Abstract
Purpose
The aim of this study is to determine the involvement of the upper gastrointestinal system (GIS) in patients diagnosed with Crohn's disease (CD), ulcerative colitis (UC), and non-inflammatory bowel disease (IBD) and to compare their differences.
Methods
This study included patients aged between 2 and 18 years who underwent colonoscopy and esophagogastroduodenoscopy (EGD) for the first time due to the prediagnosis of IBD. In EGD, samples were taken from duodenum, antrum, corpus, and esophagus; and gastritis, duodenitis, and esophagitis were identified through histopathologic examination. The data gathered the ends of the research were compared between IBD with non-IBD groups and between CD-UC with non-IBD groups, and the presence of significant differences between groups were determined.
Results
In our study, 16 patients were diagnosed with CD, 13 with UC, 3 with undeterminate colitis, and 13 with non-IBD. In the histopathological examination of the groups, GIS involvement was found in 94.1% of patients diagnosed with IBD and in 38.5% of non-IBD patients. Moreover, the difference was found to be statistically significant (p=0.032). No significant difference was found between the CD and UC groups. Gastritis was mostly observed in 93.8% of CD-diagnosed patients, 76.8% of UC-diagnosed patients, 81.2% of IBD-diagnosed patients, and 38.5% of non-IBD-diagnosed patients. On the other hand, significant differences were found between CD and non-IBD groups (p=0.03), UC and non-IBD groups (p=0.047), and IBD and non-IBD groups (p=0.03).
Conclusion
The results of the study show that gastritis was highly observed in UC- and CD-diagnosed patients than in non-IBD-diagnosed patients.
Keywords: Crohn's disease, Child, Gastritis, Ulcerative colitis
INTRODUCTION
Inflammatory bowel disease (IBD) is a lifelong and uncontrolled inflammation of the intestinal tract. In general, ulcerative colitis (UC) and Crohn's disease (CD) constitute the two main subgroups of IBD. It is unclassified colitis or undeterminate colitis (IC) clinical, endoscopic, histological cases, as well as colectomy cases, which cannot be classified. In addition, 1% to 15% of IBD cases are classified as IC cases [1,2].
In spite of intensive research, the etiology of the disease is still unclear. The uncontrolled and dysregulated inflammation occurring due to intestinal barrier dysfunction, as a result of environmental factors in patients with genetic predisposition, is suspected [3].
For the diagnosis of IBD, the evaluation of the histological data, together with anamnesis and endoscopic and radiological findings, is important. The most effective and sensitive methods regarding the differential diagnosis between UC and CD are the endoscopic investigation and histological evaluation of the endoscopic biopsy material [4,5].
CD may emerge in any region of the gastrointestinal system (GIS). UC is usually localized in the colon; however, it may also cause a mild inflammation in the terminal ileum, which is called backwash ileitis. Recent studies have shown that both UC and CD may affect the upper GIS of children and adults [6,7,8,9,10,11]. Therefore, the endoscopic evaluation of the upper GIS is essential in the extent of IBD. The Porto study group by the European Society for Paediatric Gastroenterology Hepatology and Nutrition recommends esophagogastroduodenoscopy (EGD) in all children suspected with IBD, and they also recommend the conduction of multiple biopsies and histopathological investigations on the upper GIS, namely stomach, duodenum, and esophagus [12,13]. The aim of our study is to conduct a prospective evaluation on patients suspected with IBD and to determine the involvement of upper GIS in patients diagnosed with CD, UC, and non-IBD and to compare the differences between these diseases.
MATERIALS AND METHODS
This study included patients aged between 2 and 18 years who underwent colonoscopy and EGD for the first time due to the prediagnosis of IBD in the Pediatric Gastroenterology Endoscopy Unit of Akdeniz University from June 2015 to December 2015. This study received the approval of the ethics committee (No. 279/10-06-2015). The verbal and written consents of the families were obtained prior to the endoscopic and colonoscopic examinations. Patients using proton pump inhibitors (PPI), H2-receptor antagonists, and steroids were excluded from this study, as well those patients who refused to undergo EGD although they already had colonoscopy. Futhermore, patients diagnosed with other gastrointestinal diseases, such as celiac disease and maltoma, were excluded.
During the EGD, two biopsies were taken from antrum corpus and esophagus. The specimens were taken from macroscopically normal and inflamed mucosae. After formol fixation and paraffin wax embedding, all specimens were stained with hematoxylin-eosin. To increase the chance of diagnosis, all specimens were cut for more than two times so as to reduce the size up to 5 µ. The specimens were analyzed by the pathologists experienced in IBD. Helicobacter pylori results were obtained along with the screening of the histological examination and cultivation of the biopsy specimen.
For the diagnosis of CD, UC, and IC, the diagnosis criteria of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and of the Crohn's and Colitis Foundation of America, as determined through histopathological investigation of the biopsy conducted during the colonoscopy, were used [14].
For the diagnosis of the different types of gastritis, the updated diagnosis criteria of the Sydney System was used [15]. Chronic inactive gastritis is defined as the infiltration of plasma cells and lymphocytes in the lamina propria, with the absence of neutrophils and intra-epithelial lymphocytes. Chronic active gastritis is defined as the formation of abscess of any density in the base of the intra-epithelial neutrophils or chronic gastritis. H. pylori gastritis was diagnosed with the identification of the H. pylori microorganism. H. pylori negative chronic active gastritis is defined as the absence of the histopathological demonstration (Cell Marque, Rocklin, CA, USA) of H. pylori albeit the presence of histopathological characteristics of H. pylori infection [15,16,17]. Focal enhanced gastritis is defined as the coverage of the normal mucosa with lymphocytes, macrophages, plasma cells, and occasionally neutrophils in at least one foveola or gland [18,19]. Atrophic gastritis is a histopathologic entity characterized by chronic inflammation of the gastric mucosa with loss of gastric glandular cells and replacement by intestinal-type epithelium, pyloric-type glands, and fibrous tissue. Atrophy of the gastric mucosa is the endpoint of chronic processes [20]. These histopathological criteria do not always explain the diagnosis. Several samples taken in some patients may have more than one type of gastritis, especially in samples taken from antrum and corpus.
Active duodenitis is defined as the infiltration of the duodenal mucosa by neutrophils [15]. There may be presence of erosions, but the foveolas may not show dysplasia, which is a supporting finding for the diagnosis of peptic duodenitis or duodenopathy [21].
IBD can be diagnosed by comprehensive clinical findings and pathological findings. On the other hand, if clinic, endoscopic and radiological data were insufficient, IC was diagnosed. If the histopathological findings were not in line with IBD but were matching with diseases, such as infectious colitis, eosinophilic colitis, or nonspecific colitis, the patients were diagnosed with non-IBD instead. The records of the study were used for the comparison of IBD with non-IBD patients, CD with non-IBD patients, and UC with non-IBD patients, and for the evaluation of the statistical significance of the results as well.
Statistical analysis
Statistical analysis was done with the SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). The comparison of the data was done through Mann-Whitney U-test, chi-square test, and Fisher exact test. For the definitive statistical analysis of the data, mean±standard deviation for numeric variables and numbers and percentages for the categorical data were used. The significance level was set to p<0.05.
RESULTS
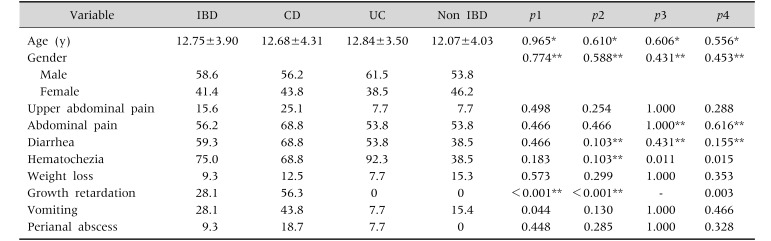
During our study, 57 patients with suspected IBD were evaluated. However, four patients due to refusal to EGD, four patients due to PPI usage, and one patient due to steroid usage were excluded. Furthermore, three patients dropped out of the study, because they were diagnosed with celiac disease after EGD. Ileocolonoscopy and gastroscopy were performed to all the remaining 45 patients. Out of 45 patients enrolled in our study, 16 of them were diagnosed with CD, 13 with UC, three with IC, and 13 with non-IBD. The non-IBD group consisted of patients with infectious colitis (eight patients), eosinophilic colitis (two patients), and nonspecific colitis (three patients). Of the patients, 58.6% of patients were males, and 41.4% were females. The male/female ratio was 1.4/1. The mean age of the patients was 12.41±3.96 years. The mean duration of the complaints in the non-IBD group was the longest, which was five months. Abdominal pain, as one of the complaints encountered during the visit, was most frequently observed in the CD group (68.8%); in addition, it was also observed in 56.2% of IBD patients and in 53.8% of UC and non-IBD patients. Abdominal pain at upper quadrants was the rarest complaint, and it was observed in 25.1% of CD patients, 15.6% of IBD patients, and in 7.7% (one patient) of UC and non-IBD patients. Diarrhea, growth retardation, and perianal abscess or fistula was most often observed in patients with CD, occurring in 68.8%, 56.3%, and 18.7% of CD patients, respectively. Hematochezia was most often observed in patients with UC (92.3%). Weight loss was most often observed in non-IBD patients (15.3%). The aforementioned percentages are presented in Table 1.
Table 1. Distribution of the Patients Regarding Their Demographic Characteristics and Presenting Symptoms.
Values are presented as mean±standard deviation, percent only, or p-value.
IBD: inflammatory bowel disease, CD: Crohn's disease, UC: ulcerative colitis.
p1: CD-UC, p2: CD-non IBD, p3: UC-non IBD, p4: IBD-non IBD.
*Mann Whitney U-test, **chi-square test, and Fisher exact test was performed for others.
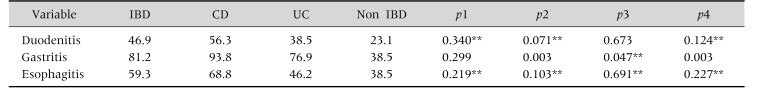
Upper GIS involvement was revealed in 94.1% of IBD patients and in 38.5% of non-IBD patients through histopathological examination. The difference between these two groups was statistically significant (p=0.032). The highest rate of duodenitis was found in the CD group (56.3%); and it was in 46.9% of IBD patients, 38.5% of UC patients, and 23.1% of non-IBD patients. The differences among the groups were not statistically significant. Similarly, the differences in esophagitis incidence among the groups were insignificant: 68.8% in CD, 59.3% in IBD, 46.2% in UC, and 38.5% in non-IBD groups.
Gastritis was most often observed in patients diagnosed with CD (93.8%). It was observed in 81.2% of IBD patients, 76.8% of UC patients, and 38.5% of non-IBD patients. Although there was no statistically significant difference between CD and UC groups, statistically significant differences were found between CD and non-IBD groups (p=0.03), UC and IBD groups (p=0.047), and IBD and non-IBD groups (p=0.03), as shown in Table 2.
Table 2. Patient Groups Divided according to Their Histopathological Results.
Values are presented as percent only or p-value.
IBD: inflammatory bowel disease, CD: Crohn's disease, UC: ulcerative colitis.
p1: CD-UC, p2: CD-non IBD, p3: UC-non IBD, p4: IBD-non IBD.
*Mann Whitney U-test, **chi-square test, and Fisher exact test was performed for others.
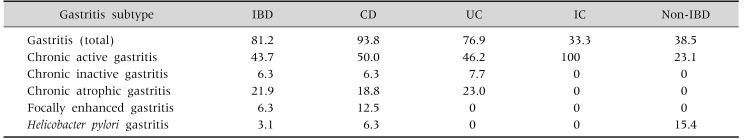
Regarding the gastritis subgroups, incidence of the chronic active gastritis was 50.0% in the CD group, 46.2% in the UC group, 43.7% in the IBD group, and 23.1% in the non-IBD group. The incidence of the chronic inactive gastritis was 6.3% in the IBD and CD groups and 7.7% (one patient) in the UC group. Focal enhanced gastritis was detected in two patients (12.5% in CD and 6.3% in IBD groups), and it was not observed in the UC and non-IBD groups. The highest incidence of the H. pylori gastritis was detected in the non-IBD group (15.4%), and H. pylori incidence was found in only one patient in the CD and IBD groups (6.3% and 3.12%, respectively) (Table 3).
Table 3. Distribution Analysis of the Gastritis Subtypes.
Values are pesented as percent.
IBD: inflammatory bowel disease, CD: Crohn's disease, UC: ulcerative colitis, IC: undeterminate colitis.
DISCUSSION
In recent years, EGD became an intervention regarded as necessary by many authors for the diagnosis of suspected IBD in children [7,8,9]. The results of our study confirmed this opinion and indicated that EGD is necessary for the diagnosis of suspected IBD in children.
In our study, we observed that upper GIS involvement was significantly higher in the IBD group as compared with the non-IBD group. However, only 15% of our patients had complaints regarding their upper intestinal system during their visits. Lemberg et al. [7] reported the involvement of upper GIS in 88.4% of their patients out of 86 children diagnosed with IBD. In the study conducted by Roka et al. [22], a statistically significant difference was found between IBD and non-IBD groups. Similarly, in a study by Hummel et al. [23], a significant difference between the IBD and non-IBD groups were found as well. In our study, we were able to find a significant difference between the IBD and non-IBD groups (94.1% and 38.5%, respectively); hence, the results of our study confirm the results of previous studies mentioned above.
The duodenitis incidence in the CD, UC, IBD, and non-IBD groups were 56.3%, 38.5%, 46.9%, and 23.1%, respectively. Although there was no significant difference, we observed a higher involvement of the CD group. Likewise, in the study of Kovacs et al. [24], the incidence of duodenitis in CD, UC, and non-IBD groups were 48%, 29%, and 31%, respectively, in which the highest incidence was found in the CD group. The results of this previous study are in line the results of our study.
The esophagitis incidence in the CD, UC, IBD, and non-IBD groups were 68.8%, 46.2%, 59.3%, and 38.5%, respectively, without any significant difference between the groups. The highest incidence found for the CD group. Thus, these findings are also in accordance with the results of Hummel et al. [23] and Sonnenberg et al. [25], in which no significant differences were detected as well.
According to the results of our study, the incidence of gastritis in the CD, UC, IBD, and non-IBD groups were 93.8%, 76.9%, 81.2%, and 38.5%, respectively. Although there was no significant difference between the CD and UC groups, the differences between CD and UC groups, UC and non-IBD groups, and IBD and non-IBD groups were statistically significant. These findings are in line with the results of the study that focused on gastritis and showed that the gastritis rates were 72.1% in CD, 69.2% in UC, and 1.4% in IC, which are significantly different from other groups [7]. They also conform with the results of the study reporting gastritis incidences of 64% in CD, 27% in UC, and 41% in non-IBD, with a significantly higher incidence [23]. Moreover, our results are also in line with the results of the study reporting gastritis incidences of 51% in CD, 33% in UC, with significantly higher rates as compared to 5% in non-IBD and also with the study reporting gastritis incidences of 69% in CD, 24% in UC, and 7% in IC, with significantly higher rates in CD and UC [23,24].
Although H. pylori negative chronic active gastritis, which is a subtype of gastritis, is encountered very rarely in a healthy population, it was observed much commonly in UC (30%) and CD patients (70%) [26]. Therefore, some investigators suggested that the presence of the H. pylori negative chronic active gastritis must be included in the definitive diagnosis of IBD [27,28,29]. The results of our study are in line with these data: the incidences of H. pylori negative chronic active gastritis were 43.7% in IBD, 50.0% in CD, 46.2% UC, and they were significantly higher in these groups as compared to the non-IBD group (23.1%).
In previous studies, the incidence of H. pylori gastritis is lower in the healthy population as compared to IBD group [30,31]. In our study, the incidence of H. pylori gastritis was 15.4% in non-IBD group, and the incidence of H. pylori gastritis was 3.1% and 6.3% in the IBD and CD groups, respectively. H. pylori gastritis was not observed in the UC group.
Numerous studies conducted on adults showed that focal enhanced gastritis (FEG) is relatively common in IBD patients [27]. The incidence of FEG, which differs among IBD patients from study to study, was up to 76% in CD patients and up to 20% in UC patients, and it was more often observed in adolescents rather than adults [25,32]. In our study on IBD patients, FEG was observed in 6.3% of CD and in 12.5% of UC patients and was not seen in non-IBD patients. These results conform to the literature.
In conclusion, the result of our study show that GIS involvement is more frequent in children diagnosed with IBD, CD, and UC than in children with non-IBD condition, and it was significantly shown that the incidence of gastritis was observed higher in UC and CD groups than in the non-IBD group.
References
- 1.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadithi M, Cazemier M, Meijer GA, Bloemena E, Felt-Bersma RJ, Mulder CJ, et al. Retrospective analysis of old-age colitis in the Dutch inflammatory bowel disease population. World J Gastroenterol. 2008;14:3183–3187. doi: 10.3748/wjg.14.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumberg RS, Strober W. Prospects for research in inflammatory bowel disease. JAMA. 2001;285:643–647. doi: 10.1001/jama.285.5.643. [DOI] [PubMed] [Google Scholar]
- 4.Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 5.Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod Pathol. 2003;16:347–358. doi: 10.1097/01.MP.0000064746.82024.D1. [DOI] [PubMed] [Google Scholar]
- 6.Tobin JM, Sinha B, Ramani P, Saleh AR, Murphy MS. Upper gastrointestinal mucosal disease in pediatric Crohn disease and ulcerative colitis: a blinded, controlled study. J Pediatr Gastroenterol Nutr. 2001;32:443–448. doi: 10.1097/00005176-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Lemberg DA, Clarkson CM, Bohane TD, Day AS. Role of esophagogastroduodenoscopy in the initial assessment of children with inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1696–1700. doi: 10.1111/j.1440-1746.2005.03954.x. [DOI] [PubMed] [Google Scholar]
- 8.Castellaneta SP, Afzal NA, Greenberg M, Deere H, Davies S, Murch SH, et al. Diagnostic role of upper gastrointestinal endoscopy in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004;39:257–261. doi: 10.1097/00005176-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Abdullah BA, Gupta SK, Croffie JM, Pfefferkorn MD, Molleston JP, Corkins MR, et al. The role of esophagogastroduodenoscopy in the initial evaluation of childhood inflammatory bowel disease: a 7-year study. J Pediatr Gastroenterol Nutr. 2002;35:636–640. doi: 10.1097/00005176-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88:995–1000. doi: 10.1136/adc.88.11.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hori K, Ikeuchi H, Nakano H, Uchino M, Tomita T, Ohda Y, et al. Gastroduodenitis associated with ulcerative colitis. J Gastroenterol. 2008;43:193–201. doi: 10.1007/s00535-007-2143-8. [DOI] [PubMed] [Google Scholar]
- 12.Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 13.Diefenbach KA, Breuer CK. Pediatric inflammatory bowel disease. World J Gastroenterol. 2006;12:3204–3212. doi: 10.3748/wjg.v12.i20.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America. Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 15.Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591–598. doi: 10.1155/2001/367832. [DOI] [PubMed] [Google Scholar]
- 16.Price AB. The Sydney system: histological division. J Gastroenterol Hepatol. 1991;6:209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 17.Hassan TMM, Al-Najjar SI, Al-Zahrani IH, Alanazi FIB, Alotibi MG. Helicobacter pylori chronic gastritis updated Sydney grading in relation to endoscopic findings and H. pylori IgG antibody: diagnostic methods. J Microsc Ultrastruct. 2016;4:167–174. doi: 10.1016/j.jmau.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrolla AA, Katz JA, Xin W. The clinical significance of focal enhanced gastritis in adults with isolated ileitis of the terminal ileum. J Gastroenterol. 2008;43:524–530. doi: 10.1007/s00535-008-2191-8. [DOI] [PubMed] [Google Scholar]
- 19.Parente F, Cucino C, Bollani S, Imbesi V, Maconi G, Bonetto S, et al. Focal gastric inflammatory infiltrates in inflammatory bowel diseases: prevalence, immunohistochemical characteristics, and diagnostic role. Am J Gastroenterol. 2000;95:705–711. doi: 10.1111/j.1572-0241.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 20.Yanaoka K, Oka M, Ohata H, Yoshimura N, Deguchi H, Mukoubayashi C, et al. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer. 2009;125:2697–2703. doi: 10.1002/ijc.24591. [DOI] [PubMed] [Google Scholar]
- 21.Day DW, Jass JR, Price AB, Shepherd NA, James M, Sloan JM, et al. Wiley InterScience (online service), editors Chronic ‘non specific’ duodenitis. In: Day DW, Morson BC, editors. Morson and Dawson's gastrointestinal pathology. 4th ed. Malden, MA: Blackwell Publishing; 2003. p. 308. [Google Scholar]
- 22.Roka K, Roma E, Stefanaki K, Panayotou I, Kopsidas G, Chouliaras G. The value of focally enhanced gastritis in the diagnosis of pediatric inflammatory bowel diseases. J Crohns Colitis. 2013;7:797–802. doi: 10.1016/j.crohns.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Hummel TZ, ten Kate FJ, Reitsma JB, Benninga MA, Kindermann A. Additional value of upper GI tract endoscopy in the diagnostic assessment of childhood IBD. J Pediatr Gastroenterol Nutr. 2012;54:753–757. doi: 10.1097/MPG.0b013e318243e3e3. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs M, Muller KE, Arato A, Lakatos PL, Kovacs JB, Varkonyi A, et al. Diagnostic yield of upper endoscopy in paediatric patients with Crohn's disease and ulcerative colitis. Subanalysis of the HUPIR registry. J Crohns Colitis. 2012;6:86–94. doi: 10.1016/j.crohns.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:39–44. doi: 10.1002/ibd.21356. [DOI] [PubMed] [Google Scholar]
- 26.Genta RM, Schuler CM, Lash RH. Helicobacter pylori-negative chronic active gastritis: a new entity or the result of widespread acid inhibition? Gastroenterology. 2008;134:858. [Google Scholar]
- 27.Kundhal PS, Stormon MO, Zachos M, Critch JN, Cutz E, Griffiths AM. Gastral antral biopsy in the differentiation of pediatric colitides. Am J Gastroenterol. 2003;98:557–561. doi: 10.1111/j.1572-0241.2003.07354.x. [DOI] [PubMed] [Google Scholar]
- 28.Ruuska T, Vaajalahti P, Arajärvi P, Mäki M. Prospective evaluation of upper gastrointestinal mucosal lesions in children with ulcerative colitis and Crohn's disease. J Pediatr Gastroenterol Nutr. 1994;19:181–186. doi: 10.1097/00005176-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Sharif F, McDermott M, Dillon M, Drumm B, Rowland M, Imrie C, et al. Focally enhanced gastritis in children with Crohn's disease and ulcerative colitis. Am J Gastroenterol. 2002;97:1415–1420. doi: 10.1111/j.1572-0241.2002.05785.x. [DOI] [PubMed] [Google Scholar]
- 30.el-Omar E, Penman I, Cruikshank G, Dover S, Banerjee S, Williams C, et al. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulphasalazine. Gut. 1994;35:1385–1388. doi: 10.1136/gut.35.10.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piodi LP, Bardella M, Rocchia C, Cesana BM, Baldassarri A, Quatrini M. Possible protective effect of 5-aminosalicylic acid on Helicobacter pylori infection in patients with inflammatory bowel disease. J Clin Gastroenterol. 2003;36:22–25. doi: 10.1097/00004836-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Prenzel F, Uhlig HH. Frequency of indeterminate colitis in children and adults with IBD - a metaanalysis. J Crohns Colitis. 2009;3:277–281. doi: 10.1016/j.crohns.2009.07.001. [DOI] [PubMed] [Google Scholar]