Abstract
Alginate-based hydrogels are widely used for the development of biomedical scaffolds in regenerative medicine. The use of sugar glass as a sacrificial template for fluidic channels fabrication within alginate scaffolds remains a challenge because of the premature dissolution of sugar by the water contained in the alginate as well as the relatively slow internal gelation rate of the alginate. Here, a new and simple method, based on a sugar glass fugitive ink loaded with calcium chloride to build sacrificial molds, is presented. We used a dual calcium cross-linking process by adding this highly soluble calcium source in the printed sugar, thus allowing the rapid gelation of a thin membrane of alginate around the sugar construct, followed by the addition of calcium carbonate and gluconic acid δ-lactone to complete the process. This innovative technique results in the rapid formation of "on-demand" alginate hydrogel with complex fluidic channels that could be used in biomedical applications such as highly vascularized scaffolds promoting pathways for nutrients and oxygen to the cells.
Keywords: Biomedical engineering, Materials science
1. Introduction
Hydrogels, such as alginate (ALG), are widely used in tissue engineering applications, mostly to create 3D scaffolds incorporating cells and other biological materials [1]. Their high fraction of water makes them similar to soft tissues, which is expected to have a good tolerance in the body. Their properties (pore sizes, gelation time, etc.) can be managed to ensure good oxygen and nutrients diffusion within the porous engineered tissue [2]. The use of ALG for soft tissue engineering is appropriate. However, one major issue arising when creating and seeding 3D scaffolds is that, for the cells to proliferate and function adequately, vascular networks are needed in the bulk and thick material to ensure adequate cell oxygenation and nutrient supply [3, 4, 5]. Without this efficient supply, necrosis is prone to occur [2]. Channels within ALG scaffolds can also be needed to control the differentiation process of stem cells through the seeding of appropriate growth factors [6, 7].
A way to achieve such network is the use of sacrificial constructs embedded within hydrogels upon casting. Few alternatives are available to produce those networks, both in terms of manufacturing method and sacrificial material used. For example, Andrew et al. used gelatin as a sacrificial mold for the creation of microfluidics networks in hydrogels [8]. The cavities were formed by heating and flushing the gelatin after the hydrogel molding [8]. Meanwhile, Bellan et al. demonstrated a technique using sugar fibers for creating capillary-like vessels using polydimethylsiloxane [9]. Lee et al. created micro networks in gelatin using thermoresponsive poly(N-isopropylacrylamide) microfibers. These microfibers are soluble in aqueous mediums at a temperature below 32 °C, which makes structure removal easier, while avoiding the use of cytotoxic solvents that might harm the cells [3, 7]. Also taking advantage of the temperature properties, Kolesky et al. used a 3D printing technique to build constructs made of Pluronic F127 in a hydrogel reservoir. The Pluronic F127 acts as a popular fugitive ink since it can be removed once the hydrogel is solid by cooling down the structure below 4 °C [4, 10, 11]. Gao et al. also used 3D bioprinting to print hollow filaments made from ALG. This technique uses a dual-ink coaxial nozzle simultaneously extruding a bioink in the external shell nozzle and a fugitive ink in the core nozzle [12]. The bioink was made from ALG combined to extracellular matrix loaded with Atorvastatin and seeded with endothelial progenitor cells. The fugitive ink was a mix of Pluronic F127 and CaCl2 solution. This allows 3D printing of cell seeded alginate tubes, and a significant amount (80%) of cells were found to still be viable after printing. However, this method does only allow printing of a single tube geometry. In the pursuit of optimal nutrients and metabolic by-products convection and exchange, and from a drug delivery perspective, branch junctions allowing more elaborate three-dimensional networks constitute a necessary objective [13], and this is not currently possible with this dual-ink coaxial bioprinting method.
Several research groups also used carbohydrate glass as a fugitive ink in an additive manufacturing process that allowed 3D printing of complex self-supporting constructs [6, 7, 14]. Among others, Miller et al. used carbohydrate to print tridimensional structures, that were subsequently cast with cell-laden hydrogels [7]. The use of carbohydrate-based mixtures (e.g. glucose, sucrose) to build these constructs provides many advantages, among which the possibility to print without having to lay any supporting material, thus allowing complex 3D structures with self-supporting branches [14]. Further advantages of this material are its limited impact on cell viability, and its solubility in aqueous solutions. These advantages facilitate the dissolution of the printed structures without the need of cytotoxic solvents. Neither are they performed in extreme conditions that could be harmful to the cells [4, 7, 14]. However, the latter advantage becomes a problem when a hydrogel such as ALG to be casted around a sugar glass structure to form a network. The gelation of the hydrogel requires a certain time, which, combined with the presence of a large amount of water in the gel, leads to an early dissolution of the sugar glass network. This results in the inability of the method to produce inner networks, maintaining their integrity, while the initial sugar glass network was sharp and precise. Miller et al. got around this challenge by coating the sugar glass constructs with poly(D-lactide-co-glycolide), which isolated the sacrificial material from the bulk material, but added an additional step [7]. With this technique, they showed cells activity around perfused channel and around gel slab, by using PEG hydrogels (with and without channels) that had laid three days in culture, and were seeded with cells of interest. Gels without channels showed cells activity only around the outer perimeter of the plain slab, while gels with channels showed cells activity on the perimeter and around the channels [7].
In this paper, we report a new method to overcome the issue of the premature dissolution of the sugar glass constructs used as temporary molds for the construction of 3D alginate scaffolds. We used a sacrificial 3D-printed construct made from a sugar glass fugitive ink loaded with CaCl2 to fabricate ALG scaffolds with small fluidic channels, without the need to use a preventive biopolymer coating. We exploited both the external and the internal gelation of ALG scaffold using a quick crosslinking process by incorporating CaCl2 within the sugar glass mixture, and a slow internal gelation process using calcium carbonate (CaCO3) nanoparticles in the ALG solution (Fig. 1). Our approach was by skillfully adding highly soluble CaCl2 into the syrup mixture so that, upon dissolution of sugar, a high amount of Ca2+ ions would be released in the ALG solution, thus forming a local and temporary membrane that ensured the integrity of fluidic channels.
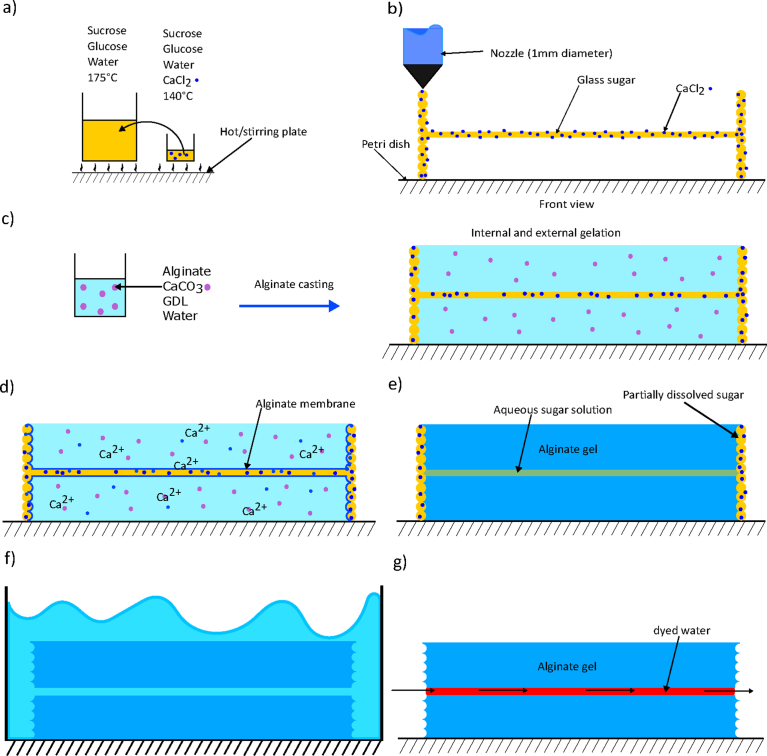
Fig. 1.
Schematic procedure. a) Sugar recipe preparation, b) 3D printing of the sugar glass construct loaded with calcium chloride, c) Preparation and casting of the alginate mixture, containing CaCO3 and GDL, (t = 0), d) Immediately after casting the alginate mixture, formation of a membrane at the sugar/ALG interface due to the release of the Ca2+ ions from the CaCl2 contained in the fugitive ink, (t = 0+), e) Completed internal gelation of alginate, (t = 600 s), f) Dissolution of the remaining sugar glass template in a demineralized water bath, g) Perfusion of the alginate gel with red food dye.
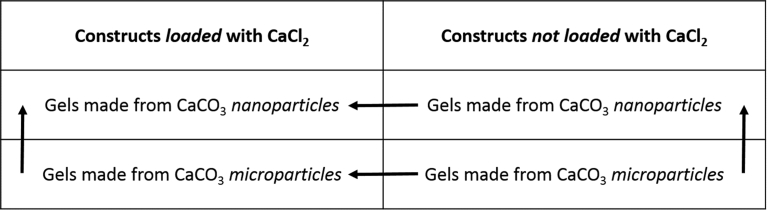
It is already known that the use of nanoparticles of CaCO3 instead of microparticles increases the gelation rate of alginate [15]. Fabrication of thin channels into the scaffold was possible because of the fast gelation induced by CaCl2 releasing Ca2+ ions as soon as the sugar began to dissolve creating a thin hydrogel membrane [16]. The gel formation rate depends on the availability of the ion Ca2+, and therefore on the capacity of a Ca2+ source to quickly dissolve once placed in aqueous conditions. Lee et al. compared the gelation kinetic of alginate with different calcium sources (calcium chloride, calcium lactate and calcium gluconate) and showed that CaCl2 provided the highest gelation rate [16]. Considering our application, CaCl2 was the best choice. On the other hand, internal gelation was obtained by the slow release of Ca2+ ions by CaCO3 nanoparticles. Since the final structure of gels obtained by external gelation alone did not provide strong, uniform and mechanically stable gels, internal gelation with the addition of CaCO3 nanoparticles combined with gluconic acid δ-lactone (GDL) was a relevant choice [17]. After short period of time, a robust gel was formed with regards to the integrity of the internal network. Our results were also compared with gels obtained from CaCO3 microparticles, where the concentration was increased, but the same GDL:CaCO3 molar ratio was kept. It resulted in a substantial decrease of the gelation rate. From these experiments, it was found that the CaCl2 loaded constructs helped in keeping the channels integrity and that the use of CaCO3 nanoparticles decreased the gelation rate. The Fig. 2 shows a schematic representation of the experiments to allow a better comprehension. The vertical arrows point at the increasing gelation rate and the horizontal arrows point at the gel that retains the best channel integrity.
Fig. 2.
Schematic representation of the comparison between the four constructs printed. The vertical arrows point at the increasing gelation rate and the horizontal arrows point at the gel that retains the best channel integrity.
2. Materials and methods
2.1. Materials
Sucrose (crystalline), Dextrose D-glucose (anhydrous) and Calcium carbonate microparticles (CaCO3, powder, certified ACS) were bought from Fisher Scientific. Calcium chloride (CaCl2, anhydrous), sodium alginate (ALG, M/G ratio = 1.56), and D-(+)-gluconic acid δ-lactone (GDL), were obtained from Sigma-Aldrich. Calcium carbonate nanoparticles (CaCO3, 15–40 nm, 97.5%) were obtained from Skyspring Nanomaterials. Demineralized water (H2O) was used in all experiments. All chemicals were used as received.
2.2. Sugar glass mixture containing CaCl2
The preparation of the main sugar glass mixture allowing good printability of the constructs was inspired from a previously reported methodology [7]. At first, sugar mix was prepared by dissolving 53 g of sucrose and 25 g of glucose in 50 ml of demineralized water. It was then stirred on a hot/stirring plate at 400RPM, and heated to 175 °C, leading to the production of a yellow syrup. The syrup was removed from the heating plate to cool down and then maintained at 130 °C. Meanwhile, a second sugar mixture was prepared by dissolving 0.15 g of calcium chloride, 2.5 g of sucrose, and 0.6 g of glucose in 4 ml of water, followed by stirring at 200RPM, and heating to 140 °C. Once the second mixture had reached 140 °C, both mixtures were mixed together and stirred to produce a homogeneous syrup. The final sugar syrup was then put into a preheated 20 ml glass syringe, and loaded in the printing head. A sugar construct without the addition of CaCl2 was prepared following the same procedure, using the main sugar-glass recipe alone.
2.3. Sugar 3D printing
As described in Bégin-Drolet et al., a commercial 3D printer (Airwolf 3D XL, USA) with a custom printing head was used to print the complex 3D sacrificial sugar glass structure [14]. The printing head maintained the glass syringe at 110 °C, while the extrusion nozzle of 1 mm of diameter was maintained at 85 °C. Two cooling nozzles were also used to rapidly cool the extruded filament, making possible the extrusion self-supporting filaments. The printer was operated using G-code commands, programmed with the Repetier-Host software (version 1.5.6), which allowed precise control of displacements, extrusion rate, temperatures, delays and air-cooling. An Arduino Mega2560 micro-controller was used to interpret commands and execute proper actions on the printer, using Marlin software. As the printer received the commands of displacements and extrusion, the syringe piston was moved at a specified rate, which extruded the syrup out of the syringe by the nozzle to form a small and uniform filament. The diameter of this filament can easily be controlled by modifying the extrusion rate or the velocity of the printing head [14]. Using the technique described above, 4 sugar glass constructs were printed, with overall dimensions of 47 mm length, 17 mm width and 13.2 mm depth. The wall thickness of the constructs was 2 mm, allowing an internal volume of approximately 8.9 ml. Two supporting branches were printed, to ensure the printability of the network itself, and then cut from every structure before the alginate casting.
2.4. Alginate scaffolds preparation
Sodium alginate was dissolved in water to prepare a solution of 4%w/v, and stirred on a hot/stirring plate for 24 hours to allow complete dissolution (ALG solution). Then, 1 ml of a 1 M of CaCO3 nanoparticles in water was added to 18 ml of the ALG solution. The solution was vigorously stirred for 20 seconds to homogenously disperse the CaCO3 nanoparticles. Finally, 2 ml of a 1 M of D-(+)-gluconic acid δ-lactone was quickly added to the ALG-CaCO3 mixture, and well stirred for 20 seconds (final alginate concentration: 3.43% w/v). The resulting solution was then carefully poured into one sugar glass construct loaded with CaCl2 and one sugar glass construct without CaCl2, ensuring no overflow. At least 10 minutes is required to the mixture to form a solid gel. ALG gel was then placed in an ultrasonic bath to free the solid alginate casting by dissolving the residual sugar mold. The remaining alginate structures were placed in a water bath at ambient temperature for approximately 18 hours to let the complete the gelation process, giving transparent robust gels, and prevent dehydration. The fabrication of ALG gels using CaCO3 microparticles was done using the same methodology, but with 1 ml of a 3 M CaCO3 microparticles solution and 3 ml of a 2 M GDL solution, resulting in a 3.27%w/v ALG gel, to compare the effect of the nano-versus microparticles of CaCO3. The same GDL: CaCO3 molar ratio was kept, but the amount of each component was increased in order to obtain a decent gelation time.
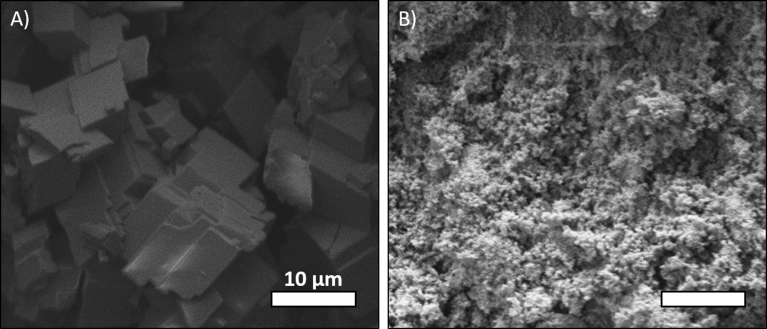
To allow a better understanding of the CaCO3 microparticle versus nanoparticle average size and size distribution, scanning electron microscopy (SEM) images were generated and are presented on the Fig. 3. As seen, there is a substantial difference between the microparticles and nanoparticles sizes. Thus, different crosslinking kinetic of the alginate hydrogel is expected because of the high surface area of the nanoparticles relative to the microparticles.
Fig. 3.
A) SEM image of the microparticles of CaCO3. B) SEM image of the nanoparticles of CaCO3. Scanning electron microscopy (SEM, Q250) was operated at 7.5 kV using a magnification of 2000.
3. Results and discussion
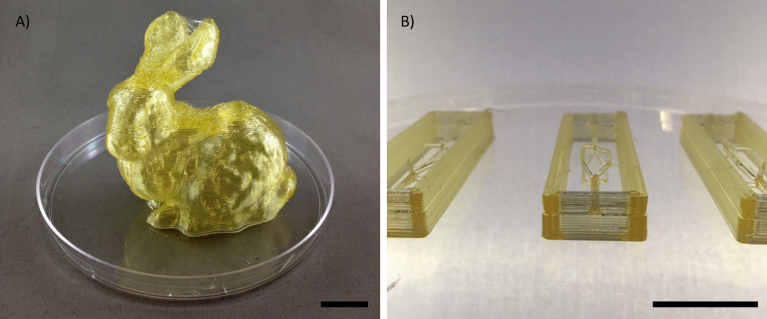
Sugar glass printing allows very precise fabrication of various 3D constructs (Fig. 4). In this work, as proof-of-concept, a 3-branch sugar glass structure, as shown in Fig. 5B, was prepared and used as sacrificial mold for alginate scaffold fabrication. The structure was supported by a sugar glass enclosure acting as the outer bound of the casting, and two supporting branches (Fig. 5A) that were cut from the mold prior to casting the ALG solution.
Fig. 4.
A) Stanford bunny. B) 4-branches sugar glass constructions. Scale bar: 10 mm.
Fig. 5.
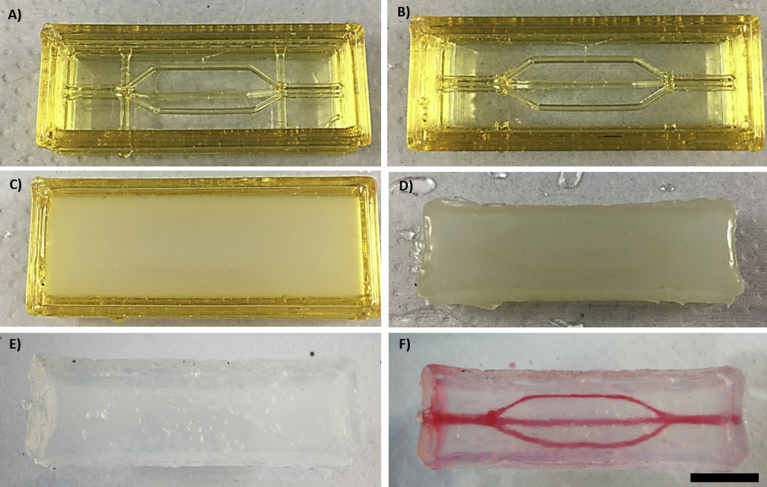
A) Sugar glass construct (loaded with CaCl2 with two supporting branches. B) Sugar glass construct (loaded with CaCl2) without the supporting branches. C) Sugar glass construct (loaded with CaCl2) filled with ALG. D) Remaining ALG gel after 10 minutes of gelation and 100 seconds of sugar dissolution in an ultrasound bath. E) Remaining alginate gel after approximately 18 hours in a demineralized water bath. F) Alginate gel immediately after the perfusion with red-dyed water. Scale bar 10 mm.
Gelation of alginate scaffold using well-known poor soluble CaCO3 takes time (>10 min) due to the slow hydrolysis rate of GDL in water which releases calcium ions under acidification [18]. Under such conditions, small filaments of sugar are completely dissolved before full gelation, hence preventing the alginate scaffold from having fluidic channels that maintain the integrity of the design. To overcome the premature dissolution of the sugar glass construct, one can either increase the gelation of alginate or delay the dissolution of sugar. To increase the gelation rate, it is known that the use of CaCO3 nanoparticles instead of microparticles is more efficient, presumably owed to the more enlarged contact surface of the particles [15]. The use of microparticles of CaCO3 allows a slow kinetic of gelation, leading to an accumulation of these microparticles at the bottom of the gel and a dissolution of the sugar network before the complete gelation. Therefore, we combined the use of CaCO3 nanoparticles and GDL (for internal gelation) with the use of CaCl2 (for external gelation). The integrity of fluidic channels was ensured by adding highly soluble CaCl2 into the syrup mixture. Thus, a high amount of Ca2+ ions was released in the ALG solution, forming a local and temporary membrane. However, this thin membrane alone was not sufficient to make a strong gel. Consequently, the addition of CaCO3-GDL to the ALG solution had the advantage to strengthen the gel within 10 minutes. Fig. 5C and D show the incorporation of the alginate solution into the sugar mold and the final product (unmold alginate scaffold) respectively. To show the success of small fluidic channels, a red dye was added to water and used to irrigate the fluidic network (Fig. 5E and F). The perfusion was performed using a 30 ml plastic syringe filled with colored water, coupled to a 2 mm luer lock cannula inserted into the inlet port of the gel. A gentle action of the piston was used to irrigate the channels with colored water. With the results presented above, it is clear that the combination of the internal and external gelation of alginate assists the formation of micro channels from a sugar glass based fugitive ink in alginate scaffold.
To analyse the effect of the calcium chloride on the channels formation, we also printed a sugar glass construct made from the main syrup recipe alone (without CaCl2), filled the construct with the ALG mixture, and let it gel. We then perfused the gel with red food-dyed water. The results are shown in Fig. 6. One of the three branches of the construct clearly dissolved itself before the complete gelation, since it is not irrigated with colored water (Fig. 6D). Furthermore, the leak at the bottom of the construct caused a drop in the alginate level and might have suppressed the sugar filament that was near the top of the construct. Therefore, the addition of calcium chloride helps to keep the integrity of the network.
Fig. 6.
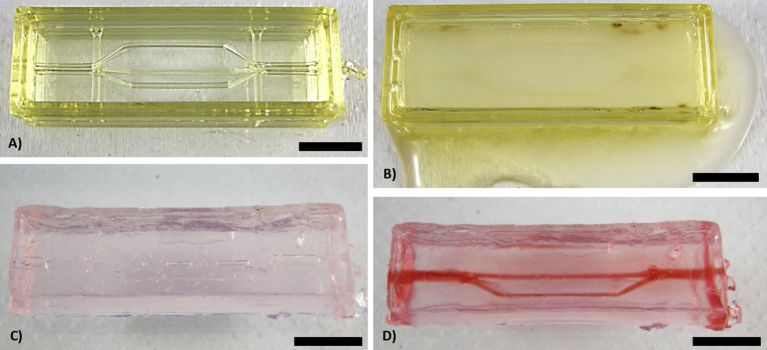
Verification of the CaCl2 impact on the channel formation. A) Sugar-glass construct not loaded with CaCl2. B) Alginate casting (with CaCO3 nanoparticles), t = 60 seconds. It is visible that the water contained in the alginate rapidly dissolved the sugar construct, leading to the leaking of the alginate at the bottom of the construct. C) Alginate gel after complete dissolution of the remaining sugar construct and complete gelation, t = 18 hours. D) Perfusion of the micro channels. One of the three intended branches is not present, which suggests that the sugar entirely dissolved itself before the complete gelation of the alginate. Scale bar: 10 mm.
To assess the impact of the nano-versus microparticles of CaCO3 in the channels formation, we printed two other sugar glass structures, one loaded with CaCl2, and the other one without CaCl2. We compared the gelation time of the gels obtained from CaCO3 microparticles to the gels obtain from CaCO3 nanoparticles. Then we compared the resulting gels made with CaCO3 microparticles, one obtained from a construct loaded with CaCl2 to another one obtained from a construct not loaded with CaCl2. Using the same casting procedure, we filled the two structures with the alginate mixture containing 1 ml of a 3 M of CaCO3 microparticles in solution and 3 ml of 2 M of a GDL solution. The alginate gelation time observed for the gels obtained from CaCO3 microparticles was approximately 25 minutes, which is longer compared to the gelation time of the gels obtained from CaCO3 nanoparticles. And this, even though the calcium concentration was increased. The remaining gels were firm, but contained lots of air bubbles, widening while the gelation process, thus, leaving non-uniform gels.
The resulting gel from CaCO3 microparticles and a CaCl2 loaded construct was firm and there were channels remaining in the material. The Fig. 7 (A–F) shows that almost no leakage occurred during the gelation process. The Fig. 7G and H show the alginate gel before and during the network's irrigation.
Fig. 7.
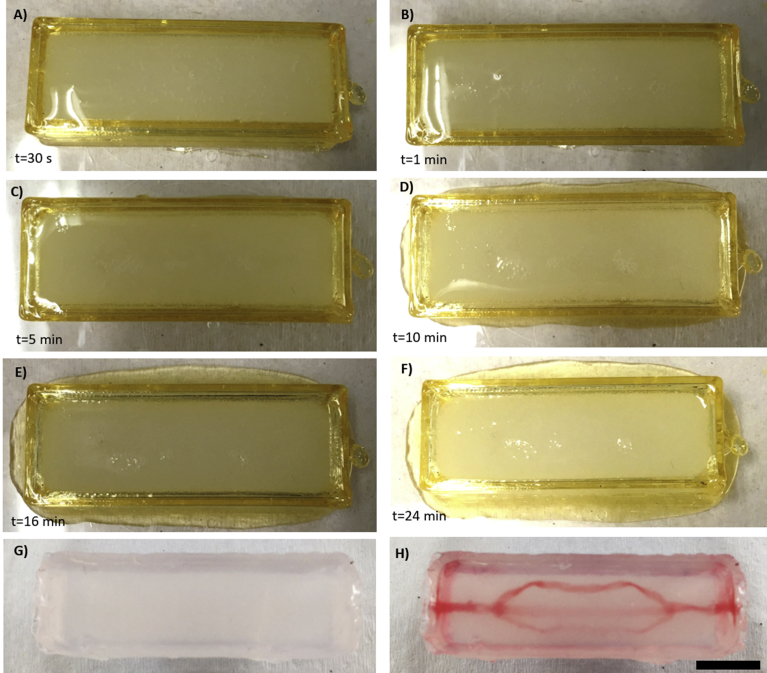
Time laps of the gelation process in a sugar-glass construct loaded with CaCl2. From A) through F), almost no leaking occurred. G) and H) represent respectively the gel before (after 18 h spent in a demineralized water bath) and immediately after the perfusion with red-dyed water. Scale bar: 10 mm.
For the gel obtained from CaCO3 microparticles and a construct not loaded with CaCl2, it is clear that the water contained in the alginate dissolved the sugar print. It is shown in the Fig. 8 that the gel leaked at the bottom of the sugar construct, lowering the alginate level, exposing the two upper filaments. Thus, none of them remained in the final solid gel. After 21 minutes, all channels were dissolved and the almost all the gel had leaked out of the sugar construct. Thus, no perfusion could be performed on this gel.
Fig. 8.
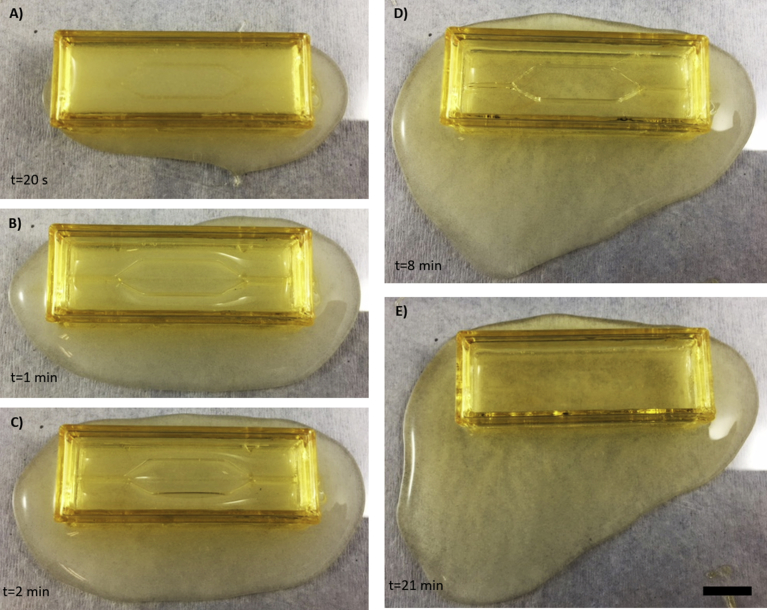
Time laps of the gelation process in a sugar-glass construct not loaded with CaCl2. From A through E, it is possible to see that the alginate mixture is leaking at the bottom of the construct over time (from 20 seconds to 21 minutes). Between 8 and 21 minutes (D) and (E), the sugar network completely dissolved itself, leaving only the outer walls. Thereby, for an alginate recipe requiring a certain laps of time, it is not possible to obtain intrinsic networks within an alginate scaffold. Scale bar: 10 mm.
Although increasing the Ca2+ ions concentration, or using CaCO3 nanoparticles increases the gelation rate, a slower rate provides more uniform gels in term of porosity and mechanical properties [17]. Moreover, in a cell seeding perspective, the amount of calcium in contact with the cells must not be too large not to harm the cells and the porosity must be controlled, to allow an adequate oxygen and nutriments diffusion through the engineered tissue. This is why the combination of the external and internal gelation of alginate is a good option, preventing the loss of the channels integrity and resulting in a strong and homogenous gel.
Finally, it is critical to mention the importance of incorporating the CaCl2-loaded syrup separately. It has been experimented here, that otherwise, dissolving the CaCl2 in the water of the main syrup recipe, calcium ions change the glass-transition temperature of the sugar, resulting in a low viscous syrup, which is harder to print, yielding sugar glass structures that are not self-supporting.
4. Conclusion
In many tissue engineering applications involving the use of a hydrogel scaffold, the inclusion of a vascularised network may constitute a significant advantage to ensure adequate oxygen and nutrient supply to the cells. Obtaining such networks using sugar-glass 3D printing is promising, but one of the main limitations is the premature sugar dissolution, which occurs before the complete gelation of the alginate hydrogel. The novelty of this study lays in the development of a technique involving the addition of CaCl2 in the fugitive ink recipe to overcome this problem by increasing the alginate gelation rate locally. The fast gelation rate of alginate in contact with Ca2+ ions from CaCl2 allows the quick formation of a thin membrane around the filaments, through external gelation, which give time to the remaining alginate to gel, through internal gelation, without losing the integrity of the network. As a proof of concept, we fabricated alginate gels with embedded channels from a sugar glass construct loaded with CaCl2. This new technique could also be applied to any other fugitive ink chemically compatible with CaCl2. Likewise, for scaffold materials other than hydrogels, other chemical accelerators could be included to fugitive inks. Further studies will imply the combination of this innovative technique and cell seeded alginate scaffolds to demonstrate the complete potential of this method.
Declarations
Author contribution statement
Gabrielle Gauvin-Rossignol: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Philippe Legros: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jean Ruel: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Marc-André Fortin: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
André Bégin-Drolet: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by NSERC.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Lee K.Y., Mooney D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J., Zheng H., Poh P.S.P., Machens H.G., Schilling A.F. Hydrogels for engineering of perfusable vascular networks. Int. J. Mol. Sci. 2015;16:15997–16016. doi: 10.3390/ijms160715997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J.B., Wang X., Faley S., Baer B., Balikov D.A., Sung H.J., Bellan L.M. Development of 3D microvascular networks within gelatin hydrogels using thermoresponsive sacrificial microfibers. Adv. Healthc. Mater. 2016;5:781–785. doi: 10.1002/adhm.201500792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulsen S.J., Miller J.S. Tissue vascularization through 3D printing: will technology bring us flow? Dev. Dyn. 2015;244:629–640. doi: 10.1002/dvdy.24254. [DOI] [PubMed] [Google Scholar]
- 5.Jain R.K., Au P., Tam J., Duda D.G., Fukumura D. Engineering vascularized tissue. Nat. Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 6.Sooppan R., Paulsen S.J., Han J., Ta A.H., Dinh P., Gaffey A.C., Venkataraman C., Trubelja A., Hung G., Miller J.S., Atluri P. In vivo anastomosis and perfusion of a three-dimensionally-printed construct containing microchannel networks. Tissue Eng. Part C Meth. 2016;22:1–7. doi: 10.1089/ten.tec.2015.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller J.S., Stevens K.R., Yang M.T., Baker B.M., Nguyen D.-H.T., Cohen D.M., Toro E., Chen A.A., Galie P.A., Yu X., Chaturvedi R., Bhatia S.N., Chen C.S. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden A.P., Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 9.Bellan L.M., Singh S.P., Henderson P.W., Porri T.J., Craighead H.G., Spector J.A. Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter. 2009;5:1354. [Google Scholar]
- 10.Kolesky D.B., Truby R.L., Gladman A.S., Busbee T.A., Homan K.A., Lewis J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 11.Wu W., Deconinck A., Lewis J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011;23:178–183. doi: 10.1002/adma.201004625. [DOI] [PubMed] [Google Scholar]
- 12.Gao G., Lee J.H., Jang J., Lee D.H., Kong J.S., Kim B.S., Choi Y.J., Jang W.B., Hong Y.J., Kwon S.M., Cho D.W. Tissue engineered bio-blood-vessels constructed using a tissue-specific bioink and 3D coaxial cell printing technique: a novel therapy for ischemic disease. Adv. Funct. Mater. 2017;27:1–12. [Google Scholar]
- 13.Sherman T.F. On connecting large vessels to small. The meaning of Murray's law. J. Gen. Physiol. 1981;78:431–453. doi: 10.1085/jgp.78.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bégin-Drolet A., Dussault M.A., Fernandez S.A., Larose-Dutil J., Leask R.L., Hoesli C.A., Ruel J. Design of a 3D printer head for additive manufacturing of sugar glass for tissue engineering applications. Addit. Manuf. 2017;15:29–39. [Google Scholar]
- 15.Paques J.P., Sagis L.M.C., van Rijn C.J.M., van der Linden E. Nanospheres of alginate prepared through w/o emulsification and internal gelation with nanoparticles of CaCO3. Food Hydrocoll. 2014;40:182–188. [Google Scholar]
- 16.Lee P., Rogers M.A. Effect of calcium source and exposure-time on basic caviar spherification using sodium alginate. Int. J. Gastron. Food Sci. 2012;1:96–100. [Google Scholar]
- 17.Kuo C.K., Ma P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 18.Liu G., Zhou H., Wu H., Chen R., Guo S. Preparation of alginate hydrogels through solution extrusion and the release behavior of different drugs. J. Biomater. Sci. Polym. Ed. 2016;27:1808–1823. doi: 10.1080/09205063.2016.1237452. [DOI] [PubMed] [Google Scholar]