Abstract
We report a meta-analysis of virtual reality (VR) interventions for anxiety and depression outcomes, as well as treatment attrition. We included randomized controlled trials comparing VR interventions, alone or in combination, to control conditions or other active psychological interventions. Effects sizes (Hedges’ g) for anxiety and depression outcomes, as post-test and follow-up, were pooled with a random-effects model. Drop-outs were compared using odds ratio (OR) with a Mantel-Haenszel model. We included 39 trials (52 comparisons). Trial risk of bias was unclear for most domains, and high for incomplete outcome data. VR-based therapies were more effective than control at post-test for anxiety, g = 0.79, 95% CI 0.57 to 1.02, and depression, g = 0.73, 95% CI 0.25 to 1.21, but not for treatment attrition, OR = 1.34, 95% CI 0.95 to 1.89. Heterogeneity was high and there was consistent evidence of small study effects. There were no significant differences between VR-based and other active interventions. VR interventions outperformed control conditions for anxiety and depression but did not improve treatment drop-out. High heterogeneity, potential publication bias, predominant use of waitlist controls, and high or uncertain risk of bias of most trials question the reliability of these effects.
Introduction
Virtual reality (VR) has garnered significant attention as a cost-effective tool for delivering psychological treatments1. Virtual reality exposure (VRE) in particular is considered an effective treatment for several anxiety disorders2, on par with in vivo exposure/IVE3,4, though doubts were expressed about the quality of this evidence5.
While many narrative reviews and commentaries focused on VR interventions, only three systematic reviews with meta-analyses examined their efficacy in randomized controlled trials/RCTs4,6,7 and they present certain shortcomings. Included trials were published through 2014 the latest, and many more trials have been conducted since, given VR technology has become more accessible. Outcomes other than anxiety were scarcely analyzed, though data on some of these has been accruing. The effects of VR interventions on treatment attrition remained unclear, with some speculation of possible superiority1,5,8, but no assessment in a meta-analysis.
Only one meta-analysis7 considered heterogeneity between effect sizes (ESs), but did so only descriptively, without providing a quantification. Assessment of quality6,7 relied on mixed and potentially inadequate tools that included items not linked to any type of trial bias (e.g., treatment fidelity)9, thereby potentially confounding the relationship between study quality and treatment effects. Only one meta-analysis7 considered publication bias, with conflicting results between the assessment methods used (Egger’s test and fail-safe N). Moreover, many VR trials are conducted on a small number of participants, which exposes meta-analyses to “small study effects”10, the notion that smaller studies show different, often larger, treatment effects than large ones. Few potential moderators were examined, with generally contradictory results regarding treatment intensity, or the type of comparison group. One yet uninvestigated potential moderator regards the involvement of developers of VR tools and interventions in the trials, as these are often for-profit developments.
Consequently, we report a meta-analysis for the effectiveness of VR-enhanced interventions in RCTs, for symptoms of anxiety and depression, as well as treatment attrition, along with assessment of risk of bias, heterogeneity, and potential moderators.
Methods
Identification and selection of studies
A literature search of PubMed, PsycInfo, EMBASE and Cochrane Central Register of Controlled Trials databases was conducted through May, 2015, updated in March, 2016 and subsequently August 2017, using the keywords “virtual reality”, “therapy”, “exposure”, “intervention”, “treatment” and a filter for randomized trials (Supplementary Method). We also searched the references from the most recent systematic reviews and meta-analyses.
Studies were included if they were a) RCTs comparing b) a VR-enhanced intervention to a control or an active psychological intervention for c) adults, d) measuring outcomes related to depression and anxiety, and e) published in peer-reviewed journals. We included studies comparing a VR-enhanced condition with controls (e.g., waitlist, placebo, treatment-as-usual) or active conditions not employing VR. Similarly to Turner & Casey (2014), the latter were defined as established interventions involving active, psychologically therapeutic mechanisms of action (e.g., CBT, IVE). No language restrictions were employed. One researcher screened all abstracts and full-texts of RCTs were recovered. Two independent researchers independently examined full-texts and selected eligible RCTs. Disagreements were resolved by discussion and consultation with a third author until consensus was reached.
Risk of bias and data extraction
We used four criteria from the Risk of Bias (RoB) assessment tool, developed by the Cochrane Collaboration11, which assesses possible sources of bias in RCTs. The following domains were rated: a) the adequate generation of allocation sequence, b) the concealment of allocation to conditions, c) the prevention of knowledge of the allocated intervention (blinding of assessors) and d) the adequate addressment of incomplete outcome data. Blinding of assessors was rated as low risk if the trial described proper methods of ensuring it or if all relevant outcome measures were self-report, thus not requiring the direct interaction with an assessor. This choice was made as we expected most outcomes to be reported on self-report scales, and there is currently no standard as to how to rate these in terms of blinding. Domain d) was assessed as low risk if there were all randomized participants were included in the analysis, either through the use on an intent-to-treat (ITT) approach or when complete data was available. We also computed an overall RoB score for each study by awarding 1 point for each bias source rated as low risk.
We extracted a series of variables from the included studies, detailed in Table 1 for further use in moderator analyses. Details about the interaction with the virtual environment were extracted from the methods sections describing the intervention or the technology used. For each trial, we noted which elements the interaction with the VR environment relied upon (e.g., visual, sound, haptic) and (2) whether or not the authors had explicitly assessed sense of presence or immersion in the trial with validated or ad hoc instruments. We also quantified the first component by tabulating the number of interaction elements each study employed, as a very crude indicator of the degree of interaction.
Table 1.
List of variables that were extracted from the included studies
| Variable name | Coding categories |
|---|---|
| Categorical variables | |
| Study location | North America (N. America) |
| Europe (EU) | |
| VR program developer as an author of the study | Yes |
| No | |
| Recruitment pool | Community volunteers |
| Clinical setting | |
| Army enlisted personnel | |
| Type of control or comparison group | Other (Placebo/Relaxation/Treatment-as-usual) |
| Waitlist | |
| CBT (cognitive-behavioral therapy) | |
| IE (imaginal exposure) | |
| IVE (in-vivo exposure) | |
| Type of VR-enhanced intervention | VRCBT (VR-enhanced CBT) |
| VRE (exposure in virtual reality) | |
| Type of anxiety disorder (only for anxiety symptoms) | Flight anxiety |
| Panic disorder | |
| PTSD | |
| Social anxiety | |
| Specific phobia | |
| Risk of bias for incomplete outcome data | High/Unclear risk of bias |
| Low risk of bias | |
| Continuous variables | |
| Publication year | |
| Number of subjects randomized to the VR-enhanced group | |
| Number of drop-outs from the VR-enhanced group | |
| Participant’s mean age | |
| Number of VR sessions | |
| Session duration | as measured in minutes |
| Overall VR therapy duration (weeks) | as measured in weeks |
| Risk of bias score | coded as the total number of criteria at low risk of bias for each included study |
| Number of elements involved in the interaction with the virtual environment | |
.
The involvement of a developer was coded using the information available in each trial, at the section of the method that described the VR therapy package used. If authors of the VR package were not listed in the original article, we independently searched the web for the specific VR program or package used in order to identify its authors. Risk of bias assessment and data extraction were performed by two independent researchers and disagreements were discussed and resolved until consensus was reached.
Meta-analyses
We computed and pooled the individual ESs with Comprehensive Meta-Analysis (CMA version 3.3.070) and Stata (Stata SE, version 15).
For anxiety and depression, we calculated the standardized mean difference (SMD) at post-test and follow-up, by subtracting the mean score of the comparison group (control or active treatment) from the mean score of the VR-enhanced group, and dividing the result by the pooled standard deviation of the two groups. Positive SMDs thus reflect superiority of the VR-enhanced condition. We report the indicator corrected for small sample bias12, Hedges’ g. We also transformed the SMD into number needed to treat (NNT), using the formula of Kraemer & Kupfer13. The NNT represents the number of patients that would have to be treated to generate one additional positive outcome14.
Given the considerable variability among outcomes measures, we grouped them into anxiety and depressive symptoms. These included all such outcomes, whether measured by general or disorder-specific scales or subscales. As anxiety outcomes were sometimes measured for individuals without an anxiety disorder, we also conducted sensitivity analyses restricted to patients with one such disorder, diagnosed with a clinical interview or by use of a cut-off at a symptom scale. When a study used multiple measures from the same category, the average ES was computed using the CMA procedure15 that assumes a correlation of 1 between outcomes. Since the correlation is probably less than 1, this approach is conservative16. ITT data were preferred where available. If means and standard were not available, we calculated the SMD from other statistics available in the study, such as t-values or exact p-values, using the standard formulae in the program15. If data was still insufficient for ES calculation, a request was sent to the study authors.
Drop-outs were defined as all randomized participants not finishing treatment, regardless of the reasons. Odds ratio (ORs) indicated the odds of participants dropping out from the VR versus the comparison group, with sub-unitary ORs indicating smaller odds for drop-out in the VR group.
We conducted separate meta-analyses for VR-enhanced therapy versus control, and respectively versus other active psychological treatments. Continuous outcomes (anxiety, depression) were pooled with a random effects model using the inverse-variance DerSimonian and Laird method17. For dichotomous outcomes, given we expected small trials, with some reporting few or no drop-outs, we used both the fixed effect Mantel-Haenszel method18,19 with a continuity correction of 0.5 for zero counts, as well as Peto’s method20, as previously recommended21,22. Trials with zero drop-outs in both arms were excluded, due to concerns they might significantly inflate bias particularly in small trials21. We conducted sensitivity analyses excluding outliers and, respectively, excluding studies with a small number (N) of participants. Outliers were defined as studies in which the pooled ES’s 95% CI was outside the 95% CI of the pooled ES (on both sides). We used an arbitrary cut-off of at least 25 randomized participants per arm to for the analysis excluding small N studies. Though power calculations might differ from trial to trial, larger N trials are at least more precise in estimating the intervention effect23.
Heterogeneity was assessed with the I2 statistic, with values of 25%, 50% and respectively 75% indicating low, moderate and high heterogeneity24. We calculated 95% confidence intervals (CI) around I2 25, using the non-central χ2-based approach26. For categorical moderators, we conducted subgroup analyses using the mixed effects model, which uses a random-effects model within subgroups and a fixed-effects one across subgroups15. For continuous moderators, meta-regression analyses employed a restricted maximum likelihood model with the Knapp-Hartung method15.
We investigated small study effects and publication bias using a variety of methods. We resorted to visual inspection of the funnel plot, and contour enhanced funnel plots27, where contour lines indicate regions where a test of treatment effects was significant for various established levels for statistical significance. We also employed statistical tests for small study effects. In the case of continuous outcomes, we conducted Egger’s test28 for the asymmetry of the funnel plot and corresponding Galbraith plots29 if the test indicated significant asymmetry. We also used the trim and fill procedure30 as a complementary method to adjust for potential publication bias or small study effects. For drop-out rates, as these were binary outcomes pooled with the ORs, we used the Harbord test31, which regresses Z/sqrt(V) against sqrt(V), where Z is the efficient score and V is Fisher’s information (the variance of Z under the null hypothesis).
Data availability
The datasets generated and analysed during the current study are available in the Figshare repository, 10.6084/m9.figshare.5675407.
Results
Selection and inclusion of studies
The search generated 1394 records (720 after duplicate removal). We excluded 374 records based on abstract inspection and examined the full-texts for 346 articles. Figure 1 reports the flowchart of the inclusion process following the PRISMA guidelines32. Subsequently, 42 trials met our inclusion criteria, six of which had insufficient data for ES calculation. Following contact with the original authors, we obtained data for one study33. For two others34,35 the author confirmed the samples overlapped with those from larger included studies. For 3 remaining trials, authors did not provide data, thus leaving a total of 39 trials in the meta-analysis (Supplementary Result).
Figure 1.
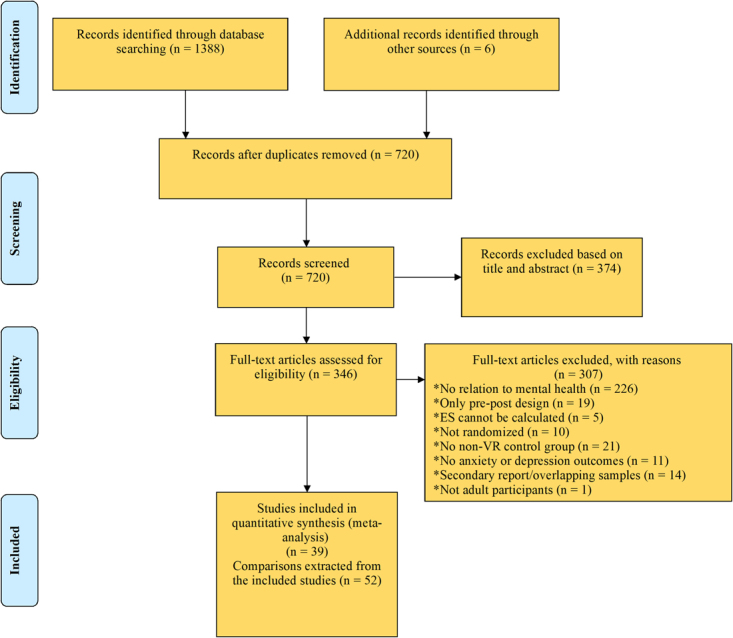
PRISMA flow-diagram of the study selection process.
Characteristics of included studies
The 39 RCTs included 52 relevant comparisons, with 869 participants in the VR-enhanced condition, and 1122 in the control or active treatments ones. The most frequent conditions were anxiety and anxiety-related (e.g., PTSD) disorders (31 studies). The most frequently used VR therapy was VRE (in 21 out of the 39 RCTs), followed by VRCBT (in 19 out of the 39 RCTs). The number of VR sessions ranged from 1 to 16. The most used VR device was the head-mounted display (HMD) (35 studies). Apart from visual feedback, the majority of studies included sound (27 studies) or some form of navigation (18 studies). Only 6 trials explicitly assessed presence or immersion in the virtual environment. In most cases, developers of the VR program used were also among the authors (27 studies) (Table 2; Supplementary Table S1).
Table 2.
Selected characteristics of included studies of VR-enhanced interventions.
| Study | Conditiona | Sampleb | Recr.c | Nrand VRd | Nsess VRe | VRweeksf | VR psy interv.g | Ctrlh | VR systemi | VR devj | Provk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson, 2013 | Social anxiety | DSM-IV-TR | Comm | 30 | 8 | 8 | VRCBT | WL; CBT | HMD | N | US |
| Banos, 2011 | Mixed disorders | DSM-IV-TR | Comm | 25 | 5 | 9 | VRCBT | CBT | VR room | Y | ES |
| Botella, 2007 | PD + AG | DSM-IV, ADIS-IV | Comb | 12 | 6 | 9 | VRCBT | WL; CBT | HMD | Y | ES |
| Botella, 2016 | Spider Phobia | DSM-IV-TR | Comm | 32 | 1 | 0.14 | VRE | IVE | HMD | Y | ES |
| Bouchard, 2016 | Social Anxiety | DSM-V | Comm | 17 | 8 | 14 | VRCBT | WL; CBT | HMD | Y | CA |
| Choi, 2005 | PD + AG | DSM-IV | Clin | 20 | 3 | 4 | VRCBT | CBT | HMD | Y | KR |
| Emmelkamp, 2002 | Acrophobia | DSM-IV/BAT | Comm | 17 | 3 | 3 | VRE | IVE | HMD | N | NL |
| Gaggioli, 2014 | Stress | VAS-A | Comm | 40 | 8 | 5 | VRCBT | WL; CBT | HMD | Y | IT |
| Garcia-Palacios, 2002 | Spider Phobia | FSQ > 97, DSM-IV | Comm | 12 | 4 | 2.5 | VRE | WL | HMD | Y | ES |
| Kampmann, 2016 | Social Anxiety | DSM-IV-TR | Comm | 20 | 7 | 5 | VRE | WL; IVE | HMD | Y | NL |
| Krijn, 2004 | Acrophobia | DSM-IV, BAT | NR | 17 | 3 | 3 | VRE | WL | HMD/CV | N | NL |
| Lau, 2010 | Ward orient | Psych diagn. | Clin | 27 | 1 | 0.14 | VRE | TAU | PC | N/R | CN |
| Malinvaud, 2016 | Tinnitus | Subj. tinnitus | Clin | 61 | 8 | 8 | VRE | CBT | HMD | Y | FR |
| Maltby, 2002 | Flight Anxiety | DSM-IV | Comm | 25 | 5 | 3 | VRE | PLB | HMD | Y | US |
| McLay, 2011 | PTSD | MINI/CAPS > 40 | Army | 10 | 8,8 | 10 | VRE | TAU | HMD | Y | US |
| McLay, 2017 | PTSD | DSM-IV | Army | 43 | 10,28 | 9 | VRE | IE | HMD | Y | US |
| Meyerbroeker, 2013 | PD + AG | DSM-IV-TR | NR | 27 | 6 | 10 | VRCBT | CBT | HMD/CV | N | NL |
| Michaliszyn, 2010 | Spider Phobia | DSM-IV, BAT | Comm | 16 | 6 | 8 | VRE | IVE | HMD | N | CA |
| Miyahira, 2012 | PTSD | CAPS, PDS | Army | 29 | 9 | 5 | VRCBT | WL | HMD | N | US |
| Muhlberger, 2001 | Flight Anxiety | DSM-IV | Comm | 15 | 1 | 0.14 | VRE | RLX | HMD | N | DE |
| Muhlberger, 2003 | Flight Anxiety | DSM-IV | Comm | 26 | 1 | 0.14 | VRCBT | CT | HMD | N | DE |
| Pelissolo, 2012 | PD + AG | DSM-IV | Clin | 43 | 12 | 12 | VRE | CBT | HMD | N | FR |
| Pitti, 2008 | PD + AG. | CIDI | Clin | 18 | 11 | 11 | VRCBT | CBT | CV | N | ES |
| Ready, 2010 | PTSD | CAPS > 60 | Clin | 6 | 10 | N/R | VRE | PLB | HMD | Y | US |
| Reger, 2016 | PTSD | DSM-IV-TR | Army | 54 | 8 | 10 | VRE | WL; IE | HMD | Y | US |
| Riva, 2003 | BED | DSM-IV | Clin | 9 | 10 | 6 | VRCBT | WL; CBT | HMD | Y | IT |
| Riva, 2006 | Severe Obesity | BMI > 41 | Clin | 57 | 9 | 6 | VRCBT | WL; CBT | HMD | Y | IT |
| Robillard, 2010 | Social Anxiety | DSM-IV-TR | NR | 14 | 16 | NR | VRCBT | WL; CBT | HMD | Y | CA |
| Rothbaum, 1995 | Acrophobia | AQ (screening) | Comm | 12 | 7 | 8 | VRE | WL | HMD | Y | US |
| Rothbaum, 2000 | Flight Anxiety | DSM-IV | Comm | 15 | 4 | 6 | VRCBT | WL; CBT | HMD | Y | US |
| Rothbaum, 2006 | Flight Anxiety | DSM-IV | Comm | 41 | 4 | 6 | VRCBT | CBT | HMD | Y | US |
| Rus-Calafell, 2013 | Flight Anxiety | DSM-IV | Comm | 7 | 6 | 3 | VRE | IE | HMD | Y | ES |
| Stetz, 2011 | Stress | PCL-M | Army | 30 | 3 | 0.42 | VRE | RLX | Screen | Y | US |
| Thompson, 2011 | Tiredness/Mood | No diagnostic | Comm | 12 | 10 | 2.5 | VRE | RLX; IE | HMD | Y | UK |
| Tortella-Feliu, 2011 | Flight Anxiety | DSM-IV | Comm | 19 | 6 | 3 | VRE | IE | HMD | Y | ES |
| Triscari, 2015 | Flight Anxiety | MCMI-III, DSM-V | Comm | 21 | 3 | 10 | VRCBT | CBT | N/R | Y | IT |
| Vincelli, 2003 | PD + AG. | DSM-IV | Clin | 4 | 8 | N/R | VRCBT | WL; CBT | HMD | Y | IT |
| Wallach, 2009 | Social Anxiety | PSA symptoms | Comm | 34 | 8 | 12 | VRCBT | WL; CBT | HMD | N | IL |
| Wiederhold, 2001 | Flight Anxiety | DSM-IV | Comm | 20 | 6 | 8 | VRE | IE | HMD | N/R | US |
aPD = panic disorder; ED = eating disorder; orient = orientation; BED = binge eating disorder; AG = agoraphobia; PTSD = post-traumatic stress disorder. Mixed disorders include PTSD, pathological grief and adjustment disorders;
bSample selection; DSM = Diagnostic and Statistical Manual of Mental Disorders; ADIS = Anxiety Disorders Interview Schedule; BAT = behavioral approach test; VAS-A = Visual Analogue Scale for Anxiety; FSQ = Fear of Spiders Questionnaire; Psych diagn. = 1st time admission in a psychiatric ward; Subj. tinnitus = subjective tinnitus; MINI = Mini-International Neuropsychiatric Interview; CAPS = Clinician Administered PTSD Scale; PDS = PTSD Diagnostic Scale; CIDI = Composite International Diagnostic Interview; BMI = Body Mass Index; AQ = Acrophobia Questionnaire; PCL = PTSD CheckList – Military Version; MCMI = Millon Clinical Multiaxial Inventory; PSA Symptoms = public speaking anxiety symptoms (psychiatric evaluation);
cComm = recruited from community samples; Clin = recruited from clinical samples; NR = not reported;
dNrand VR = number of participants randomized to the VR-enhanced treatment;
eNsess VR = number sessions of VR-enhanced treatment;
fVRweeks = the duration in weeks of the VR-enhanced treatment;
gVR psy tx = type of VR-enhanced psychological treatment; VRE = VR-enhanced exposure; VRCBT = VR-enhanced cognitive behavioral therapy;
hCtrl= control/comparison intervention; IVE = in vivo exposure; IE = imaginal exposure; RLX = relaxation; CBT = cognitive behavioral therapy; PLB = placebo; CT = cognitive therapy; WL = waitlist; TAU = treatment-as-usual;
iHMD = head-mounted display; CV = Cave-type system;
jVR dev = VR developers are among the study authors; Y = yes; N = no;
kProv, provenience; CN = China; NL = Netherlands; ES = Spain; US = United States; KR = South Korea; IT = Italy; FR = France; CA = Canada; DE = Germany; IL = Israel.
Risk of bias of the included studies
Most trials had uncertain or high risk of bias for three domains. Four RCTs had low RoB on all four domains. Nineteen studies were rated low RoB in only one domain. For sequence generation and allocation concealment, the majority of trials (27 and respectively 28) did not provide any information to enable assessment. For blinding, only seven studies employed actual blinding of outcome assessors and 25 studies used exclusively self-report measures. For incomplete outcome data, 20 studies did not employ ITT analyses, and 9 studies did not include enough information to assess this domain. For this domain, we conducted additional subgroup analysis contrasting trials with low RoB versus the rest. Trials with high and unclear RoB were combined since given the ubiquity of treatment drop-out in RCTs, the lack of any mention of ITT strategies makes it very likely that none had been employed. For 3 trials, the number of drop-outs in one arm was unclear (Table S2) (Fig. 2, Supplementary Figure S1).
Figure 2.
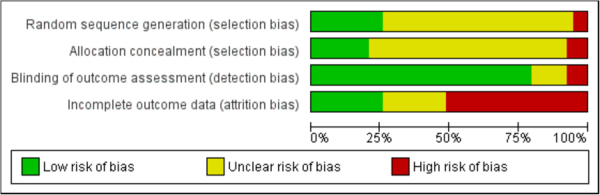
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Main results
VR-enhanced therapy compared to a control condition
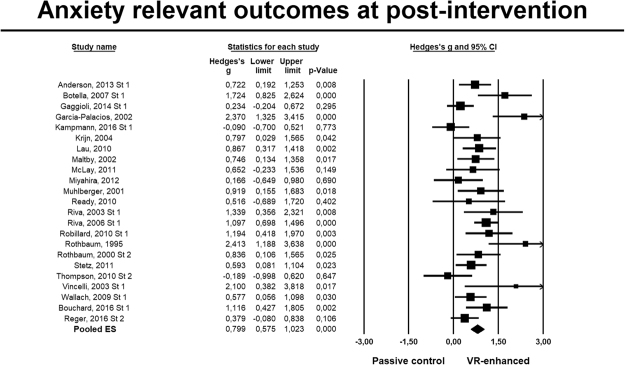
For anxiety outcomes (Fig. 3), twenty-three RCTs were pooled, g = 0.79, 95% CI 0.57 to 1.02, NNT = 2.36, with substantial heterogeneity (I2 = 59%, 95% CI 35 to 74). Analyses restricted to participants with an anxiety disorder (17 comparisons) led to slightly smaller estimates: g = 0.72, 95% CI 0.51 to 0.94, NNT = 2.56, with similarly substantial heterogeneity (I2 = 58%, 95% CI 28 to 76). Exclusion of three potential outliers led to a small decrease, g = 0.73, 95% CI 0.55 to 0.92, and reduced heterogeneity (I2 = 36%; 95% CI 0 to 63). Only 7 trials had at least 25 participants randomized in each arm. Their aggregate ES was g = 0.64, 95% CI 0.39 to 0.88, and heterogeneity was still present (I2 = 42%; 95% CI 0 to 76) (Table 3).
Figure 3.
Forest plot: Standardized mean differences post-test for VR-enhanced therapy versus control conditions for anxiety outcomes.
Table 3.
VR-enhanced therapy vs. passive control contrast, post-treatment.
| Anxiety symptoms | N | ga | 95% CI | I 2 | I2 95% CI | NNT | pb | |
|---|---|---|---|---|---|---|---|---|
| All studies | 23 | 0.79 | 0.57 to 1.02 | 59 | 35 to 74 | 2.36 | ||
| Outliers excludedc | 20 | 0.73 | 0.55 to 0.92 | 36 | 0 to 63 | 2.54 | ||
| Only studies with >25 randomized per arm | 7 | 0.64 | 0.39 to 0.88 | 42 | 0 to 76 | 2.86 | ||
| Only studies involving anxiety disorders | 17 | 0.72 | 0.51 to 0.94 | 58 | 28 to 76 | 2.56 | ||
| Subgroup analyses d | ||||||||
| Country | N. America | 11 | 0.74 | 0.49 to 1.00 | 31 | 0 to 66 | 2.50 | 0.560 |
| EU | 10 | 0.90 | 0.43 to 1.38 | 77 | 57 to 87 | 2.10 | ||
| VR program author | N | 5 | 0.64 | 0.36 to 0.93 | 0 | 0 to 79 | 2.86 | 0.292 |
| among author poole | Y | 17 | 0.87 | 0.56 to 1.18 | 68 | 48 to 81 | 2.16 | |
| Recruitmentf | Army | 4 | 0.45 | 0.15 to 0.75 | 0 | 0 to 85 | 4.00 | 0.020 |
| Clinic | 5 | 1.04 | 0.75 to 1.34 | 0 | 0 to 79 | 1.86 | ||
| Community | 11 | 0.76 | 0.38 to 1.13 | 71 | 46 to 84 | 2.44 | ||
| Control group | Other (PLB/RLX/TAU) | 7 | 0.63 | 0.38 to 0.89 | 0 | 0 to 71 | 2.91 | 0.188 |
| WL | 16 | 0.90 | 0.60 to 1.21 | 68 | 47 to 81 | 2.10 | ||
| Experimental | VRCBT | 11 | 0.87 | 0.58 to 1.16 | 52 | 5 to 76 | 2.16 | 0.536 |
| intervention | VRE | 12 | 0.73 | 0.38 to 1.07 | 64 | 34 to 81 | 2.54 | |
| Type of anxiety disorder | Flight anxiety | 3 | 0.82 | 0.42 to 1.22 | 0 | 0 to 90 | 2.28 | 0.007 |
| Panic disorder | 2 | 1.80 | 1.01 to 2.60 | 0 | N/Ai | 1.25 | ||
| PTSD | 4 | 0.39 | 0.04 to 0.74 | 0 | 0 to 85 | 4.59 | ||
| Social anxiety | 5 | 0.67 | 0.25 to 1.09 | 58 | 0 to 84 | 2.75 | ||
| Specific phobia | 3 | 1.79 | 0.64 to 2.94 | 75 | 17 to 92 | 1.25 | ||
| Incomplete outcome data RoBj | High/Unclear | 16 | 0.83 | 0.60 to 1.06 | 39 | 0 to 67 | 2.26 | 0.797 |
| Low | 7 | 0.76 | 0.26 to 1.26 | 76 | 48 to 88 | 2.44 | ||
| Depressive symptoms | ||||||||
| All studies | 10 | 0.73 | 0.25 to 1.21 | 71 | 45 to 85 | 2.54 | ||
| Outliers excludedg | 9 | 0.60 | 0.19 to 1.01 | 62 | 21 to 82 | 3.05 | ||
| Subgroup analyses | ||||||||
| Country | N. America | 5 | 0.69 | 0.25 to 1.13 | 44 | 0 to 79 | 2.67 | 0.672 |
| EU | 5 | 0.93 | −0.08 to 1.94 | 83 | 61 to 92 | 2.04 | ||
| Recruitmenth | Army | 2 | 0.32 | −0.28 to 0.92 | 45 | N/A | 5.56 | |
| Clinic | 3 | 2.21 | 0.66 to 3.77 | 67 | 0 to 90 | 1.13 | 0.066 | |
| Community | 3 | 0.23 | −0.53 to 0.99 | 73 | 8 to 92 | 7.69 | ||
| Control group | Other(PLB/RLX/TAU) | 2 | 0.87 | −0.28 to 2.03 | 54 | N/A | 2.16 | 0.814 |
| WL | 8 | 0.72 | 0.16 to 1.28 | 76 | 52 to 88 | 2.56 | ||
| Experimental | VRCBT | 6 | 1.01 | 0.34 to 1.67 | 69 | 27 to 87 | 1.91 | 0.197 |
| intervention | VRE | 4 | 0.38 | −0.29 to 1.06 | 71 | 16 to 90 | 4.72 | |
| Incomplete outcome data RoB | High/Unclear | 6 | 0.81 | 0.31 to 1.30 | 46 | 0 to 79 | 2.30 | 0.874 |
| Low | 4 | 0.72 | −0.23 to 1.68 | 85 | 62 to 94 | 2.56 | ||
aAll results are reported with Hedges’ g, using a random effects model. Positive effect indicates superiority of the VR-enhanced therapy over passive control groups.
bThe p levels in this column indicate whether the difference between the ESs in the subgroups is significant (significant results are marked with italic).
cOutliers were defined as studies in which the 95% CI was outside the 95% CI of the pooled studies (Kampmann, 2016 St.1; Garcia-Pallacios, 2002; Rothbaum, 1995).
dSubgroup analysis were conducted using a mixed effects model. Only subgroups with at least 2 studies were included.
eOne study (Lau, 2010) did not contain information about this moderator.
fTwo studies (Krijn, 2004, Robillard, 2010 St.1) did not contain information about this moderator.
gOutliers: Vincelli, 2003 St.1.
hOne study (Robillard, 2010 St.1) did not contain information about this moderator.
iConfidence intervals around I2 cannot be calculated if there are less than 3 groups.
jRoB: Risk of Bias.
For depression, ten RCTs were pooled, g = 0.73, 95% CI 0.25 to 1.21, NNT = 2.54, with high heterogeneity (I2 = 71%, 95% CI 45 to 85). Exclusion of one outlier resulted in a sizable decrease, g = 0.60, 95% CI 0.19 to 1.01, I2 = 62%. Only one trial36 had at least 25 participants randomized in each arm.
Follow-up outcomes were only reported in two RCTs for anxiety and in one for depression.
Seventeen trials reported non-zero drop-outs in at least one group and nine trials reported zero drop-outs in both groups (Supplementary Table S2). Drop-out rates did not significantly differ between the groups, with similar estimations for the Mantel-Haenszel (OR = 1.34, 95% CI 0.95 to 1.89, χ2 = 3.06, p = 0.08) (Supplementary Figure S2) and Peto methods (OR = 1.37, 95% CI 0.96 to 1.95, χ2 = 3.06, p = 0.08).
VR-enhanced therapy compared to an active condition at post-treatment and follow-up
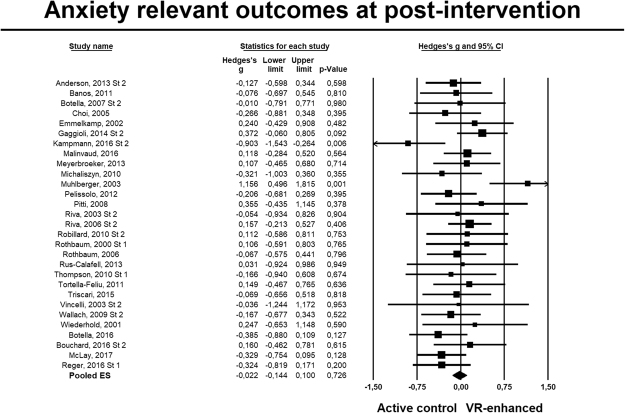
For anxiety (Fig. 4), twenty-nine RCTs were pooled, g = −0.02, 95% CI −0.14 to 0.10, with low heterogeneity (I2 = 20%, 95% CI 0 to 50). Analyses restricted to trials with participants with an anxiety disorder (23 comparisons) also resulted in non-significant effects (albeit slightly more favorable to the non-VR interventions), g = −0.10, 95% CI −0.24 to 0.04, with similar heterogeneity estimates, I2 = 26%, 95% CI 0 to 55. Results remained comparable after excluding two potential outliers, g = −0.02, 95% CI −0.13 to 0.08, I2 = 0%, and in analyses limited to trials with at least 25 participants randomized per arm, g = −0.05, 95% CI −0.19 to 0.07, I2 = 1% (Table 4).
Figure 4.
Forest plot: Standardized mean differences post-test for VR-enhanced therapy versus non-VR active psychological treatments for anxiety outcomes.
Table 4.
VR-enhanced therapy vs. active condition contrast, post-treatment.
| Anxiety symptoms | N | ga | 95% CI | I 2 | I2 95% CI | NNT | pb | |
|---|---|---|---|---|---|---|---|---|
| All studies | 29 | −0.02 | −0.14 to 0.10 | 20 | 0 to 50 | 83.33 | ||
| Outliers excludedc | 27 | −0.02 | −0.13 to 0.08 | 0 | 0 to 43 | 83.33 | ||
| Only studies with >25 randomized per arm | 12 | −0.05 | −0.19 to 0.07 | 1 | 0 to 59 | 35.71 | ||
| Only studies involving anxiety disorders | 23 | −0.10 | −0.24 to 0.04 | 26 | 0 to 55 | 17.86 | ||
| Subgroup analyses d | ||||||||
| Country | N. America | 9 | −0.12 | −0.31 to 0.06 | 0 | 0 to 65 | 14.71 | 0.198 |
| EU | 18 | 0.04 | −0.13 to 0.23 | 39 | 0 to 65 | 45.45 | ||
| VR program author | N | 8 | 0.09 | −0.21 to 0.40 | 54 | 0 to 79 | 20.00 | 0.372 |
| among author poole | Y | 20 | −0.05 | −0.18 to 0.07 | 0 | 0 to 48 | 35.71 | |
| Recruitmentf | Army | 2 | −0.32 | −0.64 to −0.005 | 0 | N/Ai | 5.56 | |
| Clinic | 7 | 0.03 | −0.17 to 0.23 | 0 | 0 to 71 | 62.50 | 0.159 | |
| Community | 17 | 0.001 | −0.19 to 0.19 | 43 | 0 to 68 | 1772.4 | ||
| Control group | CBT | 18 | 0.03 | −0.09 to 0.16 | 0 | 0 to 50 | 62.50 | 0.120 |
| IE | 6 | −0.16 | −0.41 to 0.08 | 0 | 0 to 75 | 11.11 | ||
| IVE | 4 | −0.35 | −0.78 to 0.07 | 49 | 0 to 83 | 5.10 | ||
| Experimental | VRCBT | 17 | 0.09 | −0.04 to 0.24 | 3 | 0 to 53 | 20.00 | 0.016 |
| intervention | VRE | 12 | −0.18 | −0.35 to −0.006 | 11 | 0 to 50 | 9.80 | |
| Type of anxiety disorder | Flight anxiety | 7 | 0.21 | −0.12 to 0.54 | 41 | 0 to 75 | 8.47 | 0.206 |
| Panic disorder | 6 | −0.05 | −0.32 to 0.21 | 0 | 0 to 75 | 35.71 | ||
| PTSD | 2 | −0.32 | −0.64 to −0.005 | 0 | N/A | 5.56 | ||
| Social anxiety | 5 | −0.18 | −0.52 to 0.15 | 41 | 0 to 78 | 9.80 | ||
| Specific phobia | 3 | −0.19 | −0.57 to 0.17 | 14 | 0 to 91 | 9.43 | ||
| Incomplete outcome data RoBj | High/Unclear | 20 | 0.02 | −0.11 to 0.15 | 5 | 0 to 50 | 83.33 | 0.326 |
| Low | 9 | −0.12 | −0.36 to 0.12 | 43 | 0 to 74 | 14.71 | ||
| Depressive symptoms | ||||||||
| All studies | 13 | 0.004 | −0.20 to 0.21 | 26 | 0 to 62 | 443.11 | ||
| Outliers excludedg | 12 | 0.07 | −0.10 to 0.25 | 0 | 0 to 58 | 25.00 | ||
| Only studies with >25 randomized participants per arm | 4 | −0.03 | −0.27 to 0.20 | 0 | 0 to 85 | 62.5 | ||
| Subgroup analyses | ||||||||
| Country | N. America | 3 | 0.14 | −0.19 to 0.48 | 0 | 0 to 90 | 12.82 | 0.410 |
| EU | 9 | −0.04 | −0.32 to 0.24 | 40 | 0 to 72 | 45.45 | ||
| VR program author | N | 2 | 0.03 | −0.37 to 0.43 | 0 | N/A | 62.50 | 0.901 |
| among author pool | Y | 11 | −0.001 | −0.24 to 0.24 | 37 | 0 to 69 | 1772.4 | |
| Recruitmenth | Clinic | 6 | −0.01 | −0.25 to 0.23 | 0 | 0 to 75 | 166.67 | 0.769 |
| Community | 4 | −0.12 | −0.79 to 0.55 | 76 | 32 to 91 | 14.71 | ||
| Control group | CBT | 10 | 0.08 | −0.10 to 0.28 | 0 | 0 to 62 | 21.74 | 0.777 |
| IE | 2 | 0.02 | −0.39 to 0.43 | 0 | N/A | 83.33 | ||
| Experimental | VRCBT | 8 | 0.17 | −0.07 to 0.43 | 0 | 0 to 68 | 10.42 | 0.126 |
| intervention | VRE | 5 | −0.18 | −0.57 to 0.20 | 62 | 0 to 86 | 9.80 | |
| Incomplete outcome data RoB | High/Unclear | 9 | 0.08 | −0.11 to 0.29 | 0 | 0 to 65 | 21.74 | 0.308 |
| Low | 4 | −0.25 | −0.88 to 0.37 | 70 | 15 to 90 | 7.14 | ||
aAll results are reported with Hedges’ g, using a random effects model. Negative effect indicates superiority of the active interventions over the VR-enhanced therapies.
bThe p levels in this column indicate whether the difference between the ESs in the subgroups is significant. (significant results are marked with italic).
cOutliers: Kampmann, 2016 St.2; Muhlberger, 2003.
dSubgroup analysis were conducted using a mixed effects model. Only subgroups with at least 2 studies were included.
eOne study (Wiederhold, 2001) did not contain information about this moderator.
fTwo studies (Meyerbroeker, 2013, Robillard, 2010 St2) did not contain information about this moderator.
gOutliers: Kampmann, 2016 St.2.
hOne study (Robillard, 2010 St.2) did not contain information about this moderator.
iConfidence intervals around I2 cannot be calculated if there are less than 3 groups.
jRoB: Risk of Bias.
For depression, thirteen RCTs were aggregated, g = 0.004, 95% CI: −0.20 to 0.21, with low heterogeneity (I2 = 26%, 95% CI0 to 62). Exclusion of one outlier led to similar estimations, g = 0.07, 95% CI −0.10 to 0.25, I2 = 0%, as did analyses excluding small N studies, g = −0.03, 95% CI −0.27 to 0.20, I2 = 0%.
Follow-up anxiety outcomes were reported in 15 RCTs, g = −0.07, 95% CI −0.28 to 0.13, with moderate heterogeneity (I2 = 40%, 95% CI 0 to 75). Results were similar with the exclusion of one outlier, g = −0.02, 95% CI −0.19 to 0.14, I2 = 8%. Depressive symptoms at follow-up were reported in 5 RCTs, g = −0.19, 95% CI −0.62 to 0.23, with moderate heterogeneity (I2 = 57%).
Eighteen trials reported non-zero drop-outs in at least one group and ten trials reported zero drop-outs in both groups (Supplementary Table S2). Drop-out rates did not significantly differ between the groups, with similar results for the Mantel-Haenszel (OR = 1.05, 95% CI 0.77 to 1.43, χ2 = 14.06, p = 0.66) (Supplementary Figure S3) and Peto methods (OR = 1.05, 95% CI 0.77 to 1.43, χ2 = 0.12, p = 0.72).
Subgroup and meta-regression analyses
Recruitment setting was a significant moderator for the comparison between VR-enhanced interventions and control (p = 0.02) for anxiety, with the smallest ESs for recruitment from army settings and the highest for recruitment from a clinic. The type of anxiety disorder was also a significant moderator (p < 0.01), but this result is most likely affected by the high heterogeneity present within some of the small subgroups, as shown by the very large confidence intervals around I2. Effects were very high for specific phobia (3 trials, g = 1.79, 95% CI 0.64 to 2.94) and panic disorder, though the latter was only studied in 2 trials. Effects were also high for flight anxiety (3 trials, g = 0.82, 95% CI 0.42 to 1.22). Effects were small for PTSD (4 trials, g = 0.39, 95% CI 0.04 to 0.74), and moderate for social anxiety (5 trials, g = 0.67, 95% CI 0.25 to 1.09). In the comparison with other active therapies, the type of VR intervention (VRE vs VR CBT) was a significant moderator (p = 0.02) for anxiety outcomes. In the subgroup (12 comparisons) where the VR-enhanced therapy was VRE, the non-VR intervention was slightly more effective (g = −0.18, 95% CI −0.35 to −0.006). In this subgroup, the non-VR intervention consisted of imaginal exposure (6 comparisons), CBT (2 comparisons) and in vivo exposure (4 comparisons) (Tables 3 and 4).
Univariate meta-regression indicated significant negative relationships between publication year and both anxiety (slope = −0.06, 95% CI: −0.09 to −0.03) and depression ESs (slope = −0.10, 95% CI: −0.18 to −0.02) in comparison with control conditions, which were maintained in sensitivity analyses excluding outliers. The number of elements of interaction with the virtual environment was positively associated with anxiety outcomes (slope = 0.22, 95% CI: 0.01 to 0.42), but this result did not survive in a sensitivity analysis excluding outliers. For the contrast with other active conditions, publication year, mean age and respectively RoB score were significantly related to anxiety ESs, but only the relationship with age (slope = 0.02, 95% CI: 0.006 to 0.04) survived in analyses excluding outliers.
Small study effects and publication bias
Visual inspection pointed to an asymmetrical funnel for both anxiety and depression. Contour enhanced funnel plots showed that for anxiety (Fig. 5), most of the studies with higher standard errors had results overcoming conventional statistical threshold of p < 0.05, with a considerable proportion of these even significant at the more conservative threshold of p < 0.01. Results were similar for depression (Figure S4), though the number of ESs was much smaller. Egger’s regression intercept test was statistically significant for both anxiety (intercept = 2.03, 95% CI 0.07 to 3.98, p = 0.04) and depression outcomes (intercept = 3.24, 95% CI 0.10 to 6.39, p = 0.04). Galbraith plots for anxiety (Fig. 5) evidenced the same pattern, as studies with low precision (i.e., inverse of the standard error) did not scatter randomly around the regression line, with most of them having effect estimations benefiting the VR intervention. For depression (Supplementary Figure S4) the pattern was inconclusive, probably due to the small number of studies. Finally, the Duval and Tweedie’s trim and fill procedure also pointed to small study effects for anxiety and depression. For anxiety, adjustment for potentially missing studies (n = 5), was associated with the ES decreasing from 0.79 to 0.62, whereas for depression (n = 3), it rendered the pooled ES non-significant. There was reduced indication of small study effects or publication bias for the comparison with other active treatments, with Egger’s test non-significant and no adjustment for missing studies, except for depression.
Figure 5.
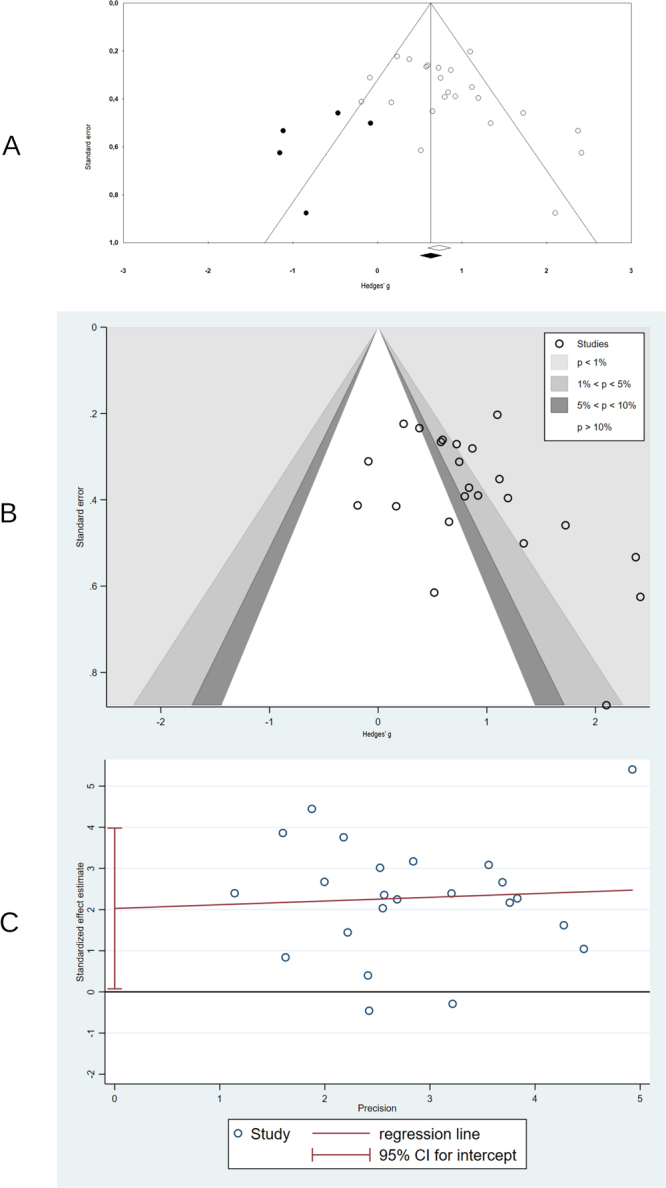
Funnel plots for comparison between VR-enhanced therapy and control conditions for anxiety outcomes: (A) Trim and fill adjusted (white circles, observed studies; black circles, imputed studies); (B) Contour-enhanced funnel plot; (C) Galbraith plot.
For drop-out rates, the Harbord test did not indicate small study effects (coeff = 0.16, 95% CI −1.92 to 2.24, p = 0.87). However, it is important to note this analysis may be biased, as it excluded studies with zero drop-out counts in both arms, which were also some of the smaller N studies (Supplementary Table S2).
Discussion
In the reported meta-analysis, we showed moderate to large effects of VR interventions compared to control conditions (e.g., waitlist, placebo, relaxation, treatment as usual), for anxiety and depression outcomes. The number of studies with follow-up evaluations was too small for a meaningful ES estimation. There was moderate to high heterogeneity and a number of studies with extreme values. Most studies had a small number of participants and there was substantial evidence of small study effects for anxiety outcomes, pointing to potential publication bias. The limited number of studies reporting on depression outcomes precluded us from drawing a meaningful conclusion about small study effects. Adjustment for funnel plot asymmetry, as well as sensitivity analyses excluding outliers or restricted to studies with a moderate number of randomized participants per arm reduced the pooled ES for anxiety, though it still remained moderate to large. Only 7 trials that reported on anxiety outcomes had randomized at least 25 participants in each arm. The persistent evidence of small study effects, as well as the significant heterogeneity, y casts doubts over the reliability of the large effects observed for anxiety25,37,38. Heterogeneity continued to remain moderate with large confidence intervals even when extreme values were excluded, showing it was not simply the by-product of a few trials. Two thirds of the studies used waitlist controls, and effect sizes were large in waitlist comparisons. Use of waitlist controls might inadvertently and artificially inflate effect sizes for both anxiety and depression outcomes39,40.
Conversely, compared with established active interventions, effect sizes were non-significant for both anxiety and depression outcomes, at post-test and follow-up. Heterogeneity was small to moderate and there was limited evidence of funnel plot asymmetry or small study effects. Sensitivity analyses excluding outliers or restricted to studies with at least 25 participants randomized in each arm produced similar estimations. There were more trials in the latter category (12) than in the comparison with control conditions (7), but these were still a minority. All but one of the trials were powered to test superiority, not equivalence or non-inferiority41, so it would be premature to construe our findings as proof of equivalent effects. Most frequently employed non-VR active interventions were IVE and CBT, both shown to be effective for anxiety and depression, thereby potentially difficult to outperform.
VR-enhanced interventions did not improve attrition, producing similar drop-out rates with control conditions and other active interventions. These findings contradict previous speculation of possible comparative benefit1,5,8. However, most trials were small and many reported zero drop-outs, sometimes in both arms, so the stability of this result needs to be considered with caution. We were not able to evidence small study effects for analyses on attrition, but this result is most likely biased by the fact studies with zero counts in both arms were excluded and many of these were also small studies.
The vast majority of RCTs of VR interventions had high or uncertain risk of bias across domains. Two previous meta-analyses6,7 examined bias using combinations of instruments, which included aspects not linked to any type of trial bias (e.g., training for providers), potentially obfuscating distorting effects. In contrast, we used the Cochrane Risk of Bias tool11, which evaluates domains likely to distort outcomes. Only four trials could be rated as low RoB on all domains considered, preventing us from reliably assessing the relationship between overall trial risk of bias and outcomes. The only RoB domain where most trials reported information was incomplete outcome data. Almost two thirds of the studies were rated as high risk of attrition bias, again questioning the reliability of the ES estimations, as exclusion of participants from RCT analyses was shown to distort outcomes42,43. In exploratory subgroup analysis, we did not find differences between studies with high/uncertain versus low RoB for incomplete outcome reporting, though the number of studies with low RoB was small, particularly in comparisons with control (7). It is possible previous assessments concluding no relationship between trial risk of bias and ESs might have been too optimistic.
Though the presence the developers of VR interventions among the author pool was not significantly associated with changes in the magnitude of the effects, it is worth underscoring the vast majority of trials did involve such a developer. For instance, for the comparison with control conditions, only five anxiety effect sizes came from independent studies, and 17 from trials involving the developer. As such, it is possible that the insufficient variability in our sample of included trials prevented us from detecting more subtle differences. Moreover, we only examined whether one of the authors had also developed the VR treatment program used, not any potential commercial involvements with VR companies, which could arguably represent a more direct conflict of interest. However, since most articles did not report this information, we could not examine it systematically.
We identified few moderators, owing to the fact most subgroups were small and affected by high heterogeneity within the group. Recruitment setting seemed to have an influence on ESs in comparisons between VR-enhanced and control conditions, with smaller effects for recruitment from army settings, but this may also be a spurious result since some of the subgroups contained a very limited number of studies. Type of anxiety diagnosis also appeared to be a significant moderator, with high effects for specific phobia and flight anxiety, and moderate or small effects for social anxiety and PTSD. It is likely that this is at least partly a spurious result, given subgroups were small and heterogeneity was high in all of them. The type of active comparison intervention used appeared to matter, with VR-enhanced exposure having slightly smaller effects than non-VR interventions. Again, the number of studies was small and this relationship could have also been confounded by other variables, such as the type of problem for which the therapy was used.
It was speculated1 that improved engagement with the virtual environment, as measured by immersion or a sense of presence, could play an important role in the effectiveness of VR. Only a modest number of trials measured immersion and presence explicitly. Even in those that did, most did not analyze these variables in relationship to treatment outcomes or found no association. We showed that the number of elements employed by the VR technology, a crude indicator of interaction, was positively related with anxiety outcomes in comparisons with passive, but not active, treatments. However, this result did not survive sensitivity analyses and could be an artefactual finding. But even for visual stimulation, though one might assume that more recent studies use very sophisticated technology, instead of stereoscopic simulations not intended for VR use, we saw no evidence to this effect. For example a 2017 trial44 relied on the same technology as similar trials from 201345 and even 200546.
Publication year was consistently negatively associated to outcomes, though reasons for this trend remained unclear. A rise in larger or lower risk of bias trials seems unlikely given we observed few such trials. The apparent decrease in effectiveness with the passing of time might also be a by-product of the early use of pilot, low powered studies where only large effects can overcome the significance threshold, a strong initial publication bias for positive findings, as well as time lag bias, whereby studies with positive results are published first and dominate the field, until the negative, but equally important, studies get published22,47. Previous meta-analyses of RCTs of VR interventions either did not consider publication bias at all4,6, or reported optimistic estimations7, based on the fail-safe N, whose use is discouraged for being unreliable and misleading22. We used a range of methods to assess funnel plot asymmetry, all of which corroborated that small studies were numerous, mostly significant and overestimated effects for comparisons with control conditions. Publication bias for positive findings, probably more prominent in the early years of studying VR interventions, is one likely cause of small study effects. We conjecture it is most likely present in the literature of VR interventions for anxiety, where most trials are concentrated.
There are several limitations to our meta-analysis. There was a high degree of heterogeneity, particularly in comparisons with control conditions. This was accompanied by very large confidence intervals around I2, even for the comparisons where heterogeneity estimates were smaller. Residual heterogeneity persisted even after sensitivity analyses were conducted, or potential moderators explored. NNTs can be useful as an ancillary clinical ES measure, but there is disagreement regarding the most adequate calculation method48, and concerns over their potential to mislead, particularly when resulting from meta-analyses, as baseline risk can vary substantially between trials49. Many of the subgroup analyses were underpowered and we were able to identify few moderators. We could not calculate effect sizes for three trials where the report did not contain enough information and the original authors did not provide the data. However, given their size and the total number of included trials, their exclusion is unlikely to have influenced estimations.
Conclusions
From the standpoint of dissemination and implementation, our results leave several open questions. Virtual reality enhanced interventions had moderate to large effects compared to control conditions, though these effects were likely inflated by several factors in the design and implementation of the trials. We could find few difference with other active interventions. These might be construed as evidence VR-enhanced interventions could be added to the armamentarium, as another effective choice available to clinicians and patients.
However, other key aspects remain unclear. Though it would be intuitive to consider VR-enhanced interventions as more cost-effective than traditional anxiety treatments, notably in vivo exposure, research substantiating this claim is missing. Moreover, it might hinge on the specific disorder targeted. For instance, for flight anxiety it may seem evident that it would be more cost-effective to conduct VR-enhanced exposure than buy a plane ticket for in vivo exposure. Conversely, for height anxiety, it could be more cost-effective to scale a flight of stairs with a patient, than to purchase a HMD system and pay for the software development of a fully immersive VR environment. Nonetheless, this kind of tailored, immersive and sophisticated technology does not seem to be used much, even in recent trials, further complicating a realistic calculation of cost-effectiveness. One might also argue VR-enhanced interventions might be particularly suitable for disorders where other active interventions have been less effective. Nonetheless, in the case of one such disorder- post-traumatic stress disorder- two recent trials36,50 failed to find additional benefits for VR interventions over non-VR treatments such as prolonged exposure, both in terms of primary outcome, as well as drop-out rates, with follow-up results actually better for the non-VR intervention.
Most importantly, many existent trials are poorly reported and exposed to bias. The effort to move forward should primarily focus on elevating the quality of VR trials. Larger trials minimizing risk of bias by prospective registration and transparent and complete reporting, as well as using credible control group, are necessary. A recent ongoing trial described in a published protocol is one such example51. Trials should also report cost-effectiveness analyses in an attempt to clarify whether and under which conditions are VR-enhanced treatments cost-effective. Finally, they should include an evaluation of the participants’ engagement with the VR environment, so as to clarify how immersive and sophisticated the system needs to be to support improved outcomes. Moreover, given the predominance of trials conducted by developers of VR treatments, independently conducted trials are also critical. It is essential that negative results are afforded journal space in order to tackle potential publication bias.
Electronic supplementary material
Acknowledgements
Liviu A. Fodor, Carmen D. Cotet and Ioana A. Cristea were supported for this work by Romanian National Authority for Scientific Research and Innovation, CNCS – UEFISCDI, project number PN-II-RU-TE-2014-4-1316 awarded to Ioana A. Cristea. Daniel David is supported by EU-FP7 ICT-2013.2.1 DREAM: Development of Robot-Enhanced Therapy for Children with Autism Spectrum Disorder (Grant No. 611391). The funder had no involvement in the study design, collection, analysis and interpretation of data, writing of the report, and in the decision to submit the article for publication.
Author Contributions
I.C. and P.C. had the original idea for this paper. L.F., C.C. and I.C. did the searches, study selection, the data extraction and analyses and wrote the first draft of the paper. All authors (L.F., C.C., P.C., S.S., D.D. and I.C.) read all versions of the text of the paper critically and contributed significantly to the content. All the authors have reviewed the present version of the manuscript and approved it for submission.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28113-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freeman D, et al. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychological medicine. 2017;47:2393–2400. doi: 10.1017/S003329171700040X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.David D, Matu S-A, David OA. New Directions in Virtual Reality-Based Therapy for Anxiety Disorders. International Journal of Cognitive Therapy. 2013;6:114–137. doi: 10.1521/ijct.2013.6.2.114. [DOI] [Google Scholar]
- 3.Gerardi M, Cukor J, Difede J, Rizzo A, Rothbaum BO. Virtual reality exposure therapy for post-traumatic stress disorder and other anxiety disorders. Current psychiatry reports. 2010;12:298–305. doi: 10.1007/s11920-010-0128-4. [DOI] [PubMed] [Google Scholar]
- 4.Opris D, et al. Virtual reality exposure therapy in anxiety disorders: a quantitative meta-analysis. Depression and anxiety. 2012;29:85–93. doi: 10.1002/da.20910. [DOI] [PubMed] [Google Scholar]
- 5.Meyerbroker K, Emmelkamp PM. Virtual reality exposure therapy in anxiety disorders: a systematic review of process-and-outcome studies. Depression and anxiety. 2010;27:933–944. doi: 10.1002/da.20734. [DOI] [PubMed] [Google Scholar]
- 6.McCann RA, et al. Virtual reality exposure therapy for the treatment of anxiety disorders: an evaluation of research quality. Journal of anxiety disorders. 2014;28:625–631. doi: 10.1016/j.janxdis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Turner WA, Casey LM. Outcomes associated with virtual reality in psychological interventions: where are we now? Clinical psychology review. 2014;34:634–644. doi: 10.1016/j.cpr.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Botella C, Serrano B, Banos RM, Garcia-Palacios A. Virtual reality exposure-based therapy for the treatment of post-traumatic stress disorder: a review of its efficacy, the adequacy of the treatment protocol, and its acceptability. Neuropsychiatric disease and treatment. 2015;11:2533–2545. doi: 10.2147/NDT.S89542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armijo-Olivo S, Fuentes J, Ospina M, Saltaji H, Hartling L. Inconsistency in the items included in tools used in general health research and physical therapy to evaluate the methodological quality of randomized controlled trials: a descriptive analysis. BMC medical research methodology. 2013;13:116. doi: 10.1186/1471-2288-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of clinical epidemiology. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 11.Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.)343, d5928, 10.1136/bmj.d5928 (2011). [DOI] [PMC free article] [PubMed]
- 12.Hedges, L. V. & Olkin, I. Statistical Methods for Meta-analysis. (Academic Press, 1985).
- 13.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biological psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. The New England journal of medicine. 1988;318:1728–1733. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 15.Borenstein, M., Hedges, L. V., Higgins, J. P. T. & Rothstein, H. R. Introduction to Meta-Analysis. (Wiley, 2009).
- 16.Scammacca N, Roberts G, Stuebing KK. Meta-Analysis With Complex Research Designs: Dealing With Dependence From Multiple Measures and Multiple Group Comparisons. Review of educational research. 2014;84:328–364. doi: 10.3102/0034654313500826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985;41:55–68. doi: 10.2307/2530643. [DOI] [PubMed] [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719–748. [PubMed] [Google Scholar]
- 20.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Progress in cardiovascular diseases. 1985;27:335–371. doi: 10.1016/S0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Pullenayegum E, Marshall JK, Iorio A, Thabane L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ open. 2016;6:e010983. doi: 10.1136/bmjopen-2015-010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins, J. P. T. & Green, S. (The Cochrane Collaboration, 2011).
- 23.IntHout J, Ioannidis JP, Borm GF, Goeman JJ. Small studies are more heterogeneous than large ones: a meta-meta-analysis. Journal of clinical epidemiology. 2015;68:860–869. doi: 10.1016/j.jclinepi.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ (Clinical research ed.) 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HETEROGI: Stata module to quantify heterogeneity in a meta-analysis (2006).
- 27.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of clinical epidemiology. 2008;61:991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Statistics in medicine. 1988;7:889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 30.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 31.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in medicine. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson T, Steffert T, Steed A, Gruzelier J. A randomized controlled trial of the effects of hypnosis with 3-D virtual reality animation on tiredness, mood, and salivary cortisol. The International journal of clinical and experimental hypnosis. 2011;59:122–142. doi: 10.1080/00207144.2011.522917. [DOI] [PubMed] [Google Scholar]
- 34.Riva G, Bacchetta M, Baruffi M, Molinari E. Virtual reality-based multidimensional therapy for the treatment of body image disturbances in obesity: a controlled study. Cyberpsychology & behavior: the impact of the Internet, multimedia and virtual reality on behavior and society. 2001;4:511–526. doi: 10.1089/109493101750527079. [DOI] [PubMed] [Google Scholar]
- 35.Riva G, Bacchetta M, Baruffi M, Molinari E. Virtual-reality-based multidimensional therapy for the treatment of body image disturbances in binge eating disorders: a preliminary controlled study. IEEE transactions on information technology in biomedicine: a publication of the IEEE Engineering in Medicine and Biology Society. 2002;6:224–234. doi: 10.1109/TITB.2002.802372. [DOI] [PubMed] [Google Scholar]
- 36.Reger GM, et al. Randomized controlled trial of prolonged exposure using imaginal exposure vs. virtual reality exposure in active duty soldiers with deployment-related posttraumatic stress disorder (PTSD) Journal of consulting and clinical psychology. 2016;84:946–959. doi: 10.1037/ccp0000134. [DOI] [PubMed] [Google Scholar]
- 37.Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. Jama. 2014;312:623–630. doi: 10.1001/jama.2014.8166. [DOI] [PubMed] [Google Scholar]
- 38.Nuesch E, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ (Clinical research ed.) 2010;341:c3515. doi: 10.1136/bmj.c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJ. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World psychiatry: official journal of the World Psychiatric Association (WPA) 2016;15:245–258. doi: 10.1002/wps.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furukawa TA, et al. Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta-analysis. Acta psychiatrica Scandinavica. 2014;130:181–192. doi: 10.1111/acps.12275. [DOI] [PubMed] [Google Scholar]
- 41.Christensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. Journal of hepatology. 2007;46:947–954. doi: 10.1016/j.jhep.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Abraha I, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. BMJ (Clinical research ed.) 2015;350:h2445. doi: 10.1136/bmj.h2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nuesch E, et al. The effects of excluding patients from the analysis in randomised controlled trials: meta-epidemiological study. BMJ (Clinical research ed.) 2009;339:b3244. doi: 10.1136/bmj.b3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouchard S, et al. Virtual reality compared with in vivo exposure in the treatment of social anxiety disorder: a three-arm randomised controlled trial. The British journal of psychiatry: the journal of mental science. 2017;210:276–283. doi: 10.1192/bjp.bp.116.184234. [DOI] [PubMed] [Google Scholar]
- 45.Anderson PL, et al. Virtual reality exposure therapy for social anxiety disorder: a randomized controlled trial. Journal of consulting and clinical psychology. 2013;81:751–760. doi: 10.1037/a0033559. [DOI] [PubMed] [Google Scholar]
- 46.Klinger E, et al. Virtual reality therapy versus cognitive behavior therapy for social phobia: a preliminary controlled study. Cyberpsychology & behavior: the impact of the Internet, multimedia and virtual reality on behavior and society. 2005;8:76–88. doi: 10.1089/cpb.2005.8.76. [DOI] [PubMed] [Google Scholar]
- 47.Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. Jama. 1998;279:281–286. doi: 10.1001/jama.279.4.281. [DOI] [PubMed] [Google Scholar]
- 48.Furukawa TA, Leucht S. How to obtain NNT from Cohen’s d: comparison of two methods. PloS one. 2011;6:e19070. doi: 10.1371/journal.pone.0019070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta-analyses–sometimes informative, usually misleading. BMJ (Clinical research ed.) 1999;318:1548–1551. doi: 10.1136/bmj.318.7197.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLay RN, et al. A Randomized, Head-to-Head Study of Virtual Reality Exposure Therapy for Posttraumatic Stress Disorder. Cyberpsychology, behavior and social networking. 2017;20:218–224. doi: 10.1089/cyber.2016.0554. [DOI] [PubMed] [Google Scholar]
- 51.Miloff A, et al. Single-session gamified virtual reality exposure therapy for spider phobia vs. traditional exposure therapy: study protocol for a randomized controlled non-inferiority trial. Trials. 2016;17:60. doi: 10.1186/s13063-016-1171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available in the Figshare repository, 10.6084/m9.figshare.5675407.