Abstract
The repetition of a stimulus leads to shorter reaction times as well as to the reduction of neural activity. Previous encounters with closely related stimuli (primes) also lead to faster and often to more accurate processing of subsequent stimuli (targets). For instance, if the prime is a name, and the target is a face, the recognition of a persons’ face is facilitated by prior presentation of his/her name. A possible explanation for this phenomenon is that the prime allows predicting the occurrence of the target. To the best of our knowledge, so far, no study tested the neural correlates of such cross-domain priming with fMRI. To fill this gap, here we used names of famous persons as primes, and congruent or incongruent faces as targets. We found that congruent primes not only reduced RT, but also lowered the BOLD signal in bilateral fusiform (FFA) and occipital (OFA) face areas. This suggests that semantic information affects not only behavioral performance, but also neural responses in relatively early processing stages of the occipito-temporal cortex. We interpret our results in the framework of predictive coding theories.
Introduction
The repeated presentation of a given stimulus leads to several behavioral and neural consequences. On the one hand, stimulus repetitions enhance performance, as indicated by shorter reaction times, an effect referred to as (immediate) repetition priming1,2. On the other hand, both macaque and human experiments have shown that stimulus repetition reduces single-cell activity3,4, the amplitude of event-related potential (ERP) components5, as well as the magnitude of the blood-oxygen level dependent (BOLD) signal in functional magnetic resonance imaging (fMRI) experiments6. This response reduction is typically referred to as repetition suppression (RS) while in the neuroimaging literature, it is often referred to as fMRI adaptation (fMRIa7).
Recently, the phenomena of RS and fMRI adaptation (fMRIa) have been connected to the process of stimulus predictions. According to the predictive coding framework, feedback connections convey prior hypotheses about the environment, and these are compared with feedforward connections by estimating an error signal, i.e., a mismatch between the predicted hypothesis and the actual sensory input8. The error signal equals to the amount of sensory evidence that cannot be “explained away” by the hypothesis. This prediction error (PE) is used to fine-tune the internal generative model through an iterative process, which occurs until the PE is eliminated. Finally, this process results in models which optimally represent the causes of sensory stimuli. Consequently, surprising/incorrectly predicted events generate larger neural activity in comparison with correctly predicted events, maximizing the efficiency of neuronal processing9–11. Given the stability of our visual environment (scenes and objects are usually constant, and change only relatively rarely12), stimulus repetition may be encoded as a ‘default’ prior (i.e. as the most fundamental form of predictions13) in the brain. Indeed, direct evidence that fMRIa is a consequence of the ‘default’ prior of repetition has been found when predictions are modulated by statistical probabilities14–19 or by explicit cues20,21. Specifically, it has been suggested that the repetition of a stimulus reduces the mismatch or PE between the expected and the actual incoming stimulus, and that it is this error reduction that is manifested in fMRIa (for a review see22). As of today, it is still unknown if and how fMRIa and the repetition priming effects, as observed in behavior, are related to each other. For example, it was suggested that repetition priming and fMRIa have different neural backgrounds23, also a dissociation of the two phenomena was found, depending on the interval between prime and target24.
Importantly, performance enhancements are not limited to the mere repetition of the same stimulus: previous encounters with similar, closely related primes also lead to faster or more accurate processing of subsequent targets (referred to as semantic or associative priming25). If the prime and target originate in different modalities (e.g., acoustic and visual), or in different stimulus domains (e.g., written text and visually presented shape), the effect is often referred to as cross-domain priming26. For example, when the prime is a name, and the target is a face25,27,28, the recognition of a familiar persons’ face is facilitated by the prior presentation of its name or by that of a related familiar person29,30.
Interestingly, so far only a few studies tested the neural background of such cross-domain behavioral priming effects and their commonalities with neural RS. As of yet, the electrophysiological data available suggests that cross-domain arrangements lead to weaker effects than domain-specific priming, and seem to occur at later stages of processing, i.e., corresponding to the N400 component in event-related potentials29–33. Interestingly, there is neuroimaging evidence that prior auditory information affected the visual processing of objects in the left fusiform gyrus34. Briefly, this region was found to be sensitive to the repetition of a given object when its name was visually or auditorily presented.
To the best of our knowledge, so far, no study used fMRI to probe the neural correlates of cross-domain effects with names priming faces. Here, we conjecture that the effect of cross-domain priming is comparable to the effects reported in predictive cueing arrangements, which have been broadly tested in fMRI experiments20,21,35–39. In such experiments, a neutral stimulus (the cue) is paired with a subsequent stimulus via learning and can, thereby, evoke perceptual expectations. The main finding of these studies is that valid cues lead to behavioral facilitations as well as to reduced BOLD responses in comparison to invalid cues. For example38, used red/blue/green lines as cues which predicted the orientation of subsequent Gabor patches and found that the BOLD signal in the middle occipital gyrus was reduced for valid as compared to invalid trials. Egner and colleagues37 associated green/blue frames with the high likelihood of face/house presentations. They found that valid cues reduced the activity of the fusiform face area (FFA) and the parahippocampal place area when compared to the invalid trials. The authors interpreted their results in the predictive coding framework and argued that feature expectation and surprise determine the visual responses more than the input, i.e., the features per se. In an fMRI study by39, letters served as predictors for faces, and the BOLD activity in the FFA was modulated as a function of the probability with which the cue was associated with the face. Another fMRI study by36, tested cross-domain priming effects through name-object associations where the names could be congruent or incongruent with a degraded and gradually revealed visual object. They found that object recognition occurred earlier for congruently than for incongruently primed objects and that the BOLD signal of the fusiform cortex was modulated by the degradation level of the stimuli. Briefly, clear stimuli induced larger neural activity for the incongruent name-object associations when compared to the congruent ones, while objects with more degradation lead to larger BOLD responses for congruently than for incongruently primed sequences36.
Here we reasoned that if cross-domain priming, similarly to these above mentioned cueing arrangements, leads to facilitated recognition of targets due to predictive processes40,41, then we should also observe fMRIa in functionally selective cortical regions for congruently primed conditions. Indeed, a recent study42 suggests that expectation can modulate repetition priming effects by expecting the repetition of a given stimulus. Here, we used names of famous persons as primes and their faces as targets in a familiarity task, while unfamiliar faces served as control stimuli. We expected that if predictions are made across domains, then we should observe fMRIa in the occipito-temporal face sensitive areas, similarly to the mere repetition of stimuli.
To anticipate our results, we indeed found that congruent primes led to lower BOLD signal in bilateral FFA and in the occipital face area (OFA). Altogether, our results suggest that semantic information, such as the names of individuals, also affect the neural responses in stimulus sensitive regions, such as OFA and FFA. We interpret our results in the predictive coding framework.
Results
Behavioural
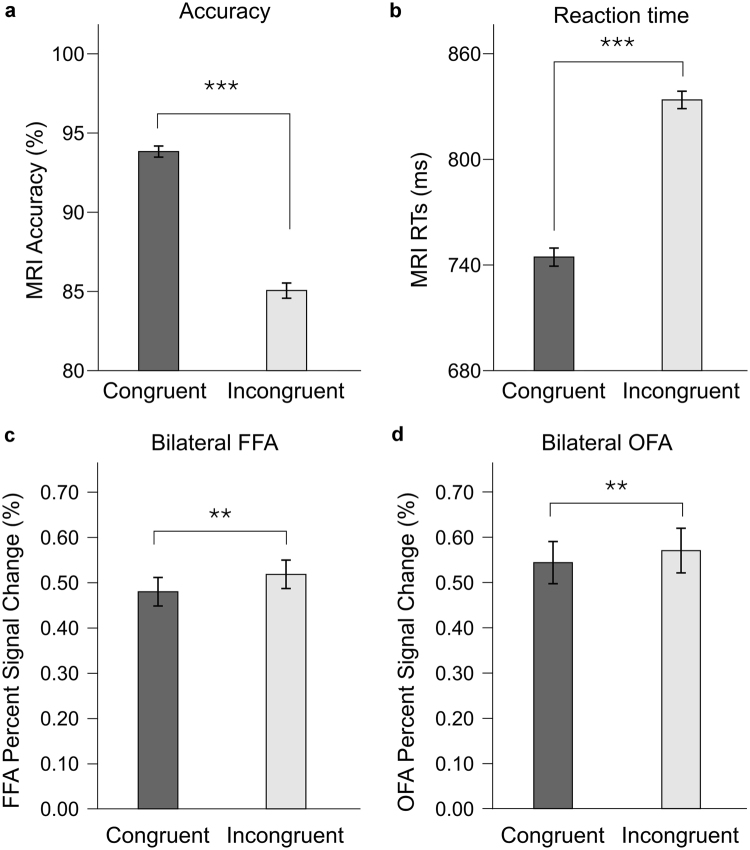
Participants needed, on average, 789 ms (±SD: 22 ms) to determine the familiarity of the presented target face. We found a strong priming effect (main effect of condition; F(1, 17) = 50.5, p < 0.000001, ηp² = 0.75; see Fig. 1a), in other words responses were faster in the Congruent as compared to the Incongruent condition.
Figure 1.
Behavioral and neuroimaging results: Mean Accuracy ((a) top left); Reaction Time ((b) top right); Percent-signal changes (±SE) are presented separately for the bilateral FFA ((c) down left) and for the bilateral OFA ((d) down right), separately for the two experimental conditions of interest (Congruent and Incongruent). ***p < 0.001, **p < 0.01.
Mean accuracy for familiarity judgment was 89% (±SD: 0.02%) across the two experimental conditions (Fig. 1a). Similarly to the reaction time (RT) results, we found a main effect of condition on accuracy as well (F(1, 17) = 26.16, p = 0.00009, ηp² = 0.61). The priming effect (i.e. main effect of condition) was due to a reduced accuracy in the Incongruent condition when compared with the Congruent one.
BOLD response
We found a main effect of condition in the FFA (F(1, 17) = 14.69, p < 0.01, ηp² = 0.46) and OFA (F(1, 17) = 8.77, p < 0.01, ηp² = 0.34) (Fig. 1b) in a way that the BOLD response was larger for the Incongruent as compared to the Congruent condition (p = 0.001 and p = 0.008; with an average signal reduction of 0.04% and 0.03%, corresponding to a relative signal reduction of 8% and 6%, for the FFA and OFA, respectively). These results suggest that cross-domain priming leads to a signal reduction in face-responsive areas, similar to the fMRIa observed for repeated face stimuli. Interestingly, there was a significant interaction between hemisphere and condition in the OFA (F(1, 17) = 4.71, p < 0.05, ηp² = 0.22), but not in the FFA (F(1, 17) = 0.47, p > 0.05, ηp² = 0.02). The post-hoc analysis of this OFA hemisphere x condition interaction revealed that the signal reduction is somewhat lower for the left OFA but nonetheless significant for both the right (p < 0.0001; average signal reduction of 0.05% and relative signal reduction of 9%) and the left (p = 0.03; average signal reduction of 0.02% and relative signal reduction of 4%) hemispheres. No other significant effects were found, as there was no main effect of hemisphere for both FFA (F(1, 17) = 1.9, p = 0.2, ηp² = 0.09) and OFA (F(1, 17) = .3, p = 0.6, ηp² = 0.02).
Whole-brain
It is possible that cross-domain priming effects also occur elsewhere in the brain. Therefore, we performed a second level, whole-brain analysis testing for a main effect of condition, using a fixed threshold of p < 0.05FWE, with a cluster size >50 voxels. The main effect of priming (Incongruent > Congruent) revealed a significant cluster of activation in the supplementary motor cortex (SMC; MNI[x, y, z]: -6, 12, 48; cluster size: 180 voxels). To confirm that no other region remained unnoticed by the commonly applied, but rather rigorous, FWE corrected threshold we also analyzed our data at a less conservative threshold (p < 0.0001uncorrected; cluster extent of >20 voxels). The Incongruent > Congruent contrast showed another significant cluster in the insula (MNI[x, y, z]: −42, 8, 6; cluster size: 28 voxels). Moreover, it is possible that the differences in brain activation may reflect changes in behaviour. Thus, differences in reaction times between the two conditions of interest (i.e. congruent and incongruent) were used as a covariate in a regression analysis. Still, no region showed a correlation between the behavioural priming and the BOLD signal reduction.
Correlation analysis of the behavioural and neuroimaging datasets
To test whether the response difference of congruent and incongruent trials is related between the behavioural and neuroimaging data, a correlation analysis was performed between the two data sets for each ROI and experimental run, separately (between and within subjects in a trial-by-trial analysis). No correlation was found between the BOLD response reduction and the behavioural priming effects (between and within subjects in a trial-by-trial analysis).
Discussion
In the current experiment, we tested prediction effects across domains driven by name-face associations. Briefly, we reasoned that if cross-domain priming induces predictive processing for congruent prime-target trials40,41, then the fMRI responses of occipito-temporal face sensitive areas should be reduced for Congruent when compared to Incongruent conditions. Our major finding is that faces of familiar persons, seen after Congruent names, led to significantly lower fMRI signal in FFA and OFA. Overall, these results clearly show that the neural activity of FFA and OFA is modulated by prior semantic information, just as these regions are affected by previously presented abstract cues20,37,39.
Several studies suggest that fMRIa is the neuronal correlate of the behaviourally observed short-term repetition priming effects43–45. Indeed, short-term repetition priming effects and fMRIa have strong similarities: Both are facilitative processes induced by identical paradigms, do not depend on retrieval, and occur with different stimulus attributes41. Yet, whether fMRIa is a neurophysiological index of priming is still under heavy debate, as there are also evidences of the dissociation of the two phenomena23,24; for a review see46,47. It is worth noting, however, that even some of the studies which report a dissociation between fMRIa and short-term repetition priming effects show reduced neural activity for primed as compared to unprimed conditions23,24. Interestingly, one of these studies manipulated short-term repetition priming and fMRIa orthogonally23. In two sessions, subjects were given pairs of stimuli that could either repeat or alternate. Some of the pairs presented had been shown in a prior session (old pairs), while other pairs were new. Their results showed BOLD signal differences between primed (old) and unprimed (new) conditions for the presentation of different stimulus pairs. Note, however, that in this study, priming was defined having previously seen a stimulus in a separate block or not. There was a large number of intervening stimuli between primes and targets. In the current study, we measured immediate priming effects with no stimuli intervening between prime and target in order to be comparable to several previous RS studies showing prediction effects14–17,19,21,38,48. The existence of two kinds of fMRIa mechanisms was proposed24, one driven by priming processes and another one driven by pure stimulus repetitions (for a review see49). Indeed, as mentioned by a recent review50, it is suggested that fMRIa is actually influenced by both low- and high-level processing mechanisms, confirming the above mentioned hypothesis24.
To the best of our knowledge, the present study is the first to investigate the relationship between fMRIa and immediate cross-domain repetition priming of face perception. Our results reveal that just like fMRIa, congruent name primes lead to lower BOLD signal in bilateral FFA and OFA when compared to incongruent name primes. Such results support a study performed by Simons and colleagues34. This study applied cross-domain and domain-specific repetition priming effects for everyday objects and authors found that the objects could be primed by images or by their acoustically presented names. Importantly however, the authors found a huge variability across subjects in their priming effects (on the left fusiform gyrus, see figure 3 of 34) and, therefore, separated the participants into two sub-groups. Those differences can be explained by the fact that no separate functional localizer images were used to identify the regions of interest.
Furthermore, the findings of the current experiment are in line with existing electrophysiological studies which show a reduction of neuronal activity for cross-domain stimulus repetitions29,30,51. Briefly, the cross-domain priming effects of these studies were weaker, had different cortical distributions and occurred at later time-windows than domain-specific stimulus repetition effects. Although the absolute signal reduction found in FFA and right OFA in the present study is comparable to that of previous studies investigating repetition of face stimuli20,21, the relative signal reduction was smaller than the one reported in the above-mentioned studies. A limitation of the current experiment relies on the fact that no direct comparison was performed between such effects, i.e. repetition priming and cross-domain repetition priming (see34 for an example). Therefore, future neuroimaging studies are necessary to compare fMRIa and the underlying mechanisms between cross-domain and domain-specific stimulus repetitions directly, ideally within the same participants and sessions.
The finding that semantic information modulates the BOLD response in FFA and OFA cannot be explained by conventional low-level response adaptation mechanisms, as there was no stimulus repetition in our paradigm. Rather, these results suggest that semantic information is fed back to face responsive areas, presumably from the visual letter-form area (LFA52), visual word-form area (WFA53–55) or anterior and medial temporal cortices. Even though the WFA and the occipito-temporal face responsive areas have different anatomical connections, there is a considerable proportion of shared connectivity between the WFA and FFA56. Additionally, another study showed that the FFA is activated by the auditory stimulation of familiar identities57, suggesting cross-domain identity representations in this area. Furthermore, novel evidences support the idea that sensory cortices receive information from domain-specific areas (via feed-forward pathways) as well as from other sensory domains, potentially via cortical feedback connections58,59; for a review see60. However, so far, the existing literature has mostly focused on the early visual cortices and their auditory sensory inputs. These studies showed that the early visual cortices receive specific, non-retinal information by auditory stimulation and/or by imagery, which induces visual representations that can be decoded via multivariate pattern analysis58,59. A common critic to these findings is that the auditory-induced visual representations could occur due to memory processes in a way that the primary visual cortex is used to restore the spatial stimulus information61. Furthermore, none of the mentioned studies found direct evidence of behavioral facilitation due to the feedback processes in these early visual cortexes. To the best of our knowledge, the current experiment provides the first direct evidence that cross-domain connections may underlie behavioral facilitation processes.
We argue that this modulation, driven by semantic information occurs because the brain is able to ‘predict’ the occurrence of a given face if provided with a congruent name previously, and that it is this prediction that leads to reduced neural activity and processing demands. Even though there is an ongoing debate on the underlying neural mechanisms of priming, in the last years, predictive coding has been used to explain several psychophysiological and neurophysiological phenomena, such as within- and cross-domain priming effects9,40,41. For example, Gotts and colleagues41 state that repetition priming occurs through enhanced neuronal synchronization, and that it is this mechanism which leads to a firing rate reduction62,63. Interestingly, Pickering and colleagues29 found smaller ERP amplitudes of the N400 for congruent name-face associations when compared to incongruent ones, as well as a shorter latency of the N400 ERP component for the primed as compared to the unprimed conditions. This ERP latency difference between Congruent and Incongruent prime-target associations can be interpreted by the synchronization theory, which states that correctly predicted inputs lead to a synchronous coupling between selective neuronal cells and regions whereas incorrectly predicted stimuli induce their asynchronous coupling41. The level of synchronization between neuronal regions might represent the update of the representation units9,10, which are expressed simultaneously throughout the hierarchy when the sensory input is similar to the priors. However, further studies are necessary to investigate the relationship between the electrophysiological and neuroimaging data, ideally applying the same paradigm within the same participants.
Here, we propose that the underlying mechanisms of cross-domain priming are similar to the ones of short-term repetition priming and repetition suppression. We argue that cross-domain priming occurs due to the formation of predictions, presumably created by feedback, cross-connections among the domain-specific areas, which encode different aspects of the input. One may speculate that the presentation of a name will generate predictions not only in face responsive areas, but, potentially, also in the auditory cortices. Additionally, as other prediction-related processes have been shown to depend on expertise15, we expect that the level of multi-sensory activations depends on the experience/familiarity with the given information. In fact, a recent study shows that short-term repetition priming as well as repetition suppression are equally modulated by environmental stability42, in other words, when repetitions were more likely and, therefore, expected, participants exhibited greater repetition priming and repetition suppression effects than when they were less likely. Future experiments should assess whether cross-domain priming effects are also modulated by such higher-level expectations.
Furthermore, we interpret this finding as a consequence of hierarchical systems on both the face perception network and prediction related processes22,64,65. It is under heavy debate currently as to which extent the face processing in the OFA is limited to low-level physical features66,67. Recent TMS evidences show that the OFA has an important role in the encoding of face identity65 and in the creation of identity-specific memory traces64. In fact, earlier case studies have already shown that an intact rOFA is crucial for identity-dependent face processing68,69. Therefore, similarly to64,65 and to certain models of face perception70, we suggest that the OFA might be crucial to face recognition by playing a role in the association of visual facial and semantic information. Current anatomical studies suggest that the semantic information, probably from the WFA reaches first the FFA and thereby the OFA56. However, specifically designed functional connectivity and transcranial magnetic stimulation studies71, using cross-domain priming paradigms will reveal the hierarchy of these feedback and feedforward pathways. Nonetheless, the fMRIa during cross-domain paradigms in the OFA/FFA suggests that these areas play a role in the association of names and faces, necessary for correct person recognition.
Interestingly, no correlation was found between the neuroimaging and bahevioural results. It is possible that the neuroimaging results are a consequence of the encountered cross-domain priming effects, rather than the cause, due to different attentional levels (as participants need less time to process a congruent face and thus, might devote less attention). Although several experiments tried to disentangle this question72–74, for reviews see75,76, the involvement of attention in priming is not yet clear77. However, the potential attentional effects in the current experiment are relatively less likely because: 1. The face stimuli were presented for a rather short duration (of 200 ms); 2. The familiarity discrimination task could not be performed until the target face appeared on the screen. 3. Participants were informed to that a name would precede a face and that their task was exclusively related to the face rather than to the name stimuli. It is worth mentioning, however, that recent findings suggest that priming resets the on-going theta-band oscillations, which have been connected to attention and predictive coding78. Therefore, one potential follow-up study could test explicitly the role of the task/attention on the observed fMRI, similarly to the stimulus repetition study of18.
Additionally, the current experimental design can be a useful tool to investigate psychiatric disorders with deficits in predictive processing, such as autism or schizophrenia79,80. However, so far, there is evidence of increased81–83 as well as decreased priming effects in schizophrenia83–85. Studies that investigated priming effects in autism show behavioral facilitation processes both for healthy and ASD participants86,87, although there is evidence that priming effects in autism are modulated by the social context88. Much less is known with regard to cross-domain priming paradigms, and these may be crucial to understand to which extent prediction processes are attenuated in such neurological conditions.
In sum, our data suggest that cross-domain priming, similarly to stimulus repetitions, leads to fMRIa in the occipito-temporal cortex and that the face perception network encodes relevant semantic information in a cross-domain name-face priming paradigm. Our findings indicate that the mechanisms by which prior information facilitates a response (behaviorally or neurally) do rely on a constant and hierarchical update of predictions, supporting predictive coding theories. Furthermore, our results also suggest that the specific content of a given sensory input and its relevancy modulates the neural processes of regions which are not a priori expected to be selective to such information.
Material and Methods
Participants
20 healthy German volunteers (with Western cultural background) participated in the fMRI experiment (7 male; 0 left-handed, mean age (±SD): 23.39 (3.42) years; 2 subjects were excluded, one due to neurological abnormality detected after the experiment and another due to low recognition of the selected famous faces). All volunteers were informed and gave a written consent form before participating. The protocol of the experiment was approved by the Ethical Committee of the Friedrich Schiller University Jena and the experiment was performed in accordance with relevant guidelines and regulations. All participants had normal or corrected to normal vision.
Stimuli and Procedure
Two groups of visual stimuli were used: faces as targets and names as prime stimuli. The face stimulus pool included 40 familiar famous (see Table 1) and 40 unfamiliar faces (downloaded from the worldwide web). The name stimuli (Arial font with size 26 in black color) corresponded to the first and the last names of the selected familiar faces (for example: Angela Merkel; see Fig. 2). Thus, in total, 40 familiar names were used in this experiment. The probability of gender was equal (i.e. 50/50% female/male) for all stimulus groups. The unfamiliar identities corresponded to Hungarian celebrities, unfamiliar to the selected group of participants (with German nationality).
Table 1.
List of famous names and faces used in the two experiments.
| Male | Female |
|---|---|
| Albert Einstein | Angela Merkel |
| Arnold Schwarzenegger | Angelina Jolie |
| Daniel Craig | Anne Hathaway |
| David Beckham | Avril Lavigne |
| David Hasselhoff | Britney Spears |
| Elvis Presley | Cameron Diaz |
| George Clooney | Emma Watson |
| George W. Bush | Heidi Klum |
| Johnny Deep | Jennifer Aniston |
| Justin Bieber | Jennifer Lawrence |
| Justin Timberlake | Jennifer Lopez |
| Leonardo DiCaprio | Julia Roberts |
| Michael Jackson | Keira Knightley |
| Pierce Brosnan | Kristen Stewart |
| Prince Harry | Natalie Portman |
| Robbie Williams | Paris Hilton |
| Rowan Atkinson | Penelope Cruz |
| Sylvester Stallone | Sarah Jessica Parker |
| Tom Cruise | Scarlett Johansson |
| Vladimir Putin | Shakira |
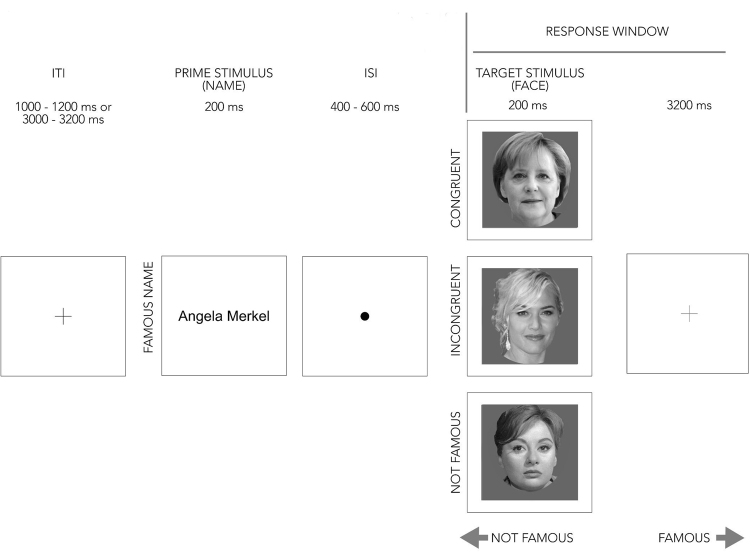
Figure 2.
Familiarity decision task for famous and unknown target faces in the fMRI experiment. A trial began with the presentation of a fixation cross, followed by the presentation of the prime stimulus, which was a name of a famous person. Following the presentation of a fixation cross a face congruent with the prime name, a face of another famous person, or an unknown person was displayed (target stimulus). The participants were instructed to make familiarity judgements (famous/not famous) for the faces. Image credits: Congruent: Armin Linnartz [CC BY-SA 3.0 de (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons (Angela Merkel, the current German Chancellor); Incongruent: Georges Biard [CC BY-SA 3.0], via Wikimedia Commons (Kate Winslet, American actress); Not famous: Fortepan/Kotnyek Antal [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons (Hédi Váradi, Hungarian actress). These images were all modified from the original (including grayscale conversion, background removal and resizing), and were not part of the actual stimulus set. Angel Merkel: File: Angela Merkel Juli 2010 - 3zu4.jpg. (2018, March 11). Wikimedia Commons, the free media repository. Retrieved 14:42, May 17, 2018 from https://commons.wikimedia.org/w/index.php?title = File:Angela_Merkel_Juli_2010_-_3zu4.jpg&oldid = 291809539. Kate Winslet: File: Kate Winslet César 2012.jpg. (2017, September 18). Wikimedia Commons, the free media repository. Retrieved 14:45, May 17, 2018 from https://commons.wikimedia.org/w/index.php?title = File:Kate_Winslet_C%C3%A9sar_2012.jpg&oldid = 258978664. Hédi Váradi. File: Váradi Hédi színművésznő. Fortepan 11996.jpg. (2017, October 26). Wikimedia Commons, the free media repository. Retrieved 14:46, May 17, 2018 from https://commons.wikimedia.org/w/index.php?title = File:V%C3%A1radi_H%C3%A9di_sz%C3%ADnm%C5%B1v%C3%A9szn%C5%91._Fortepan_11996.jpg&oldid = 264559848.
In order to create a pool of familiar faces, a prior behavioral experiment was performed to determine which famous identities were generally known by the German population. Identities that were correctly recognized by more than 85% of the participants were included in the final pool of familiar faces. The selected images fulfilled the following criteria: direct gaze and neutral facial expressions. The images were transformed in a way that the eyes of all faces were at the exact same position. Finally, the faces were converted to greyscale with equal contrast and luminance (SHINE toolbox89). Stimulus size was 3.65° in radius for the faces.
The stimuli were delivered using MATLAB R2014a (The Mathworks, Natick, MA, USA), via Psychtoolbox (Version 3.0.12) and were back-projected via an MRI-compatible LCD video projector (NEC GT 1150, NEC Deutschland GmbH, Ismaning, Germany) onto a translucent oval screen, located inside the scanner bore.
The experimental design was similar to what has been previously used by29 and90. Briefly, it consisted of a name-face cross-domain priming paradigm in which familiar, famous names primed the subsequent target faces. In the current experiment, a trial consisted of a familiar name (exposition time = 200 ms), followed by a short (400 to 600 ms with 50 ms steps) inter-stimulus interval (ISI) and a face (200 ms), which could either be unfamiliar (80 trials) or familiar (80 trials). The test faces depicted the same (Congruent) or a different (Incongruent or Unfamiliar) person as the prime names. In case of unfamiliar test faces the primes were also names of famous persons from our pool. Therefore, there were three conditions: Unfamiliar, Congruent and Incongruent. During the ISI participants saw a fixation dot. The inter-trial interval (ITI) varied between 1 s and 1.2 s (50 ms steps) or between 3 s and 3.2 s (50 ms steps). The stimulus background was always grey. Figure 2 depicts the experimental paradigm.
Importantly, the prime name itself was not predictive of the fame of the subsequent face, as it could be followed by famous or unfamous faces with equal probability. The gender of stimuli was balanced across the conditions (i.e. 50/50% male/female) and only one gender was used within a trial. In other words, the gender of the primes and targets always matched. The familiar, famous names were randomly assigned to one of the two Familiar conditions (i.e. Congruent or Incongruent) in each run.
Participants’ task was to judge whether the presented faces were familiar (i.e. famous) or unfamiliar as fast and as accurately as possible. The response time window ranged from the onset of the target stimulus until the end of the ITI.
Overall, two fMRI runs (160 trials per session) were acquired. Participants were informed that a name would precede a face and that their task was related to the face rather than the name stimuli. After the experiment, participants completed a behavioral test to assess their recognition rate of the selected familiar faces. According to these behavioral assessments, trials with false positives (i.e. when unfamiliar faces were falsely marked as famous) and misses (familiar faces that were not recognized) were removed from the statistical analysis of interest. Participants with remaining trials below 75% were also excluded from the analysis (N = 1; see64).
Imaging Parameters and Data Analysis
Imaging was performed with a 3-Tesla MR scanner (Siemens MAGNETOM Prisma fit, Erlangen, Germany). Functional, T2* weighted images were collected using an EPI sequence with the following parameters: 35 slices, 10° tilted relative to axial, TR = 2000 ms; TE = 30 ms; flip angle = 90°; 64 × 64 matrices; 3 mm isotropic voxel size. A high-resolution 3D anatomical image (T1-weighted), was acquired using a MP-RAGE sequence and the parameters were the following: TR = 2300 ms; TE = 3.03 ms; 192 slices; 1 mm isotropic voxel size. Both functional and anatomical images were acquired using a 20-channel head coil. Pre-processing and statistical analysis were conducted as described in20 using SPM12 (Welcome Department of Imaging Neuroscience, London, UK). Briefly, the functional images were slice time corrected (the 1st slice was the reference slice; 7th degree optimum B-spline transformation), realigned (in other words corrected for motion to the mean position of each experimental set; 7th degree optimum B-spline transformation) and co-registered to the structural images. Both functional and structural image sets were normalized to the MNI-152 space. Finally, the functional images were resampled to 2 × 2 × 2 mm resolution and spatially smoothed with a Gaussian kernel of 8 mm FWHM.
Two separate functional localizer runs (10.4 minutes long each, 20 sec epochs of famous faces, unfamiliar faces, chairs and Fourier randomized versions of faces, interleaved with 20 sec of blank periods, 2 Hz stimulus repetition rate; 300 ms exposure; 200 ms blank) served as basis for Regions of Interest (ROIs) detection. ROI creation was performed with MARSBAR 0.44 toolbox for SPM1291. The location of the FFA and OFA was determined individually, as an area responding more intensely to faces (famous and unfamiliar) than to chairs and Fourier randomized versions of faces (p < 0.0001UNCORRECTED). Both OFA and FFA could be identified bilaterally and reliably in 18 participants [average MNI coordinates (±SE): 1. FFA 40.9 (0.5), −46.9 (1.4), −20 (0.9) and −40.2 (0.7), −50.7 (0.9), −21.2 (0.6); average cluster size (±SE): 54 (2) and 53 (3) voxels; 2. OFA 41.8 (0.9), −77.3 (1.2), −8.8 (1.4) and −39.2 (1.0), −75.2 (1.8), −9.8 (1.8); average cluster size (±SE): 54 (3) and 54 (3) voxels, for right and left hemisphere, respectively].
The mean time series of all voxels within the ROIs (4 mm spheres around the MNI coordinate) were calculated and extracted from the event-related sessions using MARSBAR and custom made scripts. The convolution of the 3 experimental conditions (Congruent, Incongruent, Unfamiliar) with the canonical hemodynamic response function (HRF) of SPM12 served to define the predictors for a General Linear Model (GLM) analysis of the data.
Statistical Analyses
Trials with unfamiliar face identities were considered as fillers, necessary for observing priming effects and were modelled, but excluded from the final analysis. Therefore, there are two conditions of interest: Congruent and Incongruent.
Repeated measures ANOVAs were performed for both behavioral and ROI data. Separate statistical analyses were conducted for the reaction times (RT) and accuracy of the recognition task with condition (2, Congruent and Incongruent; averaged across runs) as a factor. Similarly, separate repeated measures ANOVAs were performed for the FFA and OFA activity separately with hemisphere (2, Right and Left) and condition (2, Congruent and Incongruent) as factors. Post-hoc analyses were executed using Fisher LSD tests.
Data Availability
The relevant datasets generated during the current study are available online from the OpenNeuro platform with the following DOI: 10.18112/openneuro.ds001357.v1.
Acknowledgements
This work was supported by a Deutsche Forschungsgemeinschaft Grant (KO 3918/5-1).
Author Contributions
C.A., P.K., G.A., S.T. and G.K. wrote the main manuscript. C.A., G.A. and G.K. designed the experiments. P.K. programmed the experiment. R.M. and C.A. acquired the data and performed the analysis with guidance of G.K. G.A. prepared the stimuli and the figures. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jacoby, L. L. & Brooks, L. R. Nonanalytic Cognition: Memory, Perception, and Concept Learning. In Psychology of Learning and Motivation (ed Bower, G. H.) 18, 1–47 (Academic Press, 1984).
- 2.Schacter DL. Priming and Multiple Memory Systems: Perceptual Mechanisms of ImplicitMemory. J. Cogn. Neurosci. 1992;4:244–256. doi: 10.1162/jocn.1992.4.3.244. [DOI] [PubMed] [Google Scholar]
- 3.Gross CG, Rocha-Miranda CE, Bender DB. Visual properties of neurons in inferotemporal cortex of the Macaque. J. Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- 4.Gross CG, Bender DB, Gerstein GL. Activity of inferior temporal neurons in behaving monkeys. Neuropsychologia. 1979;17:215–229. doi: 10.1016/0028-3932(79)90012-5. [DOI] [PubMed] [Google Scholar]
- 5.Schweinberger SR, Neumann MF. Repetition effects in human ERPs to faces. Cortex J. Devoted Study Nerv. Syst. Behav. 2016;80:141–153. doi: 10.1016/j.cortex.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Krekelberg B, Boynton GM, van Wezel RJA. Adaptation: from single cells to BOLD signals. Trends Neurosci. 2006;29:250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol. (Amst.) 2001;107:293–321. doi: 10.1016/S0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 8.Friston K. Prediction, perception and agency. Int. J. Psychophysiol. 2012;83:248–252. doi: 10.1016/j.ijpsycho.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friston K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friston K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 11.Friston K, Kiebel S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1211–1221. doi: 10.1098/rstb.2008.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong DW, Atick JJ. Statistics of natural time-varying images. Netw. Comput. Neural Syst. 1995;6:345–358. doi: 10.1088/0954-898X_6_3_003. [DOI] [Google Scholar]
- 13.Pajani A, Kouider S, Roux P, Gardelle V. de. Unsuppressible Repetition Suppression and exemplar-specific Expectation Suppression in the Fusiform Face Area. Sci. Rep. 2017;7:160. doi: 10.1038/s41598-017-00243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grotheer M, Hermann P, Vidnyánszky Z, Kovács G. Repetition probability effects for inverted faces. NeuroImage. 2014;102(Part 2):416–423. doi: 10.1016/j.neuroimage.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Grotheer M, Kovács G. Repetition Probability Effects Depend on Prior Experiences. J. Neurosci. 2014;34:6640–6646. doi: 10.1523/JNEUROSCI.5326-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovács G, Iffland L, Vidnyánszky Z, Greenlee MW. Stimulus repetition probability effects on repetition suppression are position invariant for faces. NeuroImage. 2012;60:2128–2135. doi: 10.1016/j.neuroimage.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Kovács G, Kaiser D, Kaliukhovich DA, Vidnyánszky Z, Vogels R. Repetition Probability Does Not Affect fMRI Repetition Suppression for Objects. J. Neurosci. 2013;33:9805–9812. doi: 10.1523/JNEUROSCI.3423-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson J, Smith AT. fMRI Repetition Suppression: Neuronal Adaptation or Stimulus Expectation? Cereb. Cortex. 2012;22:567–576. doi: 10.1093/cercor/bhr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summerfield C, Trittschuh EH, Monti JM, Mesulam M-M, Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat. Neurosci. 2008;11:1004–1006. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amado C, et al. The contribution of surprise to the prediction based modulation of fMRI responses. Neuropsychologia. 2016;84:105–112. doi: 10.1016/j.neuropsychologia.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Grotheer M, Kovács G. The relationship between stimulus repetitions and fulfilled expectations. Neuropsychologia. 2015;67:175–182. doi: 10.1016/j.neuropsychologia.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Grotheer M, Kovács G. Can predictive coding explain repetition suppression? Cortex. 2016;80:113–124. doi: 10.1016/j.cortex.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Ganel T, et al. The relationship between fMRI adaptation and repetition priming. NeuroImage. 2006;32:1432–1440. doi: 10.1016/j.neuroimage.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 24.Epstein RA, Parker WE, Feiler AM. Two kinds of FMRI repetition suppression? Evidence for dissociable neural mechanisms. J. Neurophysiol. 2008;99:2877–2886. doi: 10.1152/jn.90376.2008. [DOI] [PubMed] [Google Scholar]
- 25.Bruce V, Valentine T. Identity priming in the recognition of familiar faces. Br. J. Psychol. 1985;76:373–383. doi: 10.1111/j.2044-8295.1985.tb01960.x. [DOI] [PubMed] [Google Scholar]
- 26.Spence C. Crossmodal correspondences: A tutorial review. Atten. Percept. Psychophys. 2011;73:971–995. doi: 10.3758/s13414-010-0073-7. [DOI] [PubMed] [Google Scholar]
- 27.Calder AJ, Young AW. Self Priming: A Short term Benefit of Repetition. Q. J. Exp. Psychol. Sect. A. 1996;49:845–861. doi: 10.1080/713755666. [DOI] [Google Scholar]
- 28.Calder AJ, Young AW, Benson PJ, Perrett DI. Self priming from distinctive and caricatured faces. Br. J. Psychol. 1996;87:141–162. doi: 10.1111/j.2044-8295.1996.tb02581.x. [DOI] [Google Scholar]
- 29.Pickering EC, Schweinberger SR. N200, N250r, and N400 event-related brain potentials reveal three loci of repetition priming for familiar names. J. Exp. Psychol. Learn. Mem. Cogn. 2003;29:1298–1311. doi: 10.1037/0278-7393.29.6.1298. [DOI] [PubMed] [Google Scholar]
- 30.Schweinberger, S. How Gorbachev primed Yeltsin: Analyses of associative priming in person recognition by means of reaction times and event-related brain potentials. 22 (1996).
- 31.Jemel B, Pisani M, Rousselle L, Crommelinck M, Bruyer R. Exploring the functional architecture of person recognition system with event-related potentials in a within- and cross-domain self-priming of faces. Neuropsychologia. 2005;43:2024–2040. doi: 10.1016/j.neuropsychologia.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Wiese H, Schweinberger SR. Event-related potentials indicate different processes to mediate categorical and associative priming in person recognition. J. Exp. Psychol. Learn. Mem. Cogn. 2008;34:1246–1263. doi: 10.1037/a0012937. [DOI] [PubMed] [Google Scholar]
- 33.Wiese H, Schweinberger SR. Accessing Semantic Person Knowledge: Temporal Dynamics of Nonstrategic Categorical and Associative Priming. J. Cogn. Neurosci. 2010;23:447–459. doi: 10.1162/jocn.2010.21432. [DOI] [PubMed] [Google Scholar]
- 34.Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. NeuroImage. 2003;19:613–626. doi: 10.1016/S1053-8119(03)00096-X. [DOI] [PubMed] [Google Scholar]
- 35.Alink A, Schwiedrzik CM, Kohler A, Singer W, Muckli L. Stimulus Predictability Reduces Responses in Primary Visual Cortex. J. Neurosci. 2010;30:2960–2966. doi: 10.1523/JNEUROSCI.3730-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eger E, Henson RN, Driver J, Dolan RJ. Mechanisms of top-down facilitation in perception of visual objects studied by fMRI. Cereb. Cortex N. Y. N. 2007;1991(17):2123–2133. doi: 10.1093/cercor/bhl119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egner T, Monti JM, Summerfield C. Expectation and Surprise Determine Neural Population Responses in the Ventral Visual Stream. J. Neurosci. 2010;30:16601–16608. doi: 10.1523/JNEUROSCI.2770-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summerfield C, Koechlin E. A Neural Representation of Prior Information during Perceptual Inference. Neuron. 2008;59:336–347. doi: 10.1016/j.neuron.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Trapp S, Lepsien J, Kotz SA, Bar M. Prior probability modulates anticipatory activity in category-specific areas. Cogn. Affect. Behav. Neurosci. 2016;16:135–144. doi: 10.3758/s13415-015-0373-4. [DOI] [PubMed] [Google Scholar]
- 40.Arnal LH, Giraud A-L. Cortical oscillations and sensory predictions. Trends Cogn. Sci. 2012;16:390–398. doi: 10.1016/j.tics.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Gotts SJ, Chow CC, Martin A. Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cogn. Neurosci. 2012;3:227–237. doi: 10.1080/17588928.2012.670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olkkonen M, Aguirre GK, Epstein RA. Expectation modulates repetition priming under high stimulus variability. J. Vis. 2017;17:10–10. doi: 10.1167/17.6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunzeck N, Schütze H, Düzel E. Category-specific organization of prefrontal response-facilitation during priming. Neuropsychologia. 2006;44:1765–1776. doi: 10.1016/j.neuropsychologia.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- 45.Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat. Neurosci. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- 46.Henson RNA, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/S0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- 47.Schacter DL, Buckner RL. Priming and the Brain. Neuron. 1998;20:185–195. doi: 10.1016/S0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 48.Amado, C. et al. The contribution of surprise to the prediction based modulation of fMRI responses. Neuropsychologia10.1016/j.neuropsychologia.2016.02.003 (2016). [DOI] [PubMed]
- 49.Feuerriegel D. Selecting appropriate designs and comparison conditions in repetition paradigms. Cortex. 2016;80:196–205. doi: 10.1016/j.cortex.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Larsson J, Solomon SG, Kohn A. fMRI adaptation revisited. Cortex. 2016;80:154–160. doi: 10.1016/j.cortex.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burton AM, Kelly SW, Bruce V. Cross-domain Repetition Priming in Person Recognition. Q. J. Exp. Psychol. Sect. A. 1998;51:515–529. doi: 10.1080/713755780. [DOI] [Google Scholar]
- 52.Thesen T, et al. Sequential then interactive processing of letters and words in the left fusiform gyrus. Nat. Commun. 2012;3:1284. doi: 10.1038/ncomms2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen L, et al. The visual word form areaSpatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- 54.Cohen L, et al. Language‐specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- 55.Dehaene S. Le Clec’H, G., Poline, J.-B., Le Bihan, D. & Cohen, L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- 56.Bouhali F, et al. Anatomical Connections of the Visual Word Form Area. J. Neurosci. 2014;34:15402–15414. doi: 10.1523/JNEUROSCI.4918-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kriegstein K, von, Kleinschmidt A, Sterzer P, Giraud A-L. Interaction of Face and Voice Areas during Speaker Recognition. J. Cogn. Neurosci. 2005;17:367–376. doi: 10.1162/0898929053279577. [DOI] [PubMed] [Google Scholar]
- 58.Iurilli G, et al. Sound-driven synaptic inhibition in primary visual cortex. Neuron. 2012;73:814–828. doi: 10.1016/j.neuron.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vetter P, Smith FW, Muckli L. Decoding Sound and Imagery Content in Early Visual Cortex. Curr. Biol. 2014;24:1256–1262. doi: 10.1016/j.cub.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petro LS, Paton AT, Muckli L. Contextual modulation of primary visual cortex by auditory signals. Phil Trans R Soc B. 2017;372:20160104. doi: 10.1098/rstb.2016.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keogh R, Pearson J. The sensory strength of voluntary visual imagery predicts visual working memory capacity. J. Vis. 2014;14:7–7. doi: 10.1167/14.12.7. [DOI] [PubMed] [Google Scholar]
- 62.Bazhenov M, Stopfer M, Sejnowski TJ, Laurent G. Fast Odor Learning Improves Reliability of Odor Responses in the Locust Antennal Lobe. Neuron. 2005;46:483–492. doi: 10.1016/j.neuron.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gotts, S. Mechanisms underlying enhanced processing efficiency in neural systems (2003).
- 64.Ambrus GG, Dotzer M, Schweinberger SR, Kovács G. The occipital face area is causally involved in the formation of identity-specific face representations. Brain Struct. Funct. 2017;222:4271–4282. doi: 10.1007/s00429-017-1467-2. [DOI] [PubMed] [Google Scholar]
- 65.Ambrus GG, Windel F, Burton AM, Kovács G. Causal evidence of the involvement of the right occipital face area in face-identity acquisition. NeuroImage. 2017;148:212–218. doi: 10.1016/j.neuroimage.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 66.Rossion B, Jacques C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. NeuroImage. 2008;39:1959–1979. doi: 10.1016/j.neuroimage.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Solomon-Harris LM, Mullin CR, Steeves JKE. TMS to the “occipital face area” affects recognition but not categorization of faces. Brain Cogn. 2013;83:245–251. doi: 10.1016/j.bandc.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Bouvier SE, Engel SA. Behavioral Deficits and Cortical Damage Loci in Cerebral Achromatopsia. Cereb. Cortex. 2006;16:183–191. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- 69.Rossion B, et al. A network of occipito‐temporal face‐sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- 70.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4:223–233. doi: 10.1016/S1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 71.ECVP 2017 Abstract: Perception: SAGE Journals. Available at: http://journals.sagepub.com/page/pec/collections/ecvp-abstracts/index/ecvp-2017. (Accessed: 7th May 2018).
- 72.Vuilleumier P, Schwartz S, Duhoux S, Dolan RJ, Driver J. Selective Attention Modulates Neural Substrates of Repetition Priming and “Implicit” Visual Memory: Suppressions and Enhancements Revealed by fMRI. J. Cogn. Neurosci. 2005;17:1245–1260. doi: 10.1162/0898929055002409. [DOI] [PubMed] [Google Scholar]
- 73.Kristjánsson Á, Vuilleumier P, Schwartz S, Macaluso E, Driver J. Neural Basis for Priming of Pop-Out during Visual Search Revealed with fMRI. Cereb. Cortex. 2007;17:1612–1624. doi: 10.1093/cercor/bhl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Egner T, Hirsch J. Where Memory Meets Attention: Neural Substrates of Negative Priming. J. Cogn. Neurosci. 2005;17:1774–1784. doi: 10.1162/089892905774589226. [DOI] [PubMed] [Google Scholar]
- 75.Kristjánsson Á, Campana G. Where perception meets memory: A review of repetition priming in visual search tasks. Atten. Percept. Psychophys. 2010;72:5–18. doi: 10.3758/APP.72.1.5. [DOI] [PubMed] [Google Scholar]
- 76.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 77.Stein T, Thoma V, Sterzer P. Priming of object detection under continuous flash suppression depends on attention but not on part-whole configuration. J. Vis. 2015;15:15–15. doi: 10.1167/15.3.15. [DOI] [PubMed] [Google Scholar]
- 78.Guo, B., Lu, Z., Goold, J. E., Luo, H. & Meng, M. Fluctuations of fMRI activation patterns reveal theta-band dynamics of visual object priming. bioRxiv 148635 10.1101/148635 (2017).
- 79.Dunn MA, Gomes H, Gravel J. Mismatch negativity in children with autism and typical development. J. Autism Dev. Disord. 2008;38:52–71. doi: 10.1007/s10803-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 80.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr. Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Höschel K, Irle E. Emotional Priming of Facial Affect Identification in Schizophrenia. Schizophr. Bull. 2001;27:317–327. doi: 10.1093/oxfordjournals.schbul.a006877. [DOI] [PubMed] [Google Scholar]
- 82.Pomarol-Clotet E, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol. Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 83.Ungar L, Nestor PG, Niznikiewicz MA, Wible CG, Kubicki M. Color Stroop and negative priming in schizophrenia: An fMRI study. Psychiatry Res. Neuroimaging. 2010;181:24–29. doi: 10.1016/j.pscychresns.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andreou C, Bozikas VP, Ramnalis A, Giannakou M, Fokas K. Semantic priming in remitted patients with bipolar disorder. J. Behav. Ther. Exp. Psychiatry. 2013;44:48–52. doi: 10.1016/j.jbtep.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 85.Vinogradov S, Ober BA, Shenaut GK. Semantic priming of word pronunciation and lexical decision in schizophrenia. Schizophr. Res. 1992;8:171–181. doi: 10.1016/0920-9964(92)90033-2. [DOI] [PubMed] [Google Scholar]
- 86.Cook JL, Bird G. Atypical Social Modulation of Imitation in Autism Spectrum Conditions. J. Autism Dev. Disord. 2012;42:1045–1051. doi: 10.1007/s10803-011-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hala S, Pexman PM, Glenwright M. Priming the Meaning of Homographs in Typically Developing Children and Children with Autism. J. Autism Dev. Disord. 2007;37:329. doi: 10.1007/s10803-006-0162-6. [DOI] [PubMed] [Google Scholar]
- 88.Toichi M, Kamio Y. Verbal Association for Simple Common Words in High-Functioning Autism. J. Autism Dev. Disord. 2001;31:483–490. doi: 10.1023/A:1012216925216. [DOI] [PubMed] [Google Scholar]
- 89.Willenbockel V, et al. Controlling low-level image properties: The SHINE toolbox. Behav. Res. Methods. 2010;42:671–684. doi: 10.3758/BRM.42.3.671. [DOI] [PubMed] [Google Scholar]
- 90.Rhodes G, Tremewan T. The Simon Then Garfunkel Effect: Semantic Priming, Sensitivity, and the Modularity of Face Recognition. Cognit. Psychol. 1993;25:147–187. doi: 10.1006/cogp.1993.1004. [DOI] [Google Scholar]
- 91.Brett MC, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using the MarsBar toolbox for SPM. 2002;99:16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The relevant datasets generated during the current study are available online from the OpenNeuro platform with the following DOI: 10.18112/openneuro.ds001357.v1.