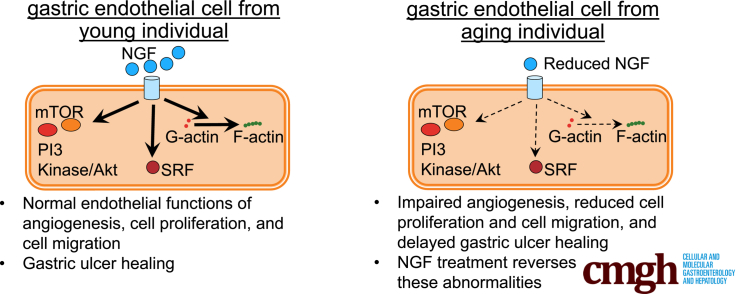
Abstract
Background & Aims
Aging gastric mucosa has increased susceptibility to injury and delayed healing owing to impaired angiogenesis, but the mechanisms are not fully known. We examined whether impairment of angiogenesis in aging gastric mucosa is caused by deficiency of nerve growth factor (NGF) in gastric endothelial cells (ECs), and whether NGF therapy could reverse this impairment.
Methods
In gastric mucosal ECs (GECs) isolated from young and aging rats we examined the following: (1) in vitro angiogenesis, (2) NGF expression, and (3) the effect of NGF treatment on angiogenesis, GEC proliferation and migration, and dependence on serum response factor. In in vivo studies in young and aging rats, we examined NGF expression in gastric mucosa and the effect of NGF treatment on angiogenesis and gastric ulcer healing. To determine human relevance, we examined NGF expression in gastric mucosal biopsy specimens of aging (≥70 y) and young (≤40 y) individuals.
Results
In cultured aging GECs, NGF expression and angiogenesis were reduced significantly by 3.0-fold and 4.1-fold vs young GECs. NGF therapy reversed impairment of angiogenesis in aging GECs, and serum response factor silencing completely abolished this response. In gastric mucosa of aging rats, NGF expression in GECs was reduced significantly vs young rats. In aging rats, local NGF treatment significantly increased angiogenesis and accelerated gastric ulcer healing. In aging human subjects, NGF expression in ECs of gastric mucosal vessels was 5.5-fold reduced vs young individuals.
Conclusions
NGF deficiency in ECs is a key mechanism underlying impaired angiogenesis and delayed ulcer healing in aging gastric mucosa. Local NGF therapy can reverse these impairments.
Keywords: Nerve Growth Factor, Angiogenesis, Endothelial Cells, Aging, Gene Therapy, Ulcer Healing
Abbreviations used in this paper: Akt, serine threonine kinase signaling protein; BrdU, bromodeoxyuridine; EC, endothelial cell; FITC, fluorescein isothiocyanate; GEC, gastric mucosal microvascular endothelial cells isolated from rats; GU, gastric ulcer; LV-GFP, lentiviral green fluorescent protein; LV-NGF, lentiviral nerve growth factor; mRNA, messenger RNA; mTOR, mammalian target of rapamycin; NGF, nerve growth factor; NSAID, nonsteroidal anti-inflammatory drug; PBS, phosphate-buffered saline; PCNA, proliferating cell nuclear antigen; PCR, polymerase chain reaction; PI3, phosphoinositide-3; siRNA, small interfering RNA; SRF, serum response factor; VEGF, vascular endothelial growth factor
Graphical abstract
See editorial on page 227.
Summary.
This study detected reduced nerve growth factor (NGF) expression within gastric endothelial cells in both elderly patients and aged rats. Reduced NGF correlated with impaired angiogenesis and delayed gastric ulcer healing in aged rats. The defects could be reversed by exogenous NGF via phosphoinositide-3 kinase/serine threonine kinase signaling protein, and mammalian target of rapamycin signaling, and was dependent on serum response factor. These data show that down-regulation of endothelial NGF expression in aging is a significant contributor to impaired gastric mucosal repair.
Our previous studies have shown that the gastric mucosa of aging individuals, which we termed aging gastric mucosa or aging gastropathy,1, 2 has increased susceptibility to injury and delayed healing owing to impaired angiogenesis,1, 3, 4, 5, 6 but the mechanisms are not fully elucidated. Angiogenesis (new blood vessel formation) is a fundamental process that is essential for reproduction, postnatal growth, and tissue injury healing.7, 8, 9, 10, 11, 12, 13 Angiogenesis is impaired in aging tissues including aging gastric mucosa and results in inadequate revascularization and delayed injury healing.3, 14, 15 Vascular endothelial growth factor (VEGF) A is a fundamental regulator of angiogenesis in general,16, 17, 18, 19 and in injured and ulcerated gastric mucosa.3, 12, 13 Our previous studies have shown that angiogenesis in vivo and in vitro is reduced dramatically in the gastric mucosa of aging rats, and showed that reduced VEGF expression in endothelial cells (ECs) is one of the mechanisms.3, 4 Nevertheless, treatment with VEGF only partly reversed impaired angiogenesis in aging gastric ECs,4 indicating an essential role for other factor(s) in addition to VEGF. Neither the identity of such a factor nor its mechanism of action has been fully explored.
Nerve growth factor (NGF), originally discovered by the 1986 Nobel Laureate Rita Levi-Montalcini20, 21 as a factor critical for growth and survival of neurons, has gained attention in recent years for its actions that extend beyond the promotion of neuronal survival and outgrowth.22, 23 One such non-canonical action of NGF is the ability to promote angiogenesis in brain capillary ECs.24 The expression of NGF in aging gastric ECs and the mechanistic role of NGF deficiency in impaired angiogenesis of aging gastric ECs are not known.
Because gastric mucosal ECs (GECs) are the key cellular targets and effectors of gastric angiogenesis, this study aimed to determine whether reduced NGF expression in aging GECs is a primary cause of the impaired angiogenesis in aging gastric mucosa, and whether NGF therapy can reverse aging-related impairment of angiogenesis in vitro. We examined the expression of NGF in ECs isolated from gastric mucosa of young and aging rats, and the cellular and molecular mechanisms of NGF action on aging GECs, including the effect of NGF treatment on GEC migration, G-actin to F-actin polymerization, stress fiber formation, proliferation, and angiogenesis. We also examined whether signaling pathways phosphoinositide-3 (PI3) kinase/serine threonine kinase signaling protein (Akt) and mammalian target of rapamycin (mTOR) are involved and the requirement of serum response factor (SRF) for these NGF actions. In in vivo studies, we examined the expression of NGF in gastric mucosa of young and aging rats, and the effect of local treatment with NGF on in vivo angiogenesis, gastric ulcer healing, and mucosal regeneration in aging rats.
Here, we show that aging GECs have significantly reduced expression of NGF and reduced angiogenesis compared with young GECs, and that NGF deficiency is a key cause of impaired angiogenesis because NGF gene therapy completely reversed this impairment. Furthermore, our study showed that NGF’s angiogenic action involves PI3 kinase/Akt and mTOR, encompasses G actin to F actin polymerization and stress fiber formation, and is critically dependent on SRF. Our in vivo studies in rats showed reduced NGF expression in ECs of blood vessels in both uninjured and ulcerated gastric mucosa of aging rats compared with young rats. In aging rats, local treatment of NGF at the base of gastric ulcers significantly increased mucosal blood flow and angiogenesis, accelerated ulcer healing, and improved mucosal regeneration. We also demonstrated the human relevance of our experimental findings by showing reduced NGF expression in gastric ECs in gastric mucosal biopsy specimens of aging (age, ≥70 y) vs young (age, ≤40 y) individuals.
Materials and Methods
Study Approval
All experimental studies in rats were approved by the institutional animal review committees: subcommittees for Animal Studies of the VA Long Beach Healthcare System (Long Beach, CA) and the Jagiellonian University Medical College (Krakow, Poland). Rats received humane care based on the National Institutes of Health recommendations outlined in the Guide for the Care and Use of Laboratory Animals. The use of archival human gastric mucosal biopsy specimens for immunostaining was approved by the Institutional Review Board of the Veterans Affairs Medical Center (Long Beach, CA). The human gastric biopsy specimens did not have any specific patient identifiers and the only inclusion criteria used were age, normal appearance on histology, and the lack of Helicobacter pylori and inflammation in mucosal biopsy specimens.
All authors had access to the study data and reviewed and approved the final manuscript.
Isolation of Gastric Mucosal ECs
GECs were isolated from Fisher F-344 rats (purchased from the National Institute on Aging, Bethesda, MD), 3 months of age (referred to as young GECs) and 24 months of age (referred to as aging GECs) using Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1/CD31) selection and magnetic bead separation as described previously.4 ECs were identified by positive staining for Factor VIII–related antigen, CD31, and VEGF-R2, and by absence of staining for the myofibroblast marker smooth muscle α-actin. ECs were grown on collagen-coated dishes in EC growth media containing fetal bovine serum, heparin, and endothelial cell growth supplements. For some studies, GECs were treated with the specific PI3 kinase inhibitor 50 μmol/L for 30 minutes; Cell Signaling Technology, Danvers, MA), the mTOR inhibitor rapamycin (10 nmol/L for 60 minutes; Cell Signaling Technology), Latrunculin B (0.5 μmol/L for 30 minutes; Invitrogen, Carlsbad, CA; inhibitor of actin polymerization), or recombinant rat NGF (10–1000 ng/mL; R&D Systems, Minneapolis, MN). Cell viability was examined using Calcein AM live cell tracking dye (10 μmol/L; Invitrogen) as described in our previous study.25 At the end of each treatment, GECs were incubated with Calcein AM (10 μmol/L) for 15 minutes at 37°C. After fixation in 4% paraformaldehyde for 20 minutes, the cells were washed, mounted on glass coverslips with Prolong Gold (Molecular Probes, Thermo Fisher Scientific, Waltham, MA), and were examined using the AxioImager2 fluorescence microscope imaging system (Carl Zeiss, Thornwood, NY). Fluorescence staining intensity was quantified using the MetaMorph 7.0 imaging system (Molecular Devices, Downington, PA). The total cell count and the number of Calcein AM stained cells were determined in 5 randomly selected fields. Cell viability was expressed as the percentage of Calcein AM–stained cells.
In Vitro Angiogenesis Assay
Endothelial tube formation on growth factor–reduced Matrigel was determined using an in vitro angiogenesis assay similar to our previous studies.4, 26 ECs were grown in complete growth medium in 60-mm tissue culture dishes until they were approximately 80% confluent. The growth medium was replaced with basal medium supplemented with 1% fetal bovine serum and antibiotics, and the cells were cultured for 18 additional hours. The cells then were trypsinized, counted, resuspended in basal medium supplemented with 1% fetal bovine serum, and seeded onto growth factor–reduced Matrigel in 48-well culture plates for up to 24 hours. Six and 24 hours later, the seeded cells were photographed using a Nikon inverted phase-contrast photomicroscope (Nikon USA, Garden City, NY) and analyzed using a video image analysis system (MetaMorph 7.0). Tube formation was quantified by measuring the total pixel length of the capillary tubes in 5 randomly selected standardized fields for each well under 200× magnification.
EC Proliferation
Cell proliferation was determined by bromodeoxyuridine (BrdU) assay using a commercially available kit (EMD Millipore Corporation, Billerica, MA) according to the manufacturer’s instructions. The total cell count and the number of BrdU-positive cells were determined in 5 randomly selected fields. Cell proliferation was expressed as the percentage of BrdU-positive cells.
Reverse-Transcription Real-Time Quantitative Polymerase Chain Reaction
Total cellular RNA was isolated from young and aging GECs using TRIzol reagent (Invitrogen). Total RNA (1 μg) was treated with deoxyribonuclease I and reverse-transcribed using the GeneAmp RNA-PCR kit (Applied Biosystems, Foster City, CA) as described in our previous studies.1, 4 The mRNA levels of NGF and β-actin were quantified by real-time polymerase chain reaction (PCR) using prevalidated QuantiTect assays (Qiagen, Valencia, CA) and the iCycler real-time PCR detection system (Bio-Rad, Hercules, CA). For negative controls, we used no template control and no reverse-transcriptase controls, which did not show any detectable mRNA expression in the reverse-transcription PCR assays. Relative mRNA levels were calculated using the 2-ΔCt method and normalized to β-actin, where ΔCt refers to the difference between threshold cycles for NGF gene and β-actin gene.
Western Blot for NGF, Akt, and Phosphorylated Akt in GECs
Proteins were isolated from GECs using standard RIPA buffer. Western blot was performed to examine the expression of NGF, phosphorylated Akt, Akt, and β actin proteins using methods described in our previous studies.1, 4 Antibodies used were as follows: NGF (1:250, sc 548; Santa Cruz Biotechnology, Santa Cruz, CA), phosphorylated Akt (1:500, 9271; Cell Signaling Technology), and Akt (1:1000, 9272; Cell Signaling Technology) or β actin (1:1000, A5316; Sigma, St. Louis, MO).
NGF Immunostaining
The expression of NGF in aging GECs was examined by immunofluorescence and immunoperoxidase staining using NGF-specific antibody (sc 548; Santa Cruz Biotechnology). Rat and human gastric mucosal specimens were immunostained with NGF-specific antibody (1:100, sc 548; Santa Cruz Biotechnology) using methods described previously in our studies.1, 27 We also performed dual-immunofluorescence staining for NGF (red) and VEGF (green) in rat GU specimens obtained 21 days after GU induction using standard methods. Briefly, tissue sections were immunostained using specific antibodies for NGF and VEGF (1:100, AF-293-NA; R&D Systems) and visualized using Alexa Fluor 488 anti-goat and Alexa Fluor 568 anti-rabbit secondary antibodies (1:200; Invitrogen) using similar methods as described in our previous study.28 Coded mucosal specimens were examined by 2 investigators with expertise in gastrointestinal pathology. ECs lining the mucosal vessels were identified easily and evaluated by these 2 investigators (AA and AST). NGF immunostaining in 10 randomly selected mucosal fields per each specimen was quantified by measuring the staining signal intensity using MetaMorph 7.0.
Gene Therapy of Aging GECs With LV-NGF
Aging GECs were transduced with LV-NGF and LV-GFP (control; to determine transfection efficiency), provided by Dr M. Tuszynski (Director, Center for Neural Repair, University of California, San Diego, CA), using methods described previously.29 GECs were plated in 12-well plates and respective wells were transduced with serial dilutions of LV-GFP and LV-NGF in medium containing polybrene linker. The LV-GFP transfection efficiency in aging GECs was determined by counting the number of transduced green fluorescent cells under epifluorescence and counting the total number of cells in the same field under low bright-field illumination. The percentage of cells showing GFP-derived fluorescence was calculated as the percentage of the total number of cells.
F-Actin and G-Actin Staining in GECs
GECs were treated with PBS or NGF (100 ng/mL) for 1 hour after pretreatment with Latrunculin B (0.5 μmol/L for 30 min), a specific inhibitor of actin polymerization. F-actin and G-actin were visualized by double staining using Alexa Green-conjugated phalloidin (2 U/100 μL; Invitrogen) to detect F-actin and Alexa Red–conjugated DNase I (5 μg/100 μL; Invitrogen) to detect G-actin, as described similarly in our previous studies.27, 30
Cell Migration Assay
We performed cell migration assays by making standardized excisions in confluent GEC monolayers and measured the number of cells that migrated into the denuded area in response to NGF and Latrunculin B. Aging GECs were seeded in 6-well plates and grown to confluence. Cell monolayers were wounded by using a razor blade as described in our previous studies.27, 30 Cells were treated with PBS or NGF (100 ng/mL) for 24 hours after pretreatment with Latrunculin B (0.5 μmol/L) for 30 minutes. Cells were stained with H&E and the migration rate was determined under an inverted microscope, as described similarly in our previous studies.27, 30
SRF Silencing
GECs were treated with either nonsilencing scrambled control (control siRNA, 100 nmol/L; Qiagen) or SRF-specific siRNA (SRF siRNA, 100 nmol/L; Qiagen) for 72 hours using methods similar to those described in our previous study.4 Transfected GECs were used to study the effects of SRF silencing on in vitro angiogenesis and cell proliferation. The siRNA transfection efficiency in GECs was determined using incorporation of Alexa Fluor 488–labeled siRNA and was 87%.
In Vivo Studies Aimed to Determine NGF Expression in Aging Rat Stomach in Response to Injury and to Determine Whether Local NGF Therapy Increases Angiogenesis and Improves GU Healing in Aging Rats
We used a well-established, in vivo model of GUs in rats to determine the expression and role of NGF in GU healing. GUs were induced in 24-month-old aging rats using local application of acetic acid similar to our previous studies.13, 31, 32 We examined the expression of NGF in ECs lining the gastric mucosal vessels in young and aging rats at baseline and 3 weeks after injury (N = 10 per group). Cell proliferation was examined by immunostaining for proliferating cell nuclear antigen (PCNA) as in a previous study33 using specific anti-PCNA antibody (1:100, MAB424; Chemicon; Millipore Sigma, Burlington, MA). The rate of PCNA-positive ECs was expressed as the ratio of positively stained ECs to the total EC count and expressed relative to that for normal gastric mucosa of young rats. We next performed in vivo studies to determine whether NGF therapy increases angiogenesis and improves gastric ulcer healing in aging rats. In separate groups of rats, 30 minutes after GU induction and once more at 3 days after GU induction, either PBS (control) or NGF (100 μg/kg body weight in 200 μL PBS, N2513; Sigma) was injected into the submucosa in 4 quadrants at the site of GU induction (N = 11 per group). Three weeks after GU induction, rats were anesthetized and mucosal blood flow in the scar and/or ulcer margin was determined by a laser Doppler flow meter (Vasomedics, Inc, St. Paul, MN) with a probe applied gently to the mucosa similarly as in our previous study.1 The GU size was measured under a dissecting microscope similarly as in our previous studies.1, 13 Gastric specimens were obtained after euthanasia and processed for histology. Quantitative histologic assessments including vascular density (number of blood vessels per mm2 of gastric mucosa or granulation tissue) and quality of mucosal regeneration were performed as described in our previous study.13 In a separate study, 30 minutes after GU induction in aging rats (N = 6), 200 μL PBS (control) or fluorescein isothiocyanate (FITC)-labeled NGF (100 μg/kg body weight in 200 μL PBS) was injected into 4 quadrants of the submucosa at the GU base. Three weeks after GU induction, rats were euthanized and gastric specimens were obtained for histology. We examined the incorporation of FITC-labeled NGF into gastric tissues under an epifluorescence microscope as described in our previous pilot study in young rats.34
Immunostaining of Human Gastric Biopsy Specimens
The use of archival human gastric mucosal biopsy specimens for immunostaining was approved by the Institutional Review Board at VA Long Beach Healthcare System. The human gastric biopsy specimens did not have any specific patient identifiers and the only inclusion criteria used were as follows: age, normal appearance on histology (absence of GU), and the lack of H pylori and inflammation. We used 10 specimens each of young individuals (age, ≤40 y) and aging individuals (age, ≥70 y), and immunostained them for NGF identically as rat sections (described earlier). Coded mucosal specimens were examined by 2 investigators (A.A. and A.S.T.) experienced in gastrointestinal histopathology. NGF immunostaining in gastric ECs of gastric blood microvessels was quantified by measuring NGF signal intensity in 10 randomly selected microvessels in each specimen using the MetaMorph 7 video analysis system.
Statistical Analysis
Data are presented as means ± SD. Statistical significance was analyzed by either the Student t test to compare data between 2 groups, 1-way analysis of variance with the Tukey multiple comparison post hoc testing for evaluating differences between multiple groups, or the Pearson correlation using Prism software (GraphPad Software, Inc, La Jolla, CA). A P value < .05 was considered statistically significant.
Results
NGF Expression Is Reduced in GECs of Aging Rats and Strongly Correlates With the Impairment of Angiogenesis in These Cells
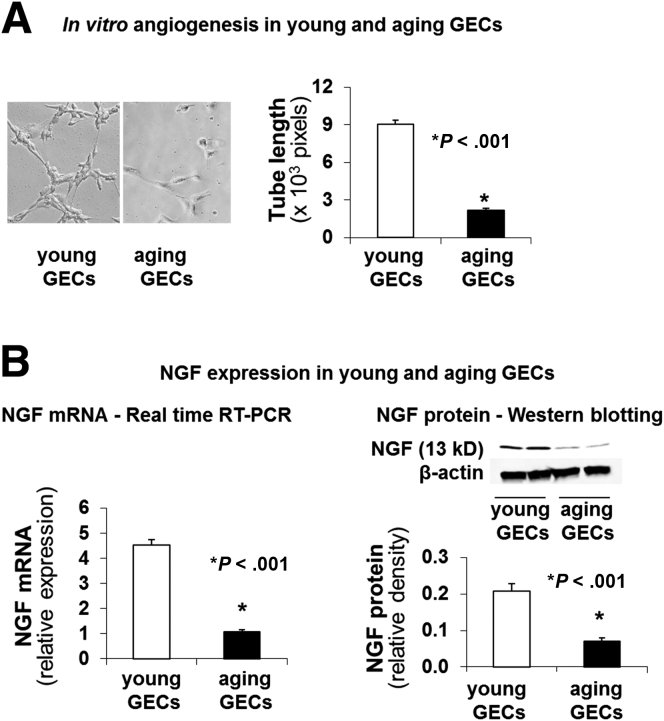
In GECs isolated from aging rats (age, 24 mo; aging GECs), in vitro angiogenesis was reduced significantly by 4.1-fold (P < .001) (Figure 1A). The expression of NGF messenger RNA (mRNA) and NGF protein was lower by 4.2-fold and 3.0-fold, respectively, vs GECs isolated from young rats (age, 3 mo; young GECs) (P < .001) (Figure 1B). These results showed that angiogenesis is impaired in aging GECs and that this impairment closely correlates with the reduced NGF protein expression in these cells (correlation coefficient: r = 0.996; P < .01).
Figure 1.
Aging GECs have reduced in vitro angiogenesis and decreased NGF expression vs young GECs. (A) Aging GECs have significantly reduced in vitro angiogenesis–endothelial tube formation vs young GECs at 6 hours. (B) Real-time reverse-transcription (RT)-PCR showed reduced NGF mRNA expression in aging GECs vs young GECs, and Western blot showed reduced NGF expression in aging GECs vs young GECs. Data are means ± SD (N = 6).
NGF Treatment Reverses Impaired Angiogenesis in Aging GECs Through PI3 Kinase/Akt and mTOR Pathways
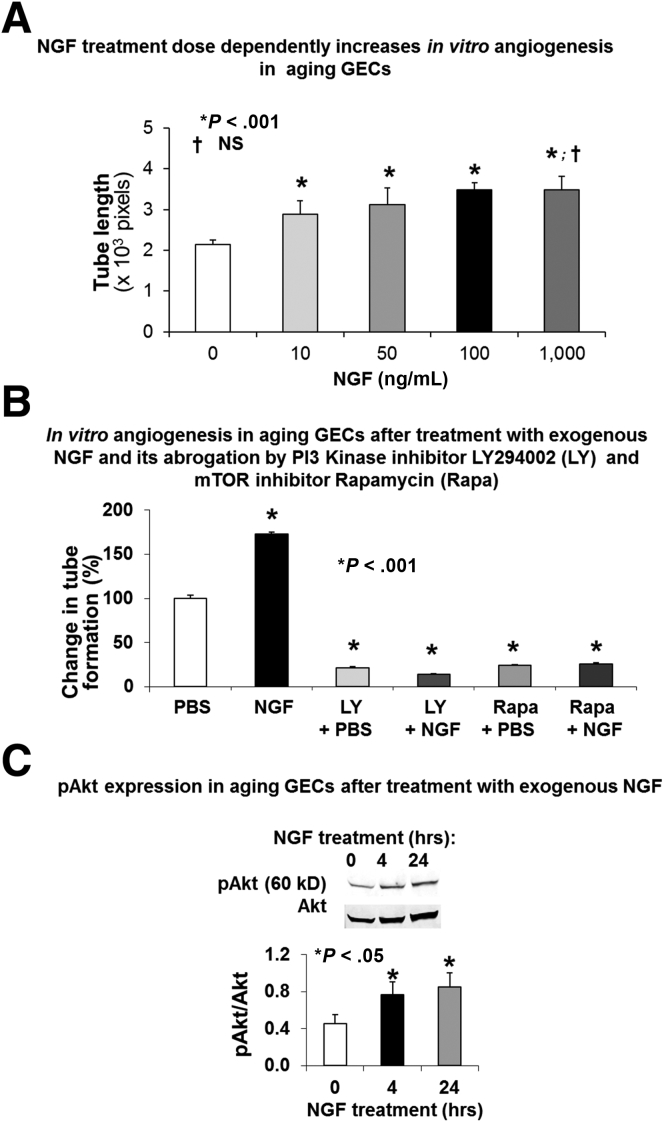
We next examined whether NGF treatment could reverse impairment of angiogenesis in aging GECs and the possible underlying mechanisms. NGF stimulated in vitro angiogenesis in aging GECs in a dose-dependent manner, and the maximal threshold dose of NGF was 100 ng/mL (Figure 2A). This NGF dose in aging GECs was similar to the maximal threshold stimulating dose of 100 ng/mL NGF that increased angiogenesis in young GECs.35 Treatment with exogenous NGF (100 ng/mL) significantly increased in vitro angiogenesis by 1.5-fold (P < .001) in aging GECs. This action of NGF was completely abolished by pretreatment with the specific PI3 kinase inhibitor LY294002, or mTOR inhibitor rapamycin (Figure 2B). Cell viability studies showed that treatment with PI3 kinase and mTOR inhibitors (at the doses used) did not affect viability of GECs. Because PI3 kinase catalyzes one of the steps upstream of Akt activation (phosphorylation), we next examined the effect of NGF treatment on Akt phosphorylation in aging GECs. Treatment of aging GECs with exogenous NGF resulted in a significant increase in Akt phosphorylation (Figure 2C).
Figure 2.
NGF treatment reverses impairment of angiogenesis in aging GECs through PI3 kinase/Akt and mTOR pathways. (A) NGF treatment dose-dependently increases in vitro angiogenesis in aging GECs. Aging GECs were treated with either PBS (control) or exogenous NGF (10, 50, 100, or 1000 ng/mL) and in vitro angiogenesis on Matrigel was examined. Data are means ± SD (N = 6). Treatment of aging GECs with NGF dose-dependently increased angiogenesis with the maximal threshold stimulating dose of 100 ng/mL at 6 hours (P < .001). There was no significant difference in angiogenesis between cells treated with NGF 100 ng/mL and 1000 ng/mL. †P > .05 (NS). (B) In vitro angiogenesis on Matrigel at 6 hours in aging GECs treated with PBS or recombinant rat NGF (100 ng/mL) with or without pretreatment with specific PI3 kinase inhibitor (LY294002; 50 μmol/L for 30 minutes), or mTOR inhibitor (rapamycin; 10 nmol/L for 60 minutes). Short-term (6 hours) treatment with exogenous NGF significantly increased in vitro angiogenesis and in aging GECs by 1.5-fold and pretreatment with LY294002, and rapamycin abolished the NGF-induced increase in angiogenesis. Data are means ± SD (N = 6). (C) Representative Western blots for phosphorylated Akt (pAkt) and Akt expression in aging GECs treated with exogenous NGF (0.1 μg/mL) for 4 and 24 hours. Treatment of aging GECs with exogenous NGF significantly increased Akt phosphorylation at 4 and 24 hours. Data are means ± SD (N = 3). The Student t test and 1-way analysis of variance with the Tukey multiple comparison test were used to determine statistical significance between 2 or multiple groups, respectively.
Impaired Angiogenesis in Aging GECs Is Owing to Reduced NGF and Can Be Reversed by NGF Gene Therapy
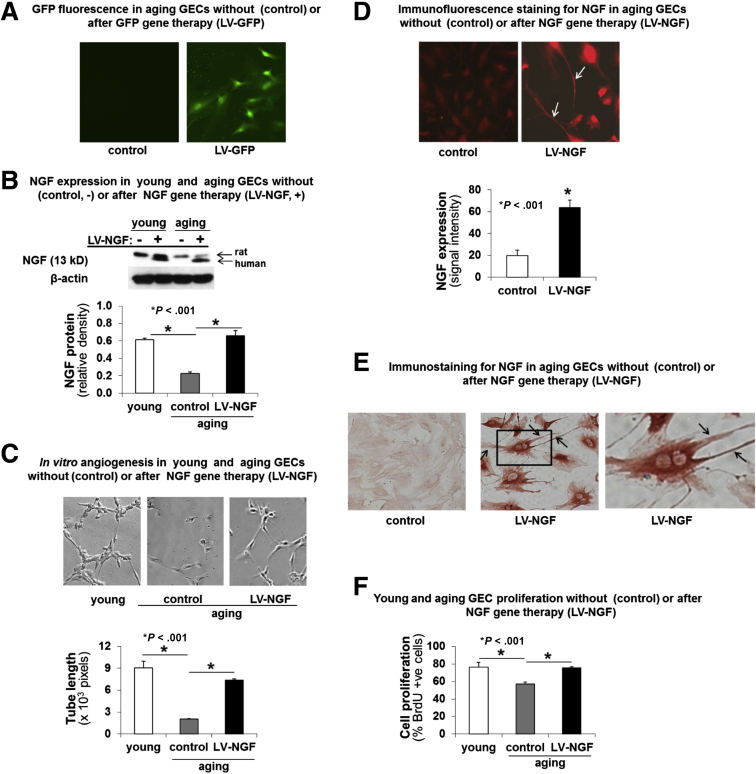
To determine a causal relationship between NGF deficiency and impaired angiogenesis in aging ECs, we examined whether overexpression of NGF could reverse the impairment of angiogenesis in aging GECs. We performed NGF gene therapy using lentiviral human NGF (LV-NGF) to increase NGF expression in aging GECs. The efficiency of transfection in aging GECs was determined using lentiviral green fluorescent protein (LV-GFP). The percentage of LV-GFP–transfected cells showing GFP-derived fluorescence (Figure 3A) was 82% ± 6%. NGF gene therapy significantly increased NGF expression in aging GECs at 72 hours after LV-NGF transduction to a level similar to that present in control (nontreated) young ECs (Figure 3B), and almost completely reversed the impaired angiogenesis in aging GECs as evidenced by more than a 3-fold increase in capillary-like endothelial tube formation (P < .001) (Figure 3C). Thus, restoring NGF levels in aging GECs to levels comparable with young GECs resulted in almost complete restoration of angiogenesis.
Figure 3.
NGF gene therapy increases NGF expression in aging GECs, reverses impaired in vitro angiogenesis, and reduced cell proliferation. Aging GECs were transduced with human LV-NGF in medium containing polybrene linker. Mock transductions in aging GECs performed using polybrene linker without LV-NGF served as negative controls (-, control) for LV-NGF gene therapy. The efficiency of transfection in aging GECs was determined using LV-GFP. (A) The percentage of LV-GFP–transfected aging GECs showing GFP-derived fluorescence was 82% ± 6%. (B) Western blot for NGF showed that after NGF gene therapy (+), aging GECs express both endogenous (rat) NGF and human NGF from gene therapy vs negative controls (-, control), which express only endogenous (rat) NGF. (C) NGF gene therapy with LV-NGF resulted in a significant 3.7-fold increase in in vitro angiogenesis at 6 hours in aging GECs vs negative controls (-, control). Panels are representative images of capillary-like tube formation. Original magnification, ×200. Data are means ± SD (N = 6). (D) Immunofluorescence staining for NGF (red fluorescence) showed that NGF gene therapy in aging GECs strongly increased the expression of NGF by 3.3-fold and induced long filopodia (arrows) vs aging GECs without gene therapy (negative controls). (E) Immunoperoxidase staining for NGF showed that NGF gene therapy of aging GECs induced a significant increase in NGF expression (brown staining) and extensive long filopodia (arrows) reflecting a change in these cells to an angiogenic phenotype; aging GECs without gene therapy (negative controls) have minimal NGF expression and lack filopodia. Data are means ± SD (N = 6). (F) Cell proliferation assessment after LV-NGF gene therapy of aging GECs. LV-NGF gene therapy of aging ECs significantly increased cell proliferation. The Student t test and 1-way analysis of variance with the Tukey multiple comparison test were used to determine statistical significance between 2 or multiple groups, respectively.
NGF gene therapy of aging GECs induced extensive long filopodia in these cells (Figure 3D and E), reflecting a switch to an angiogenic phenotype. Interestingly, NGF was strongly expressed in the filopodia, indicating its interactions with cytoskeletal proteins during filopodia formation. NGF gene therapy of aging GECs also significantly increased these cells’ proliferation vs appropriate controls (P < .001) (Figure 3F).
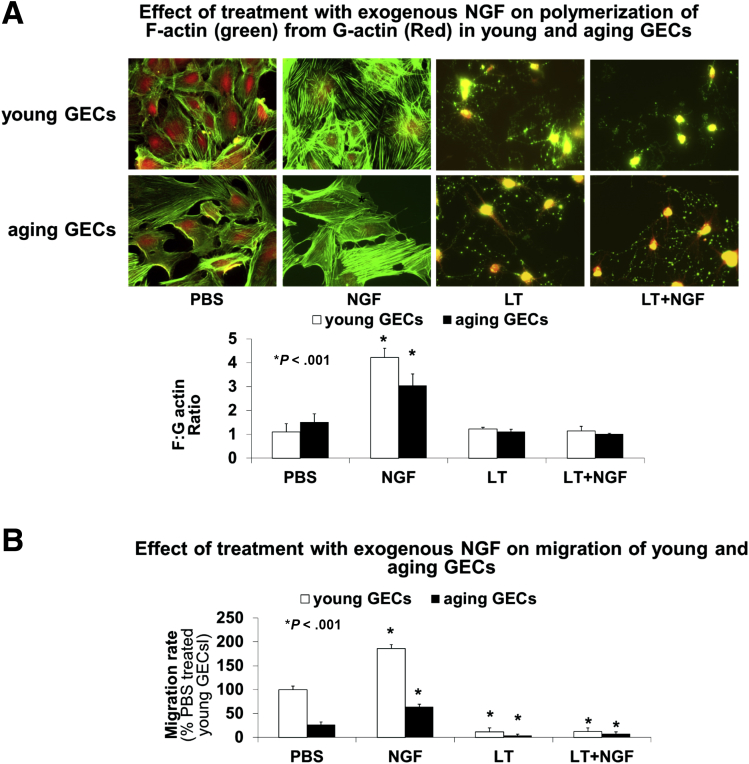
Because angiogenesis requires EC cell migration, proliferation, reorganization of cytoskeletal structures, polymerization of G-actin into F-actin, and the assembly of F-actin into stress fibers,27, 30 we next examined the effect of NGF treatment on F-actin and G-actin expression in young and aging GECs by double staining using Alexa Red–conjugated DNase I (Thermo Fisher Scientific) to detect G-actin and Alexa Green–conjugated phalloidin (Thermo Fisher Scientific) to detect F-actin. NGF treatment induced the formation of extensive F-actin stress fibers with a concurrent G-actin reduction (Figure 4A) in young and aging GECs, these effects were abolished by pretreatment with Latrunculin B. In addition, we performed cell migration assays and determined the number of cells that migrated across standardized excisions into the denuded area in response to NGF and the effect of Latrunculin B. NGF treatment increased cell migration in young and aging GECs, and this effect was inhibited by pretreatment with Latrunculin B (Figure 4B).
Figure 4.
Effect of NGF on actin polymerization and cell migration in aging GECs. Young and aging GECs were treated with PBS or NGF (100 ng/mL) for 1 hour after pretreatment with Latrunculin B (0.5 μmol/L for 30 minutes), a specific inhibitor of actin polymerization. (A) Representative photomicrographs of young and aging GECs showing visualization of F-actin and G-actin by double staining using Alexa Green–conjugated phalloidin to detect F-actin and Alexa Red–conjugated DNase I to detect G-actin. NGF treatment induced formation of extensive F-actin stress fibers and an increased F:G actin ratio in young and aging GECs. Treatment with Latrunculin B inhibited NGF-induced F-actin polymerization. (B) NGF treatment significantly increased the migration rate in young and aging GECs, but this effect was abolished by treatment with Latrunculin B. Data are means ± SD (N = 6). The Student t test and 1-way analysis of variance with the Tukey multiple comparison tests were used to determine statistical significance between 2 or multiple groups, respectively.
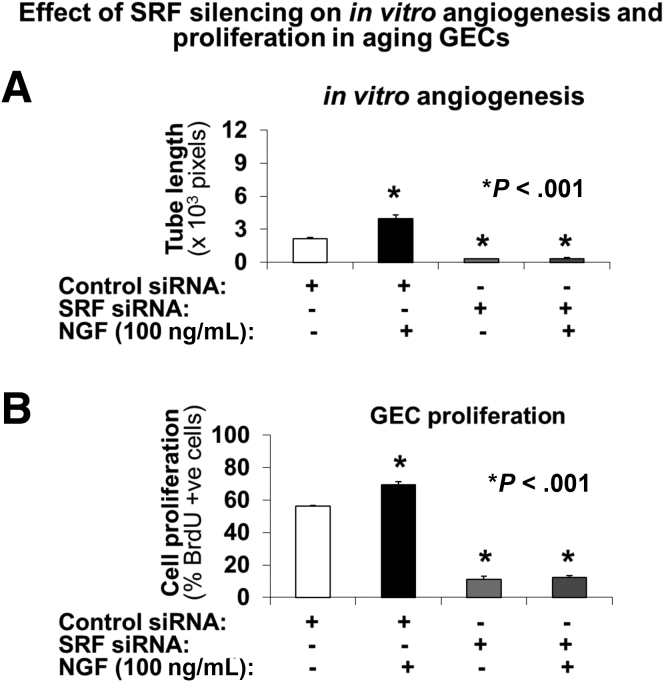
We next examined the effect of SRF on NGF-induced angiogenesis. SRF was silenced in aging GECs using specific small interfering RNA (siRNA), and the effect of SRF silencing on in vitro angiogenesis on Matrigel (Thermo Fisher Scientific) and on GEC proliferation was determined in the presence or absence of NGF. SRF silencing completely abolished NGF-induced angiogenesis (Figure 5A) and proliferation of aging GECs (Figure 5B).
Figure 5.
SRF silencing abolishes NGF-induced angiogenesis and proliferation of aging GECs. SRF was silenced in aging GECs using specific siRNA, and the effect of SRF silencing on in vitro angiogenesis on Matrigel and on GEC proliferation was determined in the presence or absence of NGF. (A) SRF silencing using specific siRNA significantly reduced in vitro angiogenesis in aging GECs (vs cells treated with scrambled [control siRNA]). This reduction in in vitro angiogenesis by SRF siRNA was not affected by treatment with exogenous recombinant rat NGF. Data are means ± SD (N = 6). (B) SRF silencing significantly reduced NGF-induced proliferation aging GECs (vs cells treated with scrambled [control siRNA]). Data are means ± SD (N = 6).
NGF Expression is Reduced In Vivo in Gastric ECs of Aging Rats and Local NGF Therapy of Gastric Ulcers in Aging Rats Increases Angiogenesis, Accelerates Gastric Ulcer Healing, and Improves Mucosal Regeneration in Gastric Ulcer Scars
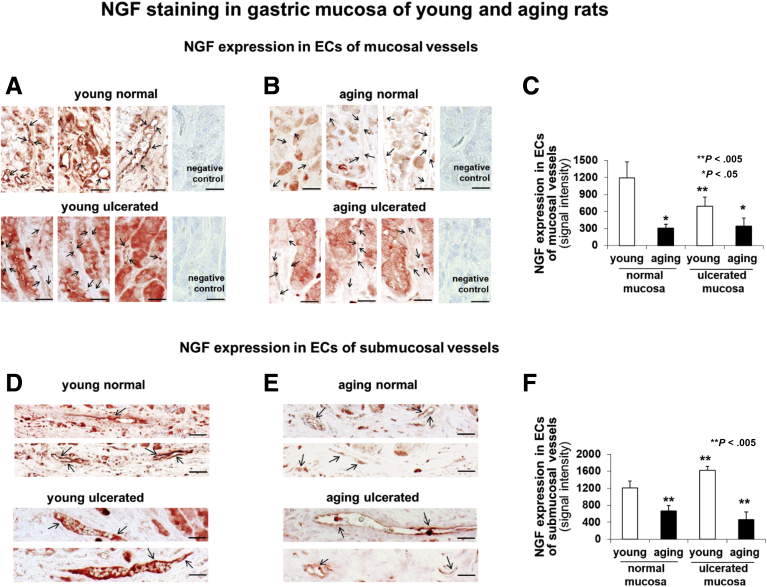
Next, we examined the expression of NGF in GECs lining gastric blood vessels in young and aging rats at baseline and 3 weeks after injury using a well-established, in vivo model of gastric ulcers (GUs) in rats (Figure 6). These studies showed that NGF expression is reduced significantly in ECs of mucosal and submucosal vessels in both normal and ulcerated gastric mucosa of aging rats compared with young rats (Figure 6C and F).
Figure 6.
NGF expression in normal and ulcerated mucosa of young and aging rats. Immunostaining for NGF in gastric mucosa of young (A and D) and aging (B and E) rats. Quantitative analysis of NGF immunostaining showed reduced NGF expression in ECs of mucosal (C) and submucosal (F) vessels in both normal and ulcerated gastric mucosa of aging rats compared to young rats. Scale bars: 20 μm. *P < .05; **P < .005. Student's t-test and one-way analysis of variance with Tukey's multiple comparison tests were used to determine statistical significance between 2 or multiple groups, respectively.
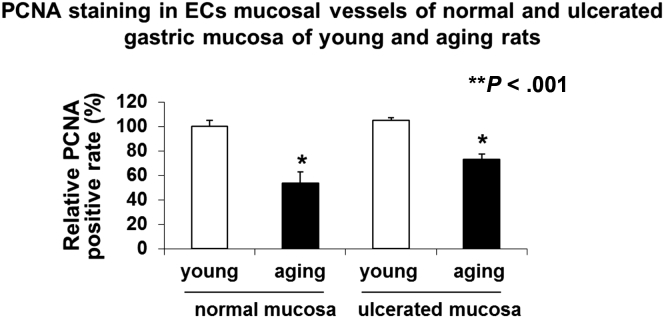
GEC proliferation in gastric mucosa of young and aging rats was examined by immunostaining for PCNA. In aging rats, GEC proliferation was reduced significantly in both normal and ulcerated gastric mucosa by 46% ± 5% and 31% ± 2%, respectively, vs young rats (Figure 7).
Figure 7.
GEC proliferation in normal and ulcerated gastric mucosa of young and aging rats determined by PCNA staining. In aging rats, the number of PCNA-positive ECs was reduced by 46% ± 5% in normal gastric mucosa and by 31% ± 2% in ECs in ulcerated gastric mucosa compared with young rats. PCNA staining in normal gastric mucosa of young rats is expressed as 100%.
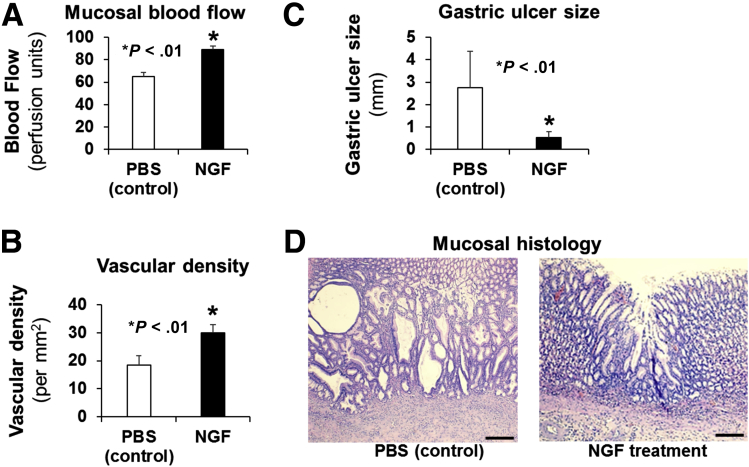
We also examined whether local treatment of GUs in aging rats with NGF protein could improve in vivo angiogenesis and accelerate GU healing. GUs were induced in aging rats and either phosphate-buffered saline (PBS) (control) or NGF (100 μg/kg body weight in 200 μL PBS) was injected into the submucosa at the site of GU induction twice (at 30 minutes and at 3 days) after GU induction, and mucosal blood flow, microvessel density, GU, or scar size and mucosal regeneration was examined 3 weeks after GU induction. This local treatment of GUs in aging rats with NGF significantly increased angiogenesis as reflected by augmented mucosal blood flow in GU scars or GU margins by 37% (vs PBS-treated control) (Figure 8A) and increased vascular density in granulation tissue (Figure 8B).
Figure 8.
Local NGF therapy of GUs in aging rats increases angiogenesis, accelerates gastric ulcer healing, and improves quality of mucosal regeneration. In aging rats, either PBS (control) or NGF (100 μg/kg body weight in 200 μL PBS) was locally injected to the ulcer base after GU induction. Mucosal blood flow, microvessel density, GU size, and mucosal regeneration was examined 3 weeks after GU induction. Local NGF treatment of GUs in aging rats (A) significantly increased mucosal blood flow in GU scars/margins by 37% vs control, and (B) increased vascular density in granulation tissue at the base of the GU scar/margin by 62% vs control, both showing increased angiogenesis. (C) NGF therapy accelerated GU healing as reflected by >5-fold reduction in GU size. (D) Mucosal histology showed better regeneration of gastric mucosa in GU scars of NGF-treated rats vs poor, disorganized, and abnormal regeneration (eg, large, dilated, irregular abnormal glands) in the control group.
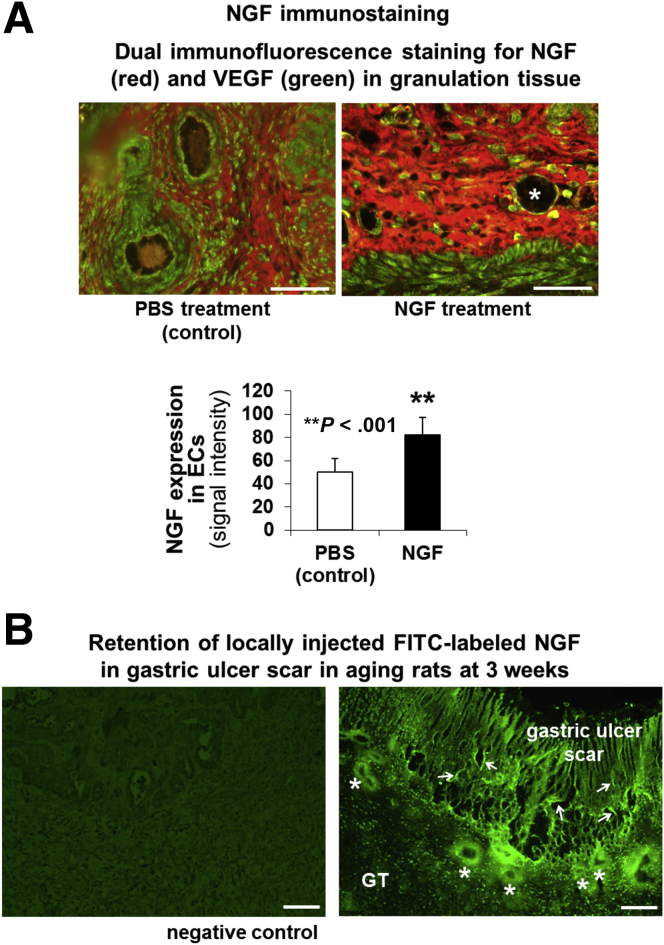
NGF treatment also dramatically accelerated GU healing as reflected by reduced size of GUs (Figure 8C) and improved quality of mucosal regeneration (Figure 8D). In the PBS-treated control group healing was impaired and regenerating glands were dilated and distorted compared with the NGF-treated group. We also examined the expression of NGF and VEGF in ulcerated gastric mucosa of rats treated with PBS and NGF. In PBS-treated control aging rats, VEGF was expressed in ECs lining the mucosal vessels, and NGF and VEGF were weakly expressed in the GU granulation tissue (Figure 9A). In aging rats treated with NGF, the expression of NGF was increased significantly in GU granulation tissue and ECs by 1.6-fold (P < .001) vs PBS treatment (Figure 9A).
Figure 9.
Local NGF therapy of GUs in aging rats increases NGF expression. (A) Dual immunostaining for NGF (red) and VEGF (green) in gastric mucosa of aging rats after local treatment with PBS (control) or NGF (100 μg/kg body weight) 3 weeks after GU induction. In PBS-treated control aging rats, VEGF was expressed modestly in ECs lining the mucosal vessels, and NGF and VEGF were expressed weakly in the GU granulation tissue. In aging rats treated with NGF, expression of NGF was increased significantly in GU granulation tissue and ECs by 1.6-fold (P < .001) vs PBS treatment. Some of the ECs lining mucosal vessels (asterisk) expressed both NGF and VEGF (yellow staining). Local NGF treatment of GUs in aging rats increased NGF expression in ECs lining the mucosal vessels. (B) Locally injected FITC-labeled NGF incorporated into the GU base and was present (retained) at 3 weeks in ECs lining submucosal blood vessels (asterisk), and mucosal microvessels (arrows) of GU scars in aging rats. Rats injected with PBS showed low-level background autofluorescence in the GU scar (negative control). Scale bars: 100 μm.
In a separate group of aging rats, we injected FITC-labeled NGF to the GU base 30 minutes after GU induction. Three weeks after GU induction, we examined the incorporation of NGF into ulcerated gastric mucosa and submucosa under an epifluorescence microscope as described in our previous pilot study.34 Locally injected FITC-labeled NGF was incorporated and retained in ECs of blood vessels of GU granulation tissue and mucosal scar in aging rats (Figure 9B).
NGF Expression Is Reduced in Gastric Mucosal ECs of Aging Individuals
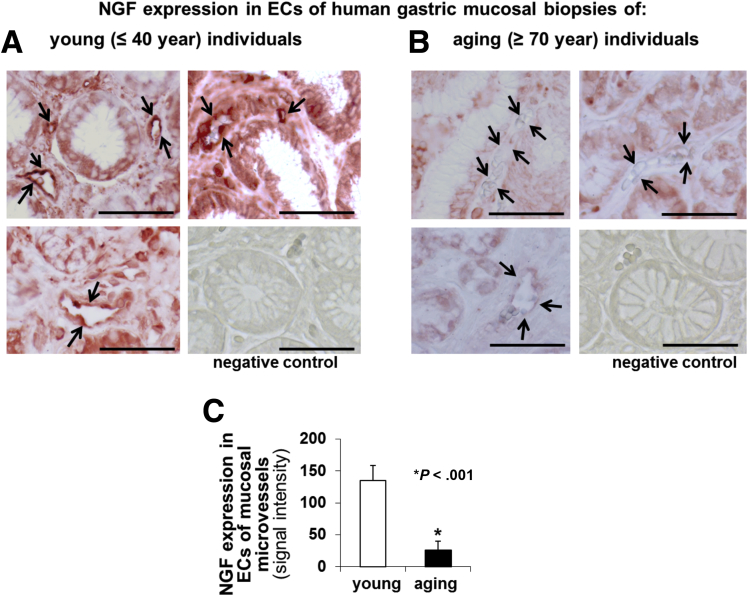
We immunostained endoscopic human gastric biopsy specimens of young individuals (age, ≤40 y) and aging individuals (age, ≥70 y) (n = 10 specimens per group) using specific NGF antibody. In the gastric mucosa of young individuals, NGF was expressed in ECs of mucosal blood vessels and in some epithelial cells (Figure 10A). In gastric specimens from aging individuals NGF signal intensity in ECs was reduced dramatically (Figure 10B) compared with gastric specimens from young individuals. Quantitative evaluation of NGF immunostaining showed a significant 5.5-fold reduction in NGF expression in ECs of mucosal vessels in the gastric mucosa of aging vs young individuals (P < .001) (Figure 10C).
Figure 10.
NGF expression is reduced in gastric ECs of aging (age, ≥70 y) vs young (age, ≤40 y) individuals. (A and B) Immunostaining for NGF in specimens of young individuals (age, ≤ 40 y) and aging individuals (age, ≥70 y) (N = 10 specimens per group). Immunostaining showed significantly reduced NGF expression in ECs lining gastric mucosal blood microvessels of (B) aging individuals (arrows) compared with that of (A) young individuals. No staining was observed in tissue sections incubated without primary antibody (negative control; lower right panels). (C) Quantitative evaluation of NGF immunostaining signal showed significantly (5.5-fold) reduced NGF expression in ECs of mucosal vessels in gastric mucosa of aging vs young individuals. Scale bars: 100 μm. The Student t test was used to determine statistical significance.
Discussion
Angiogenesis is impaired in aging tissues including aging gastric mucosa and results in inadequate revascularization and delayed injury healing.3, 14, 15, 36 However, the mechanisms are not fully elucidated and the role of NGF in aging-related angiogenesis impairment is not known. This study showed that ECs isolated from gastric mucosa of aging rats (aging GECs) have reduced expression of NGF and impaired angiogenesis, and, importantly, that NGF therapy restores in vitro angiogenesis in these aging ECs. Furthermore, this study also showed reduced NGF expression in GECs in gastric mucosa of aging human beings and rats, and that local NGF therapy significantly reversed both impaired angiogenesis and delayed GU healing in aging rats.
The rationale for the study on the role of NGF in impaired angiogenesis in aging stemmed from our previous study, which showed that NGF is critical for angiogenesis in young GECs. NGF silencing in young GECs using specific siRNA inhibited angiogenesis in these cells.35 Interestingly, the inhibition of angiogenesis after NGF silencing in young GECs could be reversed only by exogenous NGF but not by VEGF, indicating that the angiogenic action of NGF in gastric mucosal ECs is not dependent on VEGF.35 We also showed in young GECs that NGF increases mitochondrial membrane potential (MMP), up-regulates insulin-like growth factor 1, and protects those cells from nonsteroidal anti-inflammatory drug (NSAID)-induced injury by preventing MMP depolarization MMP and reduction in in vitro angiogenesis.25
Our present in vitro studies in aging GECs showed that NGF gene therapy increased NGF expression in these cells and dramatically reversed impaired angiogenesis. Because NGF gene therapy significantly increased NGF expression in aging GECs to a level similar to that present in control nontreated young GECs, this clearly showed a causal relationship between NGF deficiency and impaired angiogenesis in aging ECs. This cause–effect relationship between NGF expression in gastric ECs and angiogenesis is underscored further by showing in our previous study that NGF silencing in young GECs inhibited angiogenesis in these cells,35 comparable with the level of aging GECs. Furthermore, this study showed that NGF gene therapy induces extensive long filopodia in aging GECs, which reflect a switch to an angiogenic phenotype in these cells. Interestingly, NGF was strongly expressed in the filopodia, indicating its binding to and interactions with cytoskeletal elements during filopodia formation. One of the key steps during angiogenesis is the formation of filopodia, which send guidance cues that direct endothelial tube formation and branching.37, 38, 39, 40, 41 VEGF is a major inducer of filopodia in branching vessels during angiogenesis.42, 43 Our novel findings indicate that NGF can induce an angiogenic phenotype in aging GECs similar to that shown to be induced by VEGF in young ECs in previous studies.42, 43
Actin polymerization to F-actin and formation of stress fibers are critical for EC migration and endothelial tube formation, which are critical steps of angiogenesis. Here, we showed that NGF induces G-actin polymerization to F-actin and formation of actin stress fibers in aging GECs, and that these cytoskeletal rearrangements are critical for NGF’s angiogenic action. Latrunculin B, which inhibits G-actin polymerization, abolished NGF-induced in vitro angiogenesis in aging GECs. This shows that NGF stimulates polymerization of G-actin into F-actin and the formation of stress fibers in GECs that are critical for these cells’ migration and angiogenesis.
PI3 kinase/Akt signaling is critical for EC survival, cytoskeletal rearrangements (eg, lamellipodia and filopodia formation, migration, and angiogenesis),44, 45 and mTOR signaling is essential for sprouting angiogenesis in endothelial cells.46 Our present study showed that PI3 kinase/Akt and mTOR inhibitors abolished the angiogenic action of NGF in aging GECs. This clearly indicates that NGF mediates its actions on aging GECs through the PI3 kinase/Akt47 and mTOR48 signaling pathways.
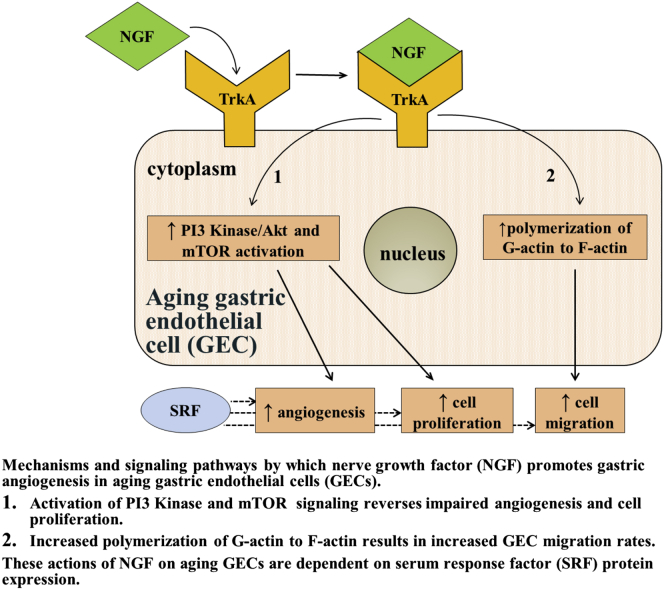
This study also explored the potential role of SRF in NGF-induced angiogenesis in aging GECs. SRF was discovered in 1986 by Treisman,49 who showed that serum induces c-fos expression in fibroblasts through activation of a transcription factor, SRF, which binds to the serum response element in the c-fos promoter. Subsequently, SRF has been shown to regulate expression of numerous genes including those involved in cytoskeletal rearrangements in several types of cells.30 Our previous study showed that SRF is a downstream mediator of VEGF signaling in ECs and is a critical requirement for VEGF-induced angiogenesis.30 In that study, the knockdown of SRF protein levels in human and rat endothelial cells abolished VEGF-induced in vitro angiogenesis, impaired endothelial cell migration and proliferation, and inhibited VEGF-induced actin polymerization and immediate early gene expression.30 Our present study showed that NGF-induced angiogenesis in aging GECs is dependent on SRF, and SRF silencing completely abolishes NGF-induced angiogenesis in these cells. A recent study showed that SRF is a major mediator of NGF-induced mitogen-activated protein kinase kinase/extracellular regulated kinase (MEK/ERK) and megakaryocytic acute leukemia (MAL) signaling, and NGF-dependent target innervation by embryonic sensory neurons.50 Thus, the present study uncovered a novel previously unrecognized role of NGF and SRF in gastric ECs and regulation of angiogenesis in aging rats (Figure 11).
Figure 11.
Diagrammatic representation of mechanisms and signaling pathways by which NGF promotes gastric angiogenesis in aging GECs. The mechanisms and signaling pathways mediating NGF’s actions on aging GECs include activation of PI3 kinase and mTOR signaling, which reverses impaired angiogenesis and cell proliferation, and polymerization of G-actin to F-actin, which increases GEC migration. These actions of NGF on aging GECs are dependent on SRF protein expression. TrkA, tropomyosin receptor kinase A (NGF receptor).
Our present in vivo studies showed that local NGF treatment of GUs in aging rats increases angiogenesis, dramatically accelerates GU healing, and improves the overall quality of mucosal regeneration within the GU scar. Furthermore, this study showed that locally injected FITC-labeled NGF incorporated into ECs of blood vessels and some epithelial cells of gastric tissues. Interestingly, NGF treatment of GUs in aging rats resulted in a more complete regeneration of gastric glandular structures in the ulcer scar than local gene therapy of GUs in young rats with a local injection of VEGF encoding naked DNA, as reported in our previous study.13 Thus, this study uncovered a novel previously unrecognized role of NGF in gastric angiogenesis and GU healing in aging rats and provided a rationale for a clinical translation.
The paradigm of NGF reversing the aging-related impairment of EC angiogenesis and cell proliferation (the 2 main characteristics of the aging EC phenotype) shown in this study in regard to GECs may apply to other types of cells and may have important implications for other aging tissues. A previous study showed that lentiviral NGF injected into the basal forebrain of aged monkeys reversed aging-related neuronal atrophy as reflected by a significant recovery in number and size of cholinergic neurons to the levels present in young monkeys.29 It is possible that the beneficial effect of NGF gene therapy in that study was owing in part to increased angiogenesis. Taken together, these findings indicate a likely role of NGF deficiency in aging of neural cells and non-neural cells (such as ECs), thus providing a greater dimension to the concept of NGF deficiency in aging tissues.
This study showed a strong expression of NGF in gastric mucosal ECs of young individuals and significantly reduced NGF expression in gastric mucosal ECs of aging individuals. This clearly indicates human relevance of our experimental findings and also can explain impaired angiogenesis and delayed healing of injured gastric mucosa in aging individuals.
Our article provides novel insight into mechanisms of impaired gastric angiogenesis in aging. Aging gastropathy and its consequences are clinically critical issues, especially because the aging US population is growing rapidly and it is estimated that by the year 2030, approximately 70 million Americans will be older than 65 years of age. Individuals older than 65 years of age have significantly increased gastric injury from aspirin and other NSAIDs and have an increased risk of tissue and organ injuries and complications of GU.51, 52, 53, 54 Prior upper gastrointestinal clinical events (eg, ulcer), age ≥65 years, and low-dose aspirin use are main risk factors for these complications.52 The risk of GU complications is 8-fold higher in individuals ≥70 years of age vs subjects younger than age 50 years.54, 55, 56, 57 Recent studies have indicated that the incidence of non–H pylori, non-NSAID ulcers (termed idiopathic ulcers) is increasing and they constitute up to 30% of all ulcers and patients with a history of idiopathic bleeding ulcers have an increased risk of recurrent ulcer bleeding and a high mortality rate.58, 59, 60 Therefore, the GU recurrence and its complications remain an important clinical issue, especially in aging and severely ill patients. Our study provides a novel insight into novel mechanisms and the regulatory role of NGF and angiogenesis in GU healing and their relation to aging-related impairment of these processes.
The stimulation of angiogenesis by NGF potentially may involve activation of other growth factors (eg, VEGF).24, 61, 62, 63, 64 Previous studies, including our own, have examined the role of other factors such as VEGF, epidermal growth factor, basic fibroblast growth factor, phosphatase and tensin homolog (PTEN), and survivin in aging, angiogenesis, and ulcer healing.1, 3, 65, 66, 67, 68, 69 Some of these factors were reviewed in our previous publications.5, 6 The novel insight into mechanisms of impaired gastric angiogenesis in aging provided in this article can be potentially applicable to other areas of mucosal healing in the gastrointestinal tract. Although we fully recognize the potential role of other growth factors and cytokines in angiogenesis, GU healing, and their impairment in aging, we focused on NGF in this study. Further studies are necessary to investigate the role of other growth factors and cytokines in angiogenesis, GU healing, and their impairment in aging.
Acknowledgments
The authors thank Dr Mark Tuszynski (Professor of Medicine, Director, Center for Neural Repair, University of California, San Diego, CA) for providing reagents and protocols for lentiviral NGF gene therapy.
Footnotes
Author contributions Amrita Ahluwalia was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis; Michael K. Jones was responsible for the analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and technical support; Neil Hoa was responsible for the acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and technical support; Ercheng Zhu was responsible for the acquisition of data and analysis and interpretation of data; Tomasz Brzozowski was responsible for the acquisition of data and analysis and interpretation of data; and Andrzej S. Tarnawski was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content, statistical analysis, obtained funding, and study supervision.
Conflicts of interest The authors disclose no conflicts. The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Funding Supported by Merit Review Award I01 BX000626-05A2 from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service (A.S.T.).
References
- 1.Tarnawski A., Pai R., Deng X., Ahluwalia A., Khomenko T., Tanigawa T., Akahoshi T., Sandor Z., Szabo S. Aging gastropathy-novel mechanisms: hypoxia, up-regulation of multifunctional phosphatase PTEN, and proapoptotic factors. Gastroenterology. 2007;133:1938–1947. doi: 10.1053/j.gastro.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Laine L., Takeuchi K., Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 3.Ahluwalia A., Jones M.K., Deng X., Sandor Z., Szabo S., Tarnawski A.S. An imbalance between VEGF and endostatin underlies impaired angiogenesis in gastric mucosa of aging rats. Am J Physiol Gastrointest Liver Physiol. 2013;305:G325–G332. doi: 10.1152/ajpgi.00127.2013. [DOI] [PubMed] [Google Scholar]
- 4.Ahluwalia A., Jones M.K., Tarnawski A.S. Key role of endothelial importin-alpha in VEGF expression and gastric angiogenesis: novel insight into aging gastropathy. Am J Physiol Gastrointest Liver Physiol. 2014;306:G338–G345. doi: 10.1152/ajpgi.00382.2013. [DOI] [PubMed] [Google Scholar]
- 5.Tarnawski A.S., Ahluwalia A., Jones M.K. Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J Gastroenterol Hepatol. 2014;29(Suppl 4):112–123. doi: 10.1111/jgh.12734. [DOI] [PubMed] [Google Scholar]
- 6.Tarnawski A.S., Ahluwalia A., Jones M.K. Increased susceptibility of aging gastric mucosa to injury: the mechanisms and clinical implications. World J Gastroenterol. 2014;20:4467–4482. doi: 10.3748/wjg.v20.i16.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J., D'Amore P.A. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 9.Jain R.K. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 11.Tarnawski A.S. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50(Suppl 1):S24–S33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 12.Jones M.K., Itani R.M., Wang H., Tomikawa M., Sarfeh I.J., Szabo S., Tarnawski A.S. Activation of VEGF and Ras genes in gastric mucosa during angiogenic response to ethanol injury. Am J Physiol. 1999;276:G1345–G1355. doi: 10.1152/ajpgi.1999.276.6.G1345. [DOI] [PubMed] [Google Scholar]
- 13.Jones M.K., Kawanaka H., Baatar D., Szabo I.L., Tsugawa K., Pai R., Koh G.Y., Kim I., Sarfeh I.J., Tarnawski A.S. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001;121:1040–1047. doi: 10.1053/gast.2001.29308. [DOI] [PubMed] [Google Scholar]
- 14.Edelberg J.M., Reed M.J. Aging and angiogenesis. Front Biosci. 2003;8:s1199–s1209. doi: 10.2741/1166. [DOI] [PubMed] [Google Scholar]
- 15.Hoenig M.R., Bianchi C., Rosenzweig A., Sellke F.W. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med. 2008;8:754–767. doi: 10.2174/156652408786733685. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw. 2009;20:158–163. doi: 10.1684/ecn.2009.0170. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005;94:209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara N., Kerbel R.S. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 20.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 21.Levi-Montalcini R. The nerve growth factor and the neuroscience chess board. Prog Brain Res. 2004;146:525–527. [PubMed] [Google Scholar]
- 22.Madduri S., Papaloizos M., Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res. 2009;65:88–97. doi: 10.1016/j.neures.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Freeman R.S., Burch R.L., Crowder R.J., Lomb D.J., Schoell M.C., Straub J.A., Xie L. NGF deprivation-induced gene expression: after ten years, where do we stand? Prog Brain Res. 2004;146:111–126. doi: 10.1016/S0079-6123(03)46008-1. [DOI] [PubMed] [Google Scholar]
- 24.Nico B., Mangieri D., Benagiano V., Crivellato E., Ribatti D. Nerve growth factor as an angiogenic factor. Microvasc Res. 2008;75:135–141. doi: 10.1016/j.mvr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Ahluwalia A., Jones M.K., Hoa N., Tarnawski A.S. NGF protects endothelial cells from indomethacin-induced injury through activation of mitochondria and upregulation of IGF-1. Cell Signal. 2017;40:22–29. doi: 10.1016/j.cellsig.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Jones M.K., Wang H., Peskar B.M., Levin E., Itani R.M., Sarfeh I.J., Tarnawski A.S. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 27.Chai J., Baatar D., Tarnawski A. Serum response factor promotes re-epithelialization and muscular structure restoration during gastric ulcer healing. Gastroenterology. 2004;126:1809–1818. doi: 10.1053/j.gastro.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Coron E., Mosnier J.F., Ahluwalia A., Le Rhun M., Galmiche J.P., Tarnawski A.S., Matysiak-Budnik T. Colonic mucosal biopsies obtained during confocal endomicroscopy are pre-stained with fluorescein in vivo and are suitable for histologic evaluation. Endoscopy. 2012;44:148–153. doi: 10.1055/s-0031-1291534. [DOI] [PubMed] [Google Scholar]
- 29.Nagahara A.H., Bernot T., Moseanko R., Brignolo L., Blesch A., Conner J.M., Ramirez A., Gasmi M., Tuszynski M.H. Long-term reversal of cholinergic neuronal decline in aged non-human primates by lentiviral NGF gene delivery. Exp Neurol. 2009;215:153–159. doi: 10.1016/j.expneurol.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai J., Jones M.K., Tarnawski A.S. Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 2004;18:1264–1266. doi: 10.1096/fj.03-1232fje. [DOI] [PubMed] [Google Scholar]
- 31.Tarnawski A., Hollander D., Stachura J., Krause W.J., Eltorai M., Dabros W., Gergely H. Vascular and microvascular changes–key factors in the development of acetic acid-induced gastric ulcers in rats. J Clin Gastroenterol. 1990;12(Suppl 1):S148–S157. doi: 10.1097/00004836-199001001-00025. [DOI] [PubMed] [Google Scholar]
- 32.Pai R., Ohta M., Itani R.M., Sarfeh I.J., Tarnawski A.S. Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology. 1998;114:706–713. doi: 10.1016/s0016-5085(98)70584-0. [DOI] [PubMed] [Google Scholar]
- 33.Imura S., Miyake H., Izumi K., Tashiro S., Uehara H. Correlation of vascular endothelial cell proliferation with microvessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in hepatocellular carcinoma. J Med Invest. 2004;51:202–209. doi: 10.2152/jmi.51.202. [DOI] [PubMed] [Google Scholar]
- 34.Tanigawa T., Ahluwalia A., Watanabe T., Arakawa T., Tarnawski A.S. Nerve growth factor injected into the gastric ulcer base incorporates into endothelial, neuronal, glial and epithelial cells: implications for angiogenesis, mucosal regeneration and ulcer healing. J Physiol Pharmacol. 2015;66:617–621. [PubMed] [Google Scholar]
- 35.Ahluwalia A., Jones M.K., Brzozowski T., Tarnawski A.S. Nerve growth factor is critical requirement for in vitro angiogenesis in gastric endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2016;311:G981–G987. doi: 10.1152/ajpgi.00334.2016. [DOI] [PubMed] [Google Scholar]
- 36.Engevik A.C., Feng R., Choi E., White S., Bertaux-Skeirik N., Li J., Mahe M.M., Aihara E., Yang L., DiPasquale B., Oh S., Engevik K.A., Giraud A.S., Montrose M.H., Medvedovic M., Helmrath M.A., Goldenring J.R., Zavros Y. The development of spasmolytic polypeptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell Mol Gastroenterol Hepatol. 2016;2:605–624. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmeliet P., Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 38.De Smet F., Segura I., De Bock K., Hohensinner P.J., Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 39.Wakayama Y., Fukuhara S., Ando K., Matsuda M., Mochizuki N. Cdc42 mediates Bmp-induced sprouting angiogenesis through Fmnl3-driven assembly of endothelial filopodia in zebrafish. Dev Cell. 2015;32:109–122. doi: 10.1016/j.devcel.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Gupton S.L., Gertler F.B. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 41.Lamalice L., Le Boeuf F., Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 42.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phng L.K., Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Dimmeler S., Zeiher A.M. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 45.Jiang B.H., Liu L.Z. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. 2008;8:19–26. doi: 10.2174/156800908783497122. [DOI] [PubMed] [Google Scholar]
- 46.Farhan M.A., Carmine-Simmen K., Lewis J.D., Moore R.B., Murray A.G. Endothelial cell mTOR complex-2 regulates sprouting angiogenesis. PLoS One. 2015;10:e0135245. doi: 10.1371/journal.pone.0135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park M.J., Kwak H.J., Lee H.C., Yoo D.H., Park I.C., Kim M.S., Lee S.H., Rhee C.H., Hong S.I. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J Biol Chem. 2007;282:30485–30496. doi: 10.1074/jbc.M701081200. [DOI] [PubMed] [Google Scholar]
- 48.Cao G.F., Liu Y., Yang W., Wan J., Yao J., Wan Y., Jiang Q. Rapamycin sensitive mTOR activation mediates nerve growth factor (NGF) induced cell migration and pro-survival effects against hydrogen peroxide in retinal pigment epithelial cells. Biochem Biophys Res Commun. 2011;414:499–505. doi: 10.1016/j.bbrc.2011.09.094. [DOI] [PubMed] [Google Scholar]
- 49.Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987;6:2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wickramasinghe S.R., Alvania R.S., Ramanan N., Wood J.N., Mandai K., Ginty D.D. Serum Response Factor Mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laine L. Review article: gastrointestinal bleeding with low-dose aspirin - what's the risk? Aliment Pharmacol Ther. 2006;24:897–908. doi: 10.1111/j.1365-2036.2006.03077.x. [DOI] [PubMed] [Google Scholar]
- 52.Laine L., Curtis S.P., Cryer B., Kaur A., Cannon C.P. Risk factors for NSAID-associated upper GI clinical events in a long-term prospective study of 34 701 arthritis patients. Aliment Pharmacol Ther. 2010;32:1240–1248. doi: 10.1111/j.1365-2036.2010.04465.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee M., Feldman M. The aging stomach: implications for NSAID gastropathy. Gut. 1997;41:425–426. doi: 10.1136/gut.41.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patrono C., Garcia Rodriguez L.A., Landolfi R., Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 55.Greenwald D.A. Aging, the gastrointestinal tract, and risk of acid-related disease. Am J Med. 2004;117(Suppl 5A):8S–13S. doi: 10.1016/j.amjmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Pilotto A. Aging and upper gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18(Suppl):73–81. doi: 10.1016/j.bpg.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 57.Lee H.L., Han D.S., Kim J.B., Kim J.P., Jeon Y.C., Sohn J.H., Hahm J.S. [Importance of age and other risk factors in NSAID-induced gastropathy] Korean J Gastroenterol. 2004;44:246–251. [PubMed] [Google Scholar]
- 58.Iijima K., Kanno T., Koike T., Shimosegawa T. Helicobacter pylori-negative, non-steroidal anti-inflammatory drug: negative idiopathic ulcers in Asia. World J Gastroenterol. 2014;20:706–713. doi: 10.3748/wjg.v20.i3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon H., Kim S.G., Jung H.C., Song I.S. High recurrence rate of idiopathic peptic ulcers in long-term follow-up. Gut Liver. 2013;7:175–181. doi: 10.5009/gnl.2013.7.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong G.L., Wong V.W., Chan Y., Ching J.Y., Au K., Hui A.J., Lai L.H., Chow D.K., Siu D.K., Lui Y.N., Wu J.C., To K.F., Hung L.C., Chan H.L., Sung J.J., Chan F.K. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009;137:525–531. doi: 10.1053/j.gastro.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Bocker-Meffert S., Rosenstiel P., Rohl C., Warneke N., Held-Feindt J., Sievers J., Lucius R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci. 2002;43:2021–2026. [PubMed] [Google Scholar]
- 62.Lazarovici P., Marcinkiewicz C., Lelkes P.I. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr Pharm Des. 2006;12:2609–2622. doi: 10.2174/138161206777698738. [DOI] [PubMed] [Google Scholar]
- 63.Jadhao C.S., Bhatwadekar A.D., Jiang Y., Boulton M.E., Steinle J.J., Grant M.B. Nerve growth factor promotes endothelial progenitor cell-mediated angiogenic responses. Invest Ophthalmol Vis Sci. 2012;53:2030–2037. doi: 10.1167/iovs.11-8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka A., Wakita U., Kambe N., Iwasaki T., Matsuda H. An autocrine function of nerve growth factor for cell cycle regulation of vascular endothelial cells. Biochem Biophys Res Commun. 2004;313:1009–1014. doi: 10.1016/j.bbrc.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 65.Majumdar A.P. Regulation of gastrointestinal mucosal growth during aging. J Physiol Pharmacol. 2003;54(Suppl 4):143–154. [PubMed] [Google Scholar]
- 66.Rongo C. Epidermal growth factor and aging: a signaling molecule reveals a new eye opening function. Aging (Albany NY) 2011;3:896–905. doi: 10.18632/aging.100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brzozowski T., Konturek P.C., Konturek S.J., Schuppan D., Drozdowicz D., Kwiecien S., Majka J., Nakamura T., Hahn E. Effect of local application of growth factors on gastric ulcer healing and mucosal expression of cyclooxygenase-1 and -2. Digestion. 2001;64:15–29. doi: 10.1159/000048835. [DOI] [PubMed] [Google Scholar]
- 68.Ernst H., Konturek P.C., Hahn E.G., Stosiek H.P., Brzozowski T., Konturek S.J. Effect of local injection with basic fibroblast growth factor (bFGF) and neutralizing antibody to bFGF on gastric ulcer healing, gastric secretion, angiogenesis and gastric blood flow. J Physiol Pharmacol. 2001;52:377–390. [PubMed] [Google Scholar]
- 69.Florkiewicz R.Z., Ahluwalia A., Sandor Z., Szabo S., Tarnawski A.S. Gastric mucosal injury activates bFGF gene expression and triggers preferential translation of high molecular weight bFGF isoforms through CUG-initiated, non-canonical codons. Biochem Biophys Res Commun. 2011;409:494–499. doi: 10.1016/j.bbrc.2011.05.033. [DOI] [PubMed] [Google Scholar]