Summary
The cytokine interleukin (IL)‐6 is a major therapeutic target for the treatment of various inflammatory and autoimmune diseases. While IL‐6 receives considerable attention in studies of innate and adaptive immunity, the IL‐6‐related family member IL‐27 is recognized increasingly for its effects on cellular proliferation, differentiation and leucocyte effector functions. Both cytokines activate responses in myeloid and stromal tissue cells, where they direct the transition from innate to adaptive immunity. However, they are identified frequently as lymphokines that control responses in T cells and B cells. In this regard, IL‐27 often opposes the action of IL‐6. Here, we will review the role of IL‐6 and IL‐27 in inflammation, with a particular focus on inflammatory arthritis, and discuss their importance in the diagnosis, stratification and treatment of autoimmune disease.
Keywords: arthritis, cytokine receptors, cytokines, inflammation, rheumatoid arthritis
The interleukin (IL)‐6 family of cytokines
All members of the interleukin (IL)‐6 family share a common 130 kDa glycoprotein signal‐transducing receptor (gp130, CD130). In this regard, receptors for IL‐11, oncostatin‐M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin‐1 (CT‐1), leukaemia inhibitory factor (LIF) and the cardiotrophin‐like cytokine (CLC) all utilize gp130 to transmit cytokine responses. These IL‐6‐related cytokines are related structurally and each contain four long α‐helical chains, which are arranged in an up–up–down–down topography 1. In contrast, the IL‐6‐related cytokine IL‐27 is a heterodimeric cytokine consisting of two independent subunits termed IL‐27p28 (also known as IL‐30) and Epstein–Barr virus‐induced gene 3 (EBI3) 2. IL‐27 therefore resembles IL‐12 (which comprises IL‐12p40 and IL‐12p35) and the related IL‐23 (IL‐23p19 and IL‐12p40) and IL‐35 (IL‐12p40 and EBI3) inflammatory cytokines (Fig. 1) 3. However, IL‐27 also shares several characteristics common to the IL‐6 cytokine family. First, the receptor complex for IL‐27 contains the IL‐27 receptor‐α [IL‐27Rα, also known as IL‐27 receptor subunit (WSX‐1) and type I T cell cytokine receptor (TCCR)] subunit, together with gp130 4. While gp130 is expressed universally in all tissues and organs, IL‐27Rα is restricted mainly to lymphocytes, monocytes and osteoclasts 1, 3. The cognate α‐subunit of the IL‐6 receptor (IL‐6R, CD126) also shows a similarly restricted pattern of expression and is found on hepatocytes, leucocyte subsets and megakaryocytes 5. Secondly, EBI3 shares close sequence identity with IL‐6R 3, 6. In this regard, the soluble IL‐6R when bound to IL‐6 resembles a heterodimeric cytokine reminiscent of IL‐12, IL‐23, IL‐27 and IL‐35 5, 6, 7. Thirdly, IL‐6 and IL‐27 receptor activation leads to signalling through the latent transcription factors signal transducer and activator of transcription‐1 (STAT1) and STAT3 8. IL‐27 is, however, the only member of the IL‐6‐related cytokine family to signal predominantly via STAT1 instead of STAT3 1, 3. Consequently IL‐6 and IL‐27 elicit both common and distinct biological outcomes, and IL‐27 can often limit IL‐6/STAT3‐driven events.
Figure 1.
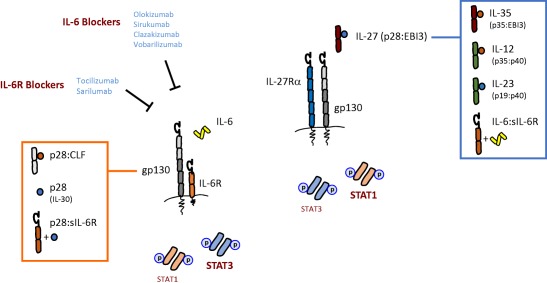
The biological relationship between interleukin (IL)‐6 and IL‐27. The illustration shows the composition of the IL‐6 and IL‐27 receptor complexes, and identifies the signal transducer and activator of transcription (STAT) factors triggered by both cytokines. Note the inclusion of gp130 in both receptors, and preferential induction of STAT1 and STAT3 activity (bold text). For the IL‐6 receptor cassette the reader should note the various IL‐6 and IL‐6R blocking therapies currently in clinical development or clinical utility. Proteins displayed in the orange box indicate biological entities that have been reported to engage with the IL‐6 receptor, albeit at low affinity. Cytokines listed in the blue box showcase the protein composition of IL‐27 related heterodimeric cytokines. Common subunits are colour‐coded. The IL‐6 : sIL‐6R (and that of p28 : sIL‐6R) complex is not stable, however, and the cytokine‐receptor undergoes association and reassociation (indicated by the + symbol).
The inflammatory significance of IL‐6 and IL‐27
Based on the biological properties of IL‐6, this cytokine was originally named interferon β2, hepatocyte‐stimulating factor, cytotoxic T cell differentiation factor, B cell differentiation factor and B cell stimulatory factor‐2. These broad definitions identify IL‐6 as a lymphokine and activator of the acute phase response 5. However, clinical experience with the blocking anti‐IL‐6R monoclonal antibody tocilizumab has helped to unearth roles for IL‐6 in the control of lipid, glucose and iron metabolism, regulation of the neuroendocrine system and changes in psychological wellbeing that include pain, fatigue, mood and depression (Fig. 2) 5. Thus, IL‐6 often displays features of a hormone. In contrast, IL‐27 is associated primarily with the control of innate and adaptive immunity to infection 3, 9. IL‐27 was first recognized as a proinflammatory cytokine due to its ability to support the development of interferon (IFN)‐γ‐secreting T helper (Th) cells. For example, IL‐27 promotes expression of IFN‐γ, the transcriptional master regulator T‐bet, STAT1 and IL‐12Rβ2 2, 8, 10, 11, 12. These activities closely resemble those of IL‐12. However, subsequent studies have shown that IL‐27 is a negative regulator of IL‐2 and can restrict development of immune responses (Fig. 3) 13, 14, 15, 16, 17, 18. For example, IL‐27 is required for the development of T‐bet+ and C‐X‐C motif chemokine receptor 3 (CXCR3)+ regulatory T cell (Treg) populations following T helper type 1 (Th1)‐mediated inflammation using Toxoplasma gondii‐challenged IL‐27R‐deficient mice 14. In this regard, the anti‐inflammatory properties of type I IFNs are attributed largely to the up‐regulation of IL‐27 and the subsequent promotion of IL‐10 19, 20, 21. Consistent with these observations, prominent immunosuppressive roles were discovered for IL‐27 through investigations in mouse models of chronic infection and autoimmunity 22, 23, 24, 25, 26. In the absence of a regulatory IL‐27 signal, IL‐27R‐deficient mice developed profound or lethal T cell‐mediated pathology 13, 16, 18, 27. In this context, IL‐27 often antagonizes the actions of IL‐6. While IL‐6 supports the development and expansion of Th cell responses 5, IL‐27 has emerged as an inhibitor of Th17 activities, and in a model of helminth infection limits Th2 responses through inhibiting GATA‐binding protein 3 (GATA3) expression (Fig. 3) 8, 18, 28. While additional investigations are required to explore fully the wider biological functions of IL‐27, emerging data also highlight potential roles for IL‐27 in the control of pain 29, myeloid cell activation 30, 31, 32, 33, 34, 35, 36 and stromal tissue responses (Fig. 2) 37, 38, 39. Thus, IL‐6 and IL‐27 contribute to inflammation and the regulation of both innate and adaptive immunity.
Figure 2.
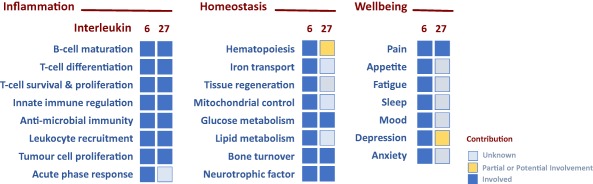
The functional properties of interleukin (IL)‐6 and IL‐27. The biological properties of IL‐6 and IL‐27 have been categorized broadly under the terms ‘inflammation’, ‘homeostasis’ and ‘wellbeing’. Defined activities have been listed for each category and the heat‐map identifies the relative contribution of IL‐6 and IL‐27 to each of these processes. The definition of the colour‐coding is listed. It should be noted that IL‐6 and IL‐27 may regulate similar or distinct outcomes in each process and the reader is referred to the text and review papers relevant to IL‐6 or IL‐27 (references 3, 5).
Figure 3.
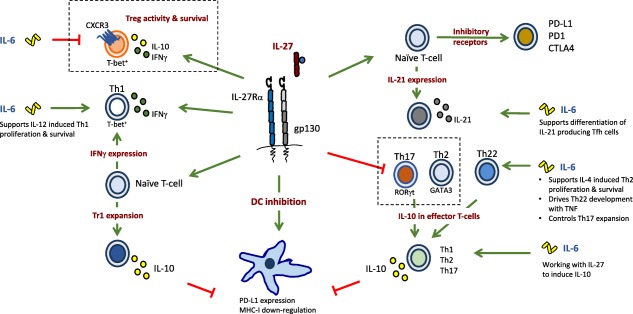
Immunomodulatory action of interleukin (IL‐27) and the interface with IL‐6. IL‐27 and IL‐6 together co‐ordinate adaptive immune responses, often with opposing biological outcomes. In an inflammatory microenvironment, and supported by accessory cytokines, IL‐6 can promote the differentiation of T helper type 1 (Th1), Th2, Th22 and Th17 cells. In contrast, IL‐27 counteracts the IL‐6‐driven expansion of Th17 cells and inhibits the development of Th2 and Th22 cells. However, IL‐6 and IL‐27 can both promote the secretion of IL‐10 in a number of effector T cell subsets, and can drive the production of IL‐21 in T helper cells. IL‐27 drives immunosuppressive effector characteristics in T cells, including the expression of the immune check‐points programmed death ligand 1 (PD‐L1), programmed death 1 (PD‐1) and cytotoxic T lymphocyte antigen‐4 (CTLA‐4). In contrast to the inhibitory action of IL‐6 on regulatory T cells (Treg cells), IL‐27 promotes the development of IL‐10‐producing T‐bet+CXCR3+ Treg cells and Tr1 cells. IL‐27 also has immunosuppressive roles at the dendritic cell (DC) : T cell synapse; for example, through promoting expression of PD‐L1 on DCs and inhibiting major histocompatibility complex (MHC)‐I expression. Boxed areas highlight opposing roles of IL‐27 and IL‐6. Figure adapted from Yoshida et al. (reference 3).
Although the receptor complex for IL‐27 signalling remains fixed, composed of IL‐27Rα and gp130, the signalling mechanisms employed by IL‐6 are highly complex (Fig. 1), and it is often challenging to understand how IL‐6 receptor signalling can elicit a diverse array of biological responses. Three very distinct forms of IL‐6 receptor signalling have now been proposed. These are termed classical IL‐6 receptor signalling, IL‐6 trans‐signalling, which is reliant upon the presence of a soluble form of IL‐6R (sIL‐6R), and a newly reported mechanism called IL‐6 trans‐presentation 5, 40, 41. In contrast, IL‐27 uses a classical IL‐27 receptor system based on the cellular expression of IL‐27Rα and gp130. However, the IL‐27p28 subunit of IL‐27 has also been reported to antagonize IL‐6‐mediated T cell responses, and can potentially bind the IL‐6R (Fig. 1) 18, 42, 43. Why does IL‐6 adopt these different forms of signalling? Here, it is important to note that IL‐6 contributions to both the regulation of immune homeostasis and inflammatory responses that are relevant to infection, trauma or injury 5. During health, classical IL‐6 receptor signalling promotes the maintenance of normal physiology. For example, IL‐6 controls various metabolic processes and tissue renewal or regeneration 5. In contrast, IL‐6 trans‐signalling is associated more widely with the regulation of inflammatory processes relevant to disease 5, 40. This distinction is not, however, black and white. In this regard, classical IL‐6 receptor signalling controls both the acute phase response and the generation of certain effector CD4 T cell populations 5. Similarly, IL‐6 trans‐signalling has been linked to processes including haematopoiesis and the rapid eye movement (REM) sleep cycle 44, 45. The newly described IL‐6 trans‐presentation mode of cell activation is a juxtacrine mechanism of IL‐6 signalling that promotes the engagement of dendritic cells with T cells 41. While further work is required to establish the precise biological significance of IL‐6 trans‐presentation, this mode of cell activation may control immunological processes in tissues that rely upon resident immune cells to mount an appropriate response to antigen challenge. These locations may include sites with immune privilege, such the brain or eye.
In the following sections, we will consider the roles of IL‐6 and IL‐27 in the progression of inflammatory disease, and focus upon their involvement in rheumatoid arthritis.
The significance of genetic polymorphisms linked with IL‐6 and IL‐27
Several lines of genetic evidence support a role for IL‐6 and IL‐27 in autoimmunity, cancer and infection. Genome‐wide association studies and analyses of genetic polymorphisms have identified several susceptibility loci relevant to IL‐6 and IL‐27 that predict a predisposition for autoimmune disease. For example, a single nucleotide polymorphism proximal to the IL6 (rs1800795) transcriptional start site is associated with an increased incidence of coronary heart disease, idiopathic juvenile arthritis and other inflammatory conditions 46, 47, 48, 49. Equally, genetic variants associated with IL6st (gp130; rs10940495) and IL6R (rs2228145) are common to patients with cardiovascular disease and rheumatoid arthritis 46, 47, 48, 49. Several polymorphisms linked with IL27 (encoding IL‐27p28; rs153109, rs181206, rs17855750) also display risk susceptibilities with asthma, certain cancers, metabolic disorders and some viral infections 50, 51, 52. For example, rs153109 is linked to more severe forms of rheumatoid arthritis 53. While additional functional genetic studies are required to determine the biological relevance of these genetic variants, several contribute to changes in cytokine or cytokine receptor expression. For example, mutations within IL6R (rs2228145) and IL6 (rs1800795) contribute to elevations in circulating sIL‐6R or IL‐6. These susceptibility loci correlate with an altered risk of cardiovascular disease, enhanced susceptibility to insulin resistance, obesity and other inflammatory complications 46, 47, 48, 49, 54, 55, 56. Comparable studies of IL‐27‐related polymorphisms require further investigation.
The therapeutic opportunities afforded by IL‐6 and IL‐27
The success of IL‐6 inhibitors in rheumatoid arthritis and related conditions illustrates the prominent role that this cytokine plays in the underlining pathology. There are now several biological drugs that target the cytokine itself (e.g. clazakizumab, olokizumab, vobarilizumab, sirukumab), the IL‐6R (e.g. tocilizumab, sarilumab) or the soluble form of IL‐6R (e.g. olamkicept) 5. Some of these are now approved for the treatment of rheumatoid arthritis, systemic juvenile arthritis, polyarticular juvenile idiopathic arthritis, giant cell arteritis and Castleman's disease. In addition, Janus kinase (Jak) inhibitors (e.g. tofacitinib, baricitinib, ruxolitinib) also impact IL‐6 receptor signalling as part of their mode of action 5, 40. While these drugs are well tolerated and offer clinical benefit, the development of sirukumab was stopped recently following a negative review from the US Food and Drug Administration (FDA). However, the anti‐inflammatory properties of IL‐27 suggests that an IL‐27 supplementation intervention may offer an alternative therapeutic strategy. For example, studies in experimental models of inflammatory arthritis and ex‐vivo culture systems show that IL‐27 inhibits the expansion of IL‐17‐secreting CD4 T cells (Th17 cells), restricts the development of ectopic lymphoid aggregates and can reduce the severity of joint damage and bone erosion 57, 58, 59. However, no clinical trials have been conducted to explore this approach in more detail. A similar strategy was adopted previously to test the anti‐arthritic properties of recombinant IL‐11, although clinical trials with recombinant IL‐11 in rheumatoid arthritis failed to reach a clinical end‐point and were suspended 60. The biology of IL‐27, and its particular influence on adaptive immune responses (Fig. 3), means that IL‐27 may be a more attractive intervention therapy.
In rheumatoid arthritis, synovial IL‐6 and IL‐27 levels correspond with differences in disease activity 57, 61, 62, 63. For example, IL‐6 expression correlates with poor disease prognosis, including elevated acute phase activity, fatigue and increased cardiovascular risk. In contrast, synovial IL‐27 levels correspond with a reduction in IL‐17 and IL‐6 and the Th17 chemoattractant CCL20 61. Thus, elevated levels of synovial IL‐27 in inflamed rheumatoid arthritis joints may reflect an effort to counteract a persistent adaptive immune response. These findings are also reflected by studies in mice. Histological assessments of joint synovitis have revealed that local IL‐27 treatment resulted in suppressed leucocyte infiltration, synovial hyperplasia, cartilage and bone erosion, vascularization and IL‐6 and IL‐17 expression in inflamed joints 58. Systemic administration of IL‐27 during collagen‐induced arthritis also reduced type II collagen‐specific antibody titres and serum levels of IL‐17 and IL‐6 57. Analysis of the peripheral immune CD4 T cell response has also revealed that IL‐27 inhibited the generation of IL‐17‐producing collagen‐specific T cells, but promoted an increase in IL‐10‐secreting CD4 T cells and suppressive Treg cells that regulate the expression of cytotoxic T lymphocyte antigen 4 (CTLA‐4) and programmed cell death protein 1 (PD‐1) 64. Interestingly, mice lacking IL‐12p35 also develop a mild form of antigen‐induced arthritis. This was attributed to an increased production of IL‐27 and IL‐10 and the expansion of Treg cells 65. In this regard, IL‐12p35 acts to suppress the action of IL‐27 during inflammatory arthritis, and blockade of IL‐27 activity was shown to restore the severity of synovitis 65. The above studies highlight the therapeutic potential of IL‐27 for the treatment of inflammatory arthritis and other diseases associated with autoimmune T cell‐mediated pathology.
IL‐6 and IL‐27 in inflammatory arthritis
IL‐6 is the archetypal member of the IL‐6‐related cytokine family. While many of these factors elicit similar biological responses in vitro, IL‐6 often displays an over‐riding influence on the same responses in vivo. Experiments in animal models show that IL‐6‐deficient mice are protected from various forms of disease 5, 40, 66. For example, the induction of inflammatory arthritis in IL‐6 deficiency is associated with an absence of synovial infiltration, synovial hyperplasia and joint damage (reviewed in Kallen 66). This is not true for other cytokines in the IL‐6 family. In models of arthritis, IL‐11 receptor‐α (IL‐11Rα)‐knock‐out (KO) mice and oncostatin M (OSM) receptor‐β (OSMRβ)‐KO mice develop disease severity comparable to wild‐type controls 67. However, there may be a context dependent caveat to this generalization 68, 69, 70, 71. For example, interleukin‐11 regulates many anti‐inflammatory outcomes in arthritis, which fuelled the aforementioned clinical trials with recombinant IL‐11 60, 72, 73. The relationship between IL‐6 and IL‐27 is different, however. In models of inflammation, IL‐27 deficiency contributes to a more active pathology that includes the development of a more severe form of synovitis and enhanced adaptive immune responses that are reflected by an increase in effector CD4 T cell numbers and antibody responses 59. Thus, IL‐6 and IL‐27 acting via a common signalling receptor subunit elicit contrasting inflammatory outcomes that influence the initiation, maintenance and severity of joint pathology. While these activities pertain primarily to the control of adaptive immunity, both cytokines have influences on stromal tissue responses to inflammation. For example, IL‐6 and IL‐27 play important roles in bone remodelling, where an imbalance between bone resorption and formation contributes to bone destruction in inflammatory arthritis 74, 75, 76, 77. Here, Il27ra‐deficient mice with experimental arthritis displayed severe synovitis and synovial hyperplasia, and an increased incidence of focal bone erosions 59. Systemic delivery of IL‐27 reversed the development of these inflammatory parameters and inhibited osteoclastogenesis 78. Notably, the action of IL‐27 on inflammation‐driven bone destruction can be both direct and indirect. For example, IL‐27 abrogates receptor activator of nuclear factor kappa‐Β ligand (RANKL) responsiveness in osteoclast precursors and suppresses signalling downstream of RANK 35. IL‐27 has also been shown to inhibit the production of RANKL in activated CD4+ T cells 79. Therefore, the impact of IL‐27 on bone turnover is not unexpected, and other IL‐6‐related cytokines, including IL‐11, OSM, leukaemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF), also control aspects of bone homeostasis 80, 81, 82, 83. In summary, both IL‐6 and IL‐27 contribute to the control of synovitis and associated changes in cartilage and bone erosion.
IL‐27 suppresses synovial ectopic lymphoid‐like structure development
IL‐6 and IL‐27 are both lymphokines that control the survival, proliferation and effector characteristics of T and B cells, and are thus poised to shape adaptive immune responses within inflamed joints. IL‐6 has long stood as a key mediator in the generation of antibody responses and the formation of germinal centre reactions 84, 85. Recent studies have also highlighted the importance of B cell‐derived IL‐6 in promoting class‐switch recombination of autoantibodies and spontaneous germinal centre formation that are required for establishing systemic lupus erythematosus in mice 86. While IL‐27 has also been shown to drive the secretion of IL‐21 in T follicular helper cells and support germinal centre function 87, the IL‐27p28 subunit can counteract IL‐6‐driven antibody responses and inhibit germinal centre development 42. Consistent with these roles, over‐expression of IL‐6 and IL‐6R in mice results in spontaneous inflammation featuring the formation of lymph node‐like structures in the lung 88. Similar lymphocytic aggregates, called ectopic lymphoid‐like structures [ELS; also called tertiary lymphoid structures (TLS)], are a histopathological hallmark of tissue inflammation in a number of autoimmune diseases, cancers and infection 89. New approaches, such as ultrasound‐directed small‐needle synovial biopsy, combined with histopathological assessment of joint inflammation, has provided new insight into disease heterogeneity in rheumatoid arthritis 90. Here, based on cellular and molecular signatures, synovitis can be classed into three pathotypes called ‘follicular’, ‘diffuse’ and ‘pauci‐immune’. While diffuse pathology is characterized by a typical random infiltration of leucocytes composed primarily of macrophages and some T cells, the follicular pathotype features highly organized and segregated aggregates of T and B cells accompanied by CD21+ follicular dendritic cell networks, active germinal centres and high endothelial venules (HEV). ELS are associated with the local priming of immune cells and autoantibody responses 91, 92.
Notably, our recent evaluation of IL27 and EBI3 expression (encoding IL‐27p28 and EBI3, respectively) in the diffuse synovial pathotype mirrored previous studies that identified heightened levels of IL‐27 in rheumatoid arthritis joint tissues compared with control osteoarthritis joints 59. However, compared to patients with diffuse pathology, the follicular form of disease was associated with reduced expression of IL‐27. Notably, IL‐27RA was expressed highly in the follicular form of rheumatoid arthritis, and cells expressing the IL‐27R were localized at ELS. These observations suggest that distinct cytokine networks govern the development of synovial pathotypes, and that the absence of a regulatory IL‐27 signal may contribute to the development of a follicular form of disease that is linked with severe local and peripheral inflammation and inferior responses to biological therapy (e.g. anti‐TNF) 93, 94, 95.
Early investigations into the endogenous role of IL‐27 in inflammatory arthritis revealed a proinflammatory role in proteoglycan‐induced arthritis 96. While this observation in IL‐27R‐deficient mice appears to contradict the therapeutic effect that has been observed following treatment with IL‐27 in experimental arthritis 57, 58, 64, 78, 97, this may reflect the importance of a robust Th1 cell response for driving the proteoglycan‐induced arthritis model. Our studies, using IL‐27R‐deficient mice in the methylated bovine serum albumin (mBSA) antigen‐induced arthritis model, revealed that these mice develop exacerbated joint inflammation, synovial hyperplasia and cartilage and bone erosion that was accompanied by elevated peripheral Th17 cell and mBSA‐specific antibody responses 59. Reflecting the observation that IL27 expression was reduced in synovial biopsies from rheumatoid arthritis patients with a follicular‐rich form of disease, IL‐27R‐deficient mice developed synovial ectopic lymphoid‐like structures that were associated with the expression of homeostatic cytokines (e.g. Lta, Ltb) and chemokines (e.g. Cxcl13, Ccl21) 59. Thus, while IL‐6 can promote ELS development in inflamed tissues, IL‐27 is a negative regulator of ELS. An inhibitory role for IL‐27 at ELS is consistent with observations in other models of inflammation that have linked heightened Th17‐type effector responses (e.g. elevated expression of IL‐17, IL‐17F, IL‐22, IL‐21) with ELS development 98, 99, 100. ELS development in antigen‐induced arthritis was associated with the local expression of IL‐17 and IL‐21 59, and effector cytokines linked with the Th17 programme (e.g. IL‐17F, IL‐21, IL‐23, IL‐22) have also been implicated in synovial lymphoid neogenesis in clinical rheumatoid arthritis 101. The inhibitory control of effector Th17‐type responses by IL‐27 may therefore offer opportunities to identify new therapeutic targets for the treatment of the follicular form of rheumatoid arthritis.
Concluding remarks
Cytokines that signal via the Jak–STAT pathway are viewed increasingly as therapeutic targets for the treatment of autoimmune diseases, infection and cancer. These include drugs that block IL‐6 (e.g. olokizumab, clazakizumab, tocilizumab), IL‐12p40 (e.g. ustekinumab), IL‐21, IL‐23p19 (e.g. risankizumab, guselkumab, tildrakizumab, mirikizumab) or granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (e.g. mavrilimumab) signalling, or members of the Jak protein family (e.g. tofacitinib, baricitinib, ruxolitinib). When considering the immunomodulatory or anti‐inflammatory properties of IL‐27 it is tempting to consider how IL‐27 intervention would supplement these therapies. Here, the capacity of IL‐27 to inhibit Th17 development, and to promote the expression of check‐point regulators and Treg activity, mirrors the therapeutic responses linked with tocilizumab treatment 3, 5. However, further investigations are required to assess the context‐dependent inflammatory activities of IL‐27. These may require clinical trials in humans. While primary clinical end‐points will undoubtedly fixate upon improvements in local tissue inflammation and damage, the wider implications of systemic inflammation are becoming equally important. For example, a metabolic shift associated with the systemic activation of T cells in PD‐1‐deficient mice was shown recently to impact the generation of brain monoamines and changes in emotional behaviour 102. In this respect, the bioactivity of IL‐27 is interesting, because IL‐27 promotes the expression of PD‐1 ligand (PD)‐L1 103. Thus, an IL‐27 intervention may offer opportunities to explore whether IL‐27 can bring about improvement in disease activity and patient wellbeing. Such strategies would be relevant to clinical indications where IL‐17 or Th17‐driven outcomes promote disease progression (e.g. psoriasis). The question is whether supplementation with recombinant IL‐27 can be used as a stand‐alone intervention or an adjunct therapy in conditions where biological drugs that target IL‐6, IL‐12, IL‐17 or IL‐23 are effective. Several of the benefits associated with IL‐6 blockade relate to the impact of therapy on altered metabolic processes (e.g. anaemia through altered iron metabolism), fatigue and patient wellbeing. It is unclear whether recombinant IL‐27 would elicit similar outcomes.
In summary, IL‐6 and IL‐27 appear to work in a co‐ordinated fashion, with IL‐27 often suppressing the action of IL‐6. These differences in biological activities reflect changes in the control of transcription factors STAT1 and STAT3 and may also relate to differences in the cytokine receptor subunits. While further work is required to appreciate fully the associations between IL‐6 and IL‐27, the current data offer interesting perspectives on how an IL‐27 intervention may supplement existing biological drug therapies against IL‐6 or members of the IL‐12 cytokine family.
Disclosures
S. A. J. has received funding support from Hoffman la Roche, GSK, Ferring Pharmaceuticals and NovImmune SA, and during the last 5 years he has acted as an advisory consultant for Roche, Chugai Pharmaceuticals, NovImmune SA, Genentech, Sanofi Regeneron, Johnson & Johnson, Janssen Pharmaceuticals, Eleven Biotherapeutics and UCB. G. W. J. has received funding from GSK and undertakes collaborative research with MedImmune. D. G. H. and A. C. declare no competing interests.
Acknowledgements
G. W. J. and S. A. J. are supported by an Arthritis Research UK Career Development Fellowship (reference 20305) and Arthritis Research UK programme grant (reference 20770) respectively. D. G. H. is supported by a Medical Research Council PhD studentship and Life Science Research Network Wales, a research initiative funded through the Welsh Government's Sêr Cymru programme.
Contributor Information
G. W. Jones, Email: JonesGW6@cardiff.ac.uk.
S. A. Jones, Email: JonesSA@cardiff.ac.uk.
References
- 1. Heinrich PC, Behrmann I, Haan S et al Principles of interleukin (IL)‐6‐type cytokine signalling and its regulation. Biochem J 2003; 374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pflanz S, Timans JC, Cheung J et al IL‐27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002; 16:779–90. [DOI] [PubMed] [Google Scholar]
- 3. Yoshida H, Hunter CA. The immunobiology of interleukin‐27. Annu Rev Immunol 2015; 33:417–43. [DOI] [PubMed] [Google Scholar]
- 4. Pflanz S, Hibbert L, Mattson J et al WSX‐1 and glycoprotein 130 constitute a signal‐transducing receptor for IL‐27. J Immunol 2004; 172:2225–31. [DOI] [PubMed] [Google Scholar]
- 5. Hunter CA, Jones SA. IL‐6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16:448–57. [DOI] [PubMed] [Google Scholar]
- 6. Jones LL, Vignali DAA. Molecular interactions within the IL‐6/IL‐12 cytokine/receptor superfamily. Immunologic Research 2011; 51:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gearing DP, Cosman D. Homology of the p40 subunit of natural killer cell stimulatory factor (NKSF) with the extracellular domain of the interleukin‐6 receptor. Cell 1991; 66:9–10. [DOI] [PubMed] [Google Scholar]
- 8. Lucas S, Ghilardi N, Li J et al IL‐27 regulates IL‐12 responsiveness of naive CD4+ T cells through Stat1‐dependent and ‐independent mechanisms. Proc Natl Acad Sci USA 2003; 100:15047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Artis D, Villarino A, Silverman M et al The IL‐27 receptor (WSX‐1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol 2004; 173:5626–34. [DOI] [PubMed] [Google Scholar]
- 10. Chen Q, Ghilardi N, Wang H et al Development of Th1‐type immune responses requires the type I cytokine receptor TCCR. Nature 2000; 407:916–20. [DOI] [PubMed] [Google Scholar]
- 11. Takeda A, Hamano S, Yamanaka A et al Cutting edge: role of IL‐27/WSX‐1 signaling for induction of T‐bet through activation of STAT1 during initial Th1 commitment. J Immunol 2003; 170:4886–90. [DOI] [PubMed] [Google Scholar]
- 12. Yoshida H, Hamano S, Senaldi G et al WSX‐1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 2001; 15:569–78. [DOI] [PubMed] [Google Scholar]
- 13. Villarino AV, Stumhofer JS, Saris CJM et al IL‐27 limits IL‐2 production during Th1 differentiation. J Immunol 2006; 176:237–47. [DOI] [PubMed] [Google Scholar]
- 14. Hall AO, Beiting DP, Tato C et al The cytokines interleukin 27 and interferon‐gamma promote distinct Treg cell populations required to limit infection‐induced pathology. Immunity 2012; 37:511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stumhofer JS, Silver JS, Laurence A et al Interleukins 27 and 6 induce STAT3‐mediated T cell production of interleukin 10. Nat Immunol 2007; 8:1363–71. [DOI] [PubMed] [Google Scholar]
- 16. Villarino A, Hibbert L, Lieberman L et al The IL‐27R (WSX‐1) is required to suppress T cell hyperactivity during infection. Immunity 2003; 19:645–55. [DOI] [PubMed] [Google Scholar]
- 17. Young A, Linehan E, Hams E et al Cutting edge: suppression of GM‐CSF expression in murine and human T cells by IL‐27. J Immunol 2012; 189:2079–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stumhofer JS, Laurence A, Wilson EH et al Interleukin 27 negatively regulates the development of interleukin 17‐producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 2006; 7:937–45. [DOI] [PubMed] [Google Scholar]
- 19. Iyer SS, Ghaffari AA, Cheng G. Lipopolysaccharide‐mediated IL‐10 transcriptional regulation requires sequential induction of type I IFNs and IL‐27 in macrophages. J Immunol 2010; 185:6599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patin EC, Jones AV, Thompson A et al IL‐27 Induced by select Candida spp. via TLR7/NOD2 signaling and IFN‐β production inhibits fungal clearance. J Immunol 2016; 197:208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clement M, Marsden M, Stacey MA et al Cytomegalovirus‐specific IL‐10‐producing CD4+ T cells are governed by type‐I IFN‐induced IL‐27 and promote virus persistence. PLOS Pathog 2016; 12: e1006050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamano S, Himeno K, Miyazaki Y et al WSX‐1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 2003; 19:657–67. [DOI] [PubMed] [Google Scholar]
- 23. Batten M, Kljavin NM, Li J et al Cutting edge: IL‐27 is a potent inducer of IL‐10 but not FoxP3 in murine T cells. J Immunol 2008; 180:2752–6. [DOI] [PubMed] [Google Scholar]
- 24. Findlay EG, Greig R, Stumhofer JS et al Essential role for IL‐27 receptor signaling in prevention of Th1‐mediated immunopathology during malaria infection. J Immunol 2010; 185:2482–92. [DOI] [PubMed] [Google Scholar]
- 25. Sun J, Dodd H, Moser EK et al CD4+ T cell help and innate‐derived IL‐27 induce Blimp‐1‐dependent IL‐10 production by antiviral CTLs. Nat Immunol 2011; 12:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu FD, Kenngott EE, Schröter MF et al Timed action of IL‐27 protects from immunopathology while preserving defense in influenza. PLOS Pathog 2014; 10: e1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Batten M, Li Ji, Yi S et al Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17‐producing T cells. Nat Immunol 2006; 7:929–36. [DOI] [PubMed] [Google Scholar]
- 28. Diveu C, McGeachy MJ, Boniface K et al IL‐27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 2009; 182:5748–56. [DOI] [PubMed] [Google Scholar]
- 29. Fonseca MM, Santa‐Cecilia F, Kusuda R et al (153) The interleukin 27 (IL‐27) protects mice from neuropathic pain development through up‐regulation of anti‐inflammatory cytokine IL‐10. J Pain 18:S14–5. [Google Scholar]
- 30. Mascanfroni ID, Yeste A, Vieira SM et al IL‐27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol 2013; 14:1054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morandi F, Di Carlo E, Ferrone S et al IL‐27 in human secondary lymphoid organs attracts myeloid dendritic cells and impairs HLA class I‐restricted antigen presentation. J Immunol 2014; 192:2634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang S, Miyazaki Y, Shinozaki Y et al Augmentation of antigen‐presenting and Th1‐promoting functions of dendritic cells by WSX‐1 (IL‐27R) deficiency. J Immunol 2007; 179:6421–8. [DOI] [PubMed] [Google Scholar]
- 33. Kalliolias GD, Gordon RA, Ivashkiv LB. Suppression of TNF‐alpha and IL‐1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL‐27. J Immunol 2010; 185:7047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalliolias GD, Ivashkiv LB. IL‐27 activates human monocytes via STAT1 and suppresses IL‐10 production but the inflammatory functions of IL‐27 are abrogated by TLRs and p38. J Immunol 2008; 180:6325–33. [DOI] [PubMed] [Google Scholar]
- 35. Kalliolias GD, Zhao B, Triantafyllopoulou A et al Interleukin‐27 inhibits human osteoclastogenesis by abrogating RANKL‐mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum 2010; 62:402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson CM, Nau GJ. Interleukin‐12 and interleukin‐27 regulate macrophage control of Mycobacterium tuberculosis . J Infect Dis 2008; 198:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dibra D, Cutrera JJ, Xia X et al Expression of WSX1 in tumors sensitizes IL‐27 signaling‐independent natural killer cell surveillance. Cancer Res 2009; 69:5505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seita J, Asakawa M, Ooehara J et al Interleukin‐27 directly induces differentiation in hematopoietic stem cells. Blood 2008; 111:1903–12. [DOI] [PubMed] [Google Scholar]
- 39. Yoshimoto T, Morishima N, Mizoguchi I et al Antiproliferative activity of IL‐27 on melanoma. J Immunol 2008; 180:6527–35. [DOI] [PubMed] [Google Scholar]
- 40. Jones SA, Scheller J, Rose‐John S. Therapeutic strategies for the clinical blockade of IL‐6/gp130 signaling. J Clin Invest 2011; 121:3375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heink S, Yogev N, Garbers C et al Trans‐presentation of IL‐6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol 2016; 18:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stumhofer JS, Tait ED, Quinn WJ et al A role for IL‐27p28 as an antagonist of gp130‐mediated signaling. Nat Immunol 2010; 11:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garbers C, Spudy B, Aparicio‐Siegmund S et al An interleukin‐6 receptor‐dependent molecular switch mediates signal transduction of the IL‐27 cytokine subunit p28 (IL‐30) via a gp130 protein receptor homodimer. J Biol Chem 2013; 288:4346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oyanedel CN, Kelemen E, Scheller J et al Peripheral and central blockade of interleukin‐6 trans‐signaling differentially affects sleep architecture. Brain Behav Immun 2015; 50:178–85. [DOI] [PubMed] [Google Scholar]
- 45. Peters M, Muller AM, Rose‐John S. Interleukin‐6 and soluble interleukin‐6 receptor: direct stimulation of gp130 and hematopoiesis. Blood 1998; 92:3495–504. [PubMed] [Google Scholar]
- 46.IL6R Genetics Consortium Emerging Risk Factors Collaboration , Sarwar N, Butterworth AS, Freitag DS et al Interleukin‐6 receptor pathways in coronary heart disease: a collaborative meta‐analysis of 82 studies. Lancet 2012; 379:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deloukas P, Kanoni S, Willenborg C et al Large‐scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013; 45:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fishman D, Faulds G, Jeffery R et al The effect of novel polymorphisms in the interleukin‐6 (IL‐6) gene on IL‐6 transcription and plasma IL‐6 levels, and an association with systemic‐onset juvenile chronic arthritis. J Clin Invest 1998; 102:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stahl E, Raychaudhuri S, Remmers EF et al Genome‐wide association study meta‐analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 2010; 42:508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Posadas‐Sánchez R, Pérez‐Hernández N, Rodríguez‐Pérez JM et al Interleukin‐27 polymorphisms are associated with premature coronary artery disease and metabolic parameters in the Mexican population: the genetics of atherosclerotic disease (GEA) Mexican study. Oncotarget 2017; 8:64459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang M, Tan X, Huang J et al Association of 3 common polymorphisms of IL‐27 gene with susceptibility to cancer in Chinese: evidence from an updated meta‐analysis of 27 studies. Med Sci Monit 2015; 21:2505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zicca E, Quirino A, Marascio N et al Interleukin 27 polymorphisms in HCV RNA positive patients: is there an impact on response to interferon therapy? BMC Infect Dis 2014; 14:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Paradowska‐Gorycka A, Raszkiewicz B, Jurkowska M et al Association of single nucleotide polymorphisms in the IL27 gene with rheumatoid arthritis. Scand J Immunol 2014; 80:298–305.] [DOI] [PubMed] [Google Scholar]
- 54. Garbers C, Monhasery N, Aparicio‐Siegmund S et al The interleukin‐6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim Biophys Acta 2014; 1842:1485–94. [DOI] [PubMed] [Google Scholar]
- 55. Esteve E, Villuendas G, Mallolas J et al Polymorphisms in the interleukin‐6 receptor gene are associated with body mass index and with characteristics of the metabolic syndrome. Clin Endocrinol (Oxf) 2006; 65:88–91. [DOI] [PubMed] [Google Scholar]
- 56. Song Y, Miyaki K, Araki J et al The interaction between the interleukin 6 receptor gene genotype and dietary energy intake on abdominal obesity in Japanese men. Metabolism 2007; 56:925–30. [DOI] [PubMed] [Google Scholar]
- 57. Niedbala W, Cai B, Wei X et al Interleukin 27 attenuates collagen‐induced arthritis. Ann Rheum Dis 2008; 67:1474–9. [DOI] [PubMed] [Google Scholar]
- 58. Pickens SR, Chamberlain ND, Volin MV et al Local expression of interleukin‐27 ameliorates collagen‐induced arthritis. Arthritis Rheum 2011; 63:2289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jones GW, Bombardieri M, Greenhill CJ et al Interleukin‐27 inhibits ectopic lymphoid‐like structure development in early inflammatory arthritis. J Exp Med 2015; 212:1793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moreland L, Gugliotti R, King K et al Results of a phase‐I/II randomized, masked, placebo‐controlled trial of recombinant human interleukin‐11 (rhIL‐11) in the treatment of subjects with active rheumatoid arthritis. Arthritis Res 2001; 3:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tanida S, Yoshitomi H, Ishikawa M et al IL‐27‐producing CD14(+) cells infiltrate inflamed joints of rheumatoid arthritis and regulate inflammation and chemotactic migration. Cytokine 2011; 55:237–44. [DOI] [PubMed] [Google Scholar]
- 62. Wong CK, Chen DP, Tam LS et al Effects of inflammatory cytokine IL‐27 on the activation of fibroblast‐like synoviocytes in rheumatoid arthritis. Arthritis Res Ther 2010; 12:R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shen H, Xia L, Xiao W et al Increased levels of interleukin‐27 in patients with rheumatoid arthritis. Arthritis Rheum 2011; 63:860–1. [DOI] [PubMed] [Google Scholar]
- 64. Moon S‐J, Park J‐S, Heo Y‐J et al In vivo action of IL‐27: reciprocal regulation of Th17 and Treg cells in collagen‐induced arthritis. Exp Mol Med 2013; 45:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vasconcellos R, Carter NA, Rosser EC et al IL‐12p35 subunit contributes to autoimmunity by limiting IL‐27‐driven regulatory responses. J Immunol 2011; 187:3402–12. [DOI] [PubMed] [Google Scholar]
- 66. Kallen K‐J. The role of transsignalling via the agonistic soluble IL‐6 receptor. Biochim Biophys Acta 2002; 1592:323–43. [DOI] [PubMed] [Google Scholar]
- 67. Wong PK, Quinn JM, Sims NA et al Interleukin‐6 modulates production of T lymphocyte‐derived cytokines in antigen‐induced arthritis and drives inflammation‐induced osteoclastogenesis. Arthritis Rheum 2006; 54:158–68. [DOI] [PubMed] [Google Scholar]
- 68. Hams E, Colmont CS, Dioszeghy V et al Oncostatin M receptor‐beta signaling limits monocytic cell recruitment in acute inflammation. J Immunol 2008; 181:2174–80. [DOI] [PubMed] [Google Scholar]
- 69. Esashi E, Ito H, Minehata K et al Oncostatin M deficiency leads to thymic hypoplasia, accumulation of apoptotic thymocytes and glomerulonephritis. Eur J Immunol 2009; 39:1664–70. [DOI] [PubMed] [Google Scholar]
- 70. West NR, Hegazy AN, Owens BMJ et al Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor‐neutralizing therapy in patients with inflammatory bowel disease. Nat Med 2017; 23:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schafer S, Viswanathan S, Widjaja AA et al IL11 is a crucial determinant of cardiovascular fibrosis. Nature 2017; 552:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hermann JA, Hall MA, Maini RN et al Important immunoregulatory role of interleukin‐11 in the inflammatory process in rheumatoid arthritis. Arthritis Rheum 1998; 41:1388–97. [DOI] [PubMed] [Google Scholar]
- 73. Walmsley M, Butler DM, Marinova‐Mutafchieva L, Feldmann M. An anti‐inflammatory role for interleukin‐11 in established murine collagen‐induced arthritis. Immunology 1998; 95:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kondo Y, Kaneko Y, Sugiura H et al Pre‐treatment interleukin‐6 levels strongly affect bone erosion progression and repair detected by magnetic resonance imaging in rheumatoid arthritis patients. Rheumatology (Oxf) 2017; 56:1089–94. [DOI] [PubMed] [Google Scholar]
- 75. Finzel S, Rech J, Schmidt S et al Interleukin‐6 receptor blockade induces limited repair of bone erosions in rheumatoid arthritis: a micro CT study. Ann Rheum Dis 2013; 72:396–400. [DOI] [PubMed] [Google Scholar]
- 76. Shukla P, Mansoori MN, Kakaji M et al Interleukin 27 (IL‐27) alleviates bone loss in estrogen‐deficient conditions by induction of early growth response‐2 gene. J Biol Chem 2017; 292:4686–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Larousserie F, Bsiri L, Dumaine V et al Frontline Science: human bone cells as a source of IL‐27 under inflammatory conditions: role of TLRs and cytokines. J Leukoc Biol 2017; 101:1289–300. [DOI] [PubMed] [Google Scholar]
- 78. Park J‐S, Jung YO, Oh H‐J et al Interleukin‐27 suppresses osteoclastogenesis via induction of interferon‐gamma. Immunology 2012; 137:326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kamiya S, Okumura M, Chiba Y et al IL‐27 suppresses RANKL expression in CD4+ T cells in part through STAT3. Immunol Lett 2011; 138:47–53. [DOI] [PubMed] [Google Scholar]
- 80. Sims NA, Quinn JM. Osteoimmunology: oncostatin M as a pleiotropic regulator of bone formation and resorption in health and disease. Bonekey Rep 2014; 3:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev 2015; 26:533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pasquin S, Sharma M, Gauchat JF. Ciliary neurotrophic factor (CNTF): new facets of an old molecule for treating neurodegenerative and metabolic syndrome pathologies. Cytokine Growth Factor Rev 2015; 26:507–15. [DOI] [PubMed] [Google Scholar]
- 83. Sims NA, Jenkins BJ, Nakamura A et al Interleukin‐11 receptor signaling is required for normal bone remodeling. J Bone Miner Res 2005; 20:1093–102. [DOI] [PubMed] [Google Scholar]
- 84. Deng C, Goluszko E, Tuzun E et al Resistance to experimental autoimmune myasthenia gravis in IL‐6‐deficient mice is associated with reduced germinal center formation and C3 production. J Immunol 2002; 169:1077–83. [DOI] [PubMed] [Google Scholar]
- 85. Kopf M, Herren S, Wiles MV et al Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med 1998; 188:1895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Arkatkar T, Du SW, Jacobs HM et al B cell‐derived IL‐6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 2017; 214:3207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Batten M, Ramamoorthi N, Kljavin NM et al IL‐27 supports germinal center function by enhancing IL‐21 production and the function of T follicular helper cells. J Exp Med 2010; 207:2895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Goya S, Matsuoka H, Mori M et al Sustained interleukin‐6 signalling leads to the development of lymphoid organ‐like structures in the lung. J Pathol 2003; 200:82–7. [DOI] [PubMed] [Google Scholar]
- 89. Jones GW, Hill DG, Jones SA. Understanding immune cells in tertiary lymphoid organ development: it is all starting to come together. Front Immunol 2016; 7:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Orr C, Sousa E, Boyle DL et al Synovial tissue research: a state‐of‐the‐art review. Nat Rev Rheumatol 2017; 13:463–75. [DOI] [PubMed] [Google Scholar]
- 91. Takemura S, Klimiuk PA, Braun A et al T cell activation in rheumatoid synovium is B cell dependent. J Immunol 2001; 167:4710–8. [DOI] [PubMed] [Google Scholar]
- 92. Humby F, Bombardieri M, Manzo A et al Ectopic lymphoid structures support ongoing production of class‐switched autoantibodies in rheumatoid synovium. PLOS Med 2009; 6: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thurlings RM, Wijbrandts CA, Mebius RE et al Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum 2008; 58:1582–9. [DOI] [PubMed] [Google Scholar]
- 94. Canete JD, Celis R, Moll C et al Clinical significance of synovial lymphoid neogenesis and its reversal after anti‐tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis 2009; 68:751–6. [DOI] [PubMed] [Google Scholar]
- 95. Dennis G Jr, Holweg CT, Kummerfeld SK et al Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther 2014; 16:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cao Y, Doodes PD, Glant TT et al IL‐27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol 2008; 180:922–30.] [DOI] [PubMed] [Google Scholar]
- 97. Rajaiah R, Puttabyatappa M, Polumuri SK et al Interleukin‐27 and interferon‐gamma are involved in regulation of autoimmune arthritis. J Biol Chem 2011; 286:2817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barone F, Nayar S, Campos J et al IL‐22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci USA 2015; 112:11024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rangel‐Moreno J, Carragher DM, de la Luz Garcia‐Hernandez M et al The development of inducible bronchus‐associated lymphoid tissue depends on IL‐17. Nat Immunol 2011; 12:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bombardieri M, Barone F, Lucchesi D et al Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol 2012; 189:3767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Canete JD, Celis R, Yeremenko N et al Ectopic lymphoid neogenesis is strongly associated with activation of the IL‐23 pathway in rheumatoid synovitis. Arthritis Res Ther 2015; 17:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Miyajima M, Zhang B, Sugiura Y et al Metabolic shift induced by systemic activation of T cells in PD‐1‐deficient mice perturbs brain monoamines and emotional behavior. Nat Immunol 2017; 18:1342–52. [DOI] [PubMed] [Google Scholar]
- 103. Hirahara K, Ghoreschi K, Yang X‐P et al Interleukin‐27 priming of T cells controls IL‐17 production in trans via induction of the ligand PD‐L1. Immunity 2012; 36:1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


