting the wall’ phenomenon [77, 78]. It is further suggested that much of the variance in response to running with LMMF and mild EIMD can be explained by the dynamic and complex interactions between the investigated psychophysiological determinants of pacing behaviour and performance during prolonged endurance exercise. Both physiological and perceptual pathways are proposed to impact performance fatigability during running with LMMF and mild EIMD via (1) amplified physiological strain and non-adaptive endocrinological distress response and (2) increase in perceived fatigability. Specifically with regards to the latter, it is hypothesised that an increase in perceived physical strain antecedes a decrease in valence, which in turn antecedes an increase in action crisis, eventually dissolving the initially aspired goal. The applied three-dimensional framework of perceived fatigability therefore provides a more comprehensive understaning of strain-perception-thinking-action coupling in centrally regulated and goal-directed exercise behvaiour than the traditional Gestalt concept of perceived exertion [79].
The extent to which the current data fit, the hypothesised cause-effect relationships has been assessed separately in a subsequent companion article using a five-step structural equation modelling procedure [80].
Additional File
Supplementary material containing findings in (1) deterioration in jump-height during the drop-jump protocol and the development of DOMS in the 84-h recovery period after experimental trials, (2) distributions of individual differences in knee extensor power output, time trial time, and time trial splits, (3) statistical details of two-way repeated measures ANOVA’s (trial × time) including follow-up tests and non-parametric ANOVA’s in perceptive variables, and (4) results of three-way mixed repeated measures ANOVA’s (trial × time × sex). (PDF 317 kb)
Acknowledgements
We would like to acknowledge (A) Nikhil Divekar for writing the code to assess jump-height, (B) Amy Mendham for conducting the ELISA test, (B) Hendriena Victor for conducting the blood lactate analyses, (C) Enduren International for sponsoring the energy and recovery drinks provided during the trials, (D) the reviewers for their constructive criticism and insightful comments, and (E) all our participants for their time and efforts.
Funding
The study has been funded by the National Research Foundation of South Africa (443920 HUB 1138) awarded to Prof Timothy D. Noakes. Dr. Venhorst has been supported by a scholarship from the German Academic Exchange Service (DAAD). The funding body played no roles in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of Data and Materials
Additional data are provided in Additional file 1. Please contact the first author for any further data requests.
Abbreviations
- ACRISS
Action Crisis Scale
- ANOVA
Analysis of variance
- BTT
Baseline time trial
- COM
Centre of mass
- CTT
Control time trial
- DOMS
Delayed onset of muscular soreness
- EDTA
Ethylenediaminetetraacetic acid
- EIMD
Exercise-induced muscle damage
- ELISA
Enzyme-linked immunosorbent assay
- FAS
Felt Arousal Scale
- FS
Feeling Scale
- FSS
Flow State Scale
- FTT
Familiarisation time trial
- ITT
Intervention time trial
- LMMF
Locomotor muscle fatigue
- PTRS
Peak treadmill running speed
- RCP
Respiratory compensation point
- SM
Supplementary material
- TOV
Take-off velocity of COM
- VAS
Visual analogue scale
- VCO2
Carbon dioxide production
- VE
Expired ventilation volume
- VO2
Oxygen uptake
- VO2peak
Peak oxygen uptake
- VT-1
First ventilatory threshold
Authors’ Contributions
AV designed the study, collected the data, analysed the data, and compiled the first draft of the manuscript. AV, DPM, and TDN contributed to the revision and final approval of the manuscript.
Ethics Approval and Consent to Participate
The study was approved by the Human Research Ethics Committee of the University of Cape Town (Reference number 092/2016), and all participants signed appropriate informed consent forms.
Consent for Publication
Not applicable
Competing Interests
The authors, Andreas Venhorst, Dominic P. Micklewright, and Timothy D. Noakes, declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Reafference is the anticipated sensory stimulation elicited by voluntary movements and is linked to efferent motor command. It thereby allows distinction between self-initiated sensory stimulation from external stimuli, so called exafference. The computation of the error difference underpins the reafference principle [81].
Corrected total-item correlation of item eight (transformation of time) was consistently negative throughout both trials indicating that item 8 loads negatively on the overall flow state construct. Removal of item 8 would have been justified and significantly improved the internal consistency estimate of reliability on average from 0.81 ± 0.06 to 0.91 ± 0.04. However, given the small sample size and the nevertheless good Cronbach’s alpha, item 8 was kept, but it is recommended that critical revision of item 8 of the flow state scale is required. For a recent critical review on flow in sports and exercise, see Swann et al. [82].
A strength of this study was the use of a custom-made handheld remote control in the investigation of maximal self-paced treadmill running behaviour. In contrast to cycling ergometer studies, where it is unclear whether participants intentionally or unintentionally change pace, runners in this study decisively had to use the remote control to adjust treadmill running speed. However, it remains unclear whether this decision was based on heuristic or rational computations, or a combination thereof [83]. The “(had to)” refers to the hypothesised driving role of valence in an affect heuristic-triggered decision-making process.
Electronic supplementary material
The online version of this article (10.1186/s40798-018-0143-2) contains supplementary material, which is available to authorized users.
Contributor Information
Andreas Venhorst, Phone: +27725894040, Email: andreas.venhorst@gmail.com.
Dominic P. Micklewright, Email: dpmick@essex.ac.uk
Timothy D. Noakes, Email: timothy.noakes@uct.ac.za
References
- 1.Millet GY, Tomazin K, Verges S, Vincent C, Bonnefoy R, Boisson R-C, et al. Neuromuscular consequences of an extreme mountain ultra-marathon. PLoS One. 2011;6:e17059. doi: 10.1371/journal.pone.0017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev. 2005;33:98–104. doi: 10.1097/00003677-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Tee JC, Bosch AN, Lambert MI. Metabolic consequences of exercise-induced muscle damage. Sports Med. 2007;37:827–836. doi: 10.2165/00007256-200737100-00001. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Yamada M, Kurakake S, Okamura N, Yamaya K, Liu Q, et al. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol. 2000;81:281–287. doi: 10.1007/s004210050044. [DOI] [PubMed] [Google Scholar]
- 5.Neubauer O, König D, Wagner K-H. Recovery after an Ironman triathlon: sustained inflammatory responses and muscular stress. Eur J Appl Physiol. 2008;104:417–426. doi: 10.1007/s00421-008-0787-6. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K, Peake J, Nosaka K, Okutsu M, Abbiss CR, Surriano R, et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman triathlon race. Eur J Appl Physiol. 2006;98:525–534. doi: 10.1007/s00421-006-0296-4. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 8.Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34:49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Peake JM, Neubauer O, Della Gatta PA, Nosaka K. Muscle damage and inflammation during recovery from exercise. J Appl Physiol. 2017;122:559–570. doi: 10.1152/japplphysiol.00971.2016. [DOI] [PubMed] [Google Scholar]
- 10.Paulsen G, Mikkelsen U, Raastad T, Peake J. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev. 2012;18:42–97. [PubMed] [Google Scholar]
- 11.Hyldahl RD. Lengthening our perspective: morphological, cellular and molecular responses to eccentric exercise. Muscle Nerve. 2014;49:155–170. doi: 10.1002/mus.24077. [DOI] [PubMed] [Google Scholar]
- 12.Marcora SM, Bosio A. Effect of exercise-induced muscle damage on endurance running performance in humans. Scand J Med Sci Sports. 2007;17:662–671. doi: 10.1111/j.1600-0838.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 13.Marcora SM, Bosio A, de Morree HM. Locomotor muscle fatigue increases cardiorespiratory responses and reduces performance during intense cycling exercise independently from metabolic stress. Am J Physiol Regul Integr Comp Physiol. 2008;294:R874–R883. doi: 10.1152/ajpregu.00678.2007. [DOI] [PubMed] [Google Scholar]
- 14.Burt D, Twist C. The effects of exercise-induced muscle damage on cycling time-trial performance. J Strength Cond. 2011;25:2185–2192. doi: 10.1519/JSC.0b013e3181e86148. [DOI] [PubMed] [Google Scholar]
- 15.Davies RC, Rowlands AV, Eston RG. Effect of exercise-induced muscle damage on ventilatory and perceived exertion responses to moderate and severe intensity cycle exercise. Eur J Appl Physiol. 2009;107:11–19. doi: 10.1007/s00421-009-1094-6. [DOI] [PubMed] [Google Scholar]
- 16.Black CD, Dobson RM. Prior eccentric exercise reduces VO2peak and ventilatory threshold but does not alter movement economy during cycling exercise. J Strength Cond Res. 2012;26:2530–2537. doi: 10.1519/JSC.0b013e31823f2838. [DOI] [PubMed] [Google Scholar]
- 17.Hughes J, Chapman P, Brown S, Johnson N, Stannard S. Indirect measures of substrate utilisation following exercise-induced muscle damage. Eur J Sport Sci. 2013;13:509–517. doi: 10.1080/17461391.2012.755570. [DOI] [PubMed] [Google Scholar]
- 18.Chen TC, Nosaka K, Lin M-J, Chen H-L, Wu C-J. Changes in running economy at different intensities following downhill running. J Sports Sci. 2009;27:1137–1144. doi: 10.1080/02640410903062027. [DOI] [PubMed] [Google Scholar]
- 19.Burt D, Lamb K, Nicholas C, Twist C. Effects of repeated bouts of squatting exercise on sub-maximal endurance running performance. Eur J Appl Physiol. 2013;113:285–293. doi: 10.1007/s00421-012-2437-2. [DOI] [PubMed] [Google Scholar]
- 20.Braun WA, Dutto DJ. The effects of a single bout of downhill running and ensuing delayed onset of muscle soreness on running economy performed 48 h later. Eur J Appl Physiol. 2003;90:29–34. doi: 10.1007/s00421-003-0857-8. [DOI] [PubMed] [Google Scholar]
- 21.Tsatalas T, Giakas G, Spyropoulos G, Sideris V, Lazaridis S, Kotzamanidis C, et al. The effects of eccentric exercise-induced muscle damage on running kinematics at different speeds. J Sports Sci. 2013;31:288–298. doi: 10.1080/02640414.2012.729135. [DOI] [PubMed] [Google Scholar]
- 22.Chen TC, Nosaka K, Tu J-H. Changes in running economy following downhill running. J Sports Sci. 2007;25:55–63. doi: 10.1080/02640410600718228. [DOI] [PubMed] [Google Scholar]
- 23.Fortes MB, di Felice U, Dolci A, Junglee NA, Crockford MJ, West L, et al. Muscle-damaging exercise increases heat strain during subsequent exercise heat stress. Med Sci Sports Exerc. 2013;45:1915–1924. doi: 10.1249/MSS.0b013e318294b0f8. [DOI] [PubMed] [Google Scholar]
- 24.Enoka RM, Duchateau J. Translating fatigue to human performance. Med Sci Sports Exerc. 2016;48:2228–2238. doi: 10.1249/MSS.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twist C, Eston RG. The effect of exercise-induced muscle damage on perceived exertion and cycling endurance performance. Eur J Appl Physiol. 2008;105:559–567. doi: 10.1007/s00421-008-0935-z. [DOI] [PubMed] [Google Scholar]
- 26.Black CD, Dobson RM. Prior eccentric exercise augments muscle pain and perception of effort during cycling exercise. Clin J Pain. 2013;29:443–449. doi: 10.1097/AJP.0b013e318262ddfe. [DOI] [PubMed] [Google Scholar]
- 27.Chrismas BCR, Taylor L, Siegler JC, Midgley AW. A reduction in maximal incremental exercise test duration 48 h post downhill run is associated with muscle damage derived exercise induced pain. Front Physiol. 2017;8:1–11. doi: 10.3389/fphys.2017.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauger AR. Factors affecting the regulation of pacing: current perspectives. Open access J Sport Med. 2014;5:209–214. doi: 10.2147/OAJSM.S38599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Morree HM, Marcora SM. Effects of isolated locomotor muscle fatigue on pacing and time trial performance. Eur J Appl Physiol. 2013;113:2371–2380. doi: 10.1007/s00421-013-2673-0. [DOI] [PubMed] [Google Scholar]
- 30.Venhorst A, Micklewright D, Noakes TD. Towards a three-dimensional framework of centrally regulated and goal-directed exercise behaviour: a narrative review. Br J Sports Med. 2017;0:1–12. doi: 10.1136/bjsports-2016-096907. [DOI] [PubMed] [Google Scholar]
- 31.Venhorst A, Micklewright D, Noakes TD. Modelling the process of falling behind and its psychophysiological consequences. Br J Sports Med. 2017;0:1–6. doi: 10.1136/bjsports-2017-097632. [DOI] [PubMed] [Google Scholar]
- 32.de Pauw K, Roelands B, Cheung SS, de Geus B, Rietjens G, Meeusen R. Guidelines to classify subject groups in sport-science research. Int. J. Sports Physiol. Perform. 2013;8:111–122. doi: 10.1123/ijspp.8.2.111. [DOI] [PubMed] [Google Scholar]
- 33.Decroix L, De Pauw K, Foster C, Meeusen R. Guidelines to classify female subject groups in sport-science research. Int J Sports Physiol Perform. 2016;11:204–213. doi: 10.1123/ijspp.2015-0153. [DOI] [PubMed] [Google Scholar]
- 34.Jackson AS, Pollock ML. Generalized equations for predicting body density of men, 1978. Br J Nutr. 2004;91:161–168. [PubMed] [Google Scholar]
- 35.Jones AM, Doust JH. A 1% treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci. 1996;14:321–327. doi: 10.1080/02640419608727717. [DOI] [PubMed] [Google Scholar]
- 36.Lucia A, Hoyos J, Pérez M, Santalla A, Earnest CP, Chicharro JL. Which laboratory variable is related with time trial performance time in the Tour de France? Br J Sports Med. 2004;38:636–640. doi: 10.1136/bjsm.2003.008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- 38.Jeukendrup AE, Wallis GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sport Med Suppl. 2005;26:28–37. doi: 10.1055/s-2004-830512. [DOI] [PubMed] [Google Scholar]
- 39.Shaw AJ, Ingham SA, Folland JP. The valid measurement of running economy in runners. Med Sci Sports Exerc. 2014;46:1968–1973. doi: 10.1249/MSS.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 40.Skurvydas A, Jascaninas J, Zachovajevas P. Changes in height of jump, maximal voluntary contraction force and low-frequency fatigue after 100 intermittent or continuous jumps with maximal intensity. Acta Physiol Scand. 2000;169:55–62. doi: 10.1046/j.1365-201x.2000.00692.x. [DOI] [PubMed] [Google Scholar]
- 41.Moir GL. Three different methods of calculating vertical jump height from force platform data in men and women. Meas Phys Educ Exerc Sci. 2008;12:207–218. doi: 10.1080/10913670802349766. [DOI] [Google Scholar]
- 42.Vickers A. Time course of muscle soreness following different types of exercise. BMC Musculoskelet Disord. 2001;4:2–5. doi: 10.1186/1471-2474-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ekkekakis P. The measurement of affect, emotion, and mood a guide for health-behavioral research. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 44.Venhorst A, Micklewright D, Noakes TD. The psychophysiological determinants of pacing behaviour and performance during prolonged endurance exercise: a performance level and competition outcome comparison. Sport Med. 2018;0:1–14. doi: 10.1007/s40279-018-0893-5. [DOI] [PubMed] [Google Scholar]
- 45.Hardy C, Rejeski W. Not what, but how one feels: the measurement of affect during exercise. J Sport Exerc Psychol. 1989;11:304–317. doi: 10.1123/jsep.11.3.304. [DOI] [Google Scholar]
- 46.Svebak S, Murgatroyd S. Metamotivational dominance: a multimethod validation of reversal theory constructs. J Pers Soc Psychol. 1985;48:107–116. doi: 10.1037/0022-3514.48.1.107. [DOI] [Google Scholar]
- 47.Herrmann M, Baur V, Brandstätter V, Hänggi J, Jäncke L. Being in two minds: the neural basis of experiencing action crises in personal long-term goals. Soc Neurosci. 2014;9:1–14. doi: 10.1080/17470919.2014.933715. [DOI] [PubMed] [Google Scholar]
- 48.Jackson SA, Csikszentmihalyi M. In: Flow in sports. Jackson SA, Csikszemtmihalyi M, editors. Champaign, IL: Human Kinetics; 1999. [Google Scholar]
- 49.Dill D, Costill D. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 50.Hopkins WG. A spreadsheet for deriving a confidence interval, mechanistic inference and clinical inference from a p value. Sportscience. 2007;11:16–20. [Google Scholar]
- 51.Wobbrock JO, Findlater L, Gergle D, Higgins JJ. The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. Proc SIGCHI Conf Hum Factors Comput Syst. 2011:143–6. 10.1145/1978942.1978963
- 52.Haouzi P, Chenuel B, Huszczuk A. Sensing vascular distension in skeletal muscle by slow conducting afferent fibers: neurophysiological basis and implication for respiratory control. J Appl Physiol. 2004;96:407–418. doi: 10.1152/japplphysiol.00597.2003. [DOI] [PubMed] [Google Scholar]
- 53.Davies RC, Rowlands AV, Poole DC, Jones AM, Eston RG. Eccentric exercise-induced muscle damage dissociates the lactate and gas exchange thresholds. J Sports Sci. 2011;29:181–189. doi: 10.1080/02640414.2010.526626. [DOI] [PubMed] [Google Scholar]
- 54.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 55.Pedersen BK. Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc. 2012;44:392–396. doi: 10.1249/MSS.0b013e31822f94ac. [DOI] [PubMed] [Google Scholar]
- 56.Reihmane D, Jurka A, Tretjakovs P, Dela F. Increase in IL-6, TNF-α, and MMP-9, but not sICAM-1, concentrations depends on exercise duration. Eur J Appl Physiol. 2013;113:851–858. doi: 10.1007/s00421-012-2491-9. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N, et al. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc. 2003;35:348–355. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- 58.Nieman DC. Marathon training and immune function. Sport. Med. 2007;37:412–415. doi: 10.2165/00007256-200737040-00036. [DOI] [PubMed] [Google Scholar]
- 59.Frankenhaeuser M. The psychophysiology of workload, stress, and health: comparison between the sexes. Ann Behav Med. 1991;13:197–204. [Google Scholar]
- 60.Dienstbier R. Arousal and physiological toughness: implications for mental and physical health. Psychol Rev. 1989;96:84–100. doi: 10.1037/0033-295X.96.1.84. [DOI] [PubMed] [Google Scholar]
- 61.Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol. 2008;586:161–173. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hureau TJ, Romer LM, Amann M. The “sensory tolerance limit”: a hypothetical construct determining exercise performance? Eur J Sport Sci Taylor Francis. 2018;18:13–24. doi: 10.1080/17461391.2016.1252428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Del Coso J, Fernandez D, Abian-Vicen J, Salinero JJ, Gonzalez-Millan C, Areces F, et al. Running pace decrease during a marathon is positively related to blood markers of muscle damage. PLoS One. 2013;8:1–7. doi: 10.1371/annotation/47fe0942-fff7-4df2-bac8-fd93bc7bb242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christian RJ, Bishop DJ, Billaut F, Girard O. The role of sense of effort on self-selected cycling power output. Front Physiol. 2014;5:1–10. doi: 10.3389/fphys.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penailillo L, Mckay K, Abbiss CR. RPE during concentric and eccentric cycling—are we measuring effort or exertion? Int. J. Sports Physiol. Perform. 2017;0:1–22. [Google Scholar]
- 66.Marcora S, Perrey S, Smirmaul BPC, Bosio A, Impellizzeri FM, Meeusen R, et al. Counterpoint: afferent feedback from fatigued locomotor muscles is not an important determinant of endurance exercise performance. J Appl Physiol. 2010;108:454–456. doi: 10.1152/japplphysiol.00976.2009a. [DOI] [PubMed] [Google Scholar]
- 67.Cabanac M. Sensory pleasure optimizes muscular work. Clin Investig Med. 2006;29:110–116. [PubMed] [Google Scholar]
- 68.Cabanac M. Exertion and pleasure from an evolutionary perspective. In: Acevedo EO, Ekkekakis P, editors. Psychobiol. Phys. Act. champaign: human kinetics. 2006. pp. 79–89. [Google Scholar]
- 69.Renfree A, Martin L, Micklewright D, St Clair Gibson A. Application of decision-making theory to the regulation of muscular work rate during self-paced competitive endurance activity. Sports Med. 2014;44:147–158. doi: 10.1007/s40279-013-0107-0. [DOI] [PubMed] [Google Scholar]
- 70.Edwards AM, Polman RCJ. Pacing and awareness: brain regulation of physical activity. Sports Med. 2013;43:1057–1064. doi: 10.1007/s40279-013-0091-4. [DOI] [PubMed] [Google Scholar]
- 71.Brandstätter V, Schüler J. Action crisis and cost–benefit thinking: a cognitive analysis of a goal-disengagement phase. J Exp Soc Psychol Elsevier Inc. 2013;49:543–553. doi: 10.1016/j.jesp.2012.10.004. [DOI] [Google Scholar]
- 72.Brandstätter V, Herrmann M, Schüler J. The struggle of giving up personal goals: affective, physiological, and cognitive consequences of an action crisis. Personal Soc Psychol Bull. 2013;39:1668–1682. doi: 10.1177/0146167213500151. [DOI] [PubMed] [Google Scholar]
- 73.Jackson SA, Eklund RC. Flow. In: Tenenbaum G, Ekund RC, Kamata A, editors. Meas. Sport Exerc. Psychol. champaign: human kinetics. 2012. pp. 349–358. [Google Scholar]
- 74.Gollwitzer P. Mindset theory of action phases. In: van Lange PA, editor. Handb. Theor. Soc. Psychol. 1. Los Angeles: Sage; 2012. pp. 526–545. [Google Scholar]
- 75.Schüler J, Langens TA. Psychological crisis in a marathon and the buffering effects of self-verbalizations. J Appl Soc Psychol. 2007;37:2319–2344. doi: 10.1111/j.1559-1816.2007.00260.x. [DOI] [Google Scholar]
- 76.Ghassemi M, Bernecker K, Herrmann M, Brandstätter V. The process of disengagement from personal goals: reciprocal influences between the experience of action crisis and appraisals of goal desirability and attainability. Personal Soc Psychol Bull. 2017;43:524–537. doi: 10.1177/0146167216689052. [DOI] [PubMed] [Google Scholar]
- 77.Buman MP, Brewer BW, Cornelius AE, Van Raalte JL, Petitpas AJ. Hitting the wall in the marathon: phenomenological characteristics and associations with expectancy, gender, and running history. Psychol Sport Exerc. 2008;9:177–190. doi: 10.1016/j.psychsport.2007.03.003. [DOI] [Google Scholar]
- 78.Buman MP, Omli JW, Giacobbi PR, Brewer BW. Experiences and coping responses of “hitting the wall” for recreational marathon runners. J Appl Sport Psychol. 2008;20:282–300. doi: 10.1080/10413200802078267. [DOI] [Google Scholar]
- 79.Smits BLM, Pepping G-J, Hettinga FJ. Pacing and decision making in sport and exercise: the roles of perception and action in the regulation of exercise intensity. Sports Med. 2014;44:763–775. doi: 10.1007/s40279-014-0163-0. [DOI] [PubMed] [Google Scholar]
- 80.Venhorst A, Micklewright D, Noakes TD. Modelling perception-action coupling in the phenomenological experience of “hitting the wall” during long-distance running with exercise induced muscle damage in highly-trained runners. Sport Med - Open. 2018;0. 10.1186/s40798-018-0136-1 [DOI] [PMC free article] [PubMed]
- 81.von Holst E, Mittelstaedt H. Das Reafferenzprinzip - Wechselwirkungen zwischen Zentralnervensystem und Peripherie. Naturwissenschaften. 1950;37:464–476. doi: 10.1007/BF00622503. [DOI] [Google Scholar]
- 82.Swann C, Piggott D, Schweickle M, Vella SA. A review of scientific progress in flow in sport and exercise: normal science, crisis, and a progressive shift. Taylor & Francis. 2018;3200:1533–1571. [Google Scholar]
- 83.Micklewright D, Kegerreis S, Raglin J, Hettinga F. Will the conscious-subconscious pacing quagmire help elucidate the mechanisms of self-paced exercise? New opportunities in dual process theory and process tracing methods. Sports Med. 2017;47:1231–1239. doi: 10.1007/s40279-016-0642-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material containing findings in (1) deterioration in jump-height during the drop-jump protocol and the development of DOMS in the 84-h recovery period after experimental trials, (2) distributions of individual differences in knee extensor power output, time trial time, and time trial splits, (3) statistical details of two-way repeated measures ANOVA’s (trial × time) including follow-up tests and non-parametric ANOVA’s in perceptive variables, and (4) results of three-way mixed repeated measures ANOVA’s (trial × time × sex). (PDF 317 kb)
Data Availability Statement
Additional data are provided in Additional file 1. Please contact the first author for any further data requests.