Abstract
Background
The etiology of non-small cell lung cancer (NSCLC) in non-smoker patients remains largely unknown. It has been widely proved that human papillomavirus (HPV) participates in the development of various cancers. Epidermal growth factor receptor (EGFR) mutation patients represent a large portion of non-smokers with NSCLC. We performed this meta-analysis to determine whether HPV infection in NSCLC tissue is associated with EGFR mutations compared with HPV negative controls.
Methods
Online databases were searched up to June 30th 2017. We included studies in which HPV detection was based on polymerase chain reaction (PCR) methods. Random effects model was used in data synthesis and the relative effects were presented as odds ratio (OR) with 95% confidence intervals (CIs).
Results
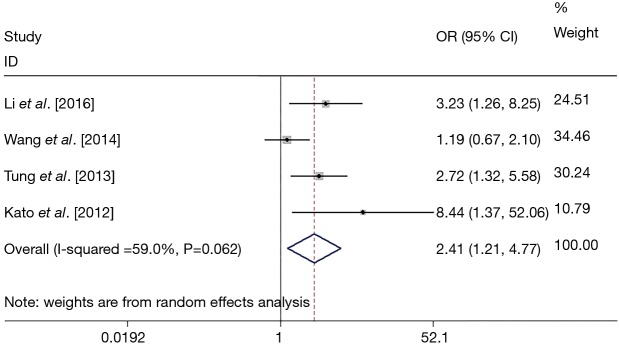
Finally, four eligible studies with a total of 498 patients from Asian countries were identified and included. The general EFGR mutation positive rate was 38.2% among all patients, and the HPV DNA detection rate (HPV subtype being involved: 16, 18, 33 and 58) was 35.3%. The presence of EGFR mutation was significantly higher in HPV-positive patients compared with HPV-negative controls (52% vs. 31%; OR =2.41, 95% CI: 1.21 to 4.77; P=0.012), with moderate heterogeneity among studies (I2=59%; P=0.062).
Conclusions
Our results suggest that HPV infection is associated with EGFR mutations in NSCLC, at least in Asian populations. Further efforts should be made on exploring the potential mechanism and the prognostic character of HPV/EGFR positive NSCLC patient.
Keywords: Human papillomavirus (HPV), non-smoking non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR) mutations
Introduction
The incidence of non-small cell lung cancer (NSCLC) is increasing rapidly worldwide. Activating mutations in the gene encoding the epidermal growth factor receptor (EGFR) protein could play a role in tumor formation and are the most intensively studied. This mutation is detected in approximately 10–20% of Caucasian and 30–50% of Asian NSCLC patients, this rate is even higher in non-smoking females (1).
Human papillomavirus (HPV) is confirmed to be an important pathogenic factor of certain benign and malignant lesions in humans. The potential mechanism may include inhibiting the regulation of the cell cycle through inactive P53 and Rb proteins, its E6 and E7 oncoproteins are able to disturb differentiation of normal epithelial and DNA synthesis (2). High-risk HPV (HR-HPV) can lead to malignant transformation by destroying genomic stability (3), which is believed to be the main cause of cervical cancers, a substantial proportion of other anogenital cancers and also oropharyngeal cancers (4,5).
Syrjanen et al. in 1979 firstly identified the association between HPV infection and bronchial squamous cell carcinoma (6). Since then a large number of reports have suggested an association between HPV and lung cancer (7,8). A meta-analysis showed that HPV infection was strongly associated with lung cancer. Furthermore, results indicated that HPV16/18 was significantly associated with NSCLC, the incidence of which was higher in non-smoking women (9). Another study directly presented evidence confirming the presence of HPV in lung cancer in never smokers and women, which plays a definite role in carcinogenesis in the disease (10). With the sympatry of HPV infection and EGFR mutation in NSCLC, it is reasonable to assume that there is probably some association between them.
Some studies have explored a potential relationship between EGFR mutation and HPV infection in NSCLC (11-14). However, limited sample size did not provide definitive conclusions. Hitherto no further evidence has been strong enough to support the relation between HPV infection and EGFR mutation in NSCLC. We therefore performed a meta-analysis of these studies to further explore this assumption.
Methods
Literature search and selection
A systematic and comprehensive literature search of online databases PubMed (National Library of Medicine, Bethesda, MD, USA), Web of Science (Thompson Scientific, Philadelphia, PA, USA), Medline, and Cochrane library was performed to identify observational studies and randomized controlled trials (RCTs) performed before July 2017 that simultaneously examined the association between HPV infection in NSCLC tissue and EGFR mutation. The search was limited to studies that had been conducted on human subjects and written in English.
References were manually reviewed to identify additional studies of interest. Several search terms and related variants were used, HPV, EGFR, NSCLC and lung cancer. The references of identified papers, previous published systematic reviews and meta-analyses were inspected to identify studies not included by the initial search.
We evaluated all searched results according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (15). The selection of original studies was based on the process of viewing titles, abstracts and full papers. Inclusion criteria were as follows: (I) studies compared HPV infection in lung tissue among NSCLC patients and non-cancer controls; (II) studies regarding the EGFR presence in HPV positive or negative patients; (III) studies detecting HPV DNA with polymerase chain reaction (PCR); (IV) histological diagnosis of cases and controls were established; (V) sufficient information was provided to calculate odds ratio (OR) with 95% confidence intervals (CIs); non-comparative studies, review articles, abstracts, case reports, editorials, expert opinions, commentary articles, and letters were excluded.
Data extraction and quality assessment
Data were extracted independently by two investigators (H Liang and Y Chen) and conflicts were adjudicated by a third investigator (W Liang). For the selected studies, information on all available variables was extracted and entered into a Microsoft Excel database. The numbers of EGFR positive patients with or without HPV infection were recorded to calculate the OR. Quality assessment of included studies was assessed using the Joanna Briggs Institute Prevalence Critical Appraisal Tool to assess the quality of the studies (16). Any disagreement was resolved via discussion among the authors.
Association between HPV infection and EGFR mutation in NSCLC
OR with 95% CIs were calculated to evaluate the relation between HPV infection and EGFR mutation in NSCLC. We used Cochran’s X2 test and I2 to examine heterogeneity among effect estimates. Statistical heterogeneity among studies was defined as I2 statistic greater than 50% (17). Fixed effects model was preferred to random effects model when there was no statistically significant heterogeneity and vice versa when there was significant heterogeneity (18). Statistical significance was taken as two-sided P<0.05.The analysis was conducted with STATA 12.0 software (Stata Corporation, College Station, TX, USA).
Results
Study selection and quality assessment
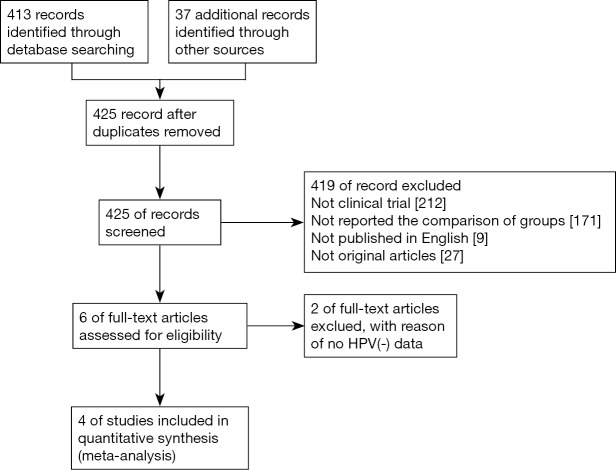
A total of 413 records were screened from previously mentioned online databases after excluding duplicates up to June 30th 2017. A manual search and inspection of the reference lists and existing reviewed articles identified 37 additional relevant studies. After an in-depth review, four full-text articles met the inclusion criteria and were considered in this analysis (Figure 1) (11-14). The contextual details and the results of the quality assessment of each study were summarized in Table 1. We defined smokers as patients who either currently smoke, or smoked in the past.
Figure 1.
Flow diagram detailing the search strategy and identification of studies used in meta-analysis.
Table 1. Characteristics of the included studies in the meta-analysis.
| Author | Year | Period | Region | Design | Female (%) | Smoker (%) | HPV species | Quality score | HPV(+) | HPV(−) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EGFR(+) | EGFR(−) | EGFR(+) | EGFR(−) | ||||||||||
| Li et al. | 2016 | 2011–2013 | Anhui, China | Retro | 46.32 | 40.00 | 16, 18, 33, 58 | 8 | 18 | 9 | 26 | 42 | |
| Wang et al. | 2014 | 2003–2011 | Taiwan | Retro | 51.43 | 40.48 | 16, 18 | 9 | 33 | 41 | 55 | 81 | |
| Tung et al. | 2013 | 1998–2004 | Taiwan | Pro | 35.10 | 43.71 | 16, 18 | 10 | 28 | 40 | 17 | 66 | |
| Kato et al. | 2012 | 2007–2008 | Kagoshima, Japan | Retro | 33.33 | 50.00 | 16, 58 | 8 | 5 | 2 | 8 | 27 | |
Retro, retrospective study; Pro, prospective study; EGFR, epidermal growth factor receptor; HPV, human papillomavirus.
The studies were conducted in three different Asian areas with periods ranging from 1998 to 2013. One article was a prospective study while the others were retrospective. A total of four studies involving 498 patients were included. The EGFR mutation positive rate was 38.15% among patients, and the HPV DNA detection rate was 35.34%. The HPV types 16, 18, 33 and 58 were included. All studies gained 8 to 10 stars in study quality assessment on a scale of 0 to 10 with Joanna Briggs Institute Prevalence Critical Appraisal Tool.
Association between HPV infection and EGFR mutation in NSCLC
In total four studies reported the correlation between EGFR mutation in NSCLC patients and HPV infection and were included for analysis. Random model was used. The EGFR mutation presented more in HPV positive patients (52%, 95% CI: 39% to 65%; P<0.001) than in negative controls (31%, 95% CI: 20% to 42%; P<0.001). The presence of EGFR mutation was significantly related to HPV infection compared with HPV negative controls (OR =2.41, 95% CI: 1.21 to 4.77; P=0.012), with significant statistical heterogeneity among studies (I2=59%; P=0.062) (Figure 2).
Figure 2.
Forest plot for pooled estimation of the correlation between EGFR mutation and HPV infection. EGFR, epidermal growth factor receptor; HPV, human papillomavirus; OR, odds ratio; CI, confidence interval.
Discussion
Lung cancer is the most common cause of cancer related deaths, among males and females (19). The epidemiology of lung cancer, however, varies across different countries, probably due to variations in the pattern of environment and gene polymorphism. Although cigarette smoking is the primary risk factor for lung cancer, non smokers account for nearly 70% of NSCLC patients. A number of studies have reported the presence of HPV in lung carcinoma. Interestingly, its detection rate appears to differ histologically and geographically. To the best of our knowledge, this is the first meta-analysis to analyze the association between HPV infection and EGFR mutation in NSCLC patients. We found a significantly higher prevalence of EGFR mutation in HPV positive NSCLC patients (52%) than controls (31%). We also observed a significant association (OR =2.41, 95% CI: 1.21 to 4.77; P=0.012) of EGFR mutation with HPV infection in NSCLC patients. In addition, the EFGR mutation positive rate was 38.15% among patients, and the HPV DNA detection rate was 35.34%.
A meta-analysis observed that prevalence of HPV in never smokers was significantly higher in East Asia (33.9%, N=392) than in Europe (14.8%, N=58, P=0.005). While the prevalence of HPV in East Asia was similar between never and ever smokers (33.9% vs. 39.2%, P=0.080), it was significantly higher in never smokers than in ever smokers (14.8% vs. 2.9%, P<0.001) in Europe (20). In addition, EGFR mutations have been demonstrated to be more common in never-smokers, women, individuals of Asian ethnicity and adenocarcinoma patients (21). Another study revealed mean HPV frequencies of 17% and 15% in Europe and the United States, respectively, with a higher frequency in Asia (35.7%) (22). Both EGFR mutations and HPV detection were more frequently observed in Asian lung cancer patients than non-Asian patients. The similar distribution between the EGFR mutation and HPV infection may imply the potential interrelation between them.
HPV mutation in NSCLC was demonstrated as a prognostic factor or a predictive factor for using tyrosine kinase inhibitor (TKI) in several studies. Li et al. (11) found patients with HPV infections (HPV+/mutant+) exhibited longer survival time when compared with the counterpart (HPV-/mutant+). Subgroup analyses for progression-free survival (PFS) stratified by treatments indicated that patients with HPV infections exhibited the longer PFS times in TKI groups, which may be a result of these patients’ positive response to TKI therapy. Another study from Japan found HR-HPV genomes in 75% (6/8) of adenocarcinomas with complete or partial response to gefitinib while the 12 patients that did not respond to gefitinib were HPV negative (23). This phenomenon could be explained by our study results that more EGFR mutations existed in HPV positive lung cancer, thus this portion of patients have a better response to EGFR-TKI and subsequently have better survival outcomes.
Several studies have explored mechanisms of the relation between HPV and EGFR mutation in NSCLC. Inflammatory-induced oxidative stress has also been linked with the development of lung adenocarcinoma and HPV infection increased DNA damage and mutation rates (24,25). A recent report indicated that the level of 8-hydroxy-2'deoxyguanosine (8-OH-dG), an oxidative stress biomarker, was closely associated with EGFR mutation in lung cancer (26) and the 8-OH-dG levels also correlated with the grade of dysplasia in HPV-associated cervical carcinogenesis (27). Thus Tung et al. explored the potential mechanism of the oncogenic effect of high risk HPV in NSCLC. They found that patients with E6-positive tumors had a greater frequency of EGFR mutations than those with E6-negative tumors (41% vs. 20%; P=0.006) and the levels of 8-OH-dG were correlated with EGFR mutations (36% vs. 16%; P=0.012).The results indicated HPV16/18 E6 expression may contribute to the occurrence of EGFR mutations in Taiwanese patients with NSCLC because of an increase in 8-OH-dG levels induced by HPV16/18 infection (12). Wu et al. (28) demonstrated that HPV-16 E6 upregulated cIAP2 via EGFR/PI3K/AKT cascade, and cIAP2 expression was positively related to EGFR mutations. Since the activation of PI3-kinase signaling was essential for HPV induced transformation (29), the EGFR/PI3K/AKT cascade may play a pivotal role in carcinogenesis of HPV-related lung cancer with EGFR mutations.
Though many studies have done much in exploring the association between HPV and EGFR positive NSCLC, there is still limit evidence supporting the NSCLC neoplasia effect of HPV. Kazemian et al. (30) identified new virus-cancer associations by searching RNA sequencing data sets from >2,000 patients, encompassing 21 cancers from The Cancer Genome Atlas (TCGA), for the presence of viral sequences. They found HPV38(+) samples contained the same 10 novel single nucleotide variations (SNVs) and a new isolate of HPV38 that appears to be integrated into the human genome. Although this study didn’t provide direct evidence on relationship between HPV and EGFR mutation in NSCLC, it provided general guidelines for computational detection and interpretation of pathogen-disease associations, which might be used in exploring the further association and mechanism between virus infection and cancer.
We acknowledge several limitations to this analysis. First, all but one of the included studies was retrospective comparisons, which provided a greater risk of potential selection and reporting bias. Second, high heterogeneity among studies (I2=59; P=0.062) still existed. Factors that could potentially explain the heterogeneity include different baseline characteristics, different sensibility of detection method and small population of each study. Last, though we observed HPV may contribute in part to EGFR mutations in NSCLC, we do not know the state of subgroup of high risk HPV in this meta-analysis.
Conclusions
Our results suggest that HPV infection is associated with EGFR mutations in NSCLC, at least in Asian populations. Further efforts should be made on exploring the potential mechanism and the prognostic character of HPV/EGFR positive NSCLC patient.
Acknowledgements
We thank Lindsey Hamblin for assistance with the language revision.
Funding: This work was supported by the following fundings: Chinese National Natural Science Foundation (grant No. 81501996); Guangdong Doctoral Launching Program (grant No. 2014A030310460); Doctoral Launching Program of Guangzhou Medical University (grant No. 2014C27); Key Project of Livelihood Technology of Guangzhou (2011Y2-00024).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Berge EM, Doebele RC. Targeted therapies in non-small cell lung cancer: emerging oncogene targets following the success of epidermal growth factor receptor. Semin Oncol 2014;41:110-25. 10.1053/j.seminoncol.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliani L, Favalli C, Syrjanen K, et al. Human papillomavirus infections in lung cancer. Detection of E6 and E7 transcripts and review of the literature. Anticancer Res 2007;27:2697-704. [PubMed] [Google Scholar]
- 3.Doorbar J. Papillomavirus life cycle organization and biomarker selection. Dis Markers 2007;23:297-313. 10.1155/2007/613150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steenbergen RD, de Wilde J, Wilting SM, et al. HPV-mediated transformation of the anogenital tract. J Clin Virol 2005;32 Suppl 1:S25-33. 10.1016/j.jcv.2004.11.019 [DOI] [PubMed] [Google Scholar]
- 5.Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467-75. 10.1158/1055-9965.EPI-04-0551 [DOI] [PubMed] [Google Scholar]
- 6.Syrjanen KJ. Condylomatous changes in neoplastic bronchial epithelium. Report of a case. Respiration 1979;38:299-304. 10.1159/000194095 [DOI] [PubMed] [Google Scholar]
- 7.Sarchianaki E, Derdas SP, Ntaoukakis M, et al. Detection and genotype analysis of human papillomavirus in non-small cell lung cancer patients. Tumour Biol 2014;35:3203-9. 10.1007/s13277-013-1419-2 [DOI] [PubMed] [Google Scholar]
- 8.Sagerup CM, Nymoen DA, Halvorsen AR, et al. Human papilloma virus detection and typing in 334 lung cancer patients. Acta Oncol 2014;53:952-7. 10.3109/0284186X.2013.879608 [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan M, Taioli E, Ragin CC. Human papillomavirus type 16 and 18 in primary lung cancers--a meta-analysis. Carcinogenesis 2009;30:1722-8. 10.1093/carcin/bgp177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae JM, Kim EH. Human papillomavirus infection and risk of lung cancer in never-smokers and women: an 'adaptive' meta-analysis. Epidemiol Health 2015;37:e2015052. 10.4178/epih/e2015052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Deng F, Qian LT, et al. Association between human papillomavirus and EGFR mutations in advanced lung adenocarcinoma. Oncol Lett 2016;12:1953-8. 10.3892/ol.2016.4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tung MC, Wu HH, Cheng YW, et al. Association of epidermal growth factor receptor mutations with human papillomavirus 16/18 E6 oncoprotein expression in non-small cell lung cancer. Cancer 2013;119:3367-76. 10.1002/cncr.28220 [DOI] [PubMed] [Google Scholar]
- 13.Kato T, Koriyama C, Khan N, et al. EGFR mutations and human papillomavirus in lung cancer. Lung Cancer 2012;78:144-7. 10.1016/j.lungcan.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 14.Wang JL, Fang CL, Wang M, et al. Human papillomavirus infections as a marker to predict overall survival in lung adenocarcinoma. Int J Cancer 2014;134:65-71. 10.1002/ijc.28349 [DOI] [PubMed] [Google Scholar]
- 15.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91-2. 10.1016/j.jcms.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 16.Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014;3:123-8. 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 20.Hasegawa Y, Ando M, Kubo A, et al. Human papilloma virus in non-small cell lung cancer in never smokers: a systematic review of the literature. Lung Cancer 2014;83:8-13. 10.1016/j.lungcan.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008;5:e185. 10.1371/journal.pmed.0050185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syrjanen K. Detection of human papillomavirus in lung cancer: systematic review and meta-analysis. Anticancer Res 2012;32:3235-50. [PubMed] [Google Scholar]
- 23.Baba M, Castillo A, Koriyama C, et al. Human papillomavirus is frequently detected in gefitinib-responsive lung adenocarcinomas. Oncol Rep 2010;23:1085-92. [DOI] [PubMed] [Google Scholar]
- 24.Taguchi A, Politi K, Pitteri SJ, et al. Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Cancer Cell 2011;20:289-99. 10.1016/j.ccr.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei L, Gravitt PE, Song H, et al. Nitric oxide induces early viral transcription coincident with increased DNA damage and mutation rates in human papillomavirus-infected cells. Cancer Res 2009;69:4878-84. 10.1158/0008-5472.CAN-08-4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawahara A, Azuma K, Hattori S, et al. The close correlation between 8-hydroxy-2'-deoxyguanosine and epidermal growth factor receptor activating mutation in non-small cell lung cancer. Hum Pathol 2010;41:951-9. 10.1016/j.humpath.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 27.Romano G, Sgambato A, Mancini R, et al. 8-hydroxy-2'-deoxyguanosine in cervical cells: correlation with grade of dysplasia and human papillomavirus infection. Carcinogenesis 2000;21:1143-7. 10.1093/carcin/21.6.1143 [DOI] [PubMed] [Google Scholar]
- 28.Wu HH, Wu JY, Cheng YW, et al. cIAP2 upregulated by E6 oncoprotein via epidermal growth factor receptor/phosphatidylinositol 3-kinase/AKT pathway confers resistance to cisplatin in human papillomavirus 16/18-infected lung cancer. Clin Cancer Res 2010;16:5200-10. 10.1158/1078-0432.CCR-10-0020 [DOI] [PubMed] [Google Scholar]
- 29.Henken FE, Banerjee NS, Snijders PJ, et al. PIK3CA-mediated PI3-kinase signalling is essential for HPV-induced transformation in vitro. Mol Cancer 2011;10:71. 10.1186/1476-4598-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazemian M, Ren M, Lin JX, et al. Possible Human Papillomavirus 38 Contamination of Endometrial Cancer RNA Sequencing Samples in The Cancer Genome Atlas Database. J Virol 2015;89:8967-73. 10.1128/JVI.00822-15 [DOI] [PMC free article] [PubMed] [Google Scholar]