Abstract
Glutathione (GSH) protects against oxidative damage in many tissues, including retinal pigment epithelium (RPE). Oxidative stress-mediated senescence and death of RPE and subsequent death of photoreceptors have been observed in age-related macular degeneration (AMD). Although the consequences of GSH depletion have been described previously, questions remain regarding the molecular mechanisms. We herein examined the downstream effects of GSH depletion on stress-induced premature senescence (SIPS) and cell death in human RPE cells. Briefly, cultured ARPE-19 cells were depleted of GSH using: (1) incubation in cystine (Cys2)-free culture medium; (2) treatment with buthionine sulphoximine (BSO, 1000 µM) to block de novo GSH synthesis for 24–48 h; or (3) treatment with erastin (10 µM for 12–24 h) to inhibit Cys2/glutamate antiporter (system xc−). These treatments decreased cell viability and increased both soluble and lipid reactive oxygen species (ROS) generation but did not affect mitochondrial ROS or mitochondrial mass. Western blot analysis revealed decreased expression of ferroptotic modulator glutathione peroxidase 4 (GPX4). Increased autophagy was apparent, as reflected by increased LC3 expression, autophagic vacuoles, and autophagic flux. In addition, GSH depletion induced SIPS, as evidenced by increased percentage of the senescence-associated β-galactosidase-positive cells, increased senescence-associated heterochromatin foci (SAHF), as well as cell cycle arrest at the G1 phase. GSH depletion-dependent cell death was prevented by selective ferroptosis inhibitors (8 μM Fer-1 and 600 nM Lip-1), iron chelator DFO (80 μM), as well as autophagic inhibitors Baf-A1 (75 nM) and 3-MA (10 mM). Inhibiting autophagy with Baf-A1 (75 nM) or 3-MA (10 mM) promoted SIPS. In contrast, inducing autophagy with rapamycin (100 nM) attenuated SIPS. Our findings suggest that GSH depletion induces ferroptosis, autophagy, and SIPS. In addition, we found that autophagy is activated in the process of ferroptosis and reduces SIPS, suggesting an essential role of autophagy in ferroptosis and SIPS.
Introduction
The eyes are exposed to constant irradiation, and therefore have extraordinary need for antioxidant protection1. RPE is particularly susceptible to oxidative stress due to its role in phagocytosis of photoreceptor outer segments2. Phagocytosed photoreceptor outer segments are highly enriched in polyunsaturated fatty acids and are the major source of intracellular ROS generation in RPE3,4. In addition, partial oxygen pressure in RPE is high due to anatomical proximity to choriocapillaries5–7. Studies using exogenous oxidants such as tert-butyl hydroperoxide (tBH) and hydrogen peroxide (H2O2) in cultured RPE cells highlight the role of oxidative stress in RPE cell death and premature senescence8–11. However, it is unclear to what extent the in vitro observations correspond to metabolic and disease processes seen in vivo12.
GSH is the most prominent antioxidant in RPE cells and is present at high concentration in retina and RPE13,14. However, the efficiency of GSH redox system declines with age, predisposing the RPE to increased oxidative-stress-mediated damage15,16. Exogenously administered GSH protects against oxidative damage in cultured human RPE17, while GSH depletion was shown to cause cell death18. However, the mechanism beneath the effect of GSH depletion-induced oxidative stress in RPE cells and the subsequent RPE damage is not well established. Therefore it is essential to investigate the effect of GSH depletion on RPE cells to further elucidate the potentially involved mechanisms.
Cell death types induced by ROS include apoptotic, autophagic, ferroptotic, and necrotic cell deaths. Earlier studies demonstrated that GSH depletion could induce apoptosis19,20 or necrosis21 in RPE cells. In other cell types such as spermatogonial cells, GSH depletion may induce autophagy22. Recent reports demonstrated that GSH depletion triggers ferroptosis in some cell types18. It is likely that variations in experimental methods, biological systems, as well as the distinct metabolic characteristics of cells contributed to the variability in reported results18,23. Ferroptosis, a form of regulated cell death initiated by lipid peroxidation, differs from other types of cell deaths at genetic, biochemical, and morphological levels24,25. Ferroptosis is regulated by distinct molecular pathways and was shown to play an important role in degenerative and neoplastic diseases26. Nonetheless, whether ferroptotic cell death is associated with the effects of GSH depletion on RPE is unknown.
Besides cell death, ROS generated from oxidative stress also triggers SIPS. SIPS is a phenomenon characterized by the irreversible cessation of the division of normal cells even in presence of nutritional and mitogenic factors27. SIPS can be caused by various factors including persistent exposure to cell stress, namely, oxidative stress or DNA-damaging mediators28,29. Documented data indicate that premature senescence of ARPE-19 cells is possibly involved in the features of AMD10,28. Previous studies reported that SIPS can be triggered by oxidative stress caused by tBH and H2O210,11; however, whether GSH depletion is linked with SIPS in RPE is unknown.
Hence, in this study, we aimed to investigated the effect of GSH depletion-induced oxidative stress in RPE cells. We also sought to explore the nature of cell death involved and examine whether RPE cells experience SIPS following GSH depletion.
Results
GSH depletion induces cell death in RPE cells
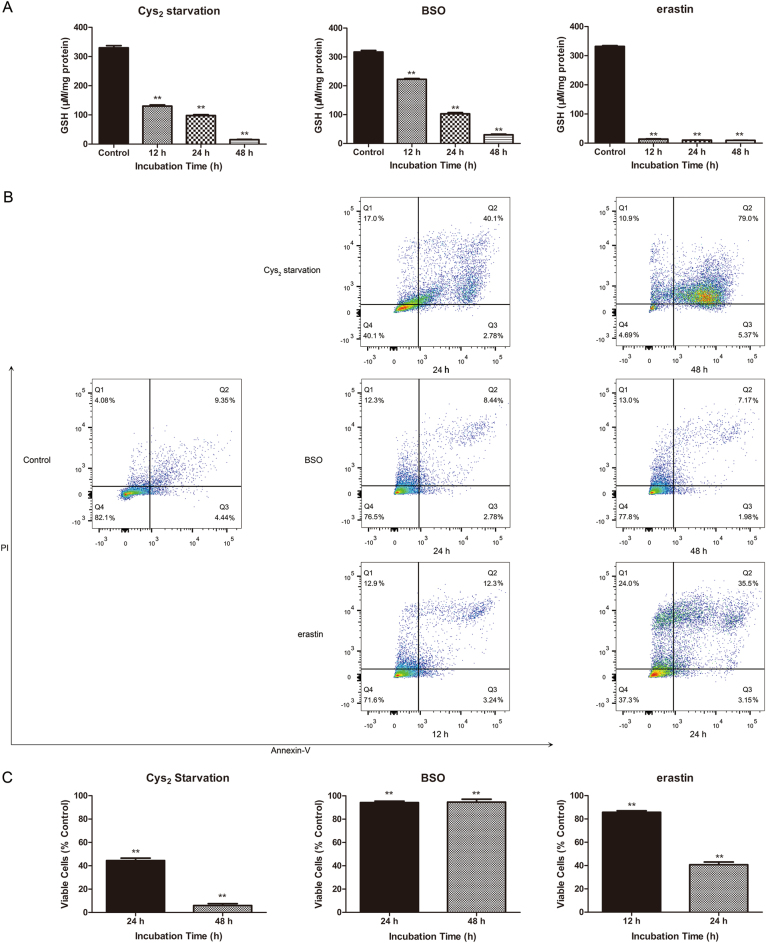
To evaluate the effect of GSH depletion on the RPE cells, we used three different approaches: depletion of Cys2 from cell culture media, treatment with buthionine sulphoximine (BSO, 1000 µM) to block de novo synthesis of GSH, or treatment with erastin (10 µM), an inhibitor of system xc− 1. GSH depletion was apparent after all three treatments (Fig. 1a), and all treatments lead to decreased cell viability, as measured by annexin V-PI assay (Fig. 1b, c).
Fig. 1. GSH depletion induces cell death in RPE cells.
a Quantification of intracellular GSH content. Cells were treated with Cys2 starvation, 1000 µM BSO or 10 µM erastin for 12, 24, and 48 h, respectively. b GSH depletion-induced cell death in ARPE-19 cells revealed by flow cytometry. The upper panel: Cys2 starvation for 24 and 48 h. The middle panel: BSO (1000 µM) treatment for 24 and 48 h. The lower panel: erastin (10 µM) treatment for 12 and 24 h. Quadrant Q4: viable cells. Numbers displayed in each quadrant represent proportion of cells. c Represent quantification of the effects depicted in (b). Data in (a) and (c) represent mean ± SD from one of three representative experiments. ** represent p < 0.01
GSH depletion causes oxidative stress and lipid peroxidation
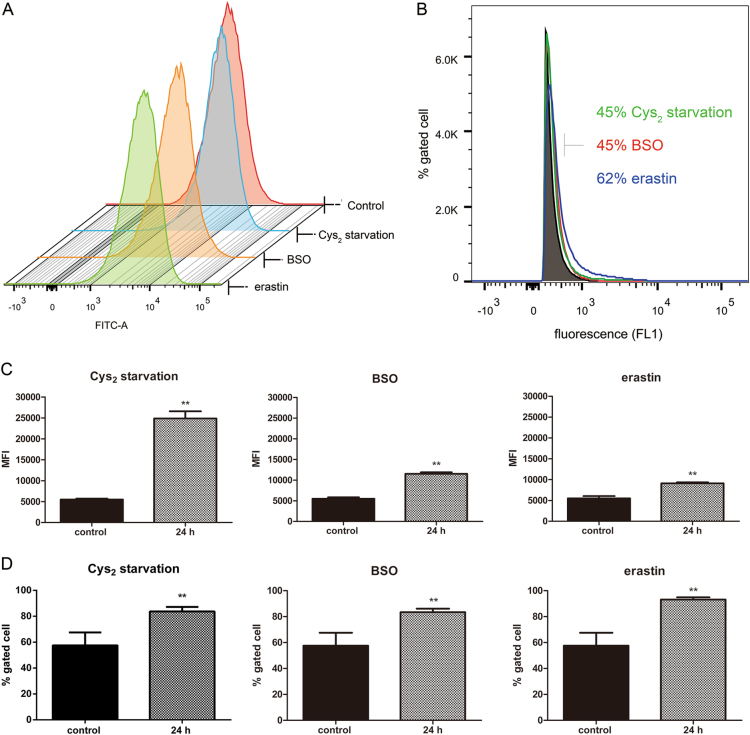
To evaluate the downstream effects of GSH depletion, we measured soluble ROS in RPE cells treated with Cys2 starvation, BSO, or erastin by flow cytometry using the fluorescent probe H2DCFDA. GSH depletion increased cytosolic ROS (Fig. 2a, c). To determine the site of ROS generation, redox-sensitive dye BODIPY 581/591 C11 was used to detect lipid ROS (Fig. 2b). Stimulation of BODIPY-loaded RPE cells with Cys2 starvation or with BSO/erastin treatment increased ROS generation as measured by increased rate of BODIPY oxidation (Fig. 2d).
Fig. 2. GSH depletion induces oxidative stress and lipid peroxidation in RPE cells.
a Cytosolic ROS production assessed in ARPE-19 cells treated for 24 h with Cys2 starvation, 1000 µM BSO, and 10 µM erastin by flow cytometry using H2DCFDA. b Lipid ROS production assessed in ARPE-19 cells treated for 24 h with Cys2 starvation, 1000 µM BSO, and 10 µM erastin by flow cytometry using C11-BODIPY. c, d represent quantified data depicted in (a, b). Data in (c, d) represent mean ± SD from one of three representative experiments. Representative data from one of three experiments are shown. ** represent p < 0.01
GSH depletion does not induce mitochondrial superoxide or alter mitochondrial mass in RPE cells
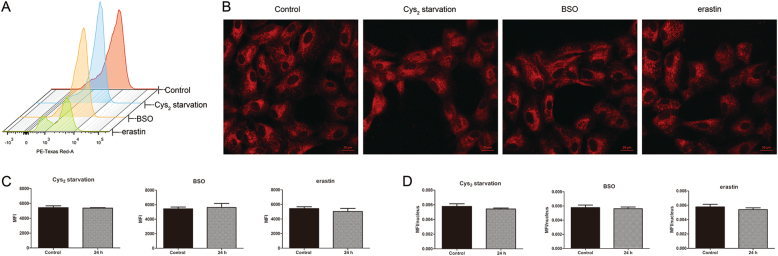
It has been suggested that mitochondria are predominant source of ROS upon H2O2-induced oxidative stress7. We next investigated whether mitochondria are the source of ROS generation in RPE cells upon GSH depletion, we used MitoSOX Red to detect mitochondrial ROS (Fig. 3a, c). Stimulation of MitoSOX-loaded RPE cells with Cys2 starvation or BSO did not cause oxidation of the fluorophore. We also failed to detect an increase in MitoSOX-sensitive mitochondrial ROS production in erastin-treated RPE cells. These observations are in line with the previous findings of Dixon et al. in HT-1080 cancer cells where treatment with erastin did not cause mitochondrial ROS production24. It has been demonstrated that oxidative stress and excessive generation of ROS affect mitochondrial morphology30. To determine if GSH depletion-induced oxidative stress impairs mitochondrial biogenesis, we used MitoTracker Red to stain live mitochondria. We calculated the mean fluorescent intensity (MFI) using ImageJ software as described in Methods. Cells subjected to Cys2 starvation or BSO and erastin coincubation did not alter MitoTracker fluorescence intensity (Fig. 3b, d). These findings further ruled out mitochondrial damage to be the source of GSH depletion-induced oxidative stress.
Fig. 3. GSH depletion does not induce mitochondrial superoxide or alter mitochondrial mass in RPE cells.
a Mitochondrial ROS assessed in ARPE-19 cells treated for 24 h with Cys2 starvation, 1000 µM BSO, and 10 µM erastin by flow cytometry using MitoSOX Red. b Mitochondrial mass was measured by MitoTracker Red dye. Scale bars: 20 μm. (c) and (d) represent quantified data depicted in (a, b). Data in (c, d) represent mean ± SD from one of three representative experiments. Representative data from one of three experiments are shown
GSH depletion causes ferroptosis in RPE cells
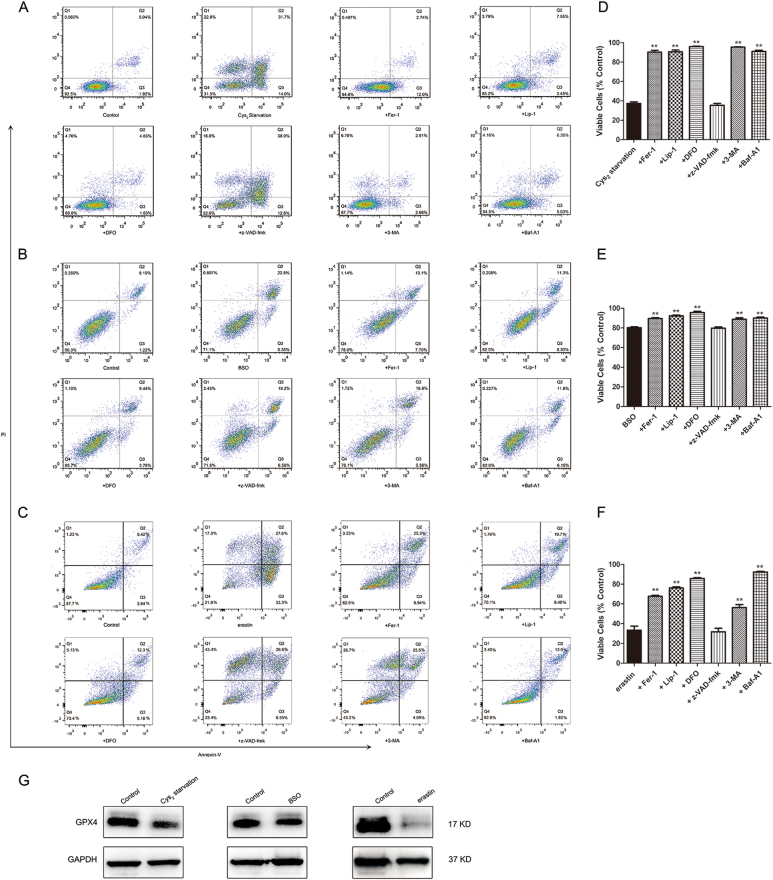
To evaluate the mechanism of cell death in GSH-depleted cells, we treated RPE cells with various cell death pathway inhibitors including ferroptosis inhibitors ferrostatin-1 (Fer-1, 8 μM), liproxstatin-1 (Lip-1, 600 nM), iron chelator deferoxamine (DFO, 80 μM), pan-caspase inhibitor z-VAD-fmk (30 μM) to inhibit apoptosis, autophagy inhibitor 3-methyladenine (3-MA, 10 mM), and lysosomal inhibitor bafilomycin A1 (Baf-A1, 75 nM). These reagents were added either in Cys2-free culture medium or coincubated with BSO or erastin at indicated concentrations. Annexin V-PI apoptosis assay was performed following the treatments to assess cell viability (Fig. 4a–c).
Fig. 4. GSH depletion induces ferroptosis in RPE cells.
a–c Cell viability detection with Annexin V/PI by flow cytometry. a Cys2 starvation with or without selected cell death inhibitors. b BSO (1000 µM) treatment with or without selected cell death inhibitors. c Erastin (10 µM) treatment with or without selected cell death inhibitors. Ferroptosis-specific inhibitor Fer-1 (8 μM), Lip-1 (600 nM), iron chelator DFO (80 μM), pan-caspase inhibitor z-VAD-fmk (30 μM), autophagic inhibitor 3-MA (10 mM), and lysosomal inhibitor Baf-A1 (75 nM) were diluted in Cys2-free culture medium or coincubated with BSO or erastin treatment at indicated dose (all stock dissolved in DMSO except 3-MA; the latter is water soluble) for 24 h. Quadrant Q4: viable cells. Numbers displayed in each quadrant represent proportion of cells. (d–f) represent quantified data depicted in (a–c). Data in (d–f) represent mean ± SD from one of three representative experiments. Representative data from one of three experiments are shown. ** represent p < 0.01. g GPX4 downregulation as assessed by immunoblotting
Cell death inhibitors achieved comparable effects in Cys2-starved or BSO/erastin-treated RPE cells. Fer-1 increased viability to 90% of cells under Cys2 starvation and in BSO-treated cells, and to 70% in erastin-treated cells (Fig. 4d–f, p < 0.01). Lip-1 increased viability to 90% under Cys2 starvation and in BSO-treated cells, and to 70% in erastin-treated RPE cells (Fig. 4d–f, p < 0.01). Similar protective effects were observed with DFO (Fig. 4d–f, p < 0.01). No rescuing effects were observed with z-VAD-fmk, suggesting that apoptosis is not induced by GSH depletion. Intriguingly, lysosomal and autophagy flux inhibitor Baf-A1 showed similar rescuing effect as DFO. Another autophagy inhibitor 3-MA also produced rescuing effects but to a smaller extent in erastin-treated cells. These findings suggest that cell death in GSH-depleted cells happens through ferroptosis and autophagy. Ferroptosis is regulated by GPX4, an enzyme that reduces lipid hydroperoxides within biological membranes26. Inactivation of GPX4 leads to accumulation of lipid ROS and induces ferroptosis18,31. We found that expression of GPX4 was reduced after Cys2 starvation and erastin treatment, further proving that ferroptosis is a primary mechanism of GSH depletion-induced cell death in RPE (Fig. 4g).
GSH depletion triggers autophagy
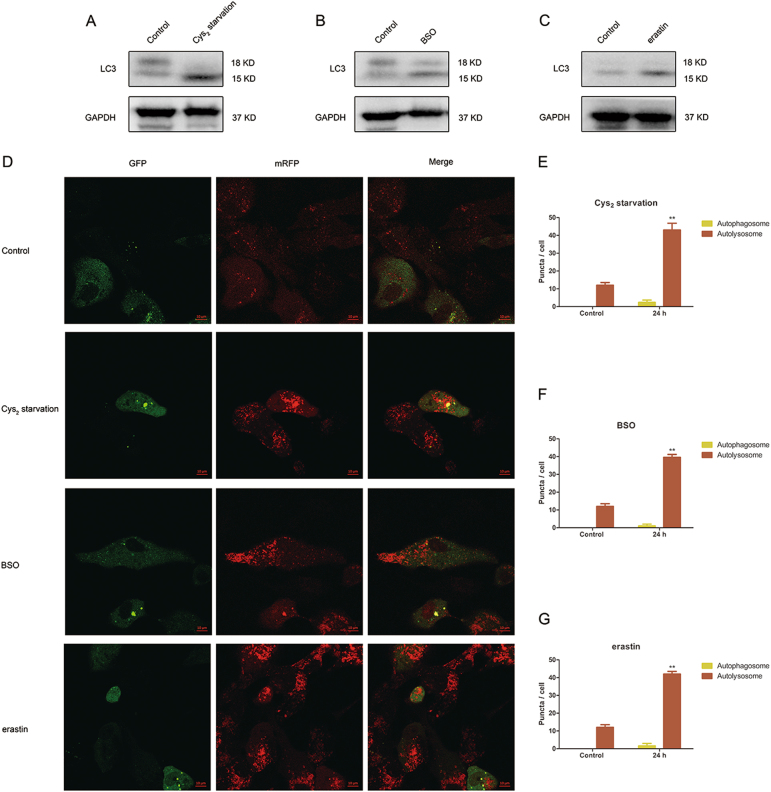
As autophagy inhibitors Baf-A1 and 3-MA protected from cell death in response to GSH depletion, we investigated whether autophagy is induced by GSH depletion. Western blot analysis of LC3II, a surrogate marker for autophagy32, revealed autophagic induction in GSH-depleted cells. In comparison to the controls, LC3-II level was increased in Cys2-starved, BSO, and erastin-treated cells (Fig. 5a–c).
Fig. 5. GSH depletion triggers autophagic activation accompanied by an increasing autophagic flux.
(a–c) represent autophagy activation in Cys2 starved, 1000 µM BSO, and 10 µM erastin-treated RPE cells, respectively, by detect LC3 using western blot. d After being exposed to Cys2 starvation milieu, 1000 µM BSO and 10 µM erastin for 24 h, representative images of ARPE-19 cells displaying LC3 puncta were immediately visualized by confocal microscopy. Number of autophagosomes represented by yellow puncta and autolysosomes represented by red puncta in merged images. Scale bar, 10 µm. (e–g) represent quantified data depicted in (d). Red puncta and yellow puncta were counted and calculated as number per cell. Data represent mean ± SD from one of three representative experiments. Representative data from one of three experiments are shown. ** represent p < 0.01
Next, we monitored autophagic flux in GSH-depleted cells by using a tandem fluorescent-tagged LC3 lentivirus mRFP-GFP-LC3 expression system. mRFP-GFP-LC3 is a ratiometric probe bound to both the inner and outer membrane of the autophagosome33. GFP signals within the inner autophagosome membrane are degraded in the autolysosomes but mRFP signals are relatively stable and thus provide a visible measure of autophagic flux status 33.
mRFP-GFP-LC3 lentivirus were transduced into RPE cells prior to treatment. Red puncta (mRFP only, representing autolysosomes) and yellow puncta (overlapping of mRFP and GFP, representing autophagosomes) were counted and presented as number per cell. GSH depletion in RPE cells increased the number of red puncta, indicating an increase in autophagic flux (Fig. 5d–g). The observed effect was least pronounced in the BSO-treated group, as shown in western blot.
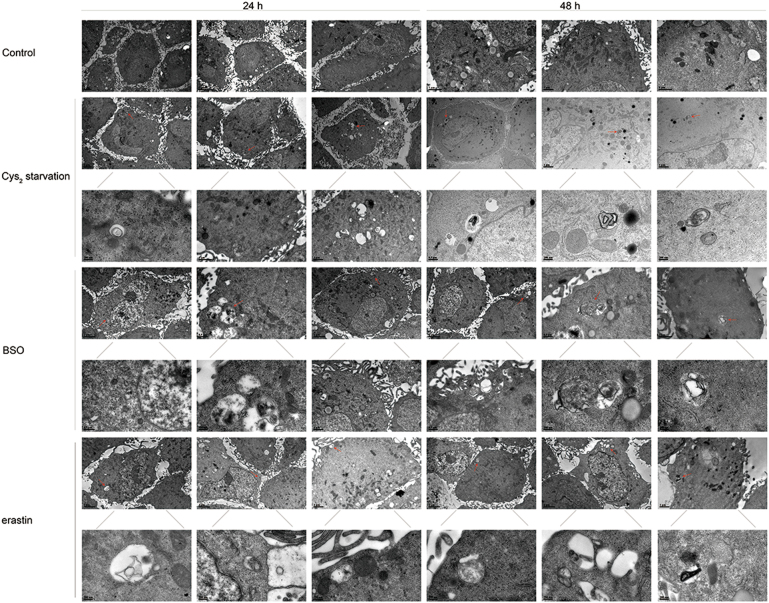
Finally, we performed transmission electron microscopy (TEM) to detect autophagic vesicles at the ultrastructural level. Autophagosomes appearing as double membrane structures were observed in cells treated with Cys2 starvation, BSO, and erastin (Fig. 6).
Fig. 6. Ultrastructure of GSH depleted RPE cells.
Representative TEM photomicrographs of ARPE-19 cells exposed to Cys2 starvation milieu, 1000 µM BSO, and 10 µM erastin for 24 and 48 h. Autophagic vacuoles are indicated by red arrows. The lower panel of each treatment shows the high magnification image of the upper panel. Scale bars are displayed in each image
GSH depletion triggers cell cycle arrest in G1/S checkpoint and induces SIPS
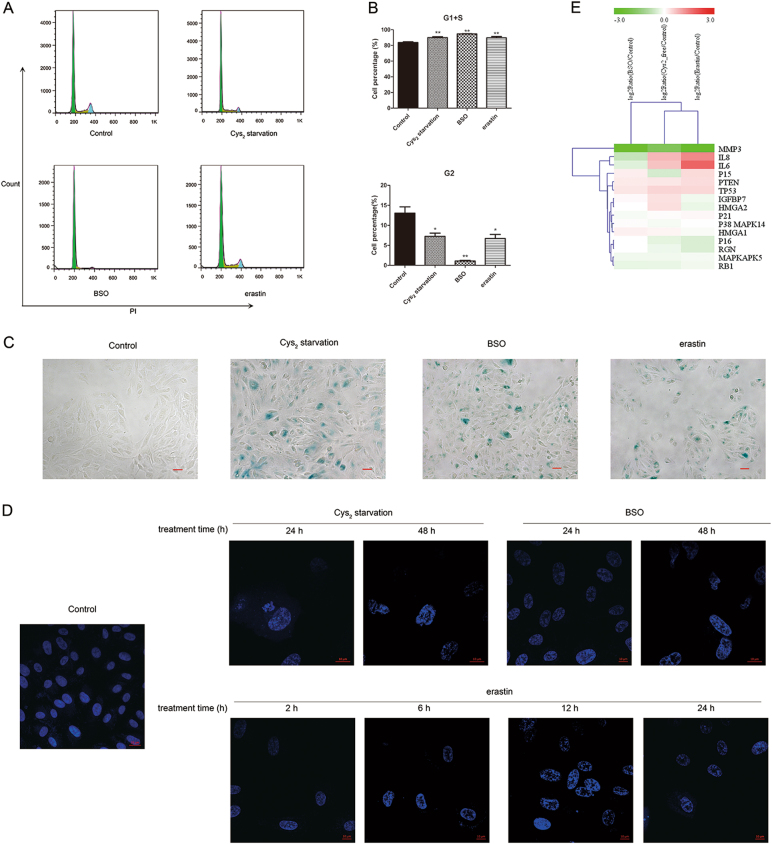
SIPS is well-characterized in cells treated with exogenous oxidants such as tBH and H2O210,11. Features of SIPS include increased senescence-associated β-galactosidase (SA-β-Gal) activity, appearance of senescence-associated heterochromatin foci (SAHF), cell growth arrest, and increased expression of senescence-associated genes11,34. To examine whether GSH depletion causes SIPS in RPE cells, we first analyzed cell cycle by flow cytometry. As shown in Fig. 7a, b, Cys2 starvation as well as BSO and erastin treatment increased the percentage of cells in the G0/G1 phase with a concomitant decrease in the G2 phases, suggesting that GSH depletion induces growth arrest in RPE cells.
Fig. 7. GSH depletion arrests cell growth and induces premature cell senescence.
a Cell cycle analysis by PI staining in cells treated with Cys2 starvation, 1000 µM BSO, and 10 µM erastin. (b) represent quantified data depicted in (a). Proportion of cells in G1 + S phase and G2 phase were calculated. Data represent mean ± SD from one of three representative experiments. Representative data from one of three experiments are shown. * represent p < 0.05, ** represent p < 0.01. c Images of senescence β-galactosidase staining. Cells were treated with Cys2 starvation, 1000 µM BSO, and 10 µM erastin for 24 h, respectively. Scale bars: 50 μm. d GSH-depleted cells were stained with DAPI to show SAHFs. e Senescence-associated gene profiles of Cys2 starved and 1000 µM BSO and 10 µM erastin-treated ARPE-19 cells. Total RNA was extracted at 24 h post treatment and assayed on BGISEQ-500 RNA-Seq
Next, SA-β-Gal staining was performed to monitor SIPS induction. GSH depletion increased the percentage of SA-β-Gal-positive cells (Fig. 7c). SAHFs in these cells were evident as large and irregularly shaped nuclear puncta (Fig. 7d). In addition, senescence-associated secreted factors, such as interleukin 6 (IL-6), interleukin 8 (IL-8), also displayed similar expression profiles, particularly in Cys2-starved and erastin-treated cells (Fig. 7e). These observations suggested that GSH depletion induces SIPS in RPE cells.
Rapamycin-induced autophagy ameliorates GSH depletion-induced premature cell senescence
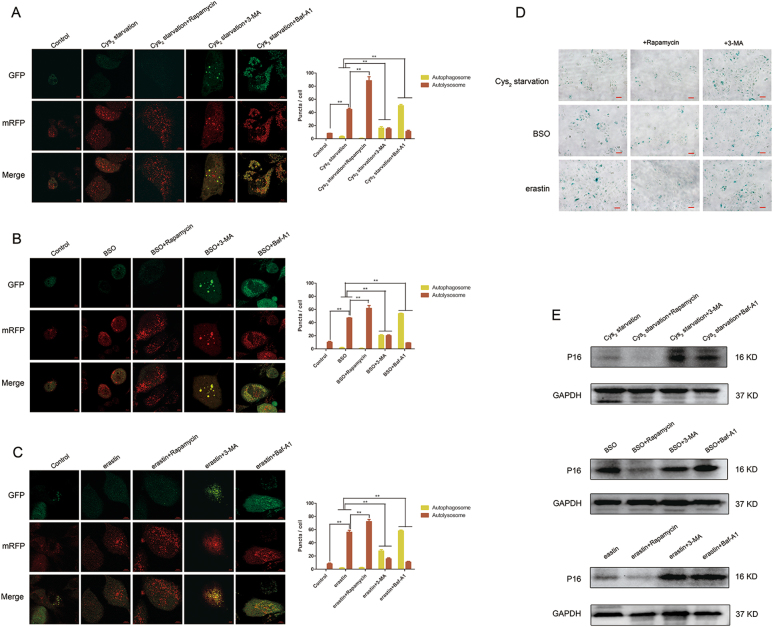
It was shown that autophagy activation especially in the presence of an increasing autophagic flux inhibits the senescence of RPE cells35–39. In our study, we observed the senescence of RPE cells despite the concomitant induction of autophagy following GSH depletion. To determine if pharmacological manipulation of autophagy effects SIPS, we treated GSH-depleted cells with the autophagic inducer rapamycin and autophagic inhibitors 3-MA and Baf-A1.
Autophagic flux tracing using tandem mRFP-GFP-LC3 probe showed that increase of autophagic flux caused by GSH depletion is augmented by rapamycin and attenuated by autophagic inhibitors 3-MA and Baf-A1 (Fig. 8a–c).
Fig. 8. Rapamycin-induced autophagy ameliorates GSH depletion-induced premature cell senescence.
a–c ARPE-19 cells were coincubated with Cys2 starvation milieu, 1000 µM BSO, and 10 µM erastin supplied with autophagic inducer rapamycin (100 nM), autophagic inhibitor 3-MA (10 mM) and lysosomal inhibitor Baf-A1 (75 nM) for 24 h; representative images of RPE cells displaying LC3 puncta were immediately visualized by confocal microscopy. Mean number of autophagosomes represented by yellow puncta in merged images and autolysosomes represented by red puncta in merged images per cell. Scale bar, 10 µm. ** represent p < 0.01. d Images of senescence β-galactosidase staining. Cells were treated with Cys2 starvation, 1000 µM BSO, and 10 µM erastin with or without 100 nM rapamycin and 10 mM 3-MA for 24 h, respectively. Scale bars: 50 μm. e Downregulation of p16 after coincubation with autophagic inducer rapamycin, while upregulation of p16 after coincubation with autophagic inhibitor 3-MA and lysosomal inhibitor Baf-A1 were detected by western blotting
SA-β-Gal staining showed that rapamycin decreased senescence, whereas 3-MA promoted senescence (Fig. 8d). Mechanistically, oxidative stress activates p16-pRb effector pathway which is thought to play a vital role in SIPS mediation11. Upregulation of p16 leads to the hypophosphorylation of the pRb protein and induction of senescence11,40. In cells depleted of GSH, p16 expression was significantly decreased by rapamycin and increased by 3-MA and Baf-A1 (Fig. 8e).
Discussion
Our results suggest that GSH depletion induces ferroptosis, autophagy, and SIPS in RPE cells. First, we characterized the mechanism of cell death observed in GSH-depleted RPE through a series of functional measurements. Second, the level of autophagy was analyzed in GSH-depleted RPE cells. Third, we found that GSH depletion induced SIPS. The interaction between autophagy and SIPS was analyzed using pharmacological autophagy modulators.
Ferroptosis is characterized by iron-dependent accumulation of lipid ROS and is distinct morphologically and mechanistically from apoptosis or other programmed cell death pathways41,42. Although the physiological function of ferroptosis remains poorly defined, it has been shown to be involved in various diseases43–45. This study is the first to describe the relationship between ferroptosis and GSH depletion in RPE cells. GSH serves as a direct antioxidant as well as an important substrate for antioxidant GPX4 to prevent lipid ROS accumulation; thus, GSH depletion ultimately leads to increased lipid peroxidation23. GSH depletion has been classified as type 1 ferroptosis inducer44,46. However, it was shown that GSH depletion induces ferroptosis in some cell types but not others23. The discrepancy may be attributed to the distinct metabolic characteristics of cells and the variations in the induction approaches used in different studies18,23. It was shown that in some cells direct inhibition of glutamate cysteine ligase (GCL) by BSO may upregulate factors involved in other antioxidative pathways (e.g. GSH-independent thioredoxin pathway), which may prevent GSH depletion-dependent oxidative stress and cell death18. In addition, some cell types can utilize transsulfuration and use methionine to biosynthesize cysteine. Thus, in these cells, Cys2 starvation or system xc− inhibitor (such as erastin) cannot induce oxidative stress and cell death26. Therefore, GSH depletion-induced oxidative stress and cell death depend on the combination of inducer and cell-specific molecular characteristics, i.e. inability to synthesize GSH and absence of alternative GSH-independent antioxidative systems.
In the current study, depriving RPE cells of the essential GSH precursor Cys2 by culturing cells in Cys2-free culture medium or blocking de novo synthesis of GSH by using GCL inhibitor BSO or blocking Cys2 uptake by pharmaceutical inhibition of system xc− caused cell death. However, BSO induced cell death to a lesser extent with slower kinetics. We demonstrated that the ferroptosis inhibitors Fer-1 and Lip-1 protected against cell death induced by GSH depletion. Fer-1 is a potent ferroptosis inhibitor that prevents accumulation of cytosolic and lipid ROS24. The ferroptosis inhibitor Lip-1 functions as a lipophilic antioxidant similar to Fer-144. In addition, we showed that iron chelator DFO also rescued cells from Cys2 starvation and BSO/erastin-induced death. While the largest percentage of intracellular iron is tightly bound to or incorporated into proteins as a cofactor or for storage, small portion of intracellular iron resides in the cytosol and intracellular organelles (e.g., lysosomes) and constitutes a redox-active liable iron pool, which regulates programmed cell death including ferroptosis42. Since DFO is membrane impermeable and accumulates in lysosomes18, we believe that it protects the cells against ferroptosis by chelating lysosomal iron as described previously 47.
Lysosomal inhibitor Baf-A1 and autophagy inhibitor 3-MA also protected against cell death induced by GSH depletion, highlighting the role of autophagy in ferroptosis. Considering the fact that Baf-A1 suppresses fusion of autophagosomes with the lysosomes39, our finding that DFO protected against ferroptosis suggests that lysosomal iron pool is involved in GSH depletion-induced cell death48. Ferroptosis is a novel programmed cell death type distinct from autophagy24. However, emerging evidence indicated that ferroptosis is an autophagic cell death process48–50. Autophagy is a highly dynamic, multistep process involving the degradation of cytoplasmic cargo through the lysosomal machinery51 and serves as a protective response mechanism under oxidative stress52. Canonical autophagy machinery plays a crucial role in ferroptosis48. Autophagy and functional lysosomes probably contribute to ferroptosis through the provision of iron 50.
Mitochondria are the main source of ROS in response to oxidants7, and play dominant roles in apoptosis53–58 and necrosis in RPE cells59,60. Mitochondria are also involved in the induction of autophagy61,62. However, in our study, oxidative stress-induced cell death was triggered by lipid peroxidation instead of the canonical mitochondrial ROS accumulation. RPE is particularly prone to lipid peroxidation owing to its persistent phagocytosis of photoreceptor outer segments. Two major lipid peroxidation products, malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), increase with aging and contribute to the pathogenesis of AMD 63–65.
GSH appears to be involved in the crosstalk between ferroptosis and autophagy. Direct inhibition of GSH synthesis triggers ferroptosis18 and autophagy22,66. On the other hand, autophagy leads to a significant decrease in intracellular GSH levels and vice versa67. The underlying mechanism of how GSH modulate the complex crosstalk between ferroptosis and autophagy remains unclear. In view of the pivotal role played by GSH in redox system and the relationship between oxidative stress and autophagy68, it is conceivable that GSH depletion-induced oxidative stress and lipid peroxidation may be a key point linking ferroptosis and autophagy in RPE cells.
GSH depletion seemed to produce contradicting effects in our study. GSH depletion induced autophagy accompanied by ferroptosis, as well as cell senescence. The relationship among GSH depletion, ferroptosis, and senescence has not been reported. In our experiments, exogenous rapamycin significantly decreased the proportion of SA-β-Gal-positive cells and p16 expression, whereas autophagy inhibitors 3-MA and Baf-A1 produced opposite effects. The crosstalk between autophagy and senescence remains poorly defined. A number of studies have provided indirect or circumstantial evidence for the collateral induction of autophagy and senescence69–72. However, other reports supported an inverse relationship35–38. Senescent cells with impaired autophagy are highly resistant to ferroptosis73. In a recently published report, autophagic flux restoration ameliorated cell senescence39. The authors claimed that autophagic flux is decreased, rather than increased, by oxidative stress triggered by exogenous oxidants H2O2. Variation in the observed results could be attributed to the main source of ROS: mitochondria in their study vs. lipid peroxidation in our study.
In conclusion, the current study demonstrated, for the first time, that GSH depletion induces ferroptosis in RPE cells. In addition, we showed that GSH depletion induces autophagy and SIPS, with autophagy being a negative regulator of SIPS. However, due to the lack of long-term observation of GSH depletion and in vivo animal experiments, it is difficult to evaluate whether ferroptosis is a bona fide autophagy activation process or a prerequisite for autophagy activation process. Further studies are required to answer these questions and dissect the exact relationship between ferroptosis, autophagy, and SIPS in RPE. Despite the limitations, this study sheds a new light on GSH depletion-induced cell death and senescence.
Material and methods
Reagents and chemicals
Erastin, rapamycin, z-VAD-fmk, 3-MA, and DFO were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fer-1, Lip-1, and Baf-A1 were purchased from Selleck Chemicals (Houston, TX, USA). BSO was purchased from Cayman Chemical (Ann Arbor, MI, USA). Anti-LC3B (D11), anti-GAPDH antibodies, and senescence β-galactosidase staining kit were purchased from Cell Signaling Technology (Danvers, MA, USA). Micro BCA Protein Assay Kit, H2DCFDA (H2-DCF, DCF), C11-BODIPY (581/591), MitoSOX™ Red Mitochondrial Superoxide Indicator, MitoTracker® Red CMXRos, and Dulbecco’s modified Eagle’s medium (High glucose, no glutamine, no methionine, no Cys2) were purchased from Thermo Scientific (Waltham, MA, USA). Dulbecco’s modified Eagle’s medium/F12 and fetal bovine serum were purchased from Gibco (Logan, UT, USA). Anti-p16-INK4A antibodies was purchased from Proteintech (Rosemont, IL, USA). Alexa Fluor® 488 annexin V/dead cell apoptosis kit was purchased from Invitrogen (Carlsbad, CA, USA). Anti-GPX4 antibody and propidium Iodide flow cytometry kit were purchased from Abcam (Cambridge, MA, USA). GSH assay kit was purchased from Beyotime Biotechnology (Nantong, Jiangsu, China).
Cell culture and STR analysis
ARPE-19 human RPE cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), and cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum at 37 °C in air containing 5% CO2. The batch of the ARPE-19 cells used in this study was validated using short tandem repeat (STR) analysis by Cobioer Biosciences (Nanjing, China). Briefly, genomic DNA was extracted from the cell pellets and amplified using GenePrint System (Promega). Amplified products were processed using the ABI3730xl Genetic Analyzer. Data were analyzed using GeneMapper4.0 software (Applied Biosystems) and then compared with the ATCC, DSMZ or JCRB databases for reference matching.
To induce intracellular GSH depletion, cells were incubated in Cys2-free medium or with BSO (1000 μM) treatment or erastin (10 µM) treatment.
GSH determination
GSH levels were determined using a colorimetric GSH assay kit according to the manufacturer’s instruction. Briefly, cellular pellets (10 µg) were mixed with 30 µl 5% metaphosphoric acid, and then frozen and thawed twice using liquid nitrogen and 37 °C water. The samples were centrifuged, and the supernatant was subjected to a GSH assay based on a kinetic enzymatic recycling method that detects the oxidation of GSH by 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) and glutathione reductase to measure the GSH content in cells74. The absorbance was measured at 412 nm with a BioTek Synergy H1 hybrid Microplate Readers. GSH content were normalized to protein concentration and expressed as µM per mg protein.
Cell viability detection with Annexin V/PI by flow cytometry
ARPE-19 cells were rinsed with PBS. The cell suspensions were washed twice with ice-cold PBS before staining using an Annexin V/PI staining kit (Alexa Fluor® 488 annexin V/Dead Cell Apoptosis Kit). Briefly, 500 µl binding buffer was added to each tube and transferred to a 1.5 ml centrifuge tube (1–5×105 cells). Then, 5 µl of Annexin V–FITC and 5 µl of propidium iodide (PI) were added, and the cells were gently vortexed. Cells were then incubated for 15 min at room temperature in the dark, and analyzed by flow cytometry (LSRFortessaTM; BD, Franklin Lakes, NJ, USA). Data were collected from at least 10,000 cells. The results were analyzed by FlowJo 7.6.2 software (Tree Star Inc., Ashland, OR, USA). Three independent experiments were conducted.
Cell cycle analysis
For cell cycle analysis, cells were harvested and fixed in 80% ethanol overnight at −20 °C, washed with PBS, and then stained with PI and 100 μg/ml RNaseA. DNA content was measured by sorting the fluorescence activated cells by flow cytometry (LSRFortessaTM; BD, Franklin Lakes, NJ, USA). Data were collected from at least 10,000 cells. The results were analyzed by FlowJo 7.6.2 software (Tree Star Inc., Ashland, OR, USA). Three independent experiments were conducted.
Measurement of ROS production
Cells were washed in prewarmed PBS and treated with 10 μM H2DCFDA for 30 min in dark. H2DCFDA is a nonfluorescent ester of the dye fluorescein that is cleaved by intracellular esterases and is entrapped within the cell as the oxidant sensitive DCF compound. ROS oxidize DCF to the fluorescent product fluorescein. The MFI was determined by flow cytometry (LSRFortessaTM; BD, Franklin Lakes, NJ, USA) in which DCF emission was recorded on channel FL1-H. Control cells were treated with H2O2 (1 mM) for 30 min as a positive control for increased ROS production. Data were collected from at least 10,000 cells. The results were analyzed by FlowJo 7.6.2 software (Tree Star Inc., Ashland, OR, USA). Three independent experiments were conducted.
Detection of mitochondrial superoxide with MitoSOX
The production of superoxide in mitochondria was visualized with MitoSOX Red (Life Technologies). Confluent cells grown on six-well plate were subjected with indicated treatments for 24 h. Before termination of treatment, cells were incubated with 5 μM MitoSOX for 15 min at 37 °C, and washed in PBS. The MFI was determined by flow cytometry (LSRFortessaTM; BD, Franklin Lakes, NJ, USA). MitoSOX emission was recorded on channels FL2-H at 585 nm. Data were collected from at least 10,000 cells. The results were analyzed by FlowJo 7.6.2 software (Tree Star Inc., Ashland, OR, USA). Three independent experiments were conducted.
Detection of lipid peroxidation
Peroxidation was examined by monitoring change in fluorescence emission of C11- BODIPY 581/591 from red to green75. Cells seeded in six-well plate were incubated with C11-BODIPY 581/591 at a final concentration of 10 µM for 30 min at 37 °C and washed three times with PBS. The MFI was determined by flow cytometry (LSRFortessaTM; BD, Franklin Lakes, NJ, USA) in which BODIPY emission was recorded on channels FL1-H at 530 nm and FL2-H at 585 nm. Data were collected from at least 10,000 cells. The results were analyzed by FlowJo 7.6.2 software (Tree Star Inc., Ashland, OR, USA). Three independent experiments were conducted.
Estimation of mitochondrial mass
Mitochondrial mass was measured by MitoTracker dye as previously described76. Cells were loaded with MitoTracker Red dye (Excitation/Emission: 579/599 nm) at 100 nM final concentration (37 °C for 15 min) mounted in Live Cell Imaging Solution (Thermo Fisher), and viewed under a laser confocal microscope (LSM 510; Zeiss, Thornwood, NY, USA). Every experiment was repeated at least three times, and representative data are shown. ImageJ software (version 1.42q) was used for quantitative analysis of the MFI.
Transmission electron microscopy
Cells were digested with 0.25% trypsin and 0.02% EDTA, washed with Hanks’ Balanced Salt Solution (HBSS). Pre-fixation was done with 2.5% glutaraldehyde phosphate (0.1 M, pH 7.4) overnight at 4 °C. Post-fixation proceeded in buffered osmium tetroxide, followed by dehydration before embedding in Epon812. Ultrathin sections (80 nm thick) were cut with an ultramicrotome (Leica, EMUC6, Germany), and then stained with uranyl acetate and lead citrate and finally examined by a Tecnai G2 Spirit TWIN transmission electron microscope (FEI, Hillsboro, OR, USA). For each condition, at least 100 cells from randomly chosen fields were observed.
SAHF detection
Cells were fixed with 4% paraformaldehyde and washed with PBS. DAPI at 300 nM concentration in PBS was added for 5 min incubation. The cells were then washed three times with PBS, drained and mounted. DAPI-stained nuclei with blue fluorescence were viewed under laser confocal microscope (LSM 510; Zeiss, Thornwood, NY, USA).
Analysis of oxidative stress-induced cellular senescence
Cell senescence assay was conducted with Senescence β-Galactosidase Staining Kit (Cell Signaling Technology) according to the manufacturer’s instruction. Hydrogen peroxide (100 μM, 90 min) was used as a positive control10. Early passage ARPE-19 cells were used as a negative control. Briefly, cells were seeded in six-well plate, growth media was removed from the cells before assay followed by a rinse with PBS. Cells were fixed with 1× Fixative Solution for 10 min at room temperature followed by a two-time rinse with PBS. Then 1 ml of the β-galactosidase staining solution was added to each well, and incubated at 37 °C overnight. Bright field mode was used to detect for the development of blue color under a Zeiss Axioscope microscope equipped with a Zeiss HRC microscope camera (×200 total magnification).
RNA-seq and analysis
Total RNA isolation, cDNA library construction, and sequencing were performed at BGI (Beijing Genomics Institute, Shenzhen, Guangdong, China) using RNA-seq technology, as previously described77,78. High-quality reads were aligned to the human reference genome (UCSC_hg38). The expression levels for each of the genes were normalized to fragments per kilobase of exon model per million mapped reads (FPKM) using a software package called RSEM79. Three biological replicates were carried out in this study. DEGseq method was used to screen for differentially expressed genes 80.
Western blotting
Cells were plated, grown to 70% confluency, and then subjected to indicated treatments. Cells were lysed at 4 °C in a radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology). Protein concentration was determined using a BCA method. Samples (30 µg protein) were resolved by 4–20% SDS-PAGE (Biofuraw™ Precast Gel, Tanon Science, Shanghai, China), transferred to polyvinylidene difluoride membranes, and incubated with one of the following antibodies: LC3 (88588s, Cell Signaling Technology, 1:1000), GPX4 (ab125066, Abcam, 1:1000), p16 (1003-1-AP, Proteintech, 1:1000). GAPDH (5174S, Cell Signaling Technology, 1:1000) was used as a loading control. Representative blots of at least two independent experiments are shown.
Lentiviral transduction of mRFP-GFP-LC3 shRNA and autophagic flux determination
Lentiviruses encoding short hairpin RNAs (shRNA) to human mRFP-GFP-LC3-puro were constructed by Hanbio Biotechnology (Hanbio, Shanghai, China). ARPE-19 cells were seeded on a 24-well plate (1×105 cells/well) for lentivirus transduction. Twenty-four hours later the culture medium was replaced with the lentivirus-containing medium (lentiviral titer 1×108 TU/mL, MOI = 3) and incubated for 6 h. Upon virus transduction, the incubation medium was removed and fresh culture medium was added. Seventy-two hours after transduction, puromycin-containing medium (2 mg/mL) was used for selection. Fifty clones were pooled, expanded and used for experiments. For autophagic flux determination, cells were viewed under a laser confocal microscope (LSM 510; Zeiss, Thornwood, NY, USA), using a GFP and RFP filter to detect autophagosomes (yellow puncta) and autolysosomes (red puncta). ImageJ software (version 1.42q) was used for count yellow and red puncta.
Statistical analysis
Statistical analysis was performed with SPSS 21.0 for Windows (IBM, Armonk, NY, USA). All data are expressed as the mean ± SD from at least three biological replicates, and comparisons between the two groups were performed with a nonparametric method (Mann−Whitney U test). A value of p < 0.05 was considered statistically significant.
Acknowledgements
This work is funded by the National Natural Science Foundation of China (81530028; 81721003), the Guangdong Province Science & Technology Plan (2014B020228002), the National Key Basic Research and 973 Development Program of China (2015CB964600) and Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program.
Authors’ contributions
Y.S. and Y.Z. conceived and designed the experiments. C.W. conducted the statistical analysis. Y.S. drafted the manuscript with inputs from Y.Z., C.W., and Y.L. All authors have seen and approved the final version of the manuscript for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by G.M. Fimia
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bridges CC, et al. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2001;42:47–54. [PubMed] [Google Scholar]
- 2.Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai J, Nelson KC, Wu M, Sternberg P, Jr., Jones DP. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000;19:205–221. doi: 10.1016/S1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 4.Yang P, Peairs JJ, Tano R, Jaffe GJ. Oxidant-mediated Akt activation in human RPE cells. Invest. Ophthalmol. Vis. Sci. 2006;47:4598–4606. doi: 10.1167/iovs.06-0140. [DOI] [PubMed] [Google Scholar]
- 5.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/S0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 6.Khandhadia S, Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev. Mol. Med. 2010;12:e34. doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 7.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp. Eye Res. 2003;76:397–403. doi: 10.1016/S0014-4835(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 8.Jiang S, et al. Increased oxidant-induced apoptosis in cultured nondividing human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2002;43:2546–2553. [PubMed] [Google Scholar]
- 9.Godley BF, Jin GF, Guo YS, Hurst JS. Bcl-2 overexpression increases survival in human retinal pigment epithelial cells exposed to H(2)O(2) Exp. Eye Res. 2002;74:663–669. doi: 10.1006/exer.2001.1146. [DOI] [PubMed] [Google Scholar]
- 10.Marazita MC, Dugour A, Marquioni-Ramella MD, Figueroa JM, Suburo AM. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biol. 2016;7:78–87. doi: 10.1016/j.redox.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu AL, et al. Subtoxic oxidative stress induces senescence in retinal pigment epithelial cells via TGF-beta release. Invest. Ophthalmol. Vis. Sci. 2009;50:926–935. doi: 10.1167/iovs.07-1003. [DOI] [PubMed] [Google Scholar]
- 12.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 13.Saxena M, Singhal SS, Awasthi YC. A specific, sensitive, and rapid method for the determination of glutathione and its application in ocular tissues. Exp. Eye Res. 1992;55:461–468. doi: 10.1016/0014-4835(92)90119-D. [DOI] [PubMed] [Google Scholar]
- 14.Huster D, et al. Subcellular compartmentation of glutathione and glutathione precursors. A high resolution immunogold analysis of the outer retina of guinea pig. Anat. Embryol. (Berl.) 1998;198:277–287. doi: 10.1007/s004290050184. [DOI] [PubMed] [Google Scholar]
- 15.Tate DJ, Jr, Newsome DA, Oliver PD. Metallothionein shows an age-related decrease in human macular retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1993;34:2348–2351. [PubMed] [Google Scholar]
- 16.Liles MR, Newsome DA, Oliver PD. Antioxidant enzymes in the aging human retinal pigment epithelium. Arch. Ophthalmol. 1991;109:1285–1288. doi: 10.1001/archopht.1991.01080090111033. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg P, et al. Protection of retinal pigment epithelium from oxidative injury by glutathione and precursors. Invest. Ophthalmol. Vis. Sci. 1993;34:3661–3668. [PubMed] [Google Scholar]
- 18.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood JP, Pergande G, Osborne NN. Prevention of glutathione depletion-induced apoptosis in cultured human RPE cells by flupirtine. Restor. Neurol. Neurosci. 1998;12:119–125. [PubMed] [Google Scholar]
- 20.Jin M, et al. Hepatocyte growth factor protects RPE cells from apoptosis induced by glutathione depletion. Invest. Ophthalmol. Vis. Sci. 2005;46:4311–4319. doi: 10.1167/iovs.05-0353. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong JS, Whiteman M, Yang H, Jones DP, Sternberg P., Jr. Cysteine starvation activates the redox-dependent mitochondrial permeability transition in retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2004;45:4183–4189. doi: 10.1167/iovs.04-0570. [DOI] [PubMed] [Google Scholar]
- 22.Mancilla H, et al. Glutathione depletion induces spermatogonial cell autophagy. J. Cell. Biochem. 2015;116:2283–2292. doi: 10.1002/jcb.25178. [DOI] [PubMed] [Google Scholar]
- 23.Dixon SJ. Ferroptosis: bug or feature? Immunol. Rev. 2017;277:150–157. doi: 10.1111/imr.12533. [DOI] [PubMed] [Google Scholar]
- 24.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stockwell BR, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toussaint O, et al. Stress-induced premature senescence or stress-induced senescence-like phenotype: one in vivo reality, two possible definitions? Sci. World J. 2002;2:230–247. doi: 10.1100/tsw.2002.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glotin AL, et al. Prematurely senescent ARPE-19 cells display features of age-related macular degeneration. Free Radic. Biol. Med. 2008;44:1348–1361. doi: 10.1016/j.freeradbiomed.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Maciel-Baron LA, et al. Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age (Dordr.) 2016;38:26. doi: 10.1007/s11357-016-9886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yagoda N, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang WS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition. Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maulucci G, et al. Quantitative analysis of autophagic flux by confocal pH-imaging of autophagic intermediates. Autophagy. 2015;11:1905–1916. doi: 10.1080/15548627.2015.1084455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000;35:927–945. doi: 10.1016/S0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 35.Kang HT, Lee KB, Kimu SY, Choi HR, Park SC. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS ONE. 2011;6:e23367. doi: 10.1371/journal.pone.0023367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drullion C, et al. Apoptosis and autophagy have opposite roles on imatinib-induced K562 leukemia cell senescence. Cell Death Dis. 2012;3:e373. doi: 10.1038/cddis.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee MJ, et al. Dehydroepiandrosterone prevents linoleic acid-induced endothelial cell senescence by increasing autophagy. Metabolism. 2015;64:1134–1145. doi: 10.1016/j.metabol.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Fujii S, et al. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology. 2012;1:630–641. doi: 10.4161/onci.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai H, et al. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy. 2017;13:99–113. doi: 10.1080/15548627.2016.1247143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, Goligorsky MS. Premature senescence of endothelial cells: Methusaleh’s dilemma. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1729–H1739. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- 41.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 43.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedmann Angeli JP, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linkermann A, et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fearnhead HO, Vandenabeele P, Vanden Berghe T. How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 2017;24:1991–1998. doi: 10.1038/cdd.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persson HL, Yu Z, Tirosh O, Eaton JW, Brunk UT. Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Radic. Biol. Med. 2003;34:1295–1305. doi: 10.1016/S0891-5849(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 48.Gao M, et al. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou W, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torii S, et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem. J. 2016;473:769–777. doi: 10.1042/BJ20150658. [DOI] [PubMed] [Google Scholar]
- 51.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szumiel I. Autophagy, reactive oxygen species and the fate of mammalian cells. Free Radic. Res. 2011;45:253–265. doi: 10.3109/10715762.2010.525233. [DOI] [PubMed] [Google Scholar]
- 53.Ferrington DA, Tran TN, Lew KL, Van Remmen H, Gregerson DS. Different death stimuli evoke apoptosis via multiple pathways in retinal pigment epithelial cells. Exp. Eye Res. 2006;83:638–650. doi: 10.1016/j.exer.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Tsao YP, Ho TC, Chen SL, Cheng HC. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci. 2006;79:545–550. doi: 10.1016/j.lfs.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 55.Zou X, et al. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012;23:994–1006. doi: 10.1016/j.jnutbio.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Kim JH, et al. Protective effect of clusterin from oxidative stress-induced apoptosis in human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2010;51:561–566. doi: 10.1167/iovs.09-3774. [DOI] [PubMed] [Google Scholar]
- 57.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp. Eye Res. 2010;90:718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho TC, et al. Activation of mitogen-activated protein kinases is essential for hydrogen peroxide -induced apoptosis in retinal pigment epithelial cells. Apoptosis. 2006;11:1899–1908. doi: 10.1007/s10495-006-9403-6. [DOI] [PubMed] [Google Scholar]
- 59.Kim MH, et al. Hydrogen peroxide-induced cell death in a human retinal pigment epithelial cell line, ARPE-19. Korean J. Ophthalmol. 2003;17:19–28. doi: 10.3341/kjo.2003.17.1.19. [DOI] [PubMed] [Google Scholar]
- 60.Li GY, Fan B, Zheng YC. Calcium overload is a critical step in programmed necrosis of ARPE-19 cells induced by high-concentration H(2)O(2) Biomed. Environ. Sci. 2010;23:371–377. doi: 10.1016/S0895-3988(10)60078-5. [DOI] [PubMed] [Google Scholar]
- 61.Scherz-Shouval R, Shvets E, Elazar Z. Oxidation as a post-translational modification that regulates autophagy. Autophagy. 2007;3:371–373. doi: 10.4161/auto.4214. [DOI] [PubMed] [Google Scholar]
- 62.Scherz-Shouval R, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Totan Y, et al. Plasma malondialdehyde and nitric oxide levels in age related macular degeneration. Br. J. Ophthalmol. 2001;85:1426–1428. doi: 10.1136/bjo.85.12.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye F, et al. Malondialdehyde induces autophagy dysfunction and VEGF secretion in the retinal pigment epithelium in age-related macular degeneration. Free Radic. Biol. Med. 2016;94:121–134. doi: 10.1016/j.freeradbiomed.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 65.Kopitz J, Holz FG, Kaemmerer E, Schutt F. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie. 2004;86:825–831. doi: 10.1016/j.biochi.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 66.Zheng X, et al. xCT deficiency induces autophagy via endoplasmic reticulum stress activated p38-mitogen-activated protein kinase and mTOR in sut melanocytes. Eur. J. Cell Biol. 2016;95:175–181. doi: 10.1016/j.ejcb.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Desideri E, Filomeni G, Ciriolo MR. Glutathione participates in the modulation of starvation-induced autophagy in carcinoma cells. Autophagy. 2012;8:1769–1781. doi: 10.4161/auto.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerland LM, et al. Association of increased autophagic inclusions labeled for beta-galactosidase with fibroblastic aging. Exp. Gerontol. 2003;38:887–895. doi: 10.1016/S0531-5565(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Autophagy may precede cellular senescence of bile ductular cells in ductular reaction in primary biliary cirrhosis. Dig. Dis. Sci. 2012;57:660–666. doi: 10.1007/s10620-011-1929-y. [DOI] [PubMed] [Google Scholar]
- 71.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Autophagy mediates the process of cellular senescence characterizing bile duct damages in primary biliary cirrhosis. Lab. Invest. 2010;90:835–843. doi: 10.1038/labinvest.2010.56. [DOI] [PubMed] [Google Scholar]
- 72.Gosselin K, et al. Senescent keratinocytes die by autophagic programmed cell death. Am. J. Pathol. 2009;174:423–435. doi: 10.2353/ajpath.2009.080332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masaldan S, et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018;14:100–115. doi: 10.1016/j.redox.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 75.Christova Y, James PS, Jones R. Lipid diffusion in sperm plasma membranes exposed to peroxidative injury from oxygen free radicals. Mol. Reprod. Dev. 2004;68:365–372. doi: 10.1002/mrd.20084. [DOI] [PubMed] [Google Scholar]
- 76.Iacovelli J, et al. PGC-1alpha induces human RPE oxidative metabolism and antioxidant capacity. Invest. Ophthalmol. Vis. Sci. 2016;57:1038–1051. doi: 10.1167/iovs.15-17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen K, et al. Methyltransferase SETD2-mediated methylation of STAT1 is critical for interferon antiviral activity. Cell. 2017;170:492–506. doi: 10.1016/j.cell.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 78.Jiang MD, Zheng Y, Wang JL, Wang YF. Drug induces depression-like phenotypes and alters gene expression profiles in Drosophila. Brain Res. Bull. 2017;132:222–231. doi: 10.1016/j.brainresbull.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinforma. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Feng Z, Wang X, Wang X, Zhang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]