Abstract
Little is known about how Borrelia burgdorferi, the Lyme disease pathogen, adapts and survives in the tick vector. We previously identified a bacterial CarD N-terminal-like (CdnL) protein, LtpA (BB0355), in B. burgdorferi that is preferably expressed at lower temperatures, which is a surrogate condition mimicking the tick portion of the enzootic cycle of B. burgdorferi. CdnL-family proteins, an emerging class of bacterial RNAP-interacting transcription factors, are essential for the viability of Mycobacterium tuberculosis and Myxococcus xanthus. Previous attempts to inactivate ltpA in B. burgdorferi have not been successful. In this study, we report the construction of a ltpA mutant in the infectious strain of B. burgdorferi, strain B31-5A4NP1. Unlike CdnL in M. tuberculosis and M. xanthus, LtpA is dispensable for the viability of B. burgdorferi. However, the ltpA mutant exhibits a reduced growth rate and a cold-sensitive phenotype. We demonstrate that LtpA positively regulates 16S rRNA expression, which contributes to the growth defects in the ltpA mutant. The ltpA mutant remains capable of infecting mice, albeit with delayed infection. Additionally, the ltpA mutant produces markedly reduced spirochetal loads in ticks and was not able to infect mice via tick infection. Overall, LtpA represents a novel regulator in the CdnL family that has an important role in the enzootic cycle of B. burgdorferi.
Introduction
Bacterial CarD N-terminal-like (CdnL) protein, a defining member of the CarD_CdnL_TRCF protein family, is part of an emerging class of bacterial RNAP-interacting transcription factors1–5. The CarD_CdnL_TRCF protein family contains two types of proteins, CarD-type and CdnL-type. CarD is a global transcriptional regulator that contains two domains: a C-terminal AT-hook DNA-binding domain resembling eukaryotic high-mobility group A (HMGA) proteins and an N-terminal transcription repair coupling factor (TRCF) domain that interacts with RNA polymerase. CdnL proteins are more common and widespread among bacterial species. They have a N-terminal TRCF domain but lack a C-terminal AT-hook DNA-binding domain2. Recent structural studies show that CdnL contains two sub-domains: CdnLNt, which consists of five β strands and interacts with RNAP, and CdnLCt, which consists of five α-helices and contains a solvent-exposed nonpolar-basic patch that is involved in DNA binding4,6–8.
Although the presence of CdnL-type CarD transcription factors is widespread among bacterial species9, our understanding of their function remains limited. In Mycobacteria, CdnL-type CarD was shown to regulate ribosomal RNA (rRNA) transcription in tuberculosis (Mtb)5, and its expression is upregulated during several stress conditions including oxidative stress, starvation, and antibiotic treatment5,10. CdnL-type CarD in Mycobacteria is essential for cell viability. Myxococcus xanthus contains both CarD and CdnL. In M. xanthus CdnL, but not CarD, is essential for cell viability2. Bacillus species appear to have two copies of CdnL proteins. The inactivation of one of the cdnL genes did not affect cell replication in B. subtilis and B. cereus but resulted in defects in the repair and outgrowth of heat-damaged spores in B. cereus11. Additionally, cdnL expression was upregulated in the vegetative cells of B. cereus in response to stress conditions12.
Borrelia burgdorferi, the Lyme disease pathogen, is maintained in nature via an enzootic cycle between ticks and mammals. Although much is known regarding how B. burgdorferi modulates its gene expression to adapt to mammalian host environments; the process of spirochetal adaptation in its tick host is poorly understood13–15. Two pathways have been identified that are critical in regulating spirochetal adaptation in ticks, the c-di-GMP signaling pathway16–21 and the (p)ppGpp stringent response pathway22–25. c-di-GMP is important for the ability of B. burgdorferi to utilize nutrients in ticks, including glycerol, chitobiose, and N-acetyl glucosamine16,20,26,27, and is involved in the chemotaxis, motility, cell envelope structure and osmolarity, all of which are important for the survival of B. burgdorferi in ticks17–20,28,29. (p)ppGpp is required for persistence in ticks and is involved in the expression of glycerol uptake and metabolism (glp) operon as well as that of other tick-associated genes such as ospA, bicA, and pncA23,25.
We previously reported that LtpA (BB0355) in B. burgdorferi is homologous to CdnL-type CarD. LtpA is most highly expressed at ambient temperature (23 ℃), which is a surrogate condition for the tick environment30. Previous attempts to generate an ltpA mutant in virulent B. burgdorferi strain 297 were unsuccessful, which has hampered the effort to elucidate the role of LtpA in the enzootic cycle of B. burgdorferi. Herein, we report the successful construction of an ltpA deletion mutant in the virulent B. burgdorferi strain B31 clone 5A4NP1. We demonstrate that LtpA is indispensable for spirochetal viability, but is required for the optimal survival of B. burgdorferi in the tick vector.
Results
LtpA is differentially expressed by temperature and growth phase
To investigate the regulation of ltpA, we examined spirochetes cultivated at different temperatures (23 or 37 ℃) and harvested at different growth phases (mid-logarithmic (107 cells/ml) or a late-logarithmic phase (108 cells/ml)). As reported previously, little to no LtpA was detected in B. burgdorferi when cultivated under conditions mimicking mammalian infection (37 ℃, late-logarithmic phase), while significant LtpA was detected in spirochetes grown in conditions mimicking tick colonization (23 ℃) (Fig. 1a)30. When harvested at the mid-logarithmic phase, LtpA was readily detected even at 37 °C, suggesting that in addition to temperature, cell density also regulates ltpA expression at 37 °C. This finding was supported by qRT-PCR results (Fig. 1b). At 23 ℃, LtpA appeared to be abundantly produced, regardless of the growth phase (Fig. 1a, b).
Fig. 1. Influence on LtpA level by temperature and growth phase.

B. burgdorferi strain 5A4NP1 was cultured in BSK-II medium at 37 °C or 23 °C. Spirochetes were collected at the mid-log phase (M, 107 spirochetes/ml) or late-log phase (L, 108 spirochetes/ml) then processed for immunoblot (a) and qPCR analyses (b). Levels of FlaB protein and flaB mRNA served as internal controls. *, p < 0.05; **, p < 0.01
Construction of the ltpA mutant and the complementation strain
To investigate the function of LtpA, we constructed an ltpA mutant using allelic exchange in a low passage, infectious strain of B. burgdorferi strain B31 clone 5A4NP1. A suicide vector pXY301R-CarD was constructed with an aadA gene (which confers streptomycin-resistance) that was flanked by upstream and downstream regions of ltpA (BB0355, Fig. 2a). pXY301R-CarD was transformed into infectious B. burgdorferi strain B31 clone 5A4NP1, where it replaced the ltpA gene. Four streptomycin- and kanamycin-resistant clones were obtained; these clones were subjected to PCR analyses for confirmation of the correct ltpA deletion (data not shown). One clone that maintained identical endogenous plasmids to those of the parental strain 5A4NP1 was chosen for complementation. For complementation, a pBSV2-derived shuttle vector with a gentamycin-resistance cassette carrying a wild-type copy of ltpA driven by an IPTG-inducible promoter, pXW006 (Fig. 2b), was transformed into the ltpA mutant. Of note, complementation using a shuttle vector harboring a constitutively expressed flaB promoter-driven ltpA was not successful despite multiple attempts (data not shown), implying that the overexpression of ltpA may be deleterious to B. burgdorferi. The loss of LtpA in the ltpA mutant and the restoration of LtpA production in the complementation strain (when grown in the presence of 1 mM IPTG) were confirmed by immunoblotting (Fig. 2c). One complementation clone that had a virtually identical endogenous plasmid profile to that of the parental strain 5A4NP1, with the exception of losing one of the cp32 plasmids, cp32-7 (Fig. 2d), was selected for further study.
Fig. 2. Construction of the ltpA mutant and the complementation strain.
a Strategy for constructing the ltpA mutant. WT: genomic structure of ltpA. pXY301R-carD: the suicide vector used for inactivation of ltpA. LtpA-mut: the ltpA mutant. b Diagram of the shuttle vector used for complementation. c B. burgdorferi strains were cultured in BSK-II medium with or without 1 mM IPTG. Spirochetes were collected at the mid-log phase (107 spirochetes/ml) then processed for immunoblotting analysis. WT wild-type B. burgdorferi strain 5A4NP1, Ltp-mut the ltpA mutant, LtpA-com the complementation strain. d Endogenous plasmid profiles of wild-type (WT) and complementation strains (LtpA-com). The ltpA mutant has an identical plasmid profile to that of the WT (not shown). * indicates the band corresponding to plasmid cp32-7 that is missing in LtpA-com
The ltpA mutant has reduced growth in vitro
The success in generating a ltpA mutant suggests that, unlike other CdnL proteins, LtpA is not essential for cell viability. To further examine whether the ltpA mutant has defects in vitro, wild-type spirochetes (WT), the ltpA mutant, and the complementation strain were cultured at 37 or 23 ℃, and their growth rates were compared. As shown in Fig. 3a, the ltpA mutant exhibited slower growth at 37 ℃ than did the wild-type or complemented strain. At 23 ℃, the ltpA mutant demonstrated a cold-sensitive phenotype, as it was viable but could hardly replicate under such conditions (Fig. 3b). These observations indicate that LtpA is required for the optimal growth of B. burgdorferi in vitro and that its function is more important at lower temperatures mimicking those typical of tick infection.
Fig. 3. Growth defects in the ltpA mutant.
WT, LtpA-mut, and LtpA-com strains were cultured in standard BSK-II medium at 37 °C (a) or 23 °C (b). The initial cell densities for the cultures at 37 °C and 23 °C were 103 spirochetes/ml and 105 spirochetes/ml, respectively. Numbers of spirochetes were enumerated daily using dark-field microscopy. Each data point is derived from the average of the data from three independent cultures. Statistical significance was calculated between LtpA-mut and the WT group. *P < 0.05
The ltpA mutant has reduced expression of 16S rRNA
It was reported that CdnL is involved in regulating ribosomal RNA transcription5. To investigate whether LtpA influences rRNA transcription in B. burgdorferi, which may account for the growth defects and the decreased growth rate, we compared the 16S rRNA levels among the wild-type, ltpA mutant, and complemented strains. The results showed that the deletion of ltpA markedly reduced the 16S rRNA levels in spirochetes grown at 23 or 37 °C (Fig. 4). The complementation of the ltpA mutant with an IPTG-inducible promoter-driven ltpA partially restored the 16S rRNA level (Fig. 4).
Fig. 4. The defect of 16S rRNA expression in the ltpA mutant.
WT, LtpA-mut, and LtpA-com strains were cultured in standard BSK-II medium at 37 °C (a) or 23 °C (b). For the culture at 37 °C, spirochetes were collected at the mid-log (107 spirochetes/ml) or stationary phases (5 × 108 spirochetes/ml) and subjected to qRT-PCR analysis. For the culture at 23 °C, spirochetes were collected at a cell density of 107 spirochetes/ml. Each data point was the average of the data from three independent cultures. *, p < 0.05; **, p < 0.01; ***, p < 0.001
The ltpA mutant exhibits delayed infection in mice
To examine the ltpA mutant’s ability to infect mice, groups of C3H/HeN mice were needle inoculated with the wild-type, ltpA mutant, or complemented strain with a dose of 1 × 105 spirochetes per mouse. Mice inoculated with the complementation strain were given IPTG via intraperitoneal (IP) injection in every two days. Ear-punch biopsies were collected and then cultured to determine the presence of spirochetes at different time points. As shown in Table 1, the mice inoculated with wild-type B. burgdorferi were culture-positive 2 weeks after inoculation, but no mice were infected when inoculated with the ltpA mutant. At 4 weeks after infection, some of the mice infected with the ltpA mutant became culture-positive. At week 7, all the mice were killed, and several mouse tissues including skin, bladder, heart, and joint were collected and cultured; seven out of the nine mice inoculated with the ltpA mutant were culture-positive (Table 1). These data demonstrated that the ltpA mutant is capable of establishing infection in mice, but it is delayed in doing so.
Table 1.
Mouse infection of the LtpA-defective mutant
| C3H/HeN | No. of infected mice/Total no. of mice | |||
|---|---|---|---|---|
| Needle infectiona | Tick bitea | |||
| 2 W | 4 W | 7 W | 7 W | |
| WT | 5/5 | 5/5 | 5/5 | 3/3 |
| LtpA-mut | 0/9 | 3/9 | 7/9 | 0/6 |
| LtpA-com | 1/3 | 3/3 | 3/3 | 1/2 |
aThe dose used for needle infection was 105 spirochetes/mouse. For tick bites, 12 infected nymphal ticks were used for each mouse. Time points for ear-punch biopsies or tissue collections were at 2 weeks (2 W), 4 weeks (4 W), or 7 weeks (7 W) after inoculation
LtpA is required for optimal survival in ticks
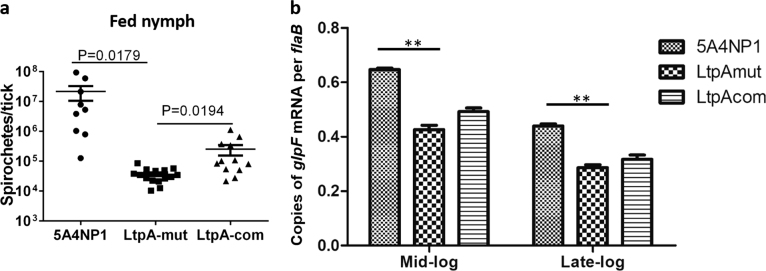
To investigate the role of LtpA in B. burgdorferi in ticks, equal amounts of the wild-type, ltpA mutant, or complemented strains of B. burgdorferi were microinjected into the midgut of nymphal ticks. The medium used for the complementation strain contained 1 mM IPTG to ensure the expression of ltpA in ticks. No differences in spirochetal numbers, as assessed using qPCR, in ticks prior to feeding was observed (data not shown). Microinjected unfed ticks were fed on naive C3H/HeN mice until fully engorged. The mice used for feeding ticks harboring the complementation strain were given IPTG daily by IP injection during the process of tick feeding. The engorged ticks were collected, and qPCR was used to assess their spirochetal loads. The results showed that the ltpA mutant produced a thousandfold lower number of spirochetes in ticks than did wild-type B. burgdorferi (Fig. 5a). The spirochetal load was partially restored in the complementation strain. These data indicate that LtpA is required for the optimal survival of ticks upon feeding.
Fig. 5. Decreased spirochetal load and glpF expression in the ltpA mutant.
a qPCR analysis of spirochetal burdens in fed nymphs. Flat nymphal ticks were first infected with equal amounts of wild-type, ltpA mutant, and complementation strains of B. burgdorferi by microinjection directly into the midgut of nymphal ticks. Ticks were then allowed to feed on naive mice, and fed nymphal ticks were collected and examined using qPCR analysis. The number of copies of the B. burgdorferi flaB gene was chosen to represent spirochete numbers. Each data point represents flaB copies in one nymph tick. b Transcript levels of glpF in WT, LtpA-mut, and LtpA-com strains as determined using qRT-PCR. RNAs were isolated from mid-logarithmic phase and stationary phase cultures grown at 37 °C in a modified BSK-glycerol medium. The glpF levels were normalized to flaB levels. Values represent the average copy number from three independent cultures. **P < 0.01
The ltpA mutant is unable to infect mice via tick bites
To examine the ltpA mutant’s ability to infect mice during tick infestation, the mice that were used to feed ticks during the above experiment (Fig. 4a) were monitored for infection. The mice infected with ticks carrying the complemented strain were given IPTG by IP injection every two days. All of the ear-punch biopsy cultures from the mice infected with ticks carrying the wild-type B. burgdorferi were positive, but all the cultures from the mice infected with ticks carrying the ltpA mutant were negative (Table 1). This finding remained true even at 7 weeks post-tick feeding (Table 1). These data suggest that, although the ltpA mutant was able to survive within the tick midgut, it was not able to infect mice via tick bites.
The glycerol uptake and metabolic pathway (encoded by the glpFKD operon) is known to be important for optimal spirochetal growth in ticks. Thus, we investigated whether LtpA has a role in glpFKG expression. The results showed that the ltpA mutant exhibited a moderate, although statistically significant, reduction in glpF expression in spirochetes compared to that in the wild-type B. burgdorferi (Fig. 5b).
Discussion
B. burgdorferi encounters drastic environmental changes when it migrates between the tick vector and the mammalian host. While major advances have been made in understanding how B. burgdorferi modulates its gene expression during mammalian host adaptation, few of the regulators required for spirochetal adaptation and survival in ticks have been identified. In this study, we focused on the CarD-like regulator in B. burgdorferi, LtpA. CarD is a new family of global transcriptional regulators. Our knowledge of the CarD family remains sparse and is largely gleaned from studies on Mycobacteria and Myxococcus. We demonstrated that LtpA has an important role in the optimal fitness of B. burgdorferi in ticks and is indispensable for the successful transmission of B. burgdorferi from ticks to mammals.
The inactivation of ltpA in virulent strains of B. burgdorferi proved challenging. Multiple attempts to generate an ltpA mutant were not successful30, and the published transposon library of B. burgdorferi also did not yield a transposon insertion within the ltpA gene31. Two factors likely contributed to our present success in constructing the ltpA mutant in this study. First, we used the limiting dilution method instead of using the semisolid agar plating method when selecting the mutant clones after transformation32–34. Second, we used the B. burgdorferi strain B31 clone 5A4NP135, rather than the strain 297 AH130 that was used previously30.
CdnL-type CarD of M. tuberculosis and CdnL of M. Xanthus are the most studied members of the CdnL family and have been demonstrated to be essential for cell viability2,5. For B. cereus, inactivation of cdnL did not affect cell replication; however, this bacterium has two copies of CdnL11. Thus, whether CdnL is required for cell viability in B. cereus remains to be determined, which requires inactivation of both copies of cdnL genes. In this regard, LtpA is unique, as it is dispensable for spirochetal viability in vitro. On the other hand, the ltpA mutant of B. burgdorferi showed growth defects, and such defects were markedly augmented at lower temperature, resulting in a cold-temperature-sensitive phenotype. Interestingly, a recent report showed that CdnL was also not essential for cell viability in Caulobacter crescentus, and the cdnL mutant used in the study was also cold-sensitive36.
The growth defects present in the ltpA mutant were likely due to decreased rRNA transcription (Fig. 4). Supporting this conclusion, the CdnL-type CarD of M. tuberculosis was reported to regulate rRNA expression5,37,38. CarD in M. tuberculosis is broadly distributed on a number of promoters and uses a minor groove wedge mechanism to stabilize the RNAP transcription complex during transcription initiation37. CdnL of C. crescentus was also shown to interact with the RNAP β subunit and to localize to at least one rRNA promoter in vivo36. A highly conserved tryptophan residue was thought to wedge into the minor groove in the upstream DNA sequence to stabilize the RNAP complex9. In addition, CdnL was shown to be capable of binding to DNA, despite lacking the AT-hook DNA-binding domain; a protein crystal structure revealed that the unique C-terminal domain of CdnL consists of a five α-helical fold, which has been shown to have DNA-binding activity in M. tuberculosis and T. thermilus4,7. CdnL in C. crescentus was also shown to interact with DNA, but not in M. xanthus6,36. LtpA lacks the conserved tryptophan residue. Whether LtpA functions via stabilizing RNAP or LtpA directly interacts with DNA to modulate gene expression remains to be determined.
Low levels of rRNA in the ltpA mutant also likely contribute to the spirochetal bacterial burden in ticks and possibly delayed infection in mice. During mouse infection, in addition to reduced growth, the ltpA mutant may have reduced infectivity. In this study, only a single dose (105 spirochetes/mouse) was given during the needle infection experiment, which precludes the calculation of the ID50 value. Nevertheless, the presence of delayed infection suggests that a higher dose of the ltpA mutant is required for infection than for the wild-type B. burgdorferi (which requires <100 spirochetes). In ticks, B. burgdorferi undergoes maximal growth to reach a high number within the tick gut during blood meal feeding, as only a small number of spirochetes are capable of penetrating through the midgut membrane into the tick hemocoel13,39. Thus, optimal growth in ticks is important for B. burgdorferi to complete the enzootic cycle. Although the ltpA mutant was capable of surviving in ticks, it had reduced spirochetal loads after tick feeding. One caveat of this study is that we did not examine the long-term survival in unfed ticks. A reduced spirochetal number in ticks is likely to be one major factors contributing to the inability to infect mice via tick infection. The reduced spirochetal number in ticks with the ltpA mutant is the result of low 16S rRNA levels; the defect in glpF expression in the ltpA mutant is another contributor. In this study, we only examined glpF expression at 37 °C, as the ltpA mutant failed to grow at 23 °C (Fig. 3b). Since glpF expression is increased by glycerol at both 23 and 37 °C (i.e., regulated similarly by glycerol in both temperature conditions), it is reasonable to postulate that LtpA also positively affected glpF expression at a lower temperature, as observed at 37 °C. In addition to reduced growth fitness in ticks, the ltpA mutant may also be defective in the process of transmission from tick midgut to the hemocoel, the salivary grand, and eventually to mice. Further understanding of how LtpA regulates gene expression and modulates spirochetal adaptation in ticks will improve our understanding of the molecular mechanisms underlying the enzootic cycle of B. burgdorferi.
Materials and methods
B. burgdorferi strains and culture conditions
B. burgdorferi strains (Table 2) were constructed using the low-passage clone LP B31 (strain 5A4NP1), kindly provided by Dr. Steve Norris. Spirochetes were cultivated in complete Barbour–Stoenner–Kelley-II (BSK-II) medium40 at 37 or 23 °C with 5% CO2.
Table 2.
Strains, plasmids, and primers used in this study
| Strains, plasmids, or primers | Description or sequence | Purpose or source |
|---|---|---|
| Strains | ||
| 5A4NP1 | Wild-type strain with bbe02 gene disrupted by Kanr | 35 |
| LtpA-mut | ltpA deletion mutant; 5A4NP1 transformed with pXY301R | This study |
| LtpA-com | ltpA complementation; LtpA-mut transformed with pXW006 | This study |
| Plasmids | ||
| pXY301R | Suicide vector for producing ltpA deletion; Strr | This study |
| pXW006 | Shuttle vector carrying IPTG-inducible ltpA gene; Genr | This study |
| Primers | ||
| qPCR-flaB-F | CAGCAATAGCTTCATCTTGGTTTG | qPCR of flaB |
| qPCR-flaB-R | ACCAGCATCTTCAGGGTCTCA | qPCR of flaB |
| Del-CarD-UF | GCGGCCGCACCATCTTGTTAATGTAAGACG | Amplification of ltpA upstream region |
| Del-CarD-UR | AGATCTCCTTAATCGTACCTACTCCATGC | Same as above |
| Del-CarD-DF | ACTAGTGTTAGCAGGGAAAAGGTAGAAGA | Amplification of ltpA downstream region |
| Del-CarD-DR | GGTACCTGAAACAAAACCAAATAAATAGG | Same as above |
| NdeI-priFX-CarDf | GCCGCATATGATGTTGTTCTCAGGAAAA | Amplification of ltpA with NdeI and AscI sites |
| AscI-priFX-CarDr | ATTAGGCGCTTACTCACTTTCCCCTAA | Same as above |
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
Spirochetes were harvested by centrifugation at 8000×g for 10 min and washed three times with PBS (pH 7.4) at 4 °C. Pellets were suspended in SDS buffer containing 50 mM Tris-HCl (pH 8), 0.3% sodium dodecyl sulfate (SDS), and 10 mM dithiothreitol (DTT). Cell lysates (5 × 107 cells per lane) were separated by 12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (GE-Healthcare, Milwaukee, WI). Membranes were blotted with antibodies against FlaB (monoclonal, 1:1000 dilution), LtpA (polyclonal, 1:2000 dilution)30,41, and then incubated with goat anti-mouse lgG-HRP secondary antibody (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) or goat anti-rat lgG-HRP secondary antibody (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA). The detection of horse radish peroxidase activity was determined using the enhanced chemiluminescence method (Thermo Pierce ECL Western Blotting Substrate, Waltham, MA) and subsequently by exposure to X-ray film.
Generation of the ltpA (bb0355) mutant and complementation strain
To construct a suicide vector for the inactivation of ltpA, regions of DNA corresponding to 1.5 kb upstream and 1.3 kb downstream of ltpA were PCR amplified from 5A4NP1 genomic DNA using the primer pair Del-CarD-UF and Del-CarD-UR, and the pair Del-CarD-DF and Del-CarD-DR, respectively (Table 2). The resulting DNA fragments were then cloned upstream and downstream of an aadA streptomycin-resistant marker within the suicide vector pXY301R16, resulting in pXY301-carD. To construct a shuttle vector for complementation, we first constructed a plasmid carrying a flaB promoter-driven ltpA. ltpA flanked by NdeI and AscI restriction sites was amplified using primers NdeI-priFX-CarDf and AscI-priFX-CarDr and cloned into pBSVG downstream of the flaB promoter. The NdeI and AatII fragment containing the flgB promoter-driven ltpA and the gentamycin-resistance cassette was cloned into the pOY112 inducible shuttle vector42, resulting in the shuttle vector used for complementation, pXW006.
Mouse infections
All tick-mouse experiments were approved by the IACUC committee of Indiana University School of Medicine under the protocol number #19792. Four-week-old C3H/HeN mice (Harlan, Indianapolis, IN) were subcutaneously inoculated with 1 × 105 spirochetes. For the mice infected with the complemented strain of the ltpA mutant, IPTG was given via IP injection (100 µl of 10 mM IPTG) every two days. The mice were killed, and ear, skin (inoculation site), bladder, heart, and joint tissues were collected 7 weeks after infection and cultivated in 2 ml of BSK-II medium (Sigma-Aldrich, St. Louis, MO) containing an antibiotic mixture of phosphomycin (2 mg/ml), rifampin (5 mg/ml), and amphotericin B (250 mg/ml) (Sigma-Aldrich, St. Louis, MO). All cultures were maintained at 37 °C and examined for the presence of spirochetes by dark-field microscopy beginning 5 days after inoculation. A single growth-positive culture was used as the criterion to determine the presence of mouse infection.
Microinjection of B. burgdorferi into nymphal ticks and mouse infection by B. burgdorferi
Microinjection and the tick-mouse experiments were approved by the IACUC committee of Indiana University School of Medicine under the protocol number #19792. I. scapularis nymphs were obtained from the Tick Rearing Facility at Oklahoma State University (Stillwater, OK). Microinjection was used to introduce spirochetes into the gut of I. scapularis nymphs as previously described34. Briefly, each B. burgdorferi variant was cultivated under normal conditions in BSK-II medium in the presence of corresponding selective antibiotics. Spirochetes were harvested by centrifugation and concentrated in BSK-II medium to a density of 3 × 108 spirochetes/mL. A total of 10 μL of the cell suspension was then loaded into a 1-mm diameter glass capillary needle (World Precision Instruments Inc., Sarasota, FL) using a microloader (Eppendorf AG, Hamburg, Germany). The bacterial suspension was then injected into the rectal aperture of unfed nymphal ticks using a FemtoJet microinjector system (Eppendorf AG, Hamburg, Germany). The parameters for injection were a pressure of 1000 hPa, injection time of 0.1 s, and a compensation pressure of 0 hPa, which delivered an average volume of 0.1 μl (~104 spirochetes). After microinjection, the ticks were placed on adult C3H/HeN mice (12 ticks per mouse), allowed to feed to repletion (4–5 days), and then collected for DNA extraction. Mouse infection was determined using the same procedure used during needle infection.
Extract DNA from ticks
DNA was isolated from engorged nymphs using the DNeasy® Blood & Tissue Kit B (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. Spirochete burdens within infected ticks were assessed by qPCR using primer pair qflaB-F/R and qTactin-F/R (Table SI). Absolute copy numbers of flab are quantified as spirochete loads in ticks.
Quantitative RT-PCR (qRT-PCR) and qPCR
RNA samples were extracted from B. burgdorferi cultures using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Three independent culture samples were used for each strain. The digestion of contaminating genomic DNA in RNA samples was performed using RNase-free DNase I (Promega, Madison, WI), and the removal of DNA was confirmed by PCR amplification of the B. burgdorferi flaB gene. cDNA was synthesized using SuperScript III reverse transcriptase with random primers (Invitrogen, Carlsbad, CA). To quantify the transcript levels of genes of interest, an absolute quantitation method was used to create a standard curve for the qPCR assay according to the manufacturer’s protocol (Strategene, La Jolla, CA). Briefly, the PCR product of the flaB gene served as a standard template. A series of tenfold dilutions (102–107copies/ml) of the standard template was prepared, and qPCR was performed to generate a standard curve by plotting the initial template quantity against the Ct values for the standards. The quantity of the targeted genes in the cDNA samples was calculated using their Ct values and the standard curve. The samples were assayed in triplicate using the ABI 7000 Sequence Detection System and GREEN PCR Master Mix (ABI, Pleasanton, CA). The levels of the target gene transcript were reported as per 100 copies of flaB.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
Acknowledgements
Funding for this work was partially provided by NIH grants AI083640 (to X.F.Y.), and the National Science Foundation of China 81171611, 81772229 (Y.L). This investigation was partially conducted in a facility with support from a research facilities improvement program grant, number C06 RR015481-01, from the National Center for Research Resources, NIH.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Yongliang Lou, Phone: 0577-86699200, Email: lyl@wmu.edu.cn.
X. Frank Yang, Phone: (317) 278-3330, Email: xfyang@iu.edu.
References
- 1.Galbis-Martinez M, Fontes M, Murillo FJ. The high-mobility group A-type protein CarD of the bacterium Myxococcus xanthus as a transcription factor for several distinct vegetative genes. Genetics. 2004;167:1585–1595. doi: 10.1534/genetics.104.029207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Moreno D, et al. CdnL, a member of the large CarD-like family of bacterial proteins, is vital for Myxococcus xanthus and differs functionally from the global transcriptional regulator CarD. Nucleic Acids Res. 2010;38:4586–4598. doi: 10.1093/nar/gkq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolas FJ, Cayuela ML, Martinez-Argudo IM, Ruiz-Vazquez RM, Murillo FJ. High mobility group I(Y)-like DNA-binding domains on a bacterial transcription factor. Proc. Natl Acad. Sci. USA. 1996;93:6881–6885. doi: 10.1073/pnas.93.14.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava DB, et al. Structure and function of CarD, an essential mycobacterial transcription factor. Proc. Natl Acad. Sci. USA. 2013;110:12619–12624. doi: 10.1073/pnas.1308270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stallings CL, et al. CarD is an essential regulator of rRNA transcription required for Mycobacterium tuberculosis persistence. Cell. 2009;138:146–159. doi: 10.1016/j.cell.2009.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallego-García A, et al. Structural insights into RNA polymerase recognition and essential function of Myxococcus xanthus CdnL. PLoS ONE. 2014;9:e108946. doi: 10.1371/journal.pone.0108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulten G, Sacchettini JC. Structure of the mtb CarD/RNAP β-Lobes complex reveals the molecular basis of interaction and presents a distinct DNA-binding domain for mtb CarD. Structure. 2013;21:1859–1869. doi: 10.1016/j.str.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur G, Dutta D, Thakur KG. Crystal structure of Mycobacterium tuberculosis CarD, an essential RNA polymerase binding protein, reveals a quasidomain‐swapped dimeric structural architecture. Protein. Struct. Funct. Bioinformatics. 2014;82:879–884. doi: 10.1002/prot.24419. [DOI] [PubMed] [Google Scholar]
- 9.Bae B, et al. CarD uses a minor groove wedge mechanism to stabilize the RNA polymerase open promoter complex. eLife. 2015;4:e08505. doi: 10.7554/eLife.08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flentie K, Garner AL, Stallings CL. Mycobacterium tuberculosis transcription machinery: ready to respond to host attacks. J. Bacteriol. 2016;198:1360–1373. doi: 10.1128/JB.00935-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warda AK, Tempelaars MH, Boekhorst J, Abee T, Groot MNN. Identification of CdnL, a putative transcriptional regulator involved in repair and outgrowth of heat-damaged Bacillus cereus spores. PLoS ONE. 2016;11:e0148670. doi: 10.1371/journal.pone.0148670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abee T, Wels M, de Been M, den Besten H. From transcriptional landscapes to the identification of biomarkers for robustness. Microb. Cell Fact. 2011;10:S9. doi: 10.1186/1475-2859-10-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev. Microbiol. 2011;65:479–499. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- 15.Caimano MJ, Drecktrah D, Kung F, Samuels DS. Interaction of the Lyme disease spirochete with its tick vector. Cell. Microbiol. 2016;18:919–927. doi: 10.1111/cmi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He M, et al. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 2011;7:e1002133. doi: 10.1371/journal.ppat.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostick JL, et al. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol. Microbiol. 2011;81:219–231. doi: 10.1111/j.1365-2958.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sultan SZ, et al. Analysis of the HD-GYP domain cyclic dimeric GMP phosphodiesterase reveals a role in motility and the enzootic life cycle of Borrelia burgdorferi. Infect. Immun. 2011;79:3273–3283. doi: 10.1128/IAI.05153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sultan SZ, Pitzer JE, Miller MR, Motaleb MA. Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophosphate in motility and virulence. Mol. Microbiol. 2010;77:128–142. doi: 10.1111/j.1365-2958.2010.07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caimano MJ, et al. Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infect. Immun. 2015;83:3043–3060. doi: 10.1128/IAI.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caimano MJ, et al. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect. Immun. 2011;79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuñé J, et al. The Leptospira interrogans lexA gene is not autoregulated. J. Bacteriol. 2005;187:5841–5845. doi: 10.1128/JB.187.16.5841-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drecktrah D, et al. The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathog. 2015;11:e1005160. doi: 10.1371/journal.ppat.1005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bugrysheva J, et al. Characterization of the stringent response and relBbu expression in Borrelia burgdorferi. J. Bacteriol. 2003;185:957–965. doi: 10.1128/JB.185.3.957-965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bugrysheva JV, et al. Characterization of the RelBbu Regulon in Borrelia burgdorferi reveals modulation of glycerol metabolism by (p)ppGpp. PLoS ONE. 2015;10:e0118063. doi: 10.1371/journal.pone.0118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pappas CJ, et al. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 2011;7:e1002102. doi: 10.1371/journal.ppat.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sze CW, et al. Study of the response regulator Rrp1 reveals its regulatory role in chitobiose utilization and virulence of Borrelia burgdorferi. Infect. Immun. 2013;81:1775–1787. doi: 10.1128/IAI.00050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak EA, Sultan SZ, Motaleb MA. The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi. Front. Cell Infect. Microbiol. 2014;4:56. doi: 10.3389/fcimb.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bontemps-Gallo S, Lawrence K, Gherardini FC. Two different virulence-related regulatory pathways in Borrelia burgdorferi are directly affected by osmotic fluxes in the blood meal of feeding Ixodes ticks. PLoS Pathog. 2016;12:e1005791. doi: 10.1371/journal.ppat.1005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XF, et al. Differential expression of a putative CarD-like transcriptional regulator, LtpA, in Borrelia burgdorferi. Infect. Immun. 2008;76:4439–4444. doi: 10.1128/IAI.00740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin T, et al. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS ONE. 2012;7:e47532. doi: 10.1371/journal.pone.0047532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louvel H, et al. Comparative and functional genomic analyses of iron transport and regulation in Leptospira spp. J. Bacteriol. 2006;188:7893–7904. doi: 10.1128/JB.00711-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuels DS, Mach K, Garon CF. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J. Bacteriol. 1994;176:6045–6049. doi: 10.1128/jb.176.19.6045-6049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawabata H, Norris SJ, Watanabe H. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 2004;72:7147–7154. doi: 10.1128/IAI.72.12.7147-7154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallego-García A, et al. Caulobacter crescentus CdnL is a non-essential RNA polymerase-binding protein whose depletion impairs normal growth and rRNA transcription. Sci. Rep. 2017;2017:43240. doi: 10.1038/srep43240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis E, Chen J, Leon K, Darst SA, Campbell EA. Mycobacterial RNA polymerase forms unstable open promoter complexes that are stabilized by CarD. Nucleic Acids Res. 2014;43:gku1231. doi: 10.1093/nar/gku1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rammohan J, Manzano AR, Garner AL, Stallings CL, Galburt EA. CarD stabilizes mycobacterial open complexes via a two-tiered kinetic mechanism. Nucleic Acids Res. 2015;43:3272–3285. doi: 10.1093/nar/gkv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunham-Ems SM, et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, et al. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 2010;6:e1001104. doi: 10.1371/journal.ppat.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang Z, Deka RK, Norgard MV. BosR (BB0647) controls the RpoN-RpoS regulatory pathway and virulence expression in Borrelia burgdorferi by a novel DNA-binding mechanism. PLoS Pathog. 2011;7:e1001272. doi: 10.1371/journal.ppat.1001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.