Abstract
The premise of medical screening is to identify clinically occult disease, facilitating intervention at an early stage with the intention of improving prognosis. Identifying solid organ malignancy before nodal or distal metastases have occurred unanimously offers the best chance of successful radical treatment, thus there is clearly a potential significant mortality benefit for successful oncological screening programmes. However, the negative consequences of screening have to be considered, particularly the impact of intervening in asymptomatic populations. Diagnostic radiology has an invaluable ability to non-invasively detect disease and has developed an essential role in several oncological screening programmes with new programmes emerging. These include the established mammography screening programme for breast carcinoma, the emerging CT screening programme for lung carcinoma and a new proposed radiological screening programme for pancreatic carcinoma. Results from published randomized controlled trials analysing the benefits of radiological screening have been convoluted and conflicting. Cancer screening remains a widely contested topic and it is a challenge for both radiologist and clinician to assess the risks and benefits at both a population and individual patient level. In this article, we discuss radiological screening and analyse the current literature on these programmes, with evaluation of recently published studies and ongoing trials.
Keywords: Radiology, screening, malignancy
Introduction
The principles of screening were first officially described in a document produced by the World Health Organisation in 1968 (1). The authors, Wilson and Junger, described “the object of screening for disease is to discover amongst the apparently well those who are in fact suffering from disease” (1). Over the past 50 years, multiple national and international screening programmes have been established. Table 1 lists all screening programmes in the UK and America. Table 2 lists all countries in the International Cancer Screening Network with variable established screening programmes for cervical, breast and colorectal malignancy. Diagnostic radiology, given its invaluable ability to non-invasively detect disease, has developed an essential role in several screening programmes and new programmes are emerging. Whilst there is tremendous potential for radiological screening, the limitations and negative consequences of intervening in asymptomatic populations have to be considered. In the original WHO document, the authors recognised potential issues with screening such as the lack of a proven cure, cost and inadequate knowledge of the principles and practice of screening. This article analyses the literature on the benefits and consequences of established radiological screening programmes for breast and lung cancer and discusses an emerging programme in screening for pancreatic cancer.
Table 1. National screening programmes in UK and USA (2-6).
| UK |
| Malignant |
| Cervical screening |
| Breast cancer screening |
| Bowel cancer screening |
| Non-malignant |
| Abdominal aortic aneurysm (AAA) screening |
| Diabetic eye screening |
| Antenatal |
| Fetal anomaly screening |
| Infectious diseases in pregnancy |
| New born and infant physical examination |
| New born blood spot |
| New born hearing |
| Sickle cell and thalassaemia |
| USA |
| Malignant |
| Breast cancer |
| Colorectal cancer and polyp screening |
| Cervical cancer |
| Uterine cancer—high-risk patients |
| Lung cancer—high-risk patients |
Table 2. International Cancer Screening Network, 33 country members including (2-6).
| Country | Nationally implemented screening programmes | ||
|---|---|---|---|
| Cervical | Breast | Colorectal | |
| Australia | ● | ● | ● |
| Belgium | – | ● | – |
| Canada | ● | ● | – |
| Czech Republic | ● | ● | ● |
| Finland | ● | ● | ● |
| France | – | ● | ● |
| Germany | – | ● | – |
| Greece | – | ● | – |
| Hungary | – | ● | ● |
| Iceland | ● | ● | – |
| Ireland | – | ● | |
| Israel | – | ● | ● |
| Italy | ● | ● | – |
| Japan | ● | ● | ● |
| Korea | ● | ● | ● |
| Luxemburg | – | ● | – |
| Netherlands | ● | ● | |
| New Zealand | ● | ● | ● |
| Norway | ● | ● | – |
| Portugal | ● | ● | – |
| Sweden | – | ● | – |
| Switzerland | – | ● | – |
| UK | ● | ● | ● |
| USA | ● | ● | ● |
Source: ICSN website.
To systematically identify relevant trial and non-trial evidence, literature searches were performed on the Medline database. Only studies published in the last 20 years [1997–2017] were analysed. For breast cancer, search terms included screening AND mammography AND breast AND cancer. For lung cancer, search terms included screening AND CT AND lung AND cancer. A small number of studies have looked at pancreatic cancer screening which we reviewed. Authors focussed on randomised controlled trial (RCT) evidence where possible.
Benefits, limitations and risks of radiological screening
Before reviewing specific screening programmes, it is important to review generic benefits and limitations of radiological screening. Some of the most successful screening programmes rely on the invasive detection of malignant pre-cursors. For example, cervical cancer screening relies on the early detection of intra-epithelial neoplasia and results in the early treatment of premalignant cells and evidence suggest this results in a reduction in invasive cancer cases. For example, a study has shown that participation in the UK cervical screening programme between the ages of 40–42 and 62–64 years reduces a woman’s risk of cervical cancer by 64% and 82% respectively over the subsequent 5 to 8 years, compared to women of the same age who don’t partake in the national screening programme (7). However, whilst there is evidence that some invasive screening techniques reduce cancer mortality, their invasive nature compared to minimally invasive techniques are less appealing to the asymptomatic invitee thus may reduce participation rates. Two recently published trials randomising bowel cancer screening patients to either CT colonography (CTC) or direct colonoscopy (8), or CTC or flexible sigmoidoscopy (9), demonstrated a higher participation rate in CTC than colonoscopy in both studies (34% vs. 27% and 30% vs. 27% respectively). Theoretically, radiological screening should be less likely to cause complications than invasive screening programmes and evidence suggests this is the case. Taking colorectal cancer screening as an example, studies have demonstrated colonic perforation to occur in 0.02% of CTC cases (10) compared to 0.06% (11) in colonoscopy and 0.09% in flexible sigmoidoscopy (12,13). Perforations in CTC cases are also less likely to require surgical intervention than those occurring in colonoscopy [32% (10) vs. 78% (14) respectively]. The potential mortality benefits from radiological screening programmes are reviewed for each tumour type in the subsequent sections.
Whilst radiological screening has demonstrated certain clear benefits, there are several limitations. Firstly, false positive results lead to unnecessary biopsies and resections with potentially lengthy follow-up in addition to the anxiety surrounding a “positive diagnosis”. Additionally, false negative studies reassure patients who may then fail to seek advice if they become symptomatic. Secondly, data can be misleading in the form of lead time bias. Lead time bias is the systematic error of apparent increased survival from detecting disease in an early stage and must be considered when analysing the success of screening programmes (15). This is the theory that whilst detection rates may improve with screening (therefore suggesting an improvement in cancer survival rates), if screening predominantly detects indolent disease, the effect on survival rates is biased. Aggressive disease (by their fast-growing nature) may arise in the years between routine screening and progress rapidly, thus missing the opportunity for early detection/treatment and biasing survival rates.
Whilst in theory these benefits and limitations could be analysed in objective RCTs, a significant difficulty in assessing trial evidence for screening programmes is that there is no study endpoint as screening is an ongoing process. Therefore, the degree of benefit has to be estimated by statistical and epidemiological methods which have created polarised discussion in academic radiology communities (16). An additional issue with trial evidence is that outcome measures (primarily mortality benefit between screened and non-screened arms) may not be apparent until many years after the initiation of the study, by which time there are multiple confounding factors. For example, improvements in oncological treatment over time, which may bias the mortality benefit of screening programmes over a long follow-up period.
Breast cancer screening
Breast cancer has the highest incidence and mortality amongst all cancers in women globally (17). There is clearly significant potential on a global scale if screening for breast cancer can reduce mortality. Mammography breast cancer screening programmes have been around for decades, introduced nationally in the UK for example in 1988. However, despite widespread implementation, there has been significant debate as to the degree of the benefits versus risks of mammography screening (16). For example, quoted rates of overdiagnosis range from 1% (18) to 52% (16,19). National Institute of Clinical Excellence (NICE) in the UK describes a mixture of benefits and harms in breast mammography screening and recognises it is impossible to give accurate estimates of lives saved as estimates vary widely (20). This is because there are conflicting opinions about the quality of the studies, the methodology used and whether benefits are outweighed by harms (20). The UK government jointly commissioned a review in 2013 with Cancer Research UK, led by a panel of independent experts (the UK Independent Panel on Breast Cancer screening) (16,21) and some of the conclusions of this are summarised below.
One of the issues with assessing evidence for breast cancer screening is that the RCTs were conducted 20–30 years ago, with more contemporary estimates of benefits coming from observational studies with heterogenous designs (21).
The International Agency for Research on Cancer published an advisory document in 2002 which pooled data from six RCTs analysing the efficacy of mammography screening. These trials randomised women within cohorts aged between 40–74 to mammography or control (22). The pooled data demonstrated that breast cancer mortality was 496/100,000 person years in the screened group vs 549/100,000 person years in the control group (22). The estimated relative risk from death from breast cancer in screened vs unscreened groups was thus shown to be 0.75 (95% CI, 0.67–0.85) (22). This analysis was used by the Advisory Committee for Breast Cancer Screening recommendations in their NHS Breast Cancer screening publication in 2006 (23). They explained the results showed a “25% reduction in mortality using an intention to treat analysis”, extrapolated to a reduction of 35% in women who are screened regularly. It was then estimated that 1,400 lives a year in the UK were saved with the NHS screening programme, or a reduction in mortality from 8 to 5.2 per 1,000 women if screened over a ten year period (23).
More recently in 2013, the UK Independent Panel on Breast Cancer Screening explained that the best evidence for mortality reduction comes from 11 RCTs on breast screening which estimate screening causes a relative 20% reduction in mortality (21). However, the panel describes certain issues with the evidence such as wide confidence intervals (11–27%), potential distortion in the trial evidence (e.g., suboptimal randomisation and adjudicating in the causes of death) and relevance of older trials to current practice (21). The panel felt more contemporaneous observational studies findings were heterogenous in design and findings, but generally results in the same direction as the trials.
Pooled data from several Swedish randomized trials (using some of the RCT data described previously) calculated a 21% relative risk reduction in mortality from screened over unscreened women over a fifteen year follow up period and revealed that the relative benefit was most significant in the 60–69-year-old age bracket (33%) (24). However, absolute numbers of breast cancer deaths were small; 511/1,864,770 in the screened groups over 584/1,688,440 in the control groups (24).
Estimates for absolute mortality benefit vary widely from 1 in 100 to 1 in 1,000 (21,25). Reasons for the variation include the age of women screened and the variation in screening/follow-up (21). The UK Independent Panel on Breast Cancer Screening extrapolated the relative risk reduction of 20% to the observed cumulative absolute risk of breast cancer mortality over the ages of 55–79 and estimated 1 breast cancer death prevented from every 235 women invited to screening and 1 life saved for every 180 women screened (21).
A study has reviewed data from the Surveillance, Epidemiology, and End Results (SEER) programme from 1975 through to 2012 to establish the success of mammography screening (26). The study concluded that since the introduction of screening, 162 extra small cancers were observed per 100,000 women. However, they estimated that only 30 of these 162 (19%) were expected to become large, assuming the underlying disease burden remained stable. Therefore, over 80% of early breast cancers are being over-diagnosed and they felt the reduction in breast cancer mortality was primarily due to improvements in treatment. The methodology of this study has been disputed. Koplan argues that their estimated incidence increase of 0.25% per year for invasive breast cancer is a sizeable underestimate (27). Data collected from the Connecticut Tumour Registry over a 40-year period shows the incidence of invasive breast cancer has increased by 1% per year since 1940. If this rate is used then the incidence of invasive breast cancer in 2008 was actually lower than what was expected by extrapolating (27). A Cochrane review published in 2011 compared mammography with no mammography screening (25). They estimated that for every 2,000 women screened over a 10-year period, one life will be saved but 10 women treated unnecessarily (25). However, another study, in part using data from the Swedish trials mentioned previously, concluded that between 2–2.5 lives were saved per women over diagnosed (Duffy 2010) (18).
Whilst diagnosing more early-stage tumours should theoretically reduce mortality, lead time bias is an important consideration. Screening works on the presumption that all early tumours lead to death, whereas some slow growing tumours may never become clinically apparent during a patient’s lifetime. Historically, breast cancer therapy is based on the size of tumour and extent of spread, however, tumour biological characteristics are now becoming more relevant to prognosis (26). It is now felt that the biology of a tumour is more important to prognosis than the size. While there is a current focus on improving mammogram resolution, should the future of breast screening take a more biological approach? While overall survival rates from breast cancer have improved since the introduction of breast screening, a large proportion of this small-observed decrease in mortality can potentially be attributed to the advances in treatment (28). This argument is further supported by a study which demonstrated that most of the reduction in breast cancer-specific mortality is explained by adjuvant therapy rather than screening mammography (29). Furthermore, the act of screening itself is not without morbidity—radiation exposure to the breast, pain and anxiety are important considerations when intervening in an asymptomatic population. There is limited evidence of a small risk of increased radiation-induced breast cancer. For example, a recent estimate is that screening women every 3 years from age 47–73 would cause 3–6 cancers per 10,000 women screened (30).
There is little evidence for the benefits of screening mammography in younger women i.e., age 40–59, as mammography is less sensitive in denser breast tissue. Miller et al. found that there was a 22% overdiagnosis rate in the 40–59 age group and there is level 1 evidence that screening mammography in women aged 40–49 does not reduce mortality (31,32).
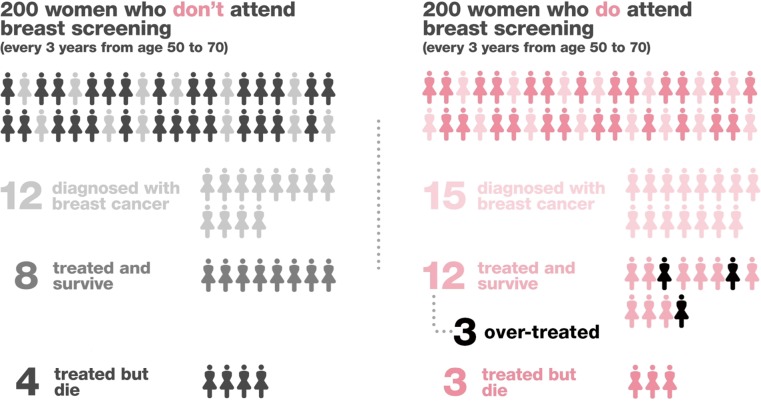
The evidence for breast cancer mammography screening is convoluted and conflicting. Whilst some studies demonstrate a significant relative risk improvement in screened vs. non-screened groups, the absolute improvement in mortality from breast cancer may be very small. Additionally, the emotional and physical burden caused by over-diagnosis and false positive results in asymptomatic women must be considered. For women aged 60–69, the evidence demonstrates more convincing benefits of screening whereas the evidence for screening the 40–59 age group is poor. It is important women are informed of both potential benefits and negative impact of participating in breast screening programmes. Several organisations and charities have tried to simplify the convoluted issues in the form of diagrams, for example, this diagram comparing screened and non-screened groups (Figure 1).
Figure 1.
Breast cancer now. Breast screening. Available at: http://breastcancernow.org/about-breast-cancer/want-to-know-about-breast-cancer/breast-screening (accessed 8.5.18).
Lung cancer screening
Lung cancer remains a significant health burden and was the leading cause of cancer death in both males and females in the UK in 2014 (33). With the success of screening breast, colorectal and cervical cancer, evidence to support a benefit of lung cancer screening has been long awaited. Identifying early stage disease in cancer has the greatest chance to improve patient survival and therefore a screening programme for lung cancer must confer this benefit above all others.
In 2014 the U.S. Preventative Services Task Force (USPSTF) made a ground-breaking recommendation of annual screening for lung cancer with low-dose computed tomography (LDCT) in high-risk individuals (34). The current UK stance is that screening should not be offered (35). However, this is imminently due for review pending results of several international trials.
Chest radiography (CXR) has been unsuccessful in yielding an intervention effect in lung cancer diagnosis or mortality (36). Since the 1990s there has been a shift towards LDCT screening. The Mayo Clinic prospective study screened annually over 5 years (37). Crucially, the majority (61%) of cancers detected were stage I disease. One of the common themes to emerge from this study was the false-positive rates in the order of 92.4–96%. With such a high degree, this invariably will generate patient anxiety with further radiation exposure and invasive procedures, as well as raising issues around cost-effectiveness. As a result of this 13 patients underwent surgery for benign disease. Despite this, no difference in mortality rates was seen when compared to the Mayo Lung Project (CXR and sputum screening) arguing against a successful screening tool (38).
The large prospective multi-national Early Lung Cancer Action (ELCA) Project showed a higher early disease detection: 85% of detected cancers were stage I with 91% undergoing surgical resection (39). The false-positive rate was much lower with approximately 12% of those screened at baseline requiring further management and in total there were 8% benign surgical interventions. However, Kaplan-Meier survival showed a predicted 10-year survival of 88% of stage I disease which appears significant compared to the 5-year survival of local disease with current detection and management of 55% (40).
The largest study to date is the randomised American National Lung Screening Trial (NLST) that recruited 53,454 individuals for LDCT at baseline and annually for 2 years with a median follow-up of 6.5 years (41). Of stage I cancers 63% were picked up at screening baseline vs. 47.6% in the CXR arm. 92.5% of the stage I cancers were treated with surgery (+/− adjuvant therapy). Most ground-breaking in this study, leading to the USPSTF recommendation, is the conclusive reduction in lung cancer mortality by 20% in screening high-risk individuals with a 6.7% reduction in overall mortality. This calculates the number needed to screen to prevent one cancer as 320 which compares favorably with breast cancer screening in England, estimated to be 400 (42). However, false positive rates remain notable at 23.3% (compared to 6.5% in the control arm). Moreover, 1.7% of those screened in the LDCT group had an invasive procedure that did not have lung cancer. The implications of this were assessed and of those who did not have a diagnosis of lung cancer but underwent an invasive diagnostic evaluation had a major complication rate of 0.06% (including 6 deaths within 60 days of an invasive diagnostic procedure) which the authors categorise as a “rare” occurrence.
The UK Lung Cancer Screening single screen protocol trial without interval screening has built on the NLST (43). In total, 85.7% stage I/II disease was detected with 83% having surgical intervention making this one of the strongest early disease detectors. There was a low benign surgical rate of 10.3% which the authors attribute to volumetric based nodule management (NLST, for instance, used diameter measurement of 4 mm for nodule assessment; little consensus is agreed on nodule assessment). The high rate of surgical interventions is comparable to NLST and ELCA. Similar results of lung cancer resectability have been shown in the ITALUNG trial (annual screening for 4 years): 85% of screen-detected lung cancers were amenable to surgical resection and this was associated with a 10% surgical resection rate for benign pathology in keeping with previous levels (44).
LDCT with the aim of reducing mortality rates over 10 years is currently ongoing in the NELSON trial and a subset analysis assessing screening interval has been carried out (45). Mayo Clinic, ELCA and NLST used annual interval screening in high-risk individuals but this raises the issue of cost, feasibility and radiation exposure. Intervals of 1, 2 and 2.5 years have been assessed. In their latest sub-analysis, a 2.5-year interval led to 60.9% stage I disease detection. This was in comparison with 75.9% with 1 year and 72.7% in the 2-year intervals. The authors noted an increase in late-stage cancer that arose in the interval period compared to the screening round: 64.3% vs. 17.3%. This increased interval therefore suggests the effect of screening is reduced and crucially lessens the number of resectable cancers.
The results from the screening rounds from the RCT Danish Lung Cancer Screening Trial provide an insight into the effects of overdiagnosis (46). Annual LDCT picked up significantly more early-stage lung cancers (stage I–IIb) compared to controls (70% vs. 33%), 37% excess early stage cancers diagnosed. However, no difference in mortality rates between groups has yet been demonstrated, contrasting NLST results. It is possible, as the authors highlight, the effect of screening may not be observed until several years of follow-up (NLST followed up individuals for a median of 6.5 years) with less incurable late-stage disease.
Early recall rates and false positive results are an undesired but unavoidable effect of screening intervention. Baseline screening in The German Lung Cancer Screening Intervention Trial (LUSI) had a rate of false positives of approximately 18% (47). However, early recall was vastly reduced in annual repeat screening rounds 2–5% to 3–4%. Baseline screening identified 73.9% of stage I cancers; by round 5 this was 71.4%. No difference has been shown in mortality for the first 2 years but from year 3 onwards preliminary data suggests the cumulative all-cause mortality is less than the control group which is in keeping with NLST findings.
In conclusion, several large observational and randomised trials have demonstrated that a significant number of early-stage disease can be detected with LDCT screening in high-risk individuals, allowing higher radical surgical resection rates, leading to increased disease-free survival. However, this comes at a cost with high false positive findings, surgical intervention for benign disease and only one conclusive study demonstrating a mortality effect. This is in addition to ionising radiation exposure with the potential for radiation-induced malignancy, particularly for those younger individuals in the screening cohort (the majority of the trials screen from 50 years). It is not clear how long individuals will need to be screened for if a programme is active. At best a 2-year interval screening plan maintains a reasonable detection rate but the associated pitfalls will be costly. If the NLST mortality results are replicated a clear benefit to lung cancer screening is likely.
Pancreatic cancer screening
Pancreatic ductal adenocarcinoma has a high mortality rate, with a 5-year survival of less than 3%. The cancer is usually clinically occult until it is at an advanced and unresectable stage. There has been no established method of detecting early disease and therefore mortality rates have remained stable since the 1970s (48).
Risk factors for pancreatic cancer include lifestyle risks such as smoking in addition to multiple genetic mutations and syndromes such as BRCA2, p16, Peutz-Jeghers syndrome and hereditary non-polyposis colorectal cancer (48).
Pancreatic cancer screening has been proposed in those with genetic risk factors and deemed “high-risk”, with the aim of detecting asymptomatic disease at an early, resectable stage, hopefully reducing mortality rates. Tumour markers in the form of serum ca19-9 are not accurate enough as an isolated screening test due to low positive predictive values. Therefore, radiological or endoscopic screening is the only viable options, e.g., MRI with MRCP or endoscopic ultrasound.
Del Chiaro et al. analysed short-term data for MRI screening in 40 high-risk patients. Fourteen patients (35%) were diagnosed with intra-ductal papillary mucinous neoplasm (IPMN) of which 2 underwent surgery. Three patients (8%) were diagnosed with ductal adenocarcinoma, all of whom underwent surgery (49).
Poruk et al. summarised seven pancreatic screening studies and found 43 of 410 high-risk screening patients underwent surgical resection of identified lesions, of which eight cases were invasive pancreatic ductal adenocarcinoma (50). This highlights the problem with screening identifying incidental lesions, e.g., intraductal papillary mucinous neoplasms (IPMNs), which require additional invasive treatment or expensive long-term follow-up, as the natural history of these potentially premalignant lesions in high-risk patients is unknown.
At present, the benefit of radiological screening for pancreatic adenocarcinoma has not been proven. However, there are hopes for the development of serum biochemical markers with high sensitivity and specificity to detect early-stage cancers, potentially before they are visible on radiological imaging, which may have positive long-term effects on morbidity and mortality from pancreatic cancer in high-risk patients.
Conclusions
Since the publication of the WHO document in 1968, multiple radiological screening programmes have been established and multiple published studies have analysed their efficacy. Whilst the evidence demonstrates the varied success of these programmes, they share common benefits and limitations. A clear attribute of radiological screening is the ability to non-invasively detect clinically occult disease. Attendance rates are likely to be higher than invasive programmes and there will be a lower complication rate; essential when considering intervening in an asymptomatic population. However, in terms of a diagnostic investigation for the underlying type and grade of cancer, radiology will always be second best to the gold standard diagnostic tool of histopathology. Radiology has developed an excellent method of detecting loco-regional and distal metastatic disease, but generally lacks the ability to differentiate between high and low grade early stage tumours. Therefore, radiological screening has the negative consequence of intervening in false positive cases which causes unnecessary mental and physical side effects for patients. Future developments will almost certainly improve the accuracy of radiology in this regard. Well-recognised bodies such as NICE in the UK, recognise the convoluted issues and heterogenous evidence behind radiological screening. Ideally, these considerations should be concisely summarized to patients prior to their acceptance in screening programmes. Ultimately, the success of a radiological screening programme in reducing mortality has to be weighed against over-diagnosis in an asymptomatic population leading to unnecessary intervention.
Acknowledgements
We would like to thank and acknowledge Breast Cancer Now charity for use of their figure (Figure 1).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Wilson JM, Jungner YG. Principles and practice of mass screening for disease. Bol Oficina Sanit Panam 1968;65:281-393. [PubMed] [Google Scholar]
- 2.NHS Choices. Screening. Available online: http://www.nhs.uk/Livewell/preventing-cancer/Pages/cancer-screening.aspx (accessed 7.5.18).
- 3.Gov.uk. Population screening programmes. Available online: https://www.gov.uk/topic/population-screening-programmes (accessed 7.5.18)
- 4.American Cancer Society. American Cancer Society guidelines for the Early Detection of Cancer. Available online: https://www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer.html (accessed 7.5.18)
- 5.National Cancer Institute. Countries participating in the International Cancer Screening Network. Available online: https://healthcaredelivery.cancer.gov/icsn/about/participants.html (accessed 7.5.18)
- 6.National Cancer Institute. Cervical Cancer Screening Programs in 19 ICSN Countries, 2012: Organization, Policies, and Program Reach. Available online: https://healthcaredelivery.cancer.gov/icsn/cervical/screening.html (accessed 7.5.18)
- 7.Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: Population based case-control study of prospectively recorded data. BMJ 2009;339:b2968. 10.1136/bmj.b2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, van de Vijver MJ, Biermann K, Thomeer M, van Leerdam ME, Fockens P, Stoker J, Kuipers EJ, Dekker E. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: A randomised controlled trial. Lancet Oncol 2012;13:55-64. 10.1016/S1470-2045(11)70283-2 [DOI] [PubMed] [Google Scholar]
- 9.Regge D, Iussich G, Segnan N, Correale L, Hassan C, Arrigoni A, Asnaghi R, Bestagini P, Bulighin G, Cassinis MC, Ederle A, Ferraris A, Galatola G, Gallo T, Gandini G, Garretti L, Martina MC, Molinar D, Montemezzi S, Morra L, Motton M, Occhipinti P, Pinali L, Soardi GA, Senore C. Comparing CT colonography and flexible sigmoidoscopy: a randomised trial within a population-based screening programme. Gut 2017;66:1434-40. 10.1136/gutjnl-2015-311278 [DOI] [PubMed] [Google Scholar]
- 10.Bellini D, Rengo M, De Cecco CN, Iafrate F, Hassan C, Laghi A. Perforation rate in CT colonography: A systematic review of the literature and meta-analysis. Eur Radiol 2014;24:1487-96. 10.1007/s00330-014-3190-1 [DOI] [PubMed] [Google Scholar]
- 11.Rutter MD, Nickerson C, Rees CJ, Patnick J, Blanks RG. Risk factors for adverse events related to polypectomy in the english bowel cancer screening programme. Endoscopy 2014;46:90-7. 10.1055/s-0033-1344987 [DOI] [PubMed] [Google Scholar]
- 12.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: A population-based study. J Natl Cancer Inst 2003;95:230-6. 10.1093/jnci/95.3.230 [DOI] [PubMed] [Google Scholar]
- 13.Sali L, Regge D. CT colonography for population screening of colorectal cancer: Hints from European trials. Br J Radiol 2016;89:20160517. 10.1259/bjr.20160517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamdani U, Naeem R, Haider F, Bansal P, Komar M, Diehl DL, Kirchner HL. Risk factors for colonoscopic perforation: A population-based study of 80118 cases. World J Gastroenterol 2013;19:3596-601. 10.3748/wjg.v19.i23.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PennState Eberly College of Science Website. Epidemiological Research Methods. Screening Biases. 2017. Available oniline: https://onlinecourses.science.psu.edu/stat507/node/74
- 16.Wallis MG. How do we manage overdiagnosis/overtreatment in breast screening? Clin Radiol 2018;73:372-80. 10.1016/j.crad.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 17.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 18.Duffy SW, Tabar L, Olsen AH, Vitak B, Allgood PC, Chen TH, Yen AM, Smith RA. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomized trial and from the Breast Screening Programme in England. J Med Screen 2010;17:25-30. 10.1258/jms.2009.009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jørgensen KJ, Gøtzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ 2009;339:b2587. 10.1136/bmj.b2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NICE. Clinical Knowledge Summaries. Available online: https://cks.nice.org.uk/breast-screening#!scenario
- 21.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: An independent review. Br J Cancer 2013;108:2205-40. 10.1038/bjc.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith RA. IARC Handbooks of Cancer Prevention, Volume 7: Breast Cancer Screening. Breast Cancer Res 2003;5:216-7. 10.1186/bcr616 [DOI] [Google Scholar]
- 23.NHSBCS. Screening for breast cancer in England: past and future. 2006. Available online: http://webarchive.nationalarchives.gov.uk/20150506220415/http://www.cancerscreening.nhs.uk//breastscreen/publications/nhsbsp61.html [DOI] [PubMed]
- 24.Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: Updated overview of the Swedish randomised trials. Lancet 2002;359:909-19. 10.1016/S0140-6736(02)08020-0 [DOI] [PubMed] [Google Scholar]
- 25.Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev 2011:CD001877. [DOI] [PubMed] [Google Scholar]
- 26.Welch HG, Prorok PC, O'Malley AJ, et al. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med 2016;375:1438-47. 10.1056/NEJMoa1600249 [DOI] [PubMed] [Google Scholar]
- 27.Kopans D: New NEJM paper offers rehash on 'overdiagnosis'. AuntMinnie.com. 2017. Available online: http://www.auntminnie.com/index.aspx?sec=sup&sub=wom&pag=dis&itemId=115330
- 28.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA 2009;302:1685-92. 10.1001/jama.2009.1498 [DOI] [PubMed] [Google Scholar]
- 29.Bell RJ. Screening mammography--early detection or over-diagnosis? Contribution from Australian data. Climacteric 2014;17 Suppl 2:66-72. 10.3109/13697137.2014.956718 [DOI] [PubMed] [Google Scholar]
- 30.Berrington de González A. Estimates of the potential risk of radiation-related cancer from screening in the UK. J Med Screen 2011;18:163-4. 10.1258/jms.2011.011073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ 2014;348:g366. 10.1136/bmj.g366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health Quality Ontario Screening mammography for women aged 40 to 49 years at average risk for breast cancer: an evidence-based analysis. Ont Health Technol Ont Health Technol Assess Ser 2007;7:1-32. [PMC free article] [PubMed] [Google Scholar]
- 33.Lung cancer statistics. Cancer Research UK. 2017. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer#heading-One
- 34.Moyer VA. Screening for lung cancer: U.S. preventive services task force recommendation statement. Ann Intern Med 2014;160:330-8. [DOI] [PubMed] [Google Scholar]
- 35.The UK NSC recommendation on Lung cancer screening in adult cigarette smokers. Available online: https://legacyscreening.phe.org.uk/lungcancer
- 36.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, Crawford ED, Fouad MN, Isaacs C, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Ragard LR, Rathmell JM, Riley TL, Wright P, Caparaso N, Hu P, Izmirlian G, Pinsky PF, Prorok PC, Kramer BS, Miller AB, Gohagan JK, Berg CD, PLCO Project Team Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306:1865-73. 10.1001/jama.2011.1591 [DOI] [PubMed] [Google Scholar]
- 37.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, Sykes AM, Aughenbaugh GL, Bungum AO, Allen KL. CT screening for lung cancer: five-year prospective experience. Radiology 2005;235:259-65. 10.1148/radiol.2351041662 [DOI] [PubMed] [Google Scholar]
- 38.Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, Prorok PC. Lung cancer mortality in the Mayo Lung Project: Impact of extended follow-up. J Natl Cancer Inst 2000;92:1308-16. 10.1093/jnci/92.16.1308 [DOI] [PubMed] [Google Scholar]
- 39.International Early Lung Cancer Action Program Investigators , Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. 10.1056/NEJMoa060476 [DOI] [PubMed] [Google Scholar]
- 40.Cancer Facts and Figures 2016. American Cancer Society. Available online: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf
- 41.National Lung Screening Trial Research Team , Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Advisory Committee on Breast Cancer Screening Screening for breast cancer in England: past and future. J Med Screen 2006;13:59-61. 10.1258/096914106777589678 [DOI] [PubMed] [Google Scholar]
- 43.Field JK, Duffy SW, Baldwin DR, Whynes DK, Devaraj A, Brain KE, Eisen T, Gosney J, Green BA, Holemans JA, Kavanagh T, Kerr KM, Ledson M, Lifford KJ, McRonald FE, Nair A, Page RD, Parmar MK, Rassl DM, Rintoul RC, Screaton NJ, Wald NJ, Weller D, Williamson PR, Yadegarfar G, Hansell DM. UK Lung Cancer RCT Pilot Screening Trial: Baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. 10.1136/thoraxjnl-2015-207140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopes Pegna A, Picozzi G, Falaschi F, Carrozzi L, Falchini M, Carozzi FM, Pistelli F, Comin C, Deliperi A, Grazzini M, Innocenti F, Maddau C, Vella A, Vaggelli L, Paci E, Mascalchi M, ITALUNG Study Research Group Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol 2013;8:866-75. 10.1097/JTO.0b013e31828f68d6 [DOI] [PubMed] [Google Scholar]
- 45.Yousaf-Khan U, van der Aalst C, de Jong PA, Heuvelmans M, Scholten E, Lammers JW, van Ooijen P, Nackaerts K, Weenink C, Groen H, Vliegenthart R, Ten Haaf K, Oudkerk M, de Koning H. Final screening round of the NELSON lung cancer screening trial: The effect of a 2.5-year screening interval. Thorax 2017;72:48-56. 10.1136/thoraxjnl-2016-208655 [DOI] [PubMed] [Google Scholar]
- 46.Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, Døssing M, Hansen H, Kofoed KF, Larsen KR, Mortensen J, Rasmussen JF, Seersholm N, Skov BG, Thorsen H, Tønnesen P, Pedersen JH. CT screening for lung cancer brings forward early disease. The randomised Danish lung cancer screening trial: Status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301. 10.1136/thoraxjnl-2011-200736 [DOI] [PubMed] [Google Scholar]
- 47.Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, Schnabel PA, Eichinger M, Optazaite DE, Puderbach M, Wielpütz M, Kauczor HU, Tremper J, Delorme S. Randomized Study on Early Detection of Lung Cancer with MSCT in Germany: Results of the First 3 Years of Follow-up After Randomization. J Thorac Oncol 2015;10:890-6. 10.1097/JTO.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 48.Pancreatic cancer statistics [Internet]. Cancer Research UK. 2017. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/pancreatic-cancer#heading-Three
- 49.Del Chiaro M, Verbeke CS, Kartalis N, Pozzi Mucelli R, Gustafsson P, Hansson J, Haas SL, Segersvärd R, Andren-Sandberg Å, Löhr JM. Short-term results of a magnetic resonance imaging-based Swedish screening program for individuals at risk for pancreatic cancer. JAMA Surg 2015;150:512-8. 10.1001/jamasurg.2014.3852 [DOI] [PubMed] [Google Scholar]
- 50.Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg 2013;257:17-26. 10.1097/SLA.0b013e31825ffbfb [DOI] [PMC free article] [PubMed] [Google Scholar]