Abstract
We evaluated 24 veterans with advanced non–small-cell lung cancer treated with pembrolizumab. Forty-one percent of patients had durable clinical benefit to therapy. We showed that veterans with high comorbidity scores responded as well as the general population to immunotherapy. An increase in absolute lymphocyte count might be associated with clinical benefit and warrants further investigation.
Background
Because of the prevalence of smoking in the veteran population, non–small-cell lung cancer (NSCLC) remains a significant cause of morbidity and mortality. The objectives of our study were to evaluate the extent of durable clinical benefit (DCB) to pembrolizumab in veterans with metastatic NSCLC and to identify clinical determinants of DCB.
Materials and Methods
Prospective clinical data on veterans receiving pembrolizumab were collected. Duration of response was calculated from the first date of infusion until date of disease progression on computed tomography scans, defined according to Response Evaluation Criteria in Solid Tumors version 1.1 (CTCAE).
Results
As of the censor date, 25 veterans consented and 24 were evaluable. The response rate was 25% (6 of 24 patients), with all achieving a partial response. Four patients received palliative radiation because of focal progression and continued to receive pembrolizumab, leading to a DCB rate of 41% (10 of 24 patients). The mean duration of response at the censor date was 12.9 months (95% confidence interval [CI], 9.9–15.9) and 2.7 months (95% CI, 1.9–4.3) for those with and without DCB, respectively. Patients without DCB had a higher pack-year smoking history (P = .007). An increase in peripheral blood absolute lymphocyte count (ALC) during therapy was seen in patients with DCB (P = .073). There were no CTCAE Grade > 3 adverse events. All immune-related adverse events occurred in patients with DCB.
Conclusion
Nearly half of the veterans exhibited DCB and pembrolizumab therapy was well tolerated. An increase in ALC from baseline and occurrence of autoimmune phenomena might be associated with DCB. Immunotherapy with pembrolizumab is a promising therapeutic strategy in veterans with advanced NSCLC.
Keywords: Checkpoint inhibitors, Immunotherapy, Lymphocyte count, NSCLC, Response rate
Introduction
Lung cancer is the second most common cancer in men as well as in women. However, it is the number one cause of cancer-associated mortality.1 Each year, approximately 40,000 incident cancers are reported in the Veterans Affairs Central Cancer Registry, accounting for approximately 3% of all cancers in the United States.2 Similar to that of the general population, lung cancer is also the second most common cancer in the veteran population, accounting for 20.9% in 2007.2 The proportion of stage IV disease at time of diagnosis is lower in the veteran population compared with the general population (46%2 and 57%,1 respectively). Variable-adjusted analyses also show that the receipt of guideline-recommended treatment for veterans with stage IV non–small-cell lung cancer (NSCLC) is 28%,3 which is similar, if not improved, compared with estimates on the basis of Surveillance, Epidemiology, and End Results Program (SEER)-Medicare data.4 Older age and coexisting comorbidities are negative predictors of treatment in both groups. Metastatic NSCLC continues to portend a dismal prognosis with an estimated 5-year survival rate of < 5% (SEER), which serves as the impetus to develop more efficacious therapeutics.
The checkpoint inhibitors pembrolizumab and nivolumab were approved in 2015 by the US Food and Drug Administration (FDA) for second-line treatment of metastatic NSCLC after progression on platinum-based therapy. The KEYNOTE-010,5 CheckMate-017,6 and CheckMate-0577 studies showed the superiority of these agents compared with docetaxel chemotherapy, which was previous standard second-line therapy. The response to checkpoint inhibitors, however, is approximately 20%8 and clinical predictors of response are not well understood.
Studies have shown that patients with smoking-related NSCLC that exhibits characteristic molecular signatures had the best response to pembrolizumab in terms of overall response and progression-free survival.9 We sought to (1) answer whether this was true in the veteran population, in whom most NSCLCs are smoking-related; and (2) evaluate factors to better characterize the population that would derive benefit from immunotherapy.
Materials and Methods
Patients
Clinical data from a small prospective trial that evaluated determinants of response to pembrolizumab therapy in veterans were analyzed. All veterans at the VA Ann Arbor Healthcare System with metastatic NSCLC who were being considered for immunotherapy and met eligibility criteria were screened for participation in this study. All staging of NSCLC was according to the American Joint Committee on Cancer (AJCC) version 7. All veterans were required to be 18 years of age or older, carry a diagnosis of metastatic NSCLC, have received at least 1 previous line of chemotherapy for metastatic disease including a platinum-based doublet, have adequate hematologic and organ function, and an adequate Eastern Cooperative Oncology Group (ECOG) performance status < 2. Patients were ineligible if their tumors carried mutations eligible for tyrosine kinase inhibitor therapy, had untreated brain metastasis, or had a history of pneumonitis. They also could not be receiving active therapy for autoimmune disease or daily glucocorticoid therapy. Programmed death-ligand 1 (PD-L1) staining using immunohistochemistry was not performed on tumor samples for several reasons: (1) PD-L1 positivity has not been consistently shown to predict response to checkpoint inhibition (see Discussion); (2) lack of adequate tumor sample for evaluation and delay in care to obtain repeat biopsy; and (3) practical consideration of significant distance traveled for patients treated at our VA. All patients signed informed consent for participation in this study. This study was approved by the VA Research and Development Committee and the VA institutional review board.
Data Collection
Data were abstracted from each patient’s medical record through the VA Computerized Patient Record System. All patients were assigned a randomized study number and personal identifiers were omitted from data analysis. The master list linking the study number to the patient’s medical record was kept secure at the Ann Arbor VA with access permitted only by the investigators and research personnel.
Monitoring
Response to therapy was determined according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST).10 All patients had baseline computed tomography (CT) scans obtained within 1 month before initiation of pembrolizumab. Serial CT scans were then obtained every 12 weeks and response was assessed at each scan by the investigators. A full history and physical examination and adverse event monitoring was completed before each infusion and routine bloodwork was also collected. If the investigators were concerned about new symptomology or physical examination findings, CT scans could be obtained earlier at their discretion. For all patients, the duration of response was defined as the length of time from date of first pembrolizumab infusion to date of last pembrolizumab infusion or date of confirmed disease progression on the basis of CT scans. For patients that were lost to follow-up, the last known follow-up date was used to calculate duration of response.
Statistics
The primary end point for response was durable clinical benefit (DCB), defined as alive and without disease progression (complete response [CR] + partial response [PR] + stable disease [SD]) at 6 months. The patients either continued to receive pembrolizumab or stopped receiving the drug but follow-up CT scans continued to show no evidence of disease progression. Patients evaluable for response received at least 1 dose of pembrolizumab. The response rate was calculated, along with a 95% confidence interval (CI) on the basis of an exact test.11 The patients were divided into those with and without DCB. Fisher exact test was used to evaluate the difference in categorical variables, whereas the Wilcoxon rank sum test was used to evaluate the difference in continuous variables.
For each patient, the absolute lymphocyte count (ALC) was obtained before each infusion of pembrolizumab and after the last infusion according to censor date. A linear mixed effect model was used to compare the ALC trajectories (log-transformed) over time between patients with and without DCB. The model includes group (with DCB/without DCB), time, and an interaction between group and time. The interaction tests the difference in the slope between those with and without DCB. Moreover, the model includes a random intercept to allow for each patient having a different intercept. A P value of < .05 was considered to be statistically significant. Statistics were calculated using SAS software (version 9.4, SAS Institute Inc, Cary, NC).
Results
Twenty-five veterans were enrolled at the Ann Arbor VA from November 2014 to June 2016. All patients received pembrolizumab, 2 mg/kg every 3 weeks. One patient died shortly after consent but before receiving therapy and 2 patients received only 1 infusion. For those 2 patients, one died suddenly on day 3 and the other had worsening performance status and declined further treatment, not believed to be related to pembrolizumab. Therefore, 24 of 25 patients were evaluable.
As of August 31, 2016, the median follow-up was 9.2 months (range, 2.1–20.9 months). Seven patients (29%) were still receiving pembrolizumab, 3 of whom had a duration of response > 6 months and therefore were included in the DCB group for analysis. For the remaining 4 patients, because they were still receiving therapy but had not yet reached the 6-month mark, they could not be classified as either with DCB or without DCB. The demographic information for all 24 patients is shown in Table 1. All patients were male. Of those 24 patients, 3 died, and 4 additional patients enrolled onto hospice.
Table 1.
Patient Demographic Characteristics (n = 24)
| Characteristic | Value |
|---|---|
| Age at Time of Diagnosis, y | |
| Median | 64 |
| Range | 52–77 |
| Race, n (%) | |
| White | 17 (70.8) |
| Black | 2 (8.3) |
| Other | 5 (20.8) |
| ECOG Performance Status, n (%) | |
| 0 | 10 (42) |
| 1 | 14 (58) |
| Distance to Ann Arbor, Miles | |
| Median | 85.4 |
| Range | 17.4–205.7 |
| Smoking Status, n (Mean Pack-Years, Range) | |
| Current | 7 (66, 22–100) |
| Former | 16 (44, 1–100) |
| Never | 1 (0) |
| Charlson Comorbidity Index, n (%) | |
| 0 | 0 |
| 1–3 | 14 (58.3) |
| ≥4 | 10 (41.7) |
| AJCC Stage at Time of Diagnosis, n (%)a | |
| I | 0 |
| II | 5 (20.8) |
| III | 10 (41.7) |
| IV | 8 (33.3) |
| Histology, n (%) | |
| Adenocarcinoma | 11 (45.8) |
| Squamous | 11 (45.8) |
| Other | 2 (8.3) |
| Previous XRT Therapy, n (%)b | |
| 0 | 6 (25) |
| <60 Gy | 7 (29.2) |
| ≥60 Gy | 11 (45.8) |
| Previous Chemotherapy Regimens, n (%) | |
| 1 | 12 (50) |
| >1 | 12 (50) |
| Development of IRAEs, n (%) | |
| Yes | 3 (12.5) |
| No | 21 (87.5) |
Abbreviations: AJCC = American Joint Committee on Cancer; ECOG = Eastern Cooperative Oncology Group; IRAE = immune-related adverse event; XRT = radiation therapy.
There was 1 patient with unknown stage at the time of diagnosis.
Dosage of previous radiation therapy for other malignancies as well as palliative radiation were included.
Response Rate
The response rate in our study was 25% (6 of 24 patients), with a 95% CI from 10% to 47%. All 6 patients had a PR according to RECIST criteria. Four patients had progression of disease but received localized radiation and continued to receive therapy because of continued clinical benefit, resulting in a DCB of 41% (10 of 24). Follow-up scans after radiation of these patients showed SD or PR. Table 2 shows the clinical course for these 4 patients including site of progression and subsequent intervention. An independent radiologist who was blinded to patient treatment course confirmed disease response.
Table 2.
Patients With Mixed Response to Pembrolizumab
| Patient | Site of Progression | Intervention | Total Duration of Response |
|---|---|---|---|
| VA001 | RLL mass after cycle 3 | XRT, 30 Gy | >9.6 mo, continues to receive pembrolizumab with SD shown on imaging |
| VA003 | RUL with SVC syndrome after cycle 5 | XRT, 30 Gy | 10.3 mo |
| VA009 | New mediastinal lymph nodes after cycle 4 | XRT, 20 Gy | 6.5 mo |
| VA015 | New brain mets after cycle 6 | WBRT, 30 Gy | 7.7 mo |
Abbreviations: mets = metastases; RLL = right lower lobe; RUL = right upper lobe; SVC = superior vena cava; WBRT = whole brain radiation therapy; XRT = radiation therapy.
Adverse Events
There was 1 CTCAE Grade 3 adverse event; this occurred in a patient (VA013) who developed hypophysitis and required hospitalization. He recovered well and continues to have no evidence of disease progression since his last pembrolizumab infusion in May 2016.
Predictors of Durable Clinical Benefit
The clinical characteristics that were evaluated between patients with and without DCB are summarized in Table 3. The average length of response for those with DCB was 12.9 months (95% CI, 9.9–15.9) and 2.7 months (95% CI, 1.8–4.0) months for those without DCB. There was no significant difference between age and Charlson comorbidity index (CCI) between the 2 groups. Patients without DCB did have a more significant pack-year smoking history than those with DCB (P = .007). With respect to other clinical predictors, there was no significant difference between the 2 groups.
Table 3.
Predictors of Durable Clinical Benefit
| Predictor | With DCB (10) | Without DCB (10) | P |
|---|---|---|---|
| Average Duration of Response, mo | 12.9 (9.9–15.9) | 2.7 (0.9–4.3) | <.001 |
| Mean Age, y | 60.8 (55.7–65.9) | 64.4 (59.4–69.4) | .09 |
| Smoking: Mean, Pack-Years | 37.6 (13.2–61) | 71.3 (50.2–92.4) | .007 |
| Mean CCI | 3.1 (1.9–5.3) | 3.3 (1.8–4.8) | .64 |
| Adenocarcinoma, n | 7 | 3 | .34 |
| >1 Previous Chemotherapy Regimen, n | 7 | 2 | .15 |
| Mean Total Radiation Received, Gy | 50 (2.4–97.6) | 39.8 (11.7–67.9) | .88 |
| Development of IRAE, n | 3 | 0 | .22 |
Values in parentheses represent 95% CIs.
Abbreviations: CCI = Charlson comorbidity index; IRAE = immune-related adverse event.
Increase in ALC
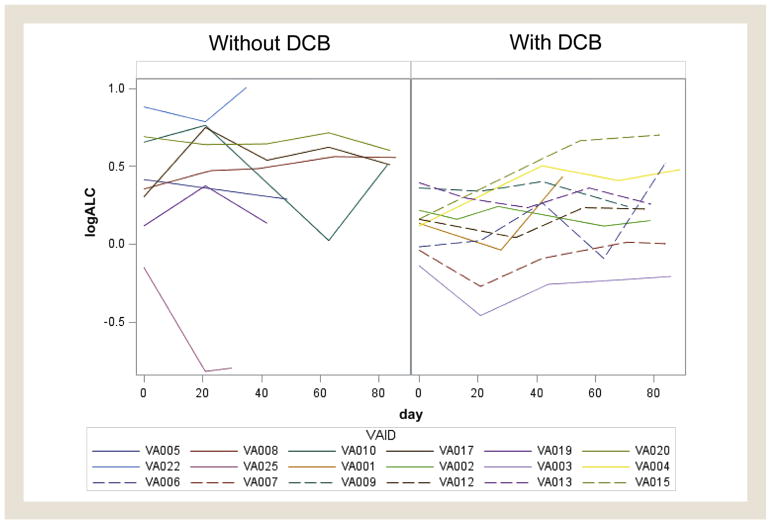
Table 4 shows the baseline ALC, ALC at 3 months, and end ALC at censor date, as well as duration of response for each patient. Mean baseline ALC (thousands per microliter) for those with DCB was 1.16 and 1.49 for those without DCB. Mean end ALC was 1.34 for those with DCB and 1.47 for those without. A linear mixed effect model of ALC trajectory over 90 days showed a P value of .073 in a comparison of difference in slope between the 2 groups (Figure 1).
Table 4.
Trend in ALC
| Patient | Duration of Response, mo | Baseline ALCa | ALC at 3 moa | End ALCa |
|---|---|---|---|---|
| With DCB | ||||
| VA004 | 20.5 | 1.12 | 1.61 | 1.41 |
| VA013 | 17.4 | 1.48 | 1.26 | 1.24 |
| VA007 | 17.4 | 0.96 | 1.11 | 1.26 |
| VA012 | 16.1 | 1.17 | 1.11 | 1.57 |
| VA002 | 13.4 | 1.24 | 1.25 | 1.61 |
| VA003b | 10.3 | 0.87 | 0.81 | 0.74 |
| VA001b | 9.6 | 1.14 | 1.43 | 1.62 |
| VA006 | 9.3 | 0.98 | 1.68 | 1.36 |
| VA015b | 7.7 | 1.17 | 1.82 | 1.34 |
| VA009b | 6.5 | 1.43 | 1.27 | 1.2 |
| Without DCB | ||||
| VA020 | 3.5 | 1.99 | 1.82 | 1.51 |
| VA010 | 2.5 | 1.92 | 1.02 | 1.66 |
| VA022 | 2.5 | 2.41 | NA | 1.98 |
| VA005c | 1.6 | 1.51 | NA | 1.33 |
| VA025 | 1.3 | 0.86 | NA | 0.45 |
| VA019 | 1.2 | 1.12 | NA | 1.09 |
| VA008 | 4.2 | 1.42 | 2.22 | 2.06 |
| VA017 | 5.6 | 1.35 | 1.66 | 1.65 |
| VA014c | 0.07 | 1.5 | NA | NA |
| VA018c | 3.4 | 0.78 | NA | NA |
VA005 received 2 doses of pembrolizumab but did not return for follow-up. VA014 died 2 days after receiving the initial dose of pembrolizumab. VA018 developed worsening performance status after the initial dose of pembrolizumab and elected to enroll onto hospice.
Abbreviations: ALC = absolute lymphocyte count; DCB = durable clinical benefit.
Reported in thousands per microliter.
Patient with progression of disease but continued receiving pembrolizumab after focal radiation and who showed DCB at 6 months.
Patients lost to follow-up after receiving ≤ 2 doses of pembrolizumab.
Figure 1. Absolute Lymphocyte Count (ALC) Trajectory Over 90 Days.
The log-transformed ALC for the first 90 days was plotted for patients with durable clinical benefit (DCB) and without DCB. A linear mixed effect model was used to evaluate the differences in slopes with P = .073.
Development of Immune-Related Adverse Events
Three patients developed immune-related adverse events (IRAEs) during pembrolizumab therapy; 2 developed thyroiditis and 1 developed hypophysitis. All 3 of these patients had durable response to therapy at 9.3, 10.3, and 17.4 months at the time of data censor.
Discussion
Despite the FDA approval of targeted tyrosine kinase inhibitors in metastatic NSCLC, only 10% of veterans present with actionable molecular subtypes of NSCLC targetable by FDA-approved therapies (personal communication, Dr Lou Fiore, Precision Oncology Program for Veterans). Therefore, there is a significant unmet need for effective therapy in this group of patients with lung cancer. To the best of our knowledge, our study is the first to evaluate response and clinical benefit of immunotherapy exclusively in veterans, and the first to describe response to immunotherapy outside of the clinical trial setting.
The response rate to pembrolizumab was 25% in our study, with a DCB rate at 6 months of 41%. Although the number of patients in our study was small, the response rate we saw suggests that veterans respond as well as the general population. Four patients developed progression of disease according to RECIST but continued to receive pembrolizumab after completing radiation with subsequent scans showing SD or PR. This practice is supported by the recently published KEYNOTE-024 study of front-line use of pembrolizumab in which patients were allowed to continue to receive therapy despite disease progression on imaging given continued clinical benefit.12 Of the 7 patients still receiving pembrolizumab, 4 have not been receiving therapy for ≥ 6 months to determine if they will be those with DCB. Among those that have had their initial 12-week scans, none have had progressive disease. Therefore, the DCB rate might be even higher than is reported. In addition, the only CTCAE Grade 3 adverse event in our study was hypophysitis, showing that veterans also tolerated this therapy.
The 2 groups were similar with respects to their CCIs. In trials using the CCI to assess comorbidity in NSCLC patients, a score ≥ 4 was considered severe comoribidty.3 Nearly half (42%) of the patients in our study had a CCI ≥ 4 and would likely not have tolerated traditional cytotoxic chemotherapy. Increased CCI was shown to have a stronger correlation with worse survival compared with ECOG performance status and a CCI > 2 was associated with early treatment dropout when cytotoxic chemotherapy was used.13
Rizvi et al have shown through whole exome sequencing in NSCLC patients treated with pembrolizumab that higher non-synonymous mutation burden in tumors was associated with improved objective response, DCB, and progression-free survival.9 This observation is consistent with the hypothesis that the efficacy of anti-programmed cell death protein-1 (PD-1) therapy is largely related to recognition of neoantigens, which result from various somatic mutations induced by carcinogens such as cigarette smoke. The sentinel study of pembrolizumab in patients with NSCLC showed that response was higher in current/former smokers compared with never-smokers.14 In contrast, in our study, patients without DCB had a significantly heavier smoking history as reflected by more pack-years, which contradicts the previous observations. However, the prevalence of smoking was very high in our population—23 of 24 patients (96%) were current/former smokers. Therefore, the extent of tobacco exposure might not be prognostic, but rather it is any exposure to smoking. In our study, it was not possible to draw any observations on the basis of 1 patient who was a never-smoker. Nonetheless, the degree of tobacco exposure in our population could account for the higher response rate that was observed.
Ku et al showed, albeit with ipilimumab in patients with melanoma, that an ALC > 1000/μL after 2 doses was significantly associated with improved clinical benefit and increased median overall survival compared with ALC <1000/μL.15 In a recently published study, Weide et al reported that a relative lymphocyte count (RLC) ≥ 17.5% was significantly associated with improved overall survival in melanoma patients treated with pembrolizumab.16 ALC after 2 doses of pembrolizumab as well as baseline RLC were also examined in our study and we did not find any correlation between ALC > 1000/μL and RLC ≥ 17.5% with DCB (results not shown). We did perform a linear mixed effect model to compare the slope of ALC between the 2 groups over a fixed time period of 90 days and found a P value of .073, which trended toward statistical significance. To the best of our knowledge, the change in lymphocyte count during pembrolizumab treatment has not been previously examined for patients with NSCLC. We have previously shown in an exceptional responder, albeit to anti-PD-L1 therapy, that a specific subset of T-cell clones expanded dramatically and persisted even months after completion of therapy (abstract presented at Immune Profiling in Health and Disease, September 2015, Seattle, WA). It would therefore be of interest to characterize the lymphocyte population in the patients with DCB to determine whether the increase in ALC is because of clonal expansion of a specific subgroup. We plan to study this in a prospective fashion and in a larger cohort. Clinically, an exuberant immune reaction and durable response to checkpoint inhibition could also be supported by the observation that all patients who developed an IRAE all had DCB to pembrolizumab. Sanlorenzo et al reported that patients who developed pembrolizumab-associated cutaneous adverse events had significantly longer progression-free survival compared with those who did not.17
There are several limitations of our current study. The number of enrolled patients was small because it was a pilot study. Therefore, many of the observed trends were not statistically significant. With a small n, it is not possible to predict whether observations would become significant with a larger population of subjects or if they occurred by chance and would not continue to hold true. The FDA approval for pembrolizumab in NSCLC is for PD–L1-positive tumors, defined using Dako 22C3 IHC staining (Agilent Technologies, Santa Clara, CA) ≥ 50%, but we did not assess PD-L1 expression in our study. Although elevated tumoral PD-L1 expression has been shown in some studies to predict response to immunotherapy,14,18 not all reveal the same correlation.6,19 Furthermore, response is also seen in tumors with 1% to 49% PD-L1 expression, which translates to clinical benefit.5,14 The objective of this study was to identify easily obtained clinical factors that could predict response to immunotherapy and limiting our patients to those with PD–L1-positive tumors could have introduced bias to our results. Of the 14 tumor samples that we had sent for mutational testing through Personal Genome Diagnostics (Baltimore, MD), 8 were insufficient to be analyzed. The median distance our patients had to travel was 85.4 miles and in our opinion, the burden of a repeat biopsy in our high-risk population and the delay of initiating care was too great. However, as PD-L1 testing becomes more widely adopted, we are planning prospective studies that include this analysis. Finally, all of our veterans received pembrolizumab, and it is not known if we can generalize our findings to all checkpoint inhibitors.
Conclusion
We have shown in this pilot study that despite a preserved performance status, veterans with recurrent/advanced NSCLC are a high-risk population because of their increased CCI. However, the response rate to pembrolizumab was as robust as that seen in clinical trials. Patients in our study also exhibited an impressive DCB. Clinical predictors that warrant further investigation include an increase in ALC and development of IRAEs. Because of the recent FDA approval for use of pembrolizumab in the first-line setting for advanced NSCLC, we believe that our observations will serve as the basis for future prospective studies in the veteran population with the goals of converting more patients to responders and integrating clinical and laboratory predictors of response.
Clinical Practice Points
Pembrolizumab is a monoclonal antibody against PD-1, approved for first-line treatment of PD–L1-positive advanced NSCLC or after disease progression on platinum-based chemotherapy.
To our knowledge, evaluation of response to checkpoint inhibitors exclusively in the veteran population has not been done in the past; furthermore, little is known about potential clinical factors that can predict response.
The rate of DCB was 41% in our study, with a response rate of 25%. An increase in ALC during therapy and development of IRAEs might be associated with benefit to therapy and are worthy of further investigation.
Despite having high comorbidity indices, veterans also tolerated therapy well without significant adverse events.
Veterans with advanced NSCLC exhibit a robust response to checkpoint inhibitor therapy.
Acknowledgments
This work was supported by the National Institutes of Health grant 4T32CA009357-34 and the VA Merit Award I01 CX001560.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177:693–701. doi: 10.7205/milmed-d-11-00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Wong ML, Hamilton N, Davoren JB, Jahan TM, Walter LC. Impact of age and comorbidity on non–small-cell lung cancer treatment in older veterans. J Clin Oncol. 2012;30:1447–55. doi: 10.1200/JCO.2011.39.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non–small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soria JC, Marabelle A, Brahmer JR, Gettinger S. Immune checkpoint modulation for non–small-cell lung cancer. Clin Cancer Res. 2015;21:2256–62. doi: 10.1158/1078-0432.CCR-14-2959. [DOI] [PubMed] [Google Scholar]
- 9.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small-cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 12.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 13.Frasci G, Lorusso V, Panza N, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non–small-cell lung cancer. J Clin Oncol. 2000;18:2529–36. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- 14.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 15.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016;22:5487–96. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206–12. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]