Abstract
Background
Surgical masks (SMs) are used to reduce bacterial shedding from the mouth, nose and face. This study aimed to investigate whether SMs may be a potential source of bacterial shedding leading to an increased risk of surgical site infection.
Methods
Bacterial contamination of the SMs was tested by making an impression of the external surface of the mask on sterile culture media immediately. We investigated the difference in bacterial counts between the SMs worn by surgeons and those placed unused in the operating room (OR), and the bacterial count variation with indicated wearing time. Moreover, the difference in bacterial counts on the external surface between the first and second layers of double-layered SMs was also assessed.
Results
The bacterial count on the surface of SMs increased with extended operating times; significant difference was found between the 4- to 6-hour and 0-hour groups (p < 0.05). When we analysed the bacterial counts from the same surgeon, a significant increase was noted in the 2-hours group. Moreover, the bacterial counts were significantly higher among the surgeons than the OR. Additionally, the bacterial count of the external surface of the second mask was significantly higher than that of the first one.
Conclusions
The source of bacterial contamination in SMs was the body surface of the surgeons rather than the OR environment. Moreover, we recommend that surgeons should change the mask after each operation, especially those beyond 2 hours. Double-layered SMs or those with excellent filtration function may also be a better alternative.
The translational potential of this article
This study provides strong evidence for the identification that SMs as source of bacterial contamination during operative procedures, which should be a cause for alarm and attention in the prevention of surgical site infection in clinical practice.
Keywords: Hospital-acquired infection, Surgical mask, Surgical site infection
Abbreviations: CDC, Center for Disease Control; CFU, Colony-Forming Unit; HAI, Hospital-Acquired Infection; SM, surgical mask; SSI, surgical site infection; TJA, Total Joint Placement
Introduction
Hospital-acquired infections rank among the top 10 leading causes of hospital deaths in the United States, and surgical site infections (SSIs) contribute to more than 20% of them [1]. It is estimated by the Center for Disease Control and Prevention that 2.7% of surgical procedures are complicated by SSI [2]. It is generally accepted that SSI is one of the most common and costly postoperative complications leading to increased morbidity, mortality, length of stay, hospital readmission and hospital costs [2], [3], [4]. Particularly in orthopaedics, SSI after total joint placement (TJA) can be devastating and a costly complication [5], [6]. Moreover, an increasing number of TJAs are performed yearly [7], the rate of which has been estimated to range from 0.2% to 2% [8]. Given its heavy economic burden on the patient and health-care system [9], it is of utmost importance to find ways to reduce SSI.
Prevention of SSIs is a goal of surgeons in the operating room (OR); controlling airborne contamination and reducing microbial shed from personnel may help decrease the incidence of SSIs. Controlling airborne contamination is not difficult, especially if a laminar flow system of ventilation is present, which can significantly purify the air and reduce bacterial load [10], [11]. In addition, a proper surgical attire, including the use of surgical gowns, sterile gloves, surgical hats and masks to the maximum extent, prevents microbial shed from the surgical personnel [12], [13]. The surgical attire aims to provide a functional barrier between the surgical team and patient. However, the efficacy of the surgical attire, such as surgical masks (SMs), in preventing SSI is often unclear [12]. Given that the overall prevalence of SSIs is low, a large number of participants or procedures must be included for a study to prove the efficacy of a particular intervention; thus, many of the current practices have limited literature support [14].
The present study discusses the role of SMs as potential sources of bacterial load, contaminating the surgical work area. For example, a previous study has evaluated the effectiveness of different headgears in preventing airborne contamination and demonstrated that bouffant hats cannot be considered superior and may be a source of contamination (hats) [15]. We hypothesised that SMs, as a tool for reducing the bacterial shedding from the mouth, nose and face, may become a potential contamination sources when worn for an extended period of time. Thus, this study aimed to answer the following three questions: (1) does the mask get contaminated if the wearing time is extended? (2) what is the source of contamination of the mask surface, surgical personnel or airborne contamination? and (3) will higher filtration reduce external surface contamination of masks?
Materials and methods
The study was performed in the OR. The study team consisted of four surgeons, a student and a microbiologist. The student cultivated the bacteria in the agar plate and counted the colony-forming units (CFUs). In this experiment, a single-blind was used, wherein the student did not know which group the SMs are from.
Forty cases of TJA were enrolled in this study. We divided the total surgical procedures into the following groups: 0- to 2-hours, 2- to 4-hours, 4- to 6-hours and no SM-used groups. After TJA, the SMs were put into sterile bags and submitted to the student. The surfaces of the SMs were cut, on an average, into three parts, and an impression was made on the sterile agar plate on a clean bench and incubated for 48 hours in an aerobic humid atmosphere at 37°C. The CFUs were then counted. We investigated the degree of contamination of SMs at different surgical stages, and the difference of bacterial counts between the SMs worn by surgeons and those unused in the OR. We also assessed the difference in the counts between the surfaces of single- and double-layered SMs.
Statistical analysis
The results are expressed as the mean ± standard deviation. Statistical differences were analysed using one-way analysis of variance followed with Dunnett post hoc test; “*” indicates a significant difference (p < 0.05), and “**” indicates a highly significant difference (p < 0.01).
Results
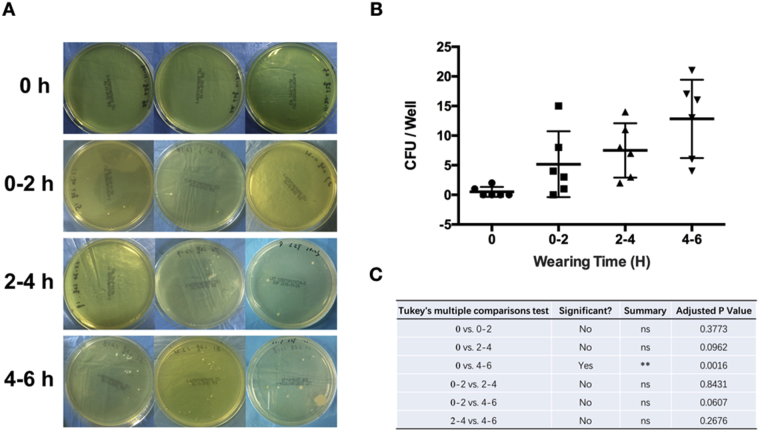
The area of sampling is shown in Figure 1. With wearing time extension, an increasing trend can be seen in the CFUs from the surface of SMs (Fig. 2A). Owing to the high variation, significance was only identified between the 0-hour and 4- to 6-hours groups (Fig. 2B). However, when we extracted the data separately and compared the CFUs from the surface of SMs used by the same surgeon, significant differences in CFU counts were observed among all extended wearing time groups (Fig. 3). These results demonstrated that the contamination of the SM surface worsens with wearing time extension. Meanwhile, a high variation existed among different surgeons.
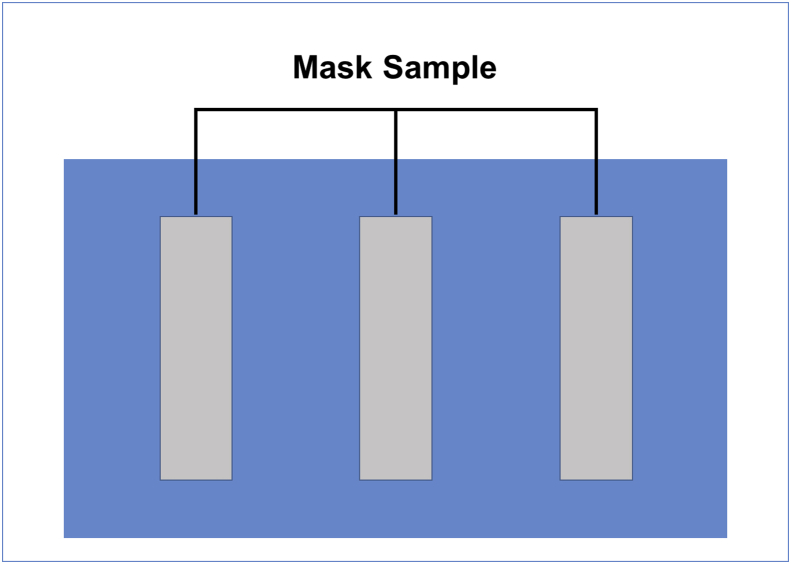
Figure 1.
Impression of mask sampling on the sterile agar plate.
Figure 2.
Mask contamination within indicated wearing times for four surgeons. (A) Representative CFUs on the agar plate; (B) analysis of the CFUs; (C) p values.
CFUs = colony-forming units.
Figure 3.
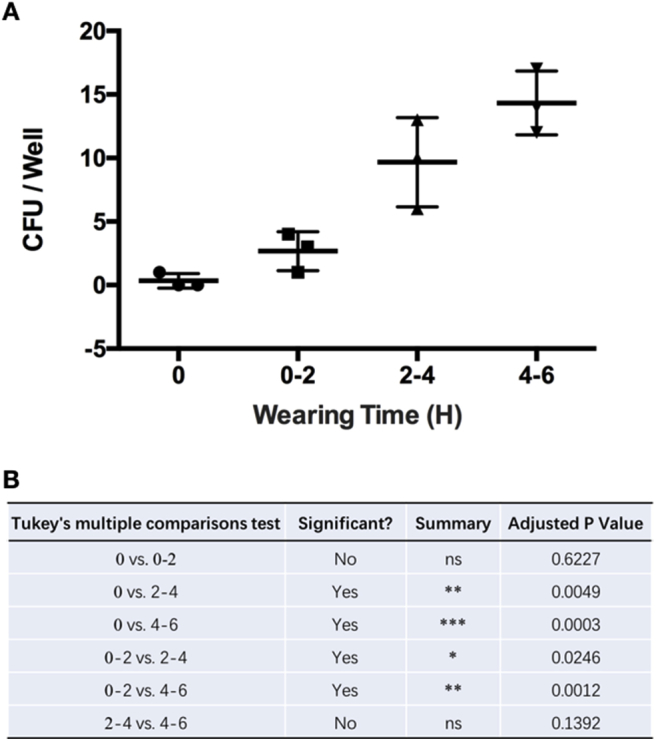
Mask contamination within indicated wearing times for the same surgeon. (A) Analysis of the CFUs. (B) p values.
CFUs = colony-forming units.
Furthermore, between surgeon-wearing and OR positioned groups, more CFUs from the surface of SMs were identified in the surgeon-wearing group (Fig. 4A and B). A significant difference could be identified in the 2-hours group, but not in the 4-hours group (Fig. 4C). These results demonstrated that the contamination of the SM surface more likely came from the surgeons themselves. With wearing time extended, the OR atmosphere could be another contamination source.
Figure 4.
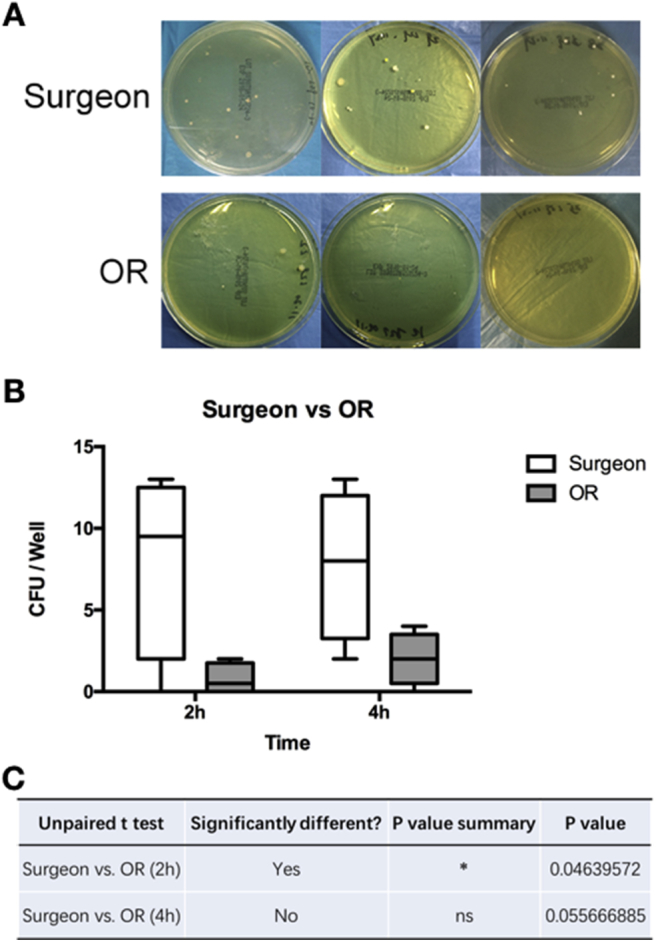
Mask contamination from the masks used by surgeon and unused masks in the OR. (A) Representative CFUs on the agar plate. (B) Analysis of the CFUs. (C) p values.
CFUs = colony-forming units; OR = operating room.
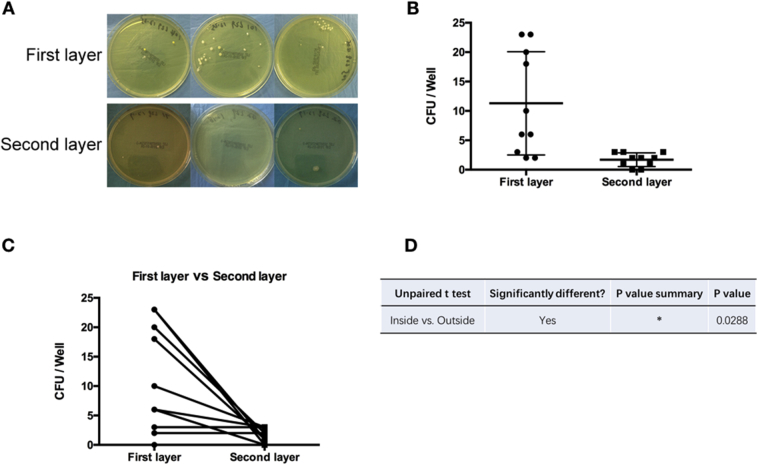
In addition, the mean of CFUs isolated from the first layer mask (close to the face) was higher compared to the second layer (far from the face) in double-layered SMs (Fig. 5). It is notable that the mean of CFUs isolated from the first layer did not have a small dispersion (Fig. 5A and B). When comparing the double-layer masks used by the same surgeon, the number of CFUs isolated from the first layer was higher compared to the second layer (Fig. 5C). These results demonstrated that double-layered SMs, which have higher filtration, could significantly reduce the surface contamination in operating work areas.
Figure 5.
Mask contamination from double-layered masks. (A) Representative CFUs on the agar plate; (B, C) analysis of the CFUs; (D) p values.
CFUs = colony-forming units.
Discussion
An increasing number of TJAs are performed yearly in orthopaedic departments. SSI is the most common complication associated with TJA, which results in a heavy economic burden on the patients and health-care system [16], [17]. Therefore, finding measures to reduce SSI is of utmost importance. The laminar flow system of ventilation and surgical attire have been used during surgical procedures in the OR over the past several decades. Over the past 50 years, the surgical attire has remained relatively unchanged. This uniform has traditionally been thought to play two roles: to protect scrubbed personnel from exposure to body fluids and to maintain the sterility of the surgical field. However, whether the measures in the prevention of bacterial shed from the surgical personnel can become the source of bacterial contamination is worth being discussed. Here, we report that the SMs may be the potential sources of bacterial contamination with the progression of surgical procedure (Supplemental Figure 1). Generally, bacterial contamination has been used as an adjunct measure of SSI, commonly measured by airborne or settled CFU counts [12].
In this present study, we firstly examined the bacterial contamination of SMs with various wearing time in the OR. The SMs were collected from four surgeons, and the mean number of CFUs showed an increased trend with extended wearing time, yet without significance. However, when we analysed the data within the same surgeon, significance could be identified almost between any two groups. Thus, we concluded that the SMs were clean before wearing and get contaminated once they were used, and the contamination became more severe with extended wearing time in the OR. Meanwhile, high variation in this assay might be because of the different hygienic practices among the surgeons.
This identification raised another question on whether the bacteria of the SMs came from the surgeon shed or from air-borne contamination. To answer this question, surgeons who wore SMs were categorised in the surgeon-wearing group, while those SMs placed separately in the OR at the same time were classified as OR positioned groups (parallel control). The results showed that the masks from the surgeon groups had more CFUs than those in the OR groups. Interestingly, it is notable that a significance could be identified in the 2-hours group than in the 4-hours group. Thus, we concluded that the bacteria on SMs was more likely from the surgeon rather than from the OR air-borne contamination, especially in the early period.
Furthermore, now that SMs could bear more bacteria with extended wearing time and could become the source of shed-induced infection during operation, masks with higher filtration might be an effective tool to reduce bacterial contamination. To verify this hypothesis, surgeons were asked to wear two SMs simultaneously. Masks closed to the faces were named the first layer, and the outer layer masks were named as the second layer. The present results indicated that the CFUs significantly declined in the second masks, which means that the double-layered masks significantly reduced the contamination of the external surface of SMs. Thus, we concluded that wearing double-layered SMs might be an effective, low cost and easy measure in the prevention of bacterial shedding during operations.
In summary, the topic of SMs in the OR has been controversial. The scientific study to support the OR policies surrounding this topic is marginal. The purpose of this study was to investigate whether the SMs is a potential source of bacterial shedding, which may lead to the understanding of the causes of SSI. Based on our research, we mainly draw three conclusions: (1) SMs could be the source of bacterial shedding with extended wearing time; thus, we recommend that surgeons must change his/her mask in every operation interval; (2) bacteria on the external surface of the SMs are more likely from surgeons, which might be related to the surgeons’ hygienic practices; thus, we recommend that surgeons must give more emphasis on face-mouth cleanliness and personal hygiene and (3) high-filtration masks, such as double-layered masks, could be an effective measure in reducing mask contamination.
Indeed, although a direct correlation between mask and SSIs has not been proven in the literature, the theory of aseptic technique is founded on the premise that a reduction in bacterial contamination will reduce the prevalence of SSI. Moreover, as the saying goes: “Do not think any virtue trivial, and so neglect it; do not think any vice trivial, and so practice it”. Especially in TJA operations, taking effective measures to limit SSI is still of utmost importance. We hope that our study could attract more attention and research on the risk factors of SSI.
There were several limitations in this study that should be noted. First, the external surface of mask was the region of interest; however, the sampling operation could increase the risk of cross-contamination. We carefully collect the used-masks, make sure that only the region of interest get in touch with sterile culture media and try our best to reduce the risk of cross-contamination. Meanwhile, we strictly adhere to the protocol of sampling operation to ensure the consistency and reliability. In addition, we also realise that there are likely numerous brands of masks that are made of different materials. Some of these might perform better than others in preventing microbial shed. Comparing specific brands of masks was beyond the scope of this study and could be considered for additional studies.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgement
This work was supported by the National Natural Science Foundation for Youths (Grant No. 81601958), Shanghai Sailing Program (Grant No. 16YF1407500), and Shanghai Jiao Tong University Medical and Engineering Cross Fund (Grant No. YG2015QN41).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jot.2018.06.002.
Contributor Information
Li Huiwu, Email: huiwu1223@163.com.
Liu Fengxiang, Email: liu_fengxiang@126.com.
Zhai Zanjing, Email: zanjing_zhai@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
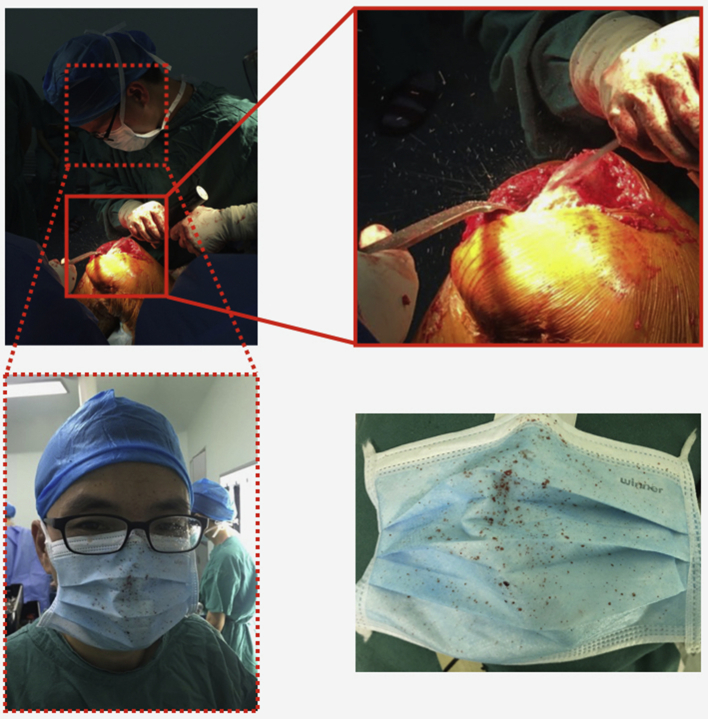
Supplemental Figure.
In the operation of total joint arthroplasty, high-speed of bone scrap could splash into the surgical mask. Thus, whether the surface of surgical mask is sterile is a question worth discussing. Especially in China, the majority of surgeons used to wear the same mask from the first operation to the last one.
References
- 1.de Lissovoy G., Fraeman K., Hutchins V., Murphy D., Song D., Vaughn B.B. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387. doi: 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Kirkland Kathryn B., Trivette Sharon L., Wilkinson William E., Sexton Daniel J. The impact of surgical-site infections in the 1990S: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 2014;20:725. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 3.Astagneau P., Rioux C., Golliot F., Brucker G., Group I.N.S. Morbidity and mortality associated with surgical site infections: results from the 1997-1999 INCISO surveillance. J Hosp Infect. 2001;48(4):267. doi: 10.1053/jhin.2001.1003. [DOI] [PubMed] [Google Scholar]
- 4.Leaper David J., van Goor H., Reilly Jacqueline, Petrosillo Nicola, Geiss Heinrich K., Torres A.J. Surgical site infection-a European perspective of incidence and economic burden. Int Wound J. 2004;1:247. doi: 10.1111/j.1742-4801.2004.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp S.L., Berbari E.F., Osmon D.R., Schroeder D.R., Hebl J.R., Horlocker T.T. The impact of anesthetic management on surgical site infections in patients undergoing total knee or total hip arthroplasty. Anesth Analg. 2015;121(5):1215. doi: 10.1213/ANE.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 6.Inacio M.C., Kritz-Silverstein D., Raman R., Macera C.A., Nichols J.F., Shaffer R.A. The impact of pre-operative weight loss on incidence of surgical site infection and readmission rates after total joint arthroplasty. J Arthroplasty. 2014;29(3):458. doi: 10.1016/j.arth.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Poultsides L.A., Ma Y., Della Valle A.G., Chiu Y.L., Sculco T.P., Memtsoudis S.G. In-hospital surgical site infections after primary hip and knee arthroplasty–incidence and risk factors. J Arthroplasty. 2013;28(3):385. doi: 10.1016/j.arth.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Pugely A.J., Martin C.T., Gao Y., Schweizer M.L., Callaghan J.J. The incidence of and risk factors for 30-day surgical site infections following primary and revision total joint arthroplasty. J Arthroplasty. 2015;30(9 Suppl):47. doi: 10.1016/j.arth.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 9.Poultsides L.A., Liaropoulos L.L., Malizos K.N. The socioeconomic impact of musculoskeletal infections. J Bone Joint Surg Am Vol. 2010;92(11):e13. doi: 10.2106/JBJS.I.01131. [DOI] [PubMed] [Google Scholar]
- 10.Scott C.C. Laminar/linear flow system of ventilation its application to medicine and surgery. Lancet. 1970;989 doi: 10.1016/s0140-6736(70)91110-4. [DOI] [PubMed] [Google Scholar]
- 11.Scott C.C., Sanderson J.T., Guthrie T.D. Choice of ventilation system for operating-theatres comparison of turbulent versus laminar/linear flow systems in operating-rooms and industrial clean rooms. Lancet. 1971;1288 doi: 10.1016/s0140-6736(71)91795-8. [DOI] [PubMed] [Google Scholar]
- 12.Salassa T.E., Swiontkowski M.F. Surgical attire and the operating room: role in infection prevention. J Bone Joint Surg Am Vol. 2014;96(17):1485. doi: 10.2106/JBJS.M.01133. [DOI] [PubMed] [Google Scholar]
- 13.Adams L.W., Aschenbrenner C.A., Houle T.T., Roy R.C. Uncovering the history of operating room attire through photographs. Anesthesiology. 2016;124(1):19. doi: 10.1097/ALN.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 14.Evans R.P. Current concepts for clean air and total joint arthroplasty: laminar airflow and ultraviolet radiation: a systematic review. Clin Orthop Relat Res. 2011;469(4):945. doi: 10.1007/s11999-010-1688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markel T.A., Gormley T., Greeley D., Ostojic J., Wise A., Rajala J. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225(5):573. doi: 10.1016/j.jamcollsurg.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Anthony C.A., Peterson R.A., Sewell D.K., Polgreen L.A., Simmering J.E., Callaghan J.J. The seasonal variability of surgical site infections in knee and hip arthroplasty. J Arthroplasty. 2018 Feb;33(2):510–514.e1. doi: 10.1016/j.arth.2017.10.043. Epub 2017 Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berbari E.F., Osmon D.R., Lahr B., Eckel-Passow J.E., Tsaras G., Hanssen A.D. The Mayo prosthetic joint infection risk score: implication for surgical site infection reporting and risk stratification. Infect Control Hosp Epidemiol. 2012;33(8):774. doi: 10.1086/666641. [DOI] [PubMed] [Google Scholar]