Abstract
In this Review, we describe the pathogenesis, diagnosis and management of primary hyperparathyroidism (PHPT), with a focus on recent advances in the field. PHPT is a common endocrine disorder that is characterized by hypercalcaemia and elevated or inappropriately normal serum levels of parathyroid hormone. Most often, the presentation of PHPT is asymptomatic in regions of the world where serum levels of calcium are routinely measured. In addition to mild hypercalcaemia, PHPT can manifest with osteoporosis and hypercalciuria as well as with vertebral fractures and nephrolithiasis, both of which can be asymptomatic. Other clinical forms of PHPT, such as classical disease and normocalcaemic PHPT, are less common. Parathyroidectomy, the only curative treatment for PHPT, is recommended in patients with symptoms and those with asymptomatic disease who are at risk of progression or have subclinical evidence of end-organ sequelae. Parathyroidectomy results in an increase in BMD and a reduction in nephrolithiasis. Various medical therapies can increase BMD or reduce serum levels of calcium, but no single drug can do both. More data are needed regarding the neuropsychological manifestations of PHPT and the pathogenetic mechanisms leading to sporadic PHPT, as well as on risk factors for complications of the disorder. Future work that advances our knowledge in these areas will improve the management of the disorder.
Primary hyperparathyroidism (PHPT) was first described approximately 90 years ago, almost simultaneously in Europe and the USA1. Since that time, the clinical presentation in the USA and Western Europe has evolved from a severe and symptomatic disease, characterized by ‘stones, bones and groans’ to one that is typically asymptomatic and incidentally discovered. Advances in diagnostics now enable us to accurately measure levels of parathyroid hormone (PTH) and image the parathyroid glands; surgical techniques have also improved. Despite these advances and the availability of medical therapies that address some of the complications of the disease, parathyroidectomy remains the only curative treatment, as was the case 90 years ago. This Review describes the pathogenesis, diagnosis and management of PHPT, with a focus on recent advances in the field.
Epidemiology and pathogenesis
PHPT is a common endocrine disorder that is characterized by hypercalcaemia and elevated or inappropriately normal levels of PTH. PHPT results from excessive secretion of PTH from one or more of the parathyroid glands. PHPT is caused by a solitary parathyroid adenoma in 80% of cases, whereas four-gland hyperplasia accounts for 10–15%, multiple adenomas for 5% and parathyroid cancer for <1% of cases. Incidence estimates for PHPT vary from ~0.4 to 82 cases per 100,000 (REFS 2–4). Before the routine measurement of serum levels of calcium in the 1970s, PHPT was a rare and symptomatic disorder. When routine evaluation of serum levels of calcium became widespread, cases of unrecognized, asymptomatic PHPT were identified, leading to an initial fivefold increase in the incidence of the disorder5. Thereafter, the incidence of PHPT declined in the USA until 1998, at which time another sharp increase was noted3,6,7, which has been attributed to the introduction of osteoporosis screening guidelines and targeted testing in those with osteoporosis7. The incidence of PHPT increases with age and is higher in women and African Americans than in men and other racial groups, respectively2. Half of all patients with PHPT are postmenopausal women, although the disorder can occur at any age8. PHPT is often diagnosed in the first decade after menopause, consistent with the known skeletal actions of oestrogen that counter the hypercalcaemic effects of excess PTH in bone.
The underlying cause of sporadic PHPT is unknown in most cases. Ionizing radiation, especially in childhood, is a risk factor9. Chronic lithium use, which decreases the sensitivity of the parathyroid glands to calcium, is also associated with the development of PHPT10. The genetic pathogenesis of PHPT is unclear in most patients. Genes regulating the cell cycle are thought to be important given the clonal nature of sporadic parathyroid adenomas. Two such genes documented as contributing to the development of PHPT are CCND1 (which encodes cyclin D1) and MEN1 (which encodes menin). Somatic mutations in MEN1 occur in 12–35% of sporadic adenomas, whereas rearrangement or overexpression of CCND1 can occur in 20–40%11,12. Recent studies have also implicated CDC73, CTNNB1, CDKN1B and AIP (which encodes the aryl hydrocarbon (AH) receptor-interacting protein) in a small percentage of adenomas13,14.
In inherited or familial forms of PHPT, which represent about 5–10% of cases, germline mutations in several causal genes have been identified15,16. The clinical features, gene products and inheritance of familial forms of PHPT are shown in TABLE 1. The following genes have been associated with familial PHPT: the tumour suppressor MEN1 in multiple endocrine neoplasia type 1 syndrome and familial isolated primary hyperparathyroidism (FIHP); the proto-oncogene RET in MEN 2A syndrome; CDKN1B in MEN 4 syndrome; inactivating mutations in CASR (which encodes the calcium-sensing receptor) in FIHP; GCM2 in FIHP17 and CDC73 in hyperparathyroidism-jaw tumour syndrome, which is also associated with an increased risk of parathyroid carcinoma. Mutations in PRUNE2 (which encodes protein prune homologue 2) have also been associated with the development of parathyroid cancer18. Other work indicates that microRNA 296 might be a novel tumour-suppressor gene in parathyroid carcinoma19. The genetics and characteristics of familial hypocalciuric hypercalcaemia (FHH), which is not considered a form of PHPT, are discussed in the following section.
Table 1 |.
| Familial syndrome | Clinical manifestations | Gene (protein) | Inheritance |
|---|---|---|---|
| MEN 1 | PHPT (95%), anterior pituitary adenomas (30%), pancreatic neuroendocrine tumours (40%); other features can include adrenal adenomas, carcinoid, lipomas, angiofibromas and collagenomas | MEN1 (menin) | Autosomal dominant |
| MEN 2A | Medullary thyroid cancer (90%), pheochromocytoma (50%), PHPT (20%) | RET (proto-oncogene c-Ret) | Autosomal dominant |
| MEN 4 | PHPT (~80%), anterior pituitary tumours (~40%), pancreatic neuroendocrine tumours; other features can include carcinoid, adrenocorticoid tumours, thyroid tumours, reproductive organ tumours and renal angiomyolipomas | CDKN1B (p27) | Autosomal dominant |
| FIHP | Isolated PHPT | • MEN1 (menin) • CASR (CASR) • GCM2 (GCM motif protein 2, also known as hGCMb) |
Autosomal dominant |
| Hyperparathyroid-jaw tumour syndrome | PHPT (80%), often parathyroid carcinoma (>15%), jaw tumours (>30%); other features can include renal abnormalities, uterine tumours, pancreatic adenocarcinoma, testicular mixed germ cells and Hürthle cell thyroid adenomas | CDC73 (also known as HRPT2; parafibromin) | Autosomal dominant |
FIHP, familial isolated primary hyperparathyroidism; MEN, multiple endocrine neoplasia; PHPT, primary hyperparathyroidism.
Pathophysiology and (differential) diagnosis
In all forms of PHPT, there is loss of normal feedback suppression of serum levels of calcium upon the synthesis and secretion of PTH, due to increased parathyroid cell mass and/or a reduction in the number of CASR proteins on parathyroid cells20. As a result, increased levels of calcium are needed to suppress PTH levels (FIG. 1). The diagnosis of PHPT is established biochemically and can be confirmed by documenting hypercal-caemia with a simultaneously elevated intact PTH level. On repeat laboratory testing, serum levels of calcium can intermittently fall into the normal range; this finding is compatible with the diagnosis of PHPT as long as a ‘recurrent pattern’ of hypercalcaemia is evident. Levels of PTH that are inappropriately normal (>20 pg/ml) in a patient with hypercalcaemia are consistent with a diagnosis of PHPT. Non-parathyroid causes of hypercalcaemia (such as malignancy or granulomatous disease) are associated with suppressed levels of PTH. Ectopic PTH secretion from a non-parathyroid tumour is extremely rare although occasionally documented in late-stage malignancies21,22.
Figure 1 |. Relationship between serum levels of calcium and PTH.
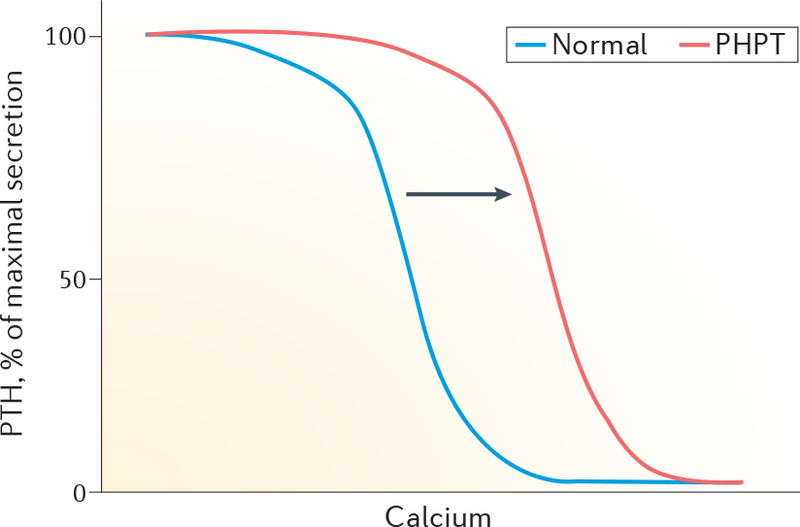
Depicted is the relationship between serum levels of calcium and PTH in patients with primary hyperparathyroidism (PHPT; red line) and normal individuals (blue line). PHPT results in a shift of the curve to the right. Increased levels of calcium are needed to suppress PTH levels in PHPT.
When assessing for PHPT, PTH levels should be measured with either an ‘intact’ second-generation PTH assay or a third-generation assay. Second-generation assays detect PTH(1–84), PTH(7–84) and other long C-terminal fragments, which are inactive fragments and/or are thought to oppose the activity of intact PTH. Unless renal failure is present, the contribution of fragments to the measured PTH value is negligible. The intact assays do not cross-react with PTH-related peptide and can reliably distinguish PHPT from hypercalcaemia of malignancy, in contrast to first-generation assays. The newer third-generation PTH assays detect the main circulating form of whole PTH(1–84) and a second PTH(1–84) molecule not detected by second-generation assays that is thought to have a post-translational modification23. Other than in renal failure, these assays do not increase the diagnostic sensitivity over second-generation assays24.
Parathyroid imaging has no role in the diagnosis of PHPT. Imaging studies assist the parathyroid surgeon in identifying the anatomic position of abnormal gland(s) when planning parathyroidectomy. Negative imaging, which is frequent in multi-glandular PHPT, is not inconsistent with the diagnosis of PHPT and does not preclude surgical cure25. Further, positive imaging is not needed to confirm the diagnosis, and false-positive tests occur often in those with concurrent nodular thyroid disease.
To differentiate PHPT from FHH, which has a similar serum biochemical profile, calculation of the fractional excretion of calcium (FeCa; calculated using a 24-hour urine sample collected off diuretics) has traditionally been used. Values below 1% are consistent with FHH, but overlap of FeCa values in FHH and PHPT occurs. As FeCa values can also be low in patients with PHPT who have coexisting vitamin D deficiency, the diagnosis of FHH should not be made until vitamin D stores are replete. FeCa can also be misleading in patients with impaired renal function (due to advanced age or renal disease). In these patients, obtaining past serum calcium levels (which should be consistently elevated) and family history of hypercalcaemia is helpful. Ultimately, if suspicion of FHH is high, mutational analysis of CASR for FHH1, as well as mutational analysis of GNA11 and AP2S1 for the diagnosis of FHH2 and FHH3, respectively, can be performed26–28. Differentiation of PHPT from FHH is important, as surgical intervention is not indicated or curative in FHH.
PHPT can be distinguished from secondary and tertiary hyperparathyroidism by its different biochemical profile (TABLE 2). Secondary hyperparathyroidism is associated with an appropriate elevation in PTH in response to a hypocalcaemic stimulus and either a frankly low or normal serum calcium level. Most commonly, secondary hyperparathyroidism is due to vitamin D deficiency, malabsorption, kidney disease or hypercalciuria. In our experience, a subset of patients with secondary hyperparathyroidism will become hypercalcaemic and will ultimately be found to have PHPT, when the underlying condition (for example, vitamin D deficiency) is corrected. In these cases, the hypercalcaemia of PHPT is said to have been ‘masked’ by the coexisting hypocalcaemic stimulus. Tertiary hyperparathyroidism describes a condition in which prolonged, severe secondary hyperparathyroidism (as in end-stage renal disease) evolves into a hypercalcaemic state due to autonomous functioning of hyperplastic parathyroid glands. Although this effect can be observed in patients on dialysis, it can also occur after renal transplant. Tertiary hyperparathyroidism is typically obvious from the history of the patient.
Table 2 |.
Biochemical features of various forms of hyperparathyroidism and FHH
| Form of hyperparathyroidism | Calcium levels | PTH levels | Phosphate levels | Urinary calcium excretion |
|---|---|---|---|---|
| Primary hyperparathyroidism | Increased | Increased or inappropriately normal | Low or low-normal | FeCA typically >1–2% |
| Secondary hyperparathyroidism | Normal or low | Increased | Dependent on cause; low or low-normal in vitamin D deficiency or malabsorption; high or high-normal in renal failure | Dependent on cause; low in vitamin D deficiency or malabsorption; high in renal calcium leak |
| Tertiary hyperparathyroidism | Increased | Increased | Variable; dependent on before or after renal transplant | Low before renal transplant |
| Normocalcaemic primary hyperparathyroidism | Normal (total & ionized) | Increased | Normal | <350 mg per 24 h29 |
| FHH | Increased | Increased or inappropriately normal; the majority have normal PTH | Normal | FeCa typically <1% |
FeCa, fractional excretion of calcium; FHH, familial hypocalciuric hypercalcaemia; PTH, parathyroid hormone
In distinction, normocalcaemic primary hyperparathyroidism (NPHPT) is a term used for those patients with normal serum albumin-corrected calcium levels and ionized calcium values with an elevated PTH level in whom all known causes of secondary hyperparathyroidism have been excluded. NPHPT was thought to be an early form of PHPT. Although data regarding the natural history of NPHPT are limited, a 2007 study found that ~19% became hypercalcaemic within 3 years of follow-up29. Thus, the term NPHPT can describe more than one natural history, with some patients maintaining the biochemical pattern of NPHPT over many years and perhaps even indefinitely.
Clinical manifestations and complications
Classical PHPT
Classical PHPT was the disease presentation almost exclusively observed before the routine measurement of serum calcium levels in the 1970s. Classical PHPT refers to a symptomatic, multi-system disorder characterized by skeletal, renal, gastrointestinal, neurological and psychiatric manifestations as well as increased mortality1. Descriptions of PHPT from the early to mid-20th century include marked hypercalcaemia (11.5–16.8 mg/dl) and frequent reports of osteitis fibrosa cystica, a skeletal condition characterized clinically by bone pain and fractures (particularly vertebral) and radiographically by demineralization, fibrosis, brown tumours and bone cysts1,30. Nephrolithiasis, nephrocalcinosis, polyuria and polydipsia as well as renal impairment were also common presenting signs and symptoms1,30; other features of classical PHPT include anorexia, constipation, peptic ulcer disease and pancreatitis, muscle weakness and associated type 2 muscle fibre atrophy, and mental disturbances as well as fatigue or lasstitude1,30.
Classical PHPT is uncommon today in the USA, Western Europe and Turkey8,31,32. In the USA, <2% of patients have osteitis fibrosa cystica, and rates of overt nephrolithiasis have steadily declined over the past 70 years from 60% to <20%1,8,33. Classical PHPT, however, remains the predominant mode of presentation in most of the Middle East, Asia and South Africa34–38. In Latin America, a 2015 report indicated that >50% of patients present with osteitis fibrosa cystica or nephrolithiasis39; other reports confirmed high rates of nephrolithiasis (>40%)40,41. This more severe form of the disease is thought to be more common in areas where severe vitamin D deficiency is endemic, although lack of routine calcium screening in these regions might also have a role in this presentation.
Asymptomatic PHPT
Today, the vast majority (>80%) of patients with PHPT in the USA and Western Europe are ‘asymptomatic’, a term used to describe those lacking the skeletal and renal manifestations described in classical PHPT. Although this term was introduced in the 1970s and 1980s to differentiate it from classical PHPT, over the years, we have come to realize that many patients with this form of the disease do indeed have manifestations of PHPT. Many have clinical complaints, whereas others have characteristic findings on testing of the target organs of the hyperparathyroid process.
Biochemical profile.
Most patients with PHPT are incidentally discovered when routine laboratory work reveals hypercalcaemia. Serum levels of calcium are mildly elevated, often within 1 mg/dl of the upper limit of normal8. PTH levels are typically within two times the upper limit of normal. Serum phosphate is often in the lower half of the normal range and less frequently frankly low due to the phosphaturic effects of PTH8. Alkaline phosphatase levels can be elevated, a reflection of increased bone resorption and compensatory formation, but most often remain within the normal range8.
The storage form of vitamin D, 25-hydroxyvitamin D (25OHD), is often in the insufficient (20–29 ng/ml) or deficient range (<20 ng/ml), whereas activated vitamin D (1,25-dihydroxyvitamin D; 1,25(OH)2D) is near the upper end of normal and sometimes frankly elevated8,42. In fact, vitamin D deficiency has been reported to be more common in patients with PHPT than in the general population43,44. Potential pathophysiological mechanisms for vitamin D deficiency in PHPT include the following: PTH enhances the conversion of 25OHD to 1,25(OH)2D by inducing the renal 1α-hydroxylase enzyme45, the half-life of 25OHD might also be shortened due to enhanced hepatic inactivation46, and chronic vitamin D deficiency could result in parathyroid hyperplasia and autonomous adenomatous change.
Whereas the biochemical profile in modern PHPT is clearly milder than in descriptions from the early and mid-20th century, recent work suggests even further evolution within the modern era42. A comparison of two PHPT cohorts recruited in the same region of the USA 20 years apart (1984–1991 and 2010–2014) revealed increased mean serum levels of 25OHD and decreased PTH levels due to self-supplementation with vitamin D in the latter cohort. Further, many cross-sectional studies have suggested an inverse correlation between serum levels of 25OHD and PTH, which indicates that low vitamin D levels might heighten PTH elevations in PHPT43,44,47–51. Additional evidence of worse hyperparathyroidism in those with vitamin D deficiency is reflected in increased serum levels of calcium, reduced levels of phosphate and increased 1,25(OH)2D levels as well as increased alkaline phosphatase levels, in some but not all studies42,43,48,49,51–57. Vitamin D treatment in PHPT is also associated with a lowering of PTH levels in observational studies and randomized controlled trials (RCTs)58,59. Thus, vitamin D deficiency, particularly when marked and prolonged, might be a risk factor for more biochemically severe PHPT.
Skeletal manifestations.
Although osteitis fibrosa cystica is rare, ample evidence exists for subclinical bone disease in PHPT. Dual-energy X-ray absorptiometry (DXA) demonstrates preferential loss of BMD at cortical sites such as the distal one-third of the forearm, whereas cancellous sites such as the lumbar spine are relatively spared60. This pattern reflects the catabolic versus anabolic effects of PTH on different skeletal compartments61. Iliac crest bone biopsy studies show a similar pattern with a reduction in cortical thickness but more favourable or similar trabecular indices in patients with PHPT versus controls61.
Estimates of the prevalence of osteoporosis in PHPT have varied in recent studies (39–62.9%)50,51,62, likely influenced by bias as a diagnosis of osteoporosis might have led to screening for PHPT. Mean T-scores in most studies are in the osteopenic range50,51. Risk factors for osteoporosis in PHPT, as in the general population, include older age and lower weight62. In contrast, vitamin D deficiency seems to have minimal effect on BMD, with only slightly reduced BMD at the one-third radius in those with low vitamin D levels; vitamin D repletion, however, might improve BMD, particularly at the spine50,51,59.
Given the BMD patterns observed with DXA, one would anticipate an increased risk of peripheral fractures but a reduction in vertebral fractures in modern PHPT Epidemiological data, however, suggest an increased risk of both vertebral and peripheral fractures63–66. The paradox of increased vertebral fracture risk despite preserved lumbar spine BMD in PHPT has remained unclear until recently. New technologies for non-invasively imaging the skeletal microarchitecture, such as high-resolution peripheral quantitative CT (HRpQCT) and the trabecular bone score (TBS), demonstrate that trabecular deterioration occurs at the spine as well as the radius and tibia67–69 (FIG. 2). Deteriorated microarchitecture has not yet been shown to be a risk factor for fracture in PHPT, but studies have implicated traditional risk factors such as older age, lower BMD and vitamin D levels, higher levels of bone turnover markers and higher PTH levels62,70. Recent work suggesting that clinically silent vertebral fractures are a common feature of PHPT led experts in the 2014 guidelines for the management of asymptomatic PHPT to recommend screening for vertebral fractures and parathyroidectomy if present62,71.
Figure 2. Trabecular deterioration in PHPT.
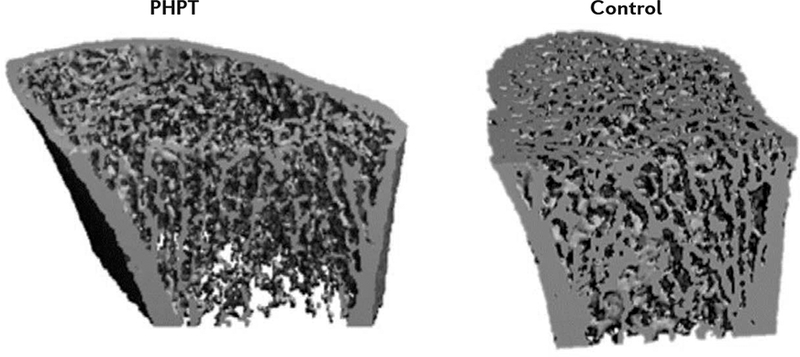
High-resolution peripheral quantitative CT images of the radius in a patient with primary hyperparathyroidism (PHPT; left) and a normal control (right). Trabecular deterioration is evident in PHPT. Reproduced with permission from REF 67, John Wiley and Sons.
This recommendation is supported by recent RCT data that suggest parathyroidectomy might reduce the risk of vertebral fractures compared with observation72.
Renal manifestations.
Today, the main renal manifestations of PHPT are hypercalciuria and nephrolithiasis73. Symptomatic nephrolithiasis is present in about 10–20% of patients8,73. Screening for asymptomatic nephrolithiasis, as recommended by the most recent (2014) guidelines for the management of asymptomatic PHPT, indicates that the prevalence is actually much higher62,71,73,74. Risk factors for nephrolithiasis include younger age and male sex, whereas degree of hypercalcaemia and hypercalciuria, PTH levels and other urinary factors have shown less consistent associations73–76. Limited data are available regarding nephrocalcinosis, but it seems to be an uncommon feature of modern PHPT, as are polyuria and polydipsia73. The prevalence of renal dysfunction (estimated glomerular filtration rate (eGFR) <60 ml/min) is low, with recent studies suggesting rates of 15–17%77–79. Neither the severity of PHPT nor having a history of nephrolithiasis was a risk factor for reduced eGFR in a 2014 study; instead, traditional risk factors, such as age, hypertension, use of antihypertensive medication and fasting glucose levels, were associated with poorer kidney function78. Longitudinal data are reassuring in this regard, as renal function remains stable in PHPT over long periods of follow-up8,80. Further, parathyroidectomy has not been shown to improve renal function, although one small study suggested an improvement in concentrating capacity8,80–83.
Neuropsychological features.
Increased serum calcium and PTH concentrations, the biochemical hallmarks of PHPT, could affect neuropsychological function. Calcium has a key role in regulating the release of neurotransmitters at synapses, and hypercalcaemia could interfere with that process. On the other hand, the long-known vascular effects of PTH could also affect cognition and mood by altering cerebrovascular function84–86. Descriptions of classical PHPT do indeed indicate neuropsychological features1,30. The extent to which these features remain a part of the modern picture of PHPT as well as the exact mechanisms underlying them is unclear. The muscle weakness and atrophy observed in classical PHPT are not seen today. A number of studies suggest, however, that even ‘mild PHPT’ (serum calcium <12 mg/dl) is associated with nonspecific symptoms such as depression, anxiety, fatigue, decreased quality of life (QoL), sleep disturbance and cognitive dysfunction. Many, but not all, observational studies have indicated these features improve after parathyroidectomy87. Three RCTs have investigated the reversibility of reduced QoL and psychiatric symptoms82,83,88. Despite being of similar design and using similar assessment tools, all three RCTs came to different conclusions: one RCT suggested parathyroidectomy prevents worsening of QoL and improves psychiatric symptoms88, another RCT indicated no benefit and the third RCT demonstrated improvement in QoL82,83. One RCT investigated changes in cognition after parathyroidectomy, but its small size precluded definitive conclusions being drawn89. At present, most experts do not recognize cognitive or psychiatric symptoms as a sole indication for parathyroidectomy. Reasons for this include the failure to clearly demonstrate reversibility in RCTs, the inability to predict which patients might improve and the lack of a clear mechanism71.
Cardiovascular manifestations.
Increased cardiovascular mortality in patients with moderate to severe PHPT has been documented in studies from Scandinavia90. Data on those with asymptomatic PHPT are limited, but several studies suggest no increase in mortality in those with mild hypercalcaemia91,92. Hypertension has long been associated with PHPT but is not reversible with parathyroidectomy90. Recent studies have investigated subclinical cardiovascular findings in PHPT, including asymptomatic coronary artery disease (CAD), valve calcification, left ventricular hypertrophy (LVH), carotid disease and vascular stiffness. When CAD is present in PHPT, it is most likely due to traditional risk factors rather than the disease itself93–95. Valve calcification, which is present in severe PHPT, has been shown to be more extensive (greater valve area) when present in those with mild PHPT than in controls96–98 and is associated with increased PTH levels, but it is not reversible with parathyroidectomy98. LVH has been associated with PHPT in many, but not all, studies90. A 2015 meta-analysis indicated that parathyroidectomy is associated with a decline in left ventricular mass and that higher levels of PTH predict a greater cardiovascular benefit; however, dissociating disease severity from study design (RCTs included individuals with lower levels of calcium and PTH than those included in observational studies) was not possible99. Conflicting data exist regarding whether intima-media thickness is increased in PHPT86,100–103. Multiple studies have reported increased vascular stiffness, sometimes associated with PTH levels, in mild PHPT, but its reversibility with parathyroidectomy is inconsistent86,104–106. Given conflicting data, most experts do not consider cardiovascular disease to be an indication for parathyroidectomy71.
Other manifestations.
Gastrointestinal symptoms, such as pancreatitis and peptic ulcer disease, do not seem to be a feature of modern PHPT, although conflicting data exist concerning an association of PHPT with constipation107,108. Patients with coeliac disease are at increased risk of developing PHPT, possibly due to chronic vitamin D deficiency109.
Normocalcaemic PHPT
Biochemical profile.
Both the Third and Fourth International Guidelines for the Management of Asymptomatic PHPT recognized NPHPT as a phenotype of PHPT24,31,110. NPHPT has a prevalence of 0.4–3.1% in community-based cohorts111. Although calcium levels are normal in this entity, PTH levels are similar or slightly lower than in the hypercalcaemic form of PHPT29,112. Phosphate levels have been reported to be higher and 1,25(OH)2D levels lower than in the hypercalcaemic variant112. By definition, ionized calcium, vitamin D levels, urinary calcium excretion and renal function must be normal to distinguish this entity from secondary hyperparathyroidism29. NPHPT can precede the development of typical hypercalcaemic PHPT, but patient series have suggested only 0.6–19% of patients go on to develop hypercalcaemia29,111,113. Higher serum calcium levels (within the normal range) and higher urinary calcium excretion as well as older age are reported to be risk factors for the development of hypercalcaemia in some studies29,113.
Skeletal, renal and other manifestations.
Although the clinical manifestations of NPHPT would be anticipated to be milder than those of hypercalcaemic PHPT, most case series suggest this is not the case, with high rates of osteoporosis, fractures and kidney stones29,114,115. This difference is likely due to selection bias, as NPHPT is often diagnosed after a clinical event such as a fracture or nephrolithiasis. Data from community cohorts suggest no differences in BMD between those with NPHPT and those with normal PTH levels, although more data are needed111. Almost no data exist regarding non-classical manifestations (such as neuropsychological and cardiovascular manifestations) in NPHPT.
Evaluation and management
Surgery
Parathyroidectomy remains the only cure for PHPT and is recommended in all symptomatic patients. The Fourth International Workshop for the Management of Asymptomatic PHPT recommended surgical intervention in patients with asymptomatic PHPT in whom evidence of subclinical end-organ (skeletal or renal) effects or risk of disease progression exists71 (TABLE 3). This recommendation includes those with serum calcium ≥1 mg/dl above the upper limit of normal; those with osteoporosis (T-score ≤−2.5) or vertebral fractures on imaging; those with eGFR <60 ml/min, severe hypercalciuria (>400 mg/day), increased risk of stones on a stone risk profile or evidence of occult nephrolithiasis or nephrocalcinosis on imaging; and those aged <50 years. Although evidence-based recommendations are lacking in NPHPT, surgery is suggested if patients become hypercalcaemic and have other indications for parathyroidectomy and should be considered in those who have disease progression regardless of hypercalcaemia (that is, worsening of BMD, fracture or kidney stones)71.
Table 3 |.
Evaluation and indications for surgery in asymptomatic PHPT
| Measure | Criteria for surgery at initial evaluation | Schedule for follow-up evaluation | Criteria for surgery at follow up |
|---|---|---|---|
| Age | <50 years | NA | <50 years |
| Serum level of calcium | ≥1 mg/dl above upper limit | Yearly | ≥1 mg/dl above upper limit |
| eGFR | <60 ml/min | Yearly | Reduction in eGFR <60 ml/min |
| 24 h urinary calcium level | >400 mg per day | Repeat if kidney stone suspected | >400 mg per day |
| Biochemical stone profile | Increased risk | Repeat if kidney stone suspected | Increased risk |
| Renal imaging | Presence of nephrolithiasis or nephrocalcinosis | Repeat if kidney stone suspected | Development of kidney stone |
| DXA (spine, hip and forearm) | T-Score ≤−2.5 | Every 1–2 years | T-Score ≤−2.5 or reduction in BMD |
| Vertebral imaging | Presence of a vertebral fracture | Repeat if vertebral fracture is suspected | Development of vertebral fracture |
DXA, dual-energy X-ray absorptiometry; eGFR, estimated glomerular filtration rate; NA, not applicable; PHPT, primary hyperparathyroidism.
Surgical guidelines from other societies are more liberal than those of the Fourth International Workshop for the Management of Asymptomatic PHPT25. The American Association of Endocrine Surgeons Guidelines additionally recommend surgery in those with cognitive or psychiatric symptoms attributable to PHPT, suggest offering parathyroidectomy to those with cardiovascular disease (other than hypertension) and consider symptoms of muscle weakness, impaired functional capacity and abnormal sleep patterns. Patient preference has an important role in decision-making; surgery is never inappropriate even in those who do not meet surgical criteria, as long as the diagnosis is secure. Finally, access to an experienced parathyroid surgeon has a role in the decision, as data suggest that surgeon volume inversely correlates with complications, cost and length of stay in hospital116,117.
Preoperative localization is necessary if a minimally invasive parathyroidectomy (MIP) is contemplated25. Imaging accuracy has improved vastly, but there remains wide variation in the sensitivity and specificity of different modalities across institutions. Cervical ultrasonography can localize parathyroid disease and assess for concomitant thyroid pathology. Technetium (99mTc) sestamibi is the dominant radioisotope in parathyroid scintigraphy. Sestamibi protocols vary (dual phase, I131 subtraction, single-photon emission CT (SPECT) or SPECT-CT); a review of the individual strengths and weaknesses of these protocols can be found elsewhere25. Sestamibi protocols have low sensitivity in multi-gland disease. Combined ultrasonography and sestamibi imaging increases localization accuracy and improves sensitivity. Although traditional CT imaging has little utility, the 4D CT protocol has emerged as a useful modality even though once again, it has limited sensitivity in multigland disease. Other technologies are recommended in more limited settings. MRI and venous sampling can be considered in cases of reoperation or difficult localization or when ionizing radiation is contraindicated. Although PET is costly and not widely available, recent data support incremental value of 18F-fluorocholine PET with CT (PET-CT) in the localization of pathologic parathyroid glands118. Preoperative fine-needle aspiration biopsy (FNAB) preoperatively is generally not recommended in PHPT and is absolutely contraindicated if parathyroid cancer is a diagnostic consideration, as FNAB can seed the operative site, leading to spread of disease. In the 5–10% of patients in whom surgery is performed for persistent or recurrent disease, two or more concordant studies should be obtained before surgery. A success rate of up to 95% can be achieved when this rule is followed, but no agreement exists as to which two studies are best in these cases119.
Bilateral neck exploration was the traditional surgical approach and has cure rates of >95% with a low risk of complications25. Improvements in imaging and the availability of intraoperative PTH monitoring allow for MIP in many centres, which reduces the extent of surgery, incision length, discomfort and recovery time and is associated with high cure rates as well25. MIP is appropriate for those with single adenomas in whom preoperative imaging has localized the culprit gland. With surgical cure of PHPT, serum biochemistries normalize, and urinary calcium levels decline120. RCTs and nonrandomized studies indicate that BMD improves robustly over the first year after parathyroidectomy with further increases over time, even in those with normal BMD8,72,83,121; limited data suggest fracture risk might also decline72. Risk of nephrolithiasis also decreases after successful parathyroidectomy122.
Postoperatively, most patients are discharged on calcium and vitamin D supplementation. Some patients remain persistently hyperparathyroid after successful parathyroid surgery (as evidenced by normalization of serum calcium levels) if they have untreated vitamin D deficiency or inadequate calcium intake (a form of ‘hungry bones’). Correction of the underlying cause of their secondary hyperparathyroidism should lead to normalization of all indices. Patients with normocalcaemic PHPT cannot be considered to be cured until and unless their PTH levels normalize, as their calcium levels were never abnormal. Patients who are not cured (those with both calcium and PTH levels that remain elevated after surgery) are said to have persistent PHPT25. A small subset of patients will be cured (with normalization of calcium and PTH levels) for a period of 6 months or more (up to many years) and then develop biochemical evidence of PHPT once again. These patients are said to have recurrent PHPT25. The incidence of recurrent PHPT is not known.
Non-surgical monitoring and management
The surgical guidelines developed by various groups imply that it is safe to monitor those who do not meet surgical guidelines25,71. Additionally, monitoring is recommended for those who refuse surgery and those who have significant comorbidities and are deemed poor surgical candidates. Very few patients fall into the latter category given the recent advances in parathyroid surgery, including MIP, which can be performed with local anaesthesia. However, those who have poor overall health that prohibits use of general anaesthesia and who are not good candidates for local anaesthesia due to aspiration risk or sleep apnoea might fall into this group. Long-term observational studies indicate that biochemistries and BMD remain stable for many years in those followed non-operatively8. However, 15-year data suggest that BMD starts to decline at cortical sites after 10 years of observation, and almost 40% of patients developed one or more indications for parathyroidectomy over 15 years of follow-up123. Regular monitoring of biochemistries and of BMD with DXA is recommended (TABLE 3) for those who choose to be observed; repeat imaging of the spine and kidney is advised when vertebral fractures or nephrolithiasis is suspected71. Guidelines for surgery during monitoring are similar to those at baseline (TABLE 3).
Some data suggest surgery is more cost-effective than medical treatment or observation of PHPT in patients who do not meet guidelines for parathyroidectomy124. However, some patients prefer observation and/or medical therapy to parathyroidectomy, even when meeting criteria for surgery. All patients who are observed should be advised to stay adequately hydrated and not to restrict dietary calcium intake. Liberal dietary calcium intake does not worsen hypercalcaemia. Conversely, restriction could exacerbate hyperparathyroidism125,126. The Fourth International Workshop recommends following the Institute of Medicine guidelines with regard to calcium intake127. Although calcium supplements are not specifically recommended in those with PHPT and osteoporosis, small doses do not seem to exacerbate hypercalcaemia or hypercalciuria if the diet is deficient128. Recent guidelines recommend restoring vitamin D to levels of 21–30 ng/ml with conservative doses of vitamin D (600–1000 IU daily) on the basis of data showing that vitamin D repletion lowers PTH levels127. Higher levels of vitamin D might be beneficial. A 2014 RCT of cholecalciferol (2,800 IU daily versus placebo) indicated that treatment increased 25OHD levels from 20 ng/ml to 37.8 ng/ml, lowered levels of PTH, and increased lumbar spine BMD without having a deleterious effect on serum or urinary calcium levels59.
Ideal medical therapy of PHPT would provide the equivalent to a medical parathyroidectomy. Such an agent would normalize serum calcium and PTH levels as well as urinary calcium excretion, increase BMD and lower fracture risk, and reduce the risk of kidney stones. Unfortunately, no currently available single drug meets all these criteria. The following medications can achieve some of these goals and might be considered in patients not having surgery in whom it is desirable to lower serum or urinary calcium levels or increase BMD (TABLE 4).
Table 4 |.
Response to medication in patients with PHPT*
| Drug | Serum calcium level | Serum PTH level | Urinary calcium excretion | BMD |
|---|---|---|---|---|
| HCTZ | No change | No change | Decreases | No data |
| Oestrogen | No change | No change | No change | Increases |
| Raloxifene | Decreases** | No change | No change | No data |
| Alendronate | No change | No change | No change | Increases |
| Cinacalcet | Decreases | Minimal decrease | No change | No change |
We are not recommending the use of these agents in PHPT (the changes described are occasionally based on small numbers of individuals).
At 8 weeks of treatment. HCTZ, hydrochlorothiazide.
Hydrochlorothiazide.
A retrospective analysis of 72 patients in 2016 suggested that thiazides might not increase serum levels of calcium in PHPT as they can do in normal individuals. Hydrochlorothiazide (12.5–50 mg daily for 3.1 years on average) was associated with a decrease in urinary calcium excretion but no change in serum levels of calcium129. Smaller and cross-sectional studies have suggested similar results, although it is unclear if hydrochlorothiazide reduces the risk of nephrolithiasis130,131. Given the heterogeneity of doses used and the absence of data from larger, (preferably) randomized trials, recommending thiazide use routinely in PHPT is premature. However, thiazides could be considered in those who refuse surgery or are poor surgical candidates but at high risk of nephrolithiasis, as long as serum levels of calcium are monitored regularly.
Oestrogen and selective oestrogen receptor modulators.
An RCT of conjugated oestrogen (0.625 mg daily plus medroxyprogesterone at 5 mg daily) versus placebo indicated that hormone-replacement therapy effectively increases BMD at all skeletal sites in patients with PHPT, with the greatest increases at the lumbar spine132. This RCT, however, did not confirm the calcium-lowering effect of earlier uncontrolled studies127. Currently, no data regarding the ability of oestrogen to lower fracture risk in patients with PHPT are available. In an RCT, raloxifene (60 mg daily compared with placebo; n = 18) decreased serum levels of bone markers and serum calcium levels after 8 weeks of treatment; BMD data were not available127.
Bisphosphonates and denosumab.
Several small RCTs have indicated that alendronate increases BMD at the lumbar spine and hip in patients with PHPT, but most studies suggest there is no change in serum biochemistries127. No data exist regarding fracture risk reduction with alendronate, and no BMD data are available in PHPT for other bisphosphonates including zoledronic acid, pamidronate or ibandronate. One small nonrandomized study of risedronate plus calcium and vitamin D versus parathyroidectomy suggested that risedronate increases BMD at the spine compared with baseline in patients with PHPT but that surgery is more effective133; no data regarding fracture risk reduction were available. No published data are available regarding the use of denosumab in PHPT. In summary, anti-resorptives can be considered in patients not undergoing parathyroidectomy who have osteoporosis, a history of fragility fracture or high fracture risk, though none are specifically approved for the treatment of PHPT.
Cinacalcet.
Cinacalcet is a type 2 calcimimetic that binds to the CASR and increases its sensitivity. Cinacalcet effectively reduces serum levels of calcium in patients with PHPT. Cinacalcet was approved for PHPT by the European Medicines Agency in 2008 and by the FDA in 2011 for the treatment of severe hypercalcaemia in patients with PHPT who are unable to undergo parathyroidectomy127. Cinacalcet maintains long-term normocalcaemia across a wide spectrum of disease severity127. Unfortunately, neither BMD nor urinary calcium excretion improves with cinacalcet treatment, and there are currently no data regarding reduction in the risk of nephrolithiasis127. Cinacalcet is also frequently associated with headache, nausea and vomiting.
Conclusions and future directions
In summary, PHPT has evolved into a disorder that is typically asymptomatic in regions of the world where serum levels of calcium are routinely measured. Manifestations of PHPT include mild hypercalcaemia, osteoporosis, hypercalciuria as well as vertebral fractures and nephrolithiasis, both of which can be subclinical. Classical PHPT and normocalcaemic disease are less common. Earlier detection of PHPT and vitamin D supplementation might have contributed to the changes in PHPT presentation in recent decades. Further evolution might continue to occur with secular trends in the measurement of serum levels of calcium and vitamin D supplementation, as well as with advances in disease detection and management.
Surgery, the only curative treatment for PHPT, is recommended for those with symptoms and is suggested for those with asymptomatic disease who are at risk of progression or have subclinical evidence of end-organ effects. Parathyroidectomy leads to an increase in BMD and a reduction in nephrolithiasis. Medical therapy for those who cannot undergo parathyroidectomy can increase BMD (for example, oestrogen and bisphosphonates) or reduce serum levels of calcium (calcimimetics), but no single drug can do both.
Looking to the future, more data are needed on the neuropsychological manifestations of PHPT to help direct surgical recommendations for patients with specific complaints, as well as on the cardiovascular effects of mild disease. Data are also needed to enable evidence-based decisions about current renal guidelines for surgery and on the applicability of current surgical criteria to NPHPT. Secular trends in the measurement of calcium levels, which might increase in the developing world and decrease in the developed world, could alter the clinical profile of the disease internationally. Finally, as the cause of most cases of sporadic PHPT is unknown, we do not understand why some patients develop skeletal, renal or other complications while others do not. Owing to the limited data regarding these mechanisms, a ‘personalized’ approach to tailor monitoring, surgical recommendations or medications to individual patients with PHPT has a limited role in the management of PHPT today. Future work that advances our knowledge in these areas will clearly improve the management of the disorder.
Key points.
Primary hyperparathyroidism (PHPT), a common endocrine disorder characterized by hypercalcaemia and elevated or inappropriately normal levels of parathyroid hormone, is diagnosed based upon biochemical evaluation
Over the past 50 years, the clinical profile of PHPT has evolved from a highly symptomatic disease to one that is most often asymptomatic, albeit with evidence of subclinical target organ involvement
Even ‘asymptomatic’ patients can have skeletal deterioration (evident upon imaging; for example, dual-energy X-ray absorptiometry), and subclinical manifestations can include osteoporosis and hypercalciuria as well as clinically silent vertebral fractures and nephrolithiasis
The diagnosis of normocalcaemic PHPT can be made after eliminating secondary causes of hyperparathyroidism; however, data are limited on its natural history and appropriate criteria for and response to surgery
Parathyroidectomy by an experienced parathyroid surgeon is recommended for patients with symptomatic disease or subclinical end-organ involvement, as no single medical therapy addresses hypercalcaemia and the skeletal and renal consequences of PHPT
Acknowledgements
The authors acknowledge the National Institiutes of Health (NIH; Grants DK074457 and DK084986 to S.J.S. and DK104105 to M.D.W.).
Footnotes
Author contributions
M.D.W. and S.J.S. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cope O The study of hyperparathyroidism at the Massachusetts General Hospital. N. Engl. J. Med 274, 1174–1182 (1966). [DOI] [PubMed] [Google Scholar]
- 2.Yeh MW et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J. Clin. Endocrinol. Metab 98, 1122–1129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wermers RA et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993–2001: an update on the changing epidemiology of the disease. J. Bone Miner. Res 21, 171–177 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Press DM et al. The prevalence of undiagnosed and unrecognized primary hyperparathyroidism: a population-based analysis from the electronic medical record. Surgery 154, 1232–1237 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Heath H 3rd, Hodgson SF & Kennedy MA Primary hyperparathyroidism. Incidence, morbidity, and potential economic impact in a community. N. Engl. J. Med 302, 189–193 (1980). [DOI] [PubMed] [Google Scholar]
- 6.Wermers RA et al. The rise and fall of primary hyperparathyroidism: a population-based study in Rochester, Minnesota, 1965–1992. Ann. Intern. Med 126, 433–440 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Griebeler ML et al. Secular trends in the incidence of primary hyperparathyroidism over five decades. Bone 73, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverberg SJ, Shane E, Jacobs TP, Siris E & Bilezikian JP A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N. Engl. J. Med 341, 1249–1255 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Rao SD et al. Hyperparathyroidism following head and neck irradiation. Arch. Intern. Med 140, 205–207 (1980). [PubMed] [Google Scholar]
- 10.Bendz H, Sjodin I, Toss G & Berglund K Hyperparathyroidism and long-term lithium therapy — a cross-sectional study and the effect of lithium withdrawal. J. Intern. Med 240, 357–365 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Newey PJ et al. Whole-exome sequencing studies of nonhereditary (sporadic) parathyroid adenomas. J. Clin. Endocrinol. Metab 97, E1995–E2005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa-Guda J & Arnold A Genetic and epigenetic changes in sporadic endocrine tumors: parathyroid tumors. Mol. Cell. Endocrinol 386, 46–54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardi E et al. Aryl hydrocarbon receptor interacting protein (AIP) mutations occur rarely in sporadic parathyroid adenomas. J. Clin. Endocrinol. Metab 98, 2800–2810 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Arnold AM & Levine MA in The Parathyroids: Basic and Clinical Concepts (ed. Bilezikian JP) 279–297 (Academic Press, 2015). [Google Scholar]
- 15.Marx SJ et al. Hyperparathyroidism in hereditary syndromes: special expressions and special managements. J. Bone Miner. Res 17 (Suppl. 2), N37–N43 (2002). [PubMed] [Google Scholar]
- 16.Thakker RV Genetics of parathyroid tumours. J. Intern. Med 280, 574–583 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Guan B et al. GCM2-activating mutations in familial isolated hyperparathyroidism. Am. J. Hum. Genet 99, 1034–1044 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu W et al. Whole-exome sequencing studies of parathyroid carcinomas reveal novel PRUNE2 mutations, distinctive mutational spectra related to APOBEC-catalyzed DNA mutagenesis and mutational enrichment in kinases associated with cell migration and invasion. J. Clin. Endocrinol. Metab 100, E360–E364 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Corbetta S et al. Differential expression of microRNAs in human parathyroid carcinomas compared with normal parathyroid tissue. Endocr. Relat. Cancer 17, 135–146 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Brown EM Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract. Res. Clin. Endocrinol. Metab 27, 333–343 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Mahoney EJ, Monchik JM, Donatini G & De Lellis R Life-threatening hypercalcemia from a hepatocellular carcinoma secreting intact parathyroid hormone: localization by sestamibi single-photon emission computed tomographic imaging. Endocr. Pract 12, 302–306 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Nussbaum SR, Gaz RD & Arnold A Hypercalcemia and ectopic secretion of parathyroid hormone by an ovarian carcinoma with rearrangement of the gene for parathyroid hormone. N. Engl. J. Med 323, 1324–1328 (1990). [DOI] [PubMed] [Google Scholar]
- 23.D’Amour P et al. Amino-terminal form of parathyroid hormone (PTH) with immunologic similarities to hPTH(1–84) is overproduced in primary and secondary hyperparathyroidism. Clin. Chem 49, 2037–2044 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Eastell R et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab 99, 3570–3579 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm SM et al. The American Association of Endocrine Surgeons Guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 151, 959–968 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Eastell R et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J. Clin. Endocrinol. Metab 94, 340–350 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Nesbit MA et al. Mutations affecting G-protein subunit α 11 in hypercalcemia and hypocalcemia. N. Engl. J. Med 368, 2476–2486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nesbit MA et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat. Genet 45, 93–97 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowe H, McMahon DJ, Rubin MR, Bilezikian JP & Silverberg SJ Normocalcemic primary hyperparathyroidism: further characterization of a new clinical phenotype. J. Clin. Endocrinol. Metab 92, 3001–3005 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Albright F, Aub J & Bauer W Hyperparathyroidism: common and polymorphic condition as illustrated by seventeen proven cases in one clinic. JAMA 102, 1276 (1934). [Google Scholar]
- 31.Silverberg SJ et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab 99, 3580–3594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usta A, Alhan E, Cinel A, Turkyilmaz S & Erem CA 20-year study on 190 patients with primary hyperparathyroidism in a developing country: Turkey experience. Int. Surg 100, 648–655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallette LE, Bilezikian JP, Heath DA & Aurbach GD Primary hyperparathyroidism: clinical and biochemical features. Med. (Baltimore) 53, 127–146 (1974). [PubMed] [Google Scholar]
- 34.Liu JM et al. Primary hyperparathyroidism: a tale of two cities revisited — New York and Shanghai. Bone Res 1, 162–169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malabu UH & Founda MA Primary hyperparathyroidism in Saudi Arabia: a review of 46 cases. Med. J. Malaysia 62, 394–397 (2007). [PubMed] [Google Scholar]
- 36.Paruk IM, Esterhuizen TM, Maharaj S, Pirie FJ & Motala AA Characteristics, management and outcome of primary hyperparathyroidism in South Africa: a single-centre experience. Postgrad. Med. J 89, 626–631 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Shah VN, Bhadada S, Bhansali A, Behera A & Mittal BR Changes in clinical and biochemical presentations of primary hyperparathyroidism in India over a period of 20 years. Indian J. Med. Res 139, 694–699 (2014). [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao L et al. The changing clinical patterns of primary hyperparathyroidism in Chinese patients: data from 2000 to 2010 in a single clinical center. J. Clin. Endocrinol. Metab 98, 721–728 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Bandeira F & Cassibba S Hyperparathyroidism and bone health. Curr. Rheumatol. Rep 17, 48 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Oliveira UE et al. Analysis of the diagnostic presentation profile, parathyroidectomy indication and bone mineral density follow-up of Brazilian patients with primary hyperparathyroidism. Braz. J. Med. Biol. Res 40, 519–526 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Spivacow FR, Martinez C & Polonsky A [Primary hyperparathyroidism: postoperative long-term evolution]. Medicina (B. Aires) 70, 408–414 (in Spanish) (2010). [PubMed] [Google Scholar]
- 42.Walker MD et al. Low vitamin D levels have become less common in primary hyperparathyroidism. Osteoporos. Int 26, 2837–2843 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moosgaard B et al. Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin. Endocrinol. (Oxf.) 63, 506–513 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Boudou P, Ibrahim F, Cormier C, Sarfati E & Souberbielle JC A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J. Endocrinol. Invest 29, 511–515 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Clements MR et al. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin. Endocrinol. (Oxf.) 37, 17–27 (1992). [DOI] [PubMed] [Google Scholar]
- 46.Clements MR et al. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Clin. Sci. (Lond.) 73, 659–664 (1987). [DOI] [PubMed] [Google Scholar]
- 47.Rao DS et al. Effect of vitamin D nutrition on parathyroid adenoma weight: pathogenetic and clinical implications. J. Clin. Endocrinol. Metab 85, 1054–1058 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Silverberg SJ, Shane E, Dempster DW & Bilezikian JP The effects of vitamin D insufficiency in patients with primary hyperparathyroidism. Am. J. Med 107, 561–567 (1999). [DOI] [PubMed] [Google Scholar]
- 49.Tassone F et al. Vitamin D status in primary hyperparathyroidism: a Southern European perspective. Clin. Endocrinol. (Oxf.) 79, 784–790 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Viccica G, Cetani F, Vignali E, Miccoli M & Marcocci C Impact of vitamin D deficiency on the clinical and biochemical phenotype in women with sporadic primary hyperparathyroidism. Endocrine 55, 256–265 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Walker MD et al. Vitamin D in primary hyperparathyroidism: effects on clinical, biochemical, and densitometric presentation. J. Clin. Endocrinol. Metab 100, 3443–3451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamashita H et al. Vitamin D status in Japanese patients with hyperparathyroidism: seasonal changes and effect on clinical presentation. World J. Surg 26, 937–941 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Ozbey N et al. Correlations between vitamin D status and biochemical/clinical and pathological parameters in primary hyperparathyroidism. World J. Surg 30, 321–326 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Jayasena CN et al. Associations of serum 25-hydroxyvitamin D with circulating PTH, phosphate and calcium in patients with primary hyperparathyroidism. Clin. Endocrinol. (Oxf.) 78, 838–843 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Inoue Y et al. Vitamin D status affects osteopenia in postmenopausal patients with primary hyperparathyroidism. Endocr. J 55, 57–65 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Moosgaard B et al. Plasma 25-hydroxyvitamin D and not 1,25-dihydroxyvitamin D is associated with parathyroid adenoma secretion in primary hyperparathyroidism: a cross-sectional study Eur. J. Endocrinol 155, 237–244 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Moosgaard B et al. Vitamin D metabolites and skeletal consequences in primary hyperparathyroidism. Clin. Endocrinol. (Oxf.) 68, 707–715 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Shah VN, Shah CS, Bhadada SK & Rao DS Effect of 25 (OH) D replacements in patients with primary hyperparathyroidism (PHPT) and coexistent vitamin D deficiency on serum 25(OH) D, calcium and PTH levels: a meta-analysis and review of literature. Clin. Endocrinol. (Oxf.) 80, 797–803 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Rolighed L et al. Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J. Clin. Endocrinol. Metab 99, 1072–1080 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Silverberg SJ et al. Skeletal disease in primary hyperparathyroidism. J. Bone Miner. Res 4, 283–291 (1989). [DOI] [PubMed] [Google Scholar]
- 61.Parisien M et al. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J. Clin. Endocrinol. Metab 70, 930–938 (1990). [DOI] [PubMed] [Google Scholar]
- 62.Cipriani C et al. Prevalence of kidney stones and vertebral fractures in primary hyperparathyroidism using imaging technology. J. Clin. Endocrinol. Metab 100, 13091315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khosla S et al. Primary hyperparathyroidism and the risk of fracture: a population-based study. J. Bone Miner. Res 14, 1700–1707 (1999). [DOI] [PubMed] [Google Scholar]
- 64.Vignali E et al. Morphometric vertebral fractures in postmenopausal women with primary hyperparathyroidism. J. Clin. Endocrinol. Metab 94, 2306–2312 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosekilde L Primary hyperparathyroidism and the skeleton. Clin. Endocrinol. (Oxf.) 69, 1–19 (2008). [DOI] [PubMed] [Google Scholar]
- 66.De Geronimo S, Romagnoli E, Diacinti D, D’Erasmo E & Minisola S The risk of fractures in postmenopausal women with primary hyperparathyroidism. Eur. J. Endocrinol 155, 415–420 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Stein EM et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J. Bone Miner. Res 28, 1029–1040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker MD et al. Effect of concomitant vitamin D deficiency or insufficiency on lumbar spine volumetric bone mineral density and trabecular bone score in primary hyperparathyroidism. Osteoporos. Int 27, 3063–3071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen S, Hauge EM, Rasmussen L, Jensen JE & Brixen K Parathyroidectomy improves bone geometry and microarchitecture in female patients with primary hyperparathyroidism. A 1-year prospective controlled study using high resolution peripheral quantitative computed tomography. J. Bone Miner. Res 27, 1150–1158 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Nordenstrom E, Westerdahl J, Lindergard B, Lindblom P & Bergenfelz A Multifactorial risk profile for bone fractures in primary hyperparathyroidism. World J. Surg 26, 1463–1467 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Bilezikian JP et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J. Clin. Endocrinol. Metab 99, 3561–3569 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lundstam K et al. Effects of parathyroidectomy versus observation on the development of vertebral fractures in mild primary hyperparathyroidism. J. Clin. Endocrinol. Metab 100, 1359–1367 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Rejnmark L, Vestergaard P & Mosekilde L Nephrolithiasis and renal calcifications in primary hyperparathyroidism. J. Clin. Endocrinol. Metab 96, 2377–2385 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Cassibba S et al. Silent renal stones in primary hyperparathyroidism: prevalence and clinical features. Endocr. Pract 20, 1137–1142 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Odvina CV et al. Biochemical characterization of primary hyperparathyroidism with and without kidney stones. Urol. Res 35, 123–128 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Berger AD et al. Patients with primary hyperparathyroidism — why do some form stones? J. Urol 181, 2141–2145 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Tassone F, Gianotti L, Emmolo I, Ghio M & Borretta G Glomerular filtration rate and parathyroid hormone secretion in primary hyperparathyroidism. J. Clin. Endocrinol. Metab 94, 4458–4461 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Walker MD et al. Predictors of renal function in primary hyperparathyroidism. J. Clin. Endocrinol. Metab 99, 1885–1892 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walker MD et al. Effect of renal function on skeletal health in primary hyperparathyroidism. J. Clin. Endocrinol. Metab 97, 1501–1507 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rao DS, Wilson RJ, Kleerekoper M & Parfitt AM Lack of biochemical progression or continuation of accelerated bone loss in mild asymptomatic primary hyperparathyroidism: evidence for biphasic disease course. J. Clin. Endocrinol. Metab 67, 1294–1298 (1988). [DOI] [PubMed] [Google Scholar]
- 81.Kristoffersson A, Backman C, Granqvist K & Jarhult J Pre- and postoperative evaluation of renal function with five different tests in patients with primary hyperparathyroidism. J. Intern. Med 227, 317–324 (1990). [DOI] [PubMed] [Google Scholar]
- 82.Ambrogini E et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J. Clin. Endocrinol. Metab 92, 3114–3121 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Bollerslev J et al. Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: a prospective, randomized trial. J. Clin. Endocrinol. Metab 92, 1687–1692 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Ogino K, Burkhoff D & Bilezikian JP The hemodynamic basis for the cardiac effects of parathyroid hormone (PTH) and PTH-related protein. Endocrinology 136, 3024–3030 (1995). [DOI] [PubMed] [Google Scholar]
- 85.Collip JB & Clark EP Further studies on the physiological action of a parathyroid hormone. J. Biol. Chem 64, 4485–507 (1925). [Google Scholar]
- 86.Walker MD et al. Carotid vascular abnormalities in primary hyperparathyroidism. J. Clin. Endocrinol. Metab 94, 3849–3856 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker MD & Silverberg SJ Parathyroidectomy in asymptomatic primary hyperparathyroidism: improves “bones” but not “psychic moans”. J. Clin. Endocrinol. Metab 92, 1613–1615 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Talpos GB et al. Randomized trial of parathyroidectomy in mild asymptomatic primary hyperparathyroidism: patient description and effects on the SF-36 health survey. Surgery 128, 1013–1020 (2000). [DOI] [PubMed] [Google Scholar]
- 89.Perrier ND et al. Prospective, randomized, controlled trial of parathyroidectomy versus observation in patients with “asymptomatic” primary hyperparathyroidism. Surgery 146, 1116–1122 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Walker MD & Silverberg SJ Cardiovascular aspects of primary hyperparathyroidism. J. Endocrinol. Invest 31, 925–931 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soreide JA et al. Survival after surgical treatment for primary hyperparathyroidism. Surgery 122, 1117–1123 (1997). [DOI] [PubMed] [Google Scholar]
- 92.Wermers RA et al. Survival after the diagnosis of hyperparathyroidism: a population-based study Am. J. Med 104, 115–122 (1998). [DOI] [PubMed] [Google Scholar]
- 93.Vestergaard P et al. Cardiovascular events before and after surgery for primary hyperparathyroidism. World J. Surg 27, 216–222 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Streeten EA et al. Coronary artery calcification in patients with primary hyperparathyroidism in comparison with control subjects from the multi-ethnic study of atherosclerosis. Endocr. Pract 14, 155–161 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Kepez A et al. Evaluation of subclinical coronary atherosclerosis in mild asymptomatic primary hyperparathyroidism patients. Int. J. Cardiovasc. Imag 25, 187–193 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Stefenelli T et al. Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am. J. Med 95, 197–202 (1993). [DOI] [PubMed] [Google Scholar]
- 97.Iwata S et al. Aortic valve calcification in mild primary hyperparathyroidism. J. Clin. Endocrinol. Metab 97, 132–137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walker MD et al. Effect of parathyroidectomy on subclinical cardiovascular disease in mild primary hyperparathyroidism. Eur. J. Endocrinol 167, 277–285 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McMahon DJ et al. Effect of parathyroidectomy upon left ventricular mass in primary hyperparathyroidism: a meta-analysis. J. Clin. Endocrinol. Metab 100, 4399–4407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nilsson IL, Aberg J, Rastad J & Lind L Endothelial vasodilatory dysfunction in primary hyperparathyroidism is reversed after parathyroidectomy. Surgery 126, 1049–1055 (1999). [DOI] [PubMed] [Google Scholar]
- 101.Kosch M et al. Studies on flow-mediated vasodilation and intima-media thickness of the brachial artery in patients with primary hyperparathyroidism. Am. J. Hypertens 13, 759–764 (2000). [DOI] [PubMed] [Google Scholar]
- 102.Lumachi F et al. Intima-media thickness measurement of the carotid artery in patients with primary hyperparathyroidism. A prospective case-control study and long-term follow-up. In Vivo 20, 887–890 (2006). [PubMed] [Google Scholar]
- 103.Fallo F et al. Ultrasound evaluation of carotid artery in primary hyperparathyroidism. J. Clin. Endocrinol. Metab 88, 2096–2099 (2003). [DOI] [PubMed] [Google Scholar]
- 104.Rosa J et al. Pulse wave velocity in primary hyperparathyroidism and effect of surgical therapy. Hypertens. Res 34, 296–300 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Schillaci G et al. Large-artery stiffness: a reversible marker of cardiovascular risk in primary hyperparathyroidism. Atherosclerosis 218, 96–101 (2011). [DOI] [PubMed] [Google Scholar]
- 106.Rubin MR, Maurer MS, McMahon DJ, Bilezikian JP & Silverberg SJ Arterial stiffness in mild primary hyperparathyroidism. J. Clin. Endocrinol. Metab 90, 3326–3330 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Ragno A et al. Chronic constipation in hypercalcemic patients with primary hyperparathyroidism. Eur. Rev. Med. Pharmacol. Sci 16, 884–889 (2012). [PubMed] [Google Scholar]
- 108.Pepe J et al. The effect of parathyroidectomy on chronic constipation in patients affected by primary hyperparathyroidism. J. Bone Miner. Metab 31, 690–694 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Ludvigsson JF et al. Primary hyperparathyroidism and celiac disease: a population-based cohort study. J. Clin. Endocrinol. Metab 97, 897–904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bilezikian JP, Khan AA & Potts JT Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J. Clin. Endocrinol. Metab 94, 335–339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cusano NE et al. Normocalcemic hyperparathyroidism and hypoparathyroidism in two community-based nonreferral populations. J. Clin. Endocrinol. Metab 98, 2734–2741 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maruani G, Hertig A, Paillard M & Houillier P Normocalcemic primary hyperparathyroidism: evidence for a generalized target-tissue resistance to parathyroid hormone. J. Clin. Endocrinol. Metab 88, 4641–4648 (2003). [DOI] [PubMed] [Google Scholar]
- 113.Rejnmark L, Amstrup AK, Mollerup CL, Heickendorff L & Mosekilde L Further insights into the pathogenesis of primary hyperparathyroidism: a nested case-control study. J. Clin. Endocrinol. Metab 98, 87–96 (2013). [DOI] [PubMed] [Google Scholar]
- 114.Tuna MM et al. Normocalcemic hyperparathyroidism is associated with complications similar to those of hypercalcemic hyperparathyroidism. J. Bone Miner. Metab 34, 331–335 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Koumakis E et al. Bone mineral density evolution after successful parathyroidectomy in patients with normocalcemic primary hyperparathyroidism. J. Clin. Endocrinol. Metab 98, 3213–3220 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Abdulla AG, Ituarte PH, Harari A, Wu JX & Yeh MW Trends in the frequency and quality of parathyroid surgery: analysis of 17,082 cases over 10 years. Ann. Surg 261, 746–750 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Stavrakis AI, Ituarte PH, Ko CY & Yeh M W Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery 142, 887–899 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Kluijfhout WP et al. Fluorine-18 fluorocholine PET-CT localizes hyperparathyroidism in patients with inconclusive conventional imaging: a multicenter study from the Netherlands. Nucl. Med. Commun 37, 1246–1252 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Udelsman R & Donovan PI Remedial parathyroid surgery: changing trends in 130 consecutive cases. Ann. Surg 244, 471–479 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rao DS, Phillips ER, Divine GW & Talpos GB Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J. Clin. Endocrinol. Metab 89, 5415–5422 (2004). [DOI] [PubMed] [Google Scholar]
- 121.Rolighed L et al. BMD improvements after operation for primary hyperparathyroidism. Langenbecks Arch. Surg 398, 113–120 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Mollerup CL et al. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ 325, 807 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rubin MR et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J. Clin. Endocrinol. Metab 93, 3462–3470 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zanocco KA, Wu JX & Yeh MW Parathyroidectomy for asymptomatic primary hyperparathyroidism: A revised cost-effectiveness analysis incorporating fracture risk reduction. Surgery 161, 16–24 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Locker FG, Silverberg SJ & Bilezikian JP Optimal dietary calcium intake in primary hyperparathyroidism. Am. J. Med 102, 543–550 (1997). [DOI] [PubMed] [Google Scholar]
- 126.Paik JM, Curhan GC & Taylor EN Calcium intake and risk of primary hyperparathyroidism in women: prospective cohort study. BMJ 345, e6390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marcocci C, Bollerslev J, Khan AA & Shoback DM Medical management of primary hyperparathyroidism: proceedings of the fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab 99, 3607–3618 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Jorde R, Szumlas K, Haug E & Sundsfjord J The effects of calcium supplementation to patients with primary hyperparathyroidism and a low calcium intake. Eur. J. Nutr 41, 258–263 (2002). [DOI] [PubMed] [Google Scholar]
- 129.Tsvetov G et al. Thiazide treatment in primary hyperparathyroidism — a new indication for an old medication? J. Clin. Endocrinol. Metab 102, 1270–1276 (2017). [DOI] [PubMed] [Google Scholar]
- 130.Riss P et al. The influence of thiazide intake on calcium and parathyroid hormone levels in patients with primary hyperparathyroidism. Clin. Endocrinol. (Oxf.) 85, 196–201 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Farquhar CW, Spathis GS, Barron JL & Levin GE Failure of thiazide diuretics to increase plasma calcium in mild primary hyperparathyroidism. Postgrad. Med. J 66, 714–716 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grey AB, Stapleton JP, Evans MC, Tatnell MA & Reid IR Effect of hormone replacement therapy on bone mineral density in postmenopausal women with mild primary hyperparathyroidism. A randomized, controlled trial. Ann. Intern. Med 125, 360–368 (1996). [DOI] [PubMed] [Google Scholar]
- 133.Tournis S et al. Effect of parathyroidectomy versus risedronate on volumetric bone mineral density and bone geometry at the tibia in postmenopausal women with primary hyperparathyroidism. J. Bone Miner. Metab 32, 151–158 (2014). [DOI] [PubMed] [Google Scholar]


