Summary
Our previous studies showed that anti‐citrate synthase (anti‐CS) immunoglobulin (Ig)M natural autoantibodies are present in healthy individuals without previous antigen stimulation, but no studies have investigated their presence in the pericardial fluid (PF). Therefore, we detected the natural anti‐CS IgG/M autoantibody levels in plasma and PF of cardiac surgery patients and investigated their relationship with cardiovascular disease‐associated bacterial pathogens. PF and blood samples of 22 coronary artery bypass graft (CABG) and 10 aortic valve replacement (AVR) patients were tested for total Ig levels, natural autoantibodies and infection‐related antibodies using enzyme‐linked immunosorbent assay (ELISA) and Luminex methods. The B cell subsets were measured by flow cytometry. The total Ig subclass levels were four to eight times lower in PF than in plasma, but the natural anti‐CS IgM autoantibodies showed a relative increase in PF. The frequency of CD19+ B lymphocytes was significantly lower in PF than in blood (P = 0·01), with a significant relative increase of B1 cells (P = 0·005). Mycoplasma pneumoniae antibody‐positive patients had significantly higher anti‐CS IgM levels. In CABG patients we found a correlation between anti‐CS IgG levels and M. pneumoniae, Chlamydia pneumoniae and Borrelia burgdorferi antibody titres. Our results provide the first evidence that natural autoantibodies are present in the PF, and they show a significant correlation with certain anti‐bacterial antibody titres in a disease‐specific manner.
Keywords: anti‐bacterial antibodies, cardiovascular disease, citrate synthase, natural autoantibodies, pericardial fluid
Introduction
There is increasing evidence that the immune system plays an important role in the development of cardiovascular diseases, especially in atherosclerosis, one of the main underlying features of cardiovascular failure 1, 2, 3, 4. However, little is known about the immunological milieu of the pericardial fluid produced by the serous membranes surrounding the heart 2, 5. Extravascular serous fluids (i.e. peritoneal, pleural and pericardial) contain cellular and humoral components of the immune system to protect the organ from microbial invasion. The peritoneal and pleural cavities and the associated serous fluids have been investigated thoroughly regarding their biochemical and immunological composition 5, 6, 7. It is not surprising that the pericardial fluid is not so well studied, because of the invasive nature of obtaining pericardial fluid during open‐heart surgery 8, 9. Thus, our goal was to study the presence of antibodies of the natural and adaptive immune systems and the corresponding B cell subsets in the pericardial fluid (PF) of patients with cardiovascular diseases undergoing cardiac surgery.
The PF lubricates the surface of the heart, and its cellular and molecular immune components may function to: (i) protect the heart and pericardium from microbial agents and (ii) facilitate of the elimination of cell and tissue debris to ensure tissue homeostasis 10, 11. Recently numerous studies have supported the role of the natural immune system, including natural autoantibodies, in the above functions as key players in the first line of defence against infections and clearance of apoptotic cells. Natural autoantibodies are present in the circulation of humans and other mammalian species without previous exposure to antigens 12, 13, and are produced by B1 cells 14, 15, 16 without somatic hypermutation; most of them are of IgM isotype. Natural autoantibodies of immunoglobulin (Ig)G isotype have also been described 17, 18, which typically bind multiple self‐ and non‐self‐antigens. These autoantibodies are often directed against highly conserved molecules and bind various ligands with relatively low affinity, and therefore they are called ‘polyreactive’. Evidence from rodents deficient in natural antibody production suggests that natural autoantibodies serve homeostatic and housekeeping functions and natural IgM autoantibodies serve as scavengers of damaged molecules and cells 10, and therefore have been implicated in the control of inflammation and autoimmune diseases 19, 20. Natural antibodies may also serve as innate‐like recognition receptors, recognizing various bacterial cell wall components or parasites 21, 22. Antibodies produced by the adaptive immune system are generally derived from T‐dependent B lymphocytes, in response to exposure to microbes or vaccination. It has been shown previously that T cells are the dominant lymphocytes in the PF 23 but less is known about the B1 and B2 cell composition of PF, which may reflect on the source of natural and infection‐related antibodies found in the PF space.
Here we investigated whether IgM and IgG natural autoantibodies are present in the PF of patients with different cardiovascular diseases undergoing open‐heart surgery. Based on previous studies, including those from our laboratory, it is known that citrate synthase (CS), a highly conserved mitochondrial inner membrane enzyme, is an autoantigen recognized by the natural immune system 24, 25, 26, 27. Heart muscle cells contain the highest number of mitochondria in body tissues, and mitochondrial enzymes can become released in different cardiovascular diseases. For this reason, we chose to investigate anti‐CS natural autoantibodies in patients with cardiovascular diseases and the levels of infection‐related antibodies with a potential role in the development of atherosclerosis, including Chlamydia pneumoniae, Mycoplasma pneumoniae, Borrelia burgdorferi, Helicobacter pylori and Yersinia enterocolitica 28, 29, 30, 31, 32, 33. We compared the plasma/PF ratios of anti‐CS IgG and IgM antibodies and correlated their levels with total and anti‐bacterial IgG/IgM antibody titres in patients with aortic valve replacement (AVR) and coronary artery bypass graft (CABG) with or without previous myocardial infarction (MI). We also investigated the frequencies of B1 and B2 cells to gain insight into the source of natural antibody production in PF. Our data provide evidence that natural autoantibodies and anti‐bacterial antibodies are present in the PF, and their correlation may suggest their importance in the development of cardiovascular diseases.
Materials and methods
Patients
Thirty‐two patients who were admitted to University of Pécs Heart Institute for elective open‐heart surgery (due to coronary or aortic valve disease) during a 1‐year period were selected for our study. Patients with known autoimmune disease and patients who underwent combined open‐heart surgery and patients whose samples could not be used due to technical issues were excluded from the study. The average age of patients was 58·8 ± 7·5 years, and the female : male ratio was 8 : 24. The patients were divided into three groups: aortic valve replacement group (AVR, n = 10), coronary artery bypass grafting patients (CABG) with previous myocardial infarction (CABG with MI, n = 10) and those who underwent CABG but had no previous myocardial infarction (CABG no MI, n = 12). The diagnosis of myocardial infarction was based on coronary angiography and echocardiography findings. Prior to surgery, all patients underwent physical examination and search for inflammatory foci, including urological, gynaecological, dental and ear, nose and throat examinations, together with chest X‐ray and abdominal ultrasound scans. The laboratory parameters the day before surgery were within the normal range [C‐reactive protein (CRP); white blood cells count (WBC); erythrocyte sedimentation rate (ESR) were included]. The study was approved by the Regional Review Board (RIGEB 3415/2009). Patients gave written informed consent to all procedures.
Pericardial fluid and peripheral blood samples
PF and blood samples were drawn simultaneously during elective open‐heart surgery. At least 3 ml of PF was drained using a syringe connected to a Vacutainer tube. At the same time, peripheral blood samples were taken in sterile, heparinized tubes (BD Vacutainer CPT™ Cell Preparation Tube; Becton Dickinson, Franklin Lakes, NJ, USA). The samples were processed within 60 min by separating the cells by centrifugation and the plasma and PF samples were aliquoted promptly and stored subsequently at −80°C until analysis.
Immunological and serological analysis
Measurement of immunoglobulin isotypes by the Luminex method
The Milliplex Map Human Isotyping Kit (Millipore Corp., Burlington, MA, USA) was used to measure human IgG subclasses (1, 2, 3 and 4), IgM and IgA in the plasma and PF samples (1 : 16 000 and 1 : 10 dilutions of plasma and PF, respectively), according to the manufacturer's instructions. Briefly, the samples and the anti‐human multi‐immunoglobulin beads were added to the wet microtitre filter plate. The plate was covered and incubated for 1 h on a plate shaker at room temperature. The plate was then incubated with anti‐human antibodies to kappa or lambda light chains. Following the vacuum removal of the fluid, the beads were suspended in sheath fluid. The reaction was measured on a Luminex 100™ IS (Luminex, Austin, TX, USA) instrument.
Measurement of anti‐CS antibodies by enzyme‐linked immunosorbent assay (ELISA)
Ninety‐six‐well polystyrene plates (Nunc, Roskilde, Denmark) were coated with 5 µg/ml citrate synthase (CS) from porcine heart (Sigma, St Louis, MO, USA) in 0·1 M bicarbonate buffer, pH 9·6 at 4°C overnight. Following the saturation of non‐specific binding sites with 0·5% gelatin (Sigma) in phosphate‐buffered saline (PBS) (pH 7·3), plasma samples were incubated in duplicate at 1 : 100 dilution and PF samples at 1 : 10 dilution in washing buffer (PBS, 0·05% Tween 20) for 1 h at room temperature. Finally, the plate was incubated with horseradish peroxidase (HRP)‐conjugated anti‐human IgG‐ or IgM‐specific secondary antibody (Dako, Glostrup, Denmark) for 1 h at room temperature. The reaction was developed with o‐phenylenediamine (Sigma) and measured using an iEMS MF microphotometer (Thermo Labsystems, Waltham, MA, USA) at 492 nm. Cut‐off values were calculated from the average of measured optical density (OD) 492 nm data. All measurements were standardized using a monoclonal anti‐citrate synthase antibody (clone 4H3‐E5) produced previously 34.
Serological tests for anti‐bacterial antibodies
Commercial ELISA kits were used to determine the anti‐bacterial antibody concentrations in the plasma and PF samples. B. burgdorferi‐specific IgG and IgM, anti‐H. pylori IgA and IgG, anti‐Y. enterocolitica IgA, IgM and IgG antibodies (Mikrogen GmBH, Neureid, Germany), anti‐C. pneumoniae and anti‐M. pneumonia IgA, IgG and IgM antibodies (Ani Labsystems Diagnostics, Vantaa, Finland) were measured by indirect ELISA tests, according to the manufacturer's instructions. Briefly, plasma samples at 1 : 100 dilution and PF samples at 1 : 20 dilution were incubated for 1 h at 37°C. The plate was then incubated with HRP‐conjugated anti‐human IgA/IgG/IgM antibodies for 30 min at 37°C. The reaction was developed with 3,3',5,5'‐tetramethylbenzidine (TMB) and measured using a Siemens BEP‐2000 ELISA reader at 450 nm Biocompare, San Francisco, CA, USA).
Measurement of B1 and B2 B cells in PF and plasma by flow cytometry
Multi‐parametric flow cytometry was performed on PF and plasma samples with antibodies specific for CD19, CD5 and CD3. To identify B cells, fluorescein isothiocyanate (FITC)‐conjugated anti‐CD19 (4G7; Becton Dickinson) were used to distinguish between B1 and B2 B cell phycoerythrin (PE)‐conjugated anti‐CD5 antibodies (L17F12; Becton Dickinson). Stained cells were detected and data acquired using a fluorescence activated cell sorter (FACS)Calibur flow cytometer (Becton Dickinson) and analysed with FCS Express 4 software (DeNovo Software, Glendale, CA, USA).
Statistical analysis
Statistical evaluation was performed using spss version 20.0 statistics package (IBM, Armonk, NY, USA). Spearman's correlation analysis and Mann–Whitney U‐tests were used as appropriate. P‐values < 0·05 were considered significant.
Results
Relative excess of anti‐CS IgM natural autoantibodies in the pericardial fluid
CS has been characterized as an autoantigen recognized by the natural immune system in humans. We have documented previously that anti‐CS IgM natural antibodies are continuously present in plasma of healthy controls and that anti‐CS IgG levels increase in patients with autoimmune diseases and heart transplantation 25, 26, 35; however, no data have been published regarding the anti‐CS antibodies in PF. The anti‐CS IgM and IgG natural autoantibodies were measured by an in‐house ELISA developed and validated in our laboratory. We detected anti‐CS IgM and IgG antibodies both in the plasma and PF of patients undergoing cardiac surgery. The anti‐CS IgG level was approximately 11‐fold higher in plasma than in PF (P < 0·001) (Fig. 1b), whereas anti‐CS IgM concentration was only approximately twofold lower in the PF than in the plasma (P < 0·001) (Fig. 1a). This reflects a relative excess of anti‐CS IgM autoantibodies in the PF of cardiac surgery patients. Similar differences in the levels of anti‐CS IgG and IgM autoantibodies were detected in the different disease groups (Table 1).
Figure 1.
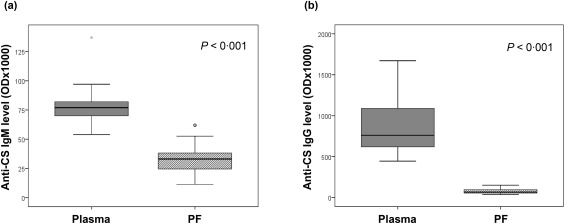
Anti‐citrate synthase (anti‐CS) natural autoantibody levels in the plasma and pericardial fluid (PF) of cardiac surgery patients. The proportion of anti‐CS immunoglobulin (Ig)M (a) antibody levels were only twofold higher (P < 0·001), while anti‐CS IgG (b) antibody levels were 11‐fold higher in the plasma than in the pericardial fluid (P < 0·001). Boxes show interquartile ranges (IQR), whiskers indicate lowest and highest values, horizontal lines represent medians, dots indicate outliers of 1.5 × interquartile range (IQR). The antibody levels were measured using enzyme‐linked immunosorbent assay (ELISA). OD = optical density; plasma, peripheral blood plasma; n = 32 patients.
Table 1.
Ratios of anti‐citrate synthase (anti‐CS) immunoglobulin (Ig)G and IgM autoantibodies in pericardial fluid and plasma of patients with different cardiac diseases
| Natural autoantibody | Disease group | PF : plasma ratio | % Plasma level |
|---|---|---|---|
| Anti‐CS IgG | AVR |
1 : 11·5 80·06 ± 8·45 : 921·60 ± 92·75 |
8·7% |
| CABG with MI |
1 : 11·5 67·95 ± 7·54 : 787·60 ± 81·80 |
8·7% | |
| CABG no MI |
1 : 11·6 76·14 ± 9·73 : 886·64 ± 107·58 |
8·6% | |
| Anti‐CS IgM | AVR |
1 : 2·3 34·14 ± 2·60 : 78·90 ± 3·31 |
43·4% |
| CABG with MI |
1 : 2·3 34·04 ± 4·48 : 78·10 ± 7·25 |
43·4% | |
| CABG no MI |
1 : 2·5 29·85 ± 3·21 : 74·00 ± 2·56 |
40·0% |
AVR = aortic valve replacement; MI = myocardial infarction; CABG = coronary artery bypass graft.
We also tested the correlation of anti‐CS IgM and IgG levels between plasma and PF in individual patients. A statistically significant correlation of anti‐CS IgM antibody concentrations (P < 0·001) (Fig. 2a) and a strong correlation in the case of anti‐CS IgG levels (P < 0·001) (Fig. 2b) was found between the plasma and PF (r 2 = 0.880 and r 2 = 0.997, respectively).
Figure 2.
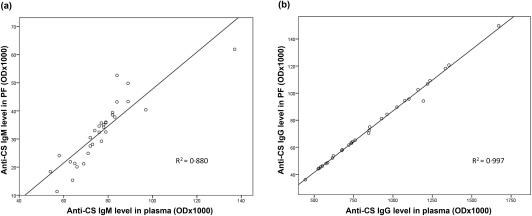
Correlation between the plasma and pericardial fluid (PF) levels of anti‐citrate synthase (anti‐CS) natural autoantibodies. Significant correlations were detected in the levels of anti‐CS immunoglobulin (Ig)M in the plasma versus PF (P < 0·001) (a), as well as in the anti‐CS IgG levels in plasma versus PF (P < 0·001) (b).
We wanted to compare the anti‐CS IgG/IgM levels to the total immunoglobulin subclass levels in plasma and PF. We found that the concentrations of all the investigated immunoglobulin isotypes were significantly lower in PF than in plasma (Table 2), but the plasma/PF ratios were different. IgM, IgG2 and IgG3 were eight times higher in plasma than in PF, while the PF levels of IgA, IgG1 and IgG4 were 6·0‐, 5·3‐ and 4·2‐fold lower than in plasma. The average IgG subclass levels in PF was 6·2 times lower than in plasma (Table 2), which reflects a lower total IgM than total IgG class levels in PF.
Table 2.
Total immunoglobulin (Ig) subclass levels and their ratios in plasma and pericardial fluid (PF) of cardiac surgery patients
| Ig isotype | Plasma mean ± s.e.m. | Pericardial fluid mean ± s.e.m. | PF : plasma ratio | % Plasma level |
|---|---|---|---|---|
| IgA (g/l) | 0·41 ± 0·13 | 0·07 ± 0·02 | 1 : 6 | 16·6% |
| IgG1 (g/l) | 1·62 ± 0·48 | 0·31 ± ·10 | 1 : 5 | 18·9% |
| IgG2 (g/l) | 4·89 ± 1·34 | 0·59 ± 0·18 | 1 : 8 | 12·5% |
| IgG3 (g/l) | 0·13 ± ,05 | 0·02 ± 0·01 | 1 : 8 | 12·5% |
| IgG4 (g/l) | 0·15 ± 0·07 | 0·04 ± 0·02 | 1 : 4 | 23·8% |
| IgM (g/l) | 0·12 ± 0·07 | 0·014 ± 0·01 | 1 : 8 | 12·5% |
s.e.m. = standard error of the mean.
B1 cell frequencies are higher in pericardial fluid
In order to gain insight into the different Ig class‐producing cells in PF we investigated and compared the ratio of B cell subgroups in blood and PF. The percentage of CD19+ B cells was significantly lower in PF than in blood (P < 0·001). Analysing the B cell subsets, we found lower frequencies of both B1 and B2 B cells in PF compared to the blood (P < 0·001 and P < 0·002, respectively) (Fig. 3), but the proportion of B1 cells in the CD19+ B cell population was significantly higher in the PF than in the blood of cardiac surgery patients (n = 32) (Table 3), which might be the source of natural anti‐CS IgM autoantibody production in the PF.
Figure 3.
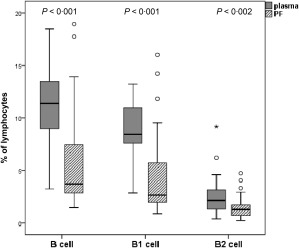
Frequencies of total CD19+ B cells and B1, B2 subpopulations in plasma and pericardial fluid (PF). The percentage of all investigated B cell groups were significantly lower in PF than in plasma samples (P < 0·001, P < 0·001 and P < 0·002, respectively). Boxes show interquartile ranges (IQR), whiskers indicate lowest and highest values, horizontal lines represent medians, dots indicate outliers of 1·5 × IQR. Frequencies of cells were measured with flow cytometer. plasma, peripheral blood plasma; n = 32 patients.
Table 3.
Percentages of B1 cells in the CD19+ B cell population in blood and PF of patients with different cardiac diseases
| Disease type | B1 cells in blood (% of CD19+ B cells) | B1 cells in PF (% of CD19+ B cells) | P‐value |
|---|---|---|---|
| CABG no MI (n = 12) | 24 ± 8·6% | 36 ± 23·8% | 0·237 |
| CABG with MI (n = 10) | 30 ± 26·1% | 48 ± 43·6% | 0·108 |
| AVR (n = 10) | 27 ± 13·9% | 51 ± 34·5% | 0·078 |
| Total (n = 32) | 27 ± 17·1% | 45 ± 34·1% | 0·005 |
Aortic valve replacement group (AVR, n = 10), coronary artery bypass grafting patients (CABG) with previous myocardial infarction (CABG with MI, n = 10), and those who underwent CABG but had no previous myocardial infarction (CABG no MI, n = 12). PF = pericardial fluid.
Anti‐CS natural autoantibody levels correlate with anti‐bacterial antibody titres in coronary artery bypass graft patients
Several infections are described to have a potential role in the development of atherosclerosis, therefore we tested the plasma and PF samples for antibodies (IgG/IgA/IgM) against five different cardiovascular disease associated bacteria, such as M. pneumoniae, Y. enterocolitica, C. pneumoniae, H. pylori and B. burgdorferi. All patients were positive for at least one anti‐bacterial antibody (IgG and/or IgA) in the plasma, while in the PF we found fewer positive samples, although their corresponding plasma sample was positive. The highest proportion of positivity for anti‐bacterial antibodies in the plasma was against M. pneumoniae (74·2%) and H. pylori (74·2%), whereas in the PF it was against H. pylori (37·0%), M. pneumoniae (14.8%) and Chlamydia pneumoniae (14·8%). No IgM positivity could be detected either in plasma or PF, which indicates the absence of an early infection.
We tested whether there was a relationship between anti‐CS IgM or IgG autoantibody levels and the presence of anti‐bacterial antibody positivity for any of the five different bacterial strains, and we found significantly elevated anti‐CS IgM levels in the plasma (P = 0·031) and an increased tendency in the PF (P = 0·076) of patients with positive M. pneumoniae (MP) antibodies (Fig. 4).
Figure 4.
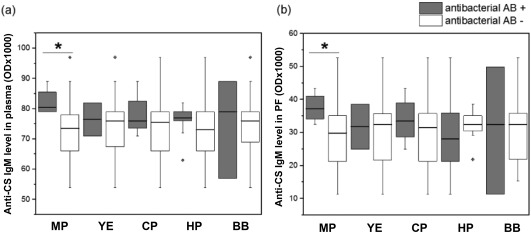
Anti‐citrate synthase (anti‐CS) immunoglobulin (Ig)M levels in plasma and pericardial fluid (PF) of patients negative or positive for various anti‐bacterial antibodies. Patients who were positive for Mycoplasma pneumoniae antibodies in the PF (and plasma) had significantly higher levels of anti‐citrate synthase IgM in their plasma (a) and an elevated tendency in the PF (b) when compared to M. pneumoniae antibody‐negative patients (P = 0·031 and P = 0·076, respectively). Boxes show interquartile ranges (IQR), whiskers indicate lowest and highest values, horizontal lines represent medians, dots indicate outliers of 1·5 × IQR, while the asterisks represent the significance (n = 32). MP = M. pneumoniae; YE = Yersinia enterocolitica; CP = Chlamydia pneumoniae; HP = Helicobacter pylori; BB = Borelia burgdorferi; plasma = peripheral blood plasma; AB = antibody.
We also tested the correlation of anti‐CS IgG and IgM antibody levels with anti‐bacterial antibody titres both in the plasma and PF of patients belonging to different disease groups. In CABG patients without previous MI there was a significant correlation between anti‐CS IgG levels and M. pneumoniae antibody titres, while in CABG patients with previous MI C. pneumoniae and B. burgdorferi antibodies correlated significantly with anti‐CS IgG levels. In patients with AVR, anti‐CS IgG did not show a correlation with any of these anti‐bacterial antibody levels. No clear‐cut correlations were observed between anti‐CS IgM and the anti‐bacterial antibody titres investigated (Table 4).
Table 4.
Correlation of citrate synthase (anti‐CS) IgG and immunoglobulin (Ig)M antibody levels with antibacterial antibody titres in plasma and PF of patients with cardiovascular diseases
| Disease group | CABG no MI | CABG with MI | AVR |
|---|---|---|---|
| Natural autoantibody | Correlating anti‐microbial antibody | ||
| Anti‐CS IgG plasma | MP IgG (plasma) (rho = 0·789; P = 0·004) | CP IgG (plasma) (rho = 0·753; P = 0·012) | No correlation |
| MP IgM (plasma) (rho = 0·802; P = 0·003) | CP IgG (PF) (rho = 0·769; P = 0·026) | ||
| MP IgG (PF) (rho = 0·579; P = 0·062) | BB IgG (plasma) (rho = 0·632; P = 0·049) | ||
| Anti‐CS IgG PF | MP IgG (plasma) (rho = 0·797; P = 0·003) | CP IgG (plasma) (rho = 0·754; P = 0·012) | No correlation |
| MP IgM (plasma) (rho = 0·829; P = 0·002) | CP IgG (PF) (rho = 0·762; P = 0·028) | ||
| MP IgG (PF) (rho = 0·611; P = 0·046) | BB IgG (plasma) (rho = 0·633; P = 0·049) | ||
| Anti‐CS IgM plasma | No correlation | YE IgM (plasma) (rho = 0·710; P = 0·021) | MP IgG (plasma) (rho = 0·780; P = 0·013) |
| Anti‐CS IgM PF | No correlation | No correlation | No correlation |
Aortic valve replacement group (AVR, n = 10), coronary artery bypass grafting patients (CABG) with previous myocardial infarction (CABG with MI, n = 10), and those who underwent CABG but had no previous myocardial infarction (CABG no MI, n = 12). MP = Mycoplasma pneumoniae; CP = Chlamydia pneumoniae; BB = Borrelia burgdorferi; YE = Yersinia enterocolitica.
Discussion
The goal of this study was to determine whether natural autoantibodies to mitochondrial citrate synthase enzyme (CS) and antibodies to certain bacteria related to atherosclerosis are present in the plasma and PF of cardiac surgery patients, and to determine the relationship of these antibodies to each other and to the total immunoglobulin class levels in plasma and PF. Our study has revealed that anti‐CS natural autoantibodies and anti‐bacterial antibodies are present not only in the plasma, but also in the PF. We also wanted to examine the frequencies of B1 and B2 B lymphocytes in PF and compare them to the percentages found in blood to gain insight into the source of natural autoantibodies in PF.
First, we determined the total immunoglobulin (IgA, IgM and IgG1–4) subclass concentrations in PF and plasma of cardiac surgery patients, and found that in PF their level is four‐ to eightfold lower than that in plasma. In contrast, when we compared the anti‐CS natural antibody levels, the anti‐CS IgM was only twofold lower, whereas the anti‐CS IgG levels were 10‐fold lower in PF than in plasma in all study groups. The increased level of anti‐CS IgM may be due to the presence of increased frequencies of B1 cells in the PF. Although the total CD19+ B cell ratio is significantly lower in PF compared to blood, the proportion of B1 cells in the CD19+ B cell population was significantly higher in the PF than in the blood of cardiac surgery patients, which suggests that B1 cells are the probable source of IgM natural autoantibodies 23, 36. The tissue‐specific signals in different tissue compartments may affect the functionally heterogeneous B1 cells differentially 37, but their shared function might be to ensure a prompt and effective local defence.
Natural antibodies may exert distinct effector functions according to their specificity and respective isotypes 2. Our data suggest that the pericardial space containing the PF is protected by the natural immune system, which may play a crucial role in tissue homeostasis by elimination of cellular and molecular debris that needs to be cleared in order to prevent the accumulation of apoptotic material 10 to control inflammation and autoimmune mechanisms 17, 19, 20. Another key and overarching function of natural antibodies is to serve as a bridge between the innate and adaptive immune systems 22 by pathogen neutralization, complement activation and antigen recruitment to secondary lymphoid organs, and for immune regulation and homeostasis 16, 22. Our data may support the notion that anti‐CS natural autoantibodies, similarly to other polyreactive natural antibodies, may serve as scavengers of dead or dying cells and microbial antigens both in plasma and in the PF 38, 39. It is probable that CS is not the only molecular constituent cleared by such mechanisms, but there could be a plethora of other self‐ and pathogen‐related molecules recognized by the natural autoantibodies destined for subsequent clearance to prevent their ‘toxicity’ to cardiomyocytes 11, 17. In mouse models of atherosclerosis, natural IgM antibodies produced by serosal B1 cells have been shown to protect from atherosclerosis, whereas antibodies produced by B2 cells promote atherosclerosis 40. Clinical data obtained from patients with systemic lupus erythematosus also support the notion that natural IgM autoantibodies may inhibit carotid atherogenesis 41.
The strong correlation of anti‐CS IgG in plasma versus PF may suggest that these antibodies may not be produced exclusively in the PF, but they could be transported from the plasma or lymphatic fluid into the pericardial space. A receptor‐mediated transcytosis of immunoglobulins 42 may underlie the transport of anti‐CS and other IgG antibodies into the PF. In addition to transcytosis, some antibodies may be synthesized locally in the pericardial space. Our data showing the relative increase of anti‐CS IgM antibody in the PF is consistent with the notion that B1 cells produce anti‐CS IgM (but not anti‐CS IgG) locally in the pericardial space.
Using immunoserological methods we measured M. pneumoniae‐, Y. enterocolitica‐, C. pneumoniae‐, H. pylori‐ and B. burgdorferi‐specific antibodies in PF and plasma because data have been published on the possible involvement of these microbes in the development of cardiovascular diseases 31. Of note, all patients were positive for antibodies to at least one bacterial species; however, some patients were negative for some of these antibodies in the PF. This may suggest that the levels of anti‐bacterial antibodies in the plasma may be generally higher than in the PF (the PF is more sequestered from the immune system than blood), resulting in antibody levels below the limit of detection in the PF. Interestingly, M. pneumoniae has been found in atherosclerotic plaques and in normal arteries and veins of patients 31, and this bacterium was also shown to be present in the PF of atherosclerotic patients 43. H. pylori has also been found in human atherosclerotic plaques, and a significant association of antibodies to this pathogen has been found in patients with coronary arteriosclerosis when compared to control subjects 43. C. pneumoniae has been detected in atherosclerotic plaques by quantitative polymerase chain reaction (qPCR) 44, and viable C. pneumoniae organisms were isolated from plaques 31. Our current data on the high frequency of anti‐microbial antibodies in the plasma and PF of patients undergoing heart surgery (atherosclerosis‐related coronary heart disease) are consistent with the potential pathogenic role of the above‐mentioned bacteria in these diseases.
Our data suggest an inter‐relationship between natural and anti‐bacterial antibodies. We found that all patients whose plasma and/or PF were positive for antibodies to M. pneumoniae had significantly higher levels of anti‐CS IgM, both in their plasma and PF. Investigating the different disease groups, in CABG patients without previous MI we found a significant correlation between anti‐CS IgG levels and M. pneumoniae antibody titres, while in CABG patients with previous MI, C. pneumoniae and B. burgdorferi antibody concentrations showed correlation with anti‐CS IgG levels. No such correlation with any of these anti‐bacterial antibody levels was observed in patients with AVR. Of note M. pneumoniae, as an intracellular microbe and an obligate parasite, requires the essential metabolites from the host cells. This bacterium can cause cell death and inflammation in the respiratory system 45, 46, resulting in the release of CS molecules from damaged cells, which then could lead to increased production of anti‐CS antibodies by the immune system. Interestingly, during its evolution M. pneumoniae has lost many of its genes, including citrate synthase 47; therefore, it is not the bacterial CS that causes the elevation of the natural anti‐CS IgM and IgG autoantibody levels. M. pneumoniae infection can have extrapulmonary manifestations, including infection of the pericardium 40, which may result in increased levels of anti‐CS antibodies in the plasma and PF. Our results provide evidence that the pericardial fluid (PF) of cardiac surgery patients contains functionally heterogeneous anti‐CS IgM and IgG natural autoantibodies. The correlation of elevated anti‐CS IgM levels with the M. pneumoniae infection was independent from the disease groups. This may reflect its general protective role as a natural autoantibody, independent of the presence or absence of atherosclerosis. In contrast, anti‐CS IgG level showed a strong correlation with higher levels of atherosclerosis‐related anti‐bacterial antibodies only in patients with coronary artery disease, which reflects its potential protective role in these infections. These data suggest that the PF is being ‘surveyed’ by the humoral components of the natural and adaptive immune systems in strong co‐operation to protect the pericardial space from accumulation of toxic molecules and various pathogenic microbes. Further studies will be needed to investigate the network of natural and anti‐bacterial autoantibodies and their role in the pathogenesis of cardiovascular diseases.
Disclosure
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Hungarian Medical Research Council (ETT 342/09) (L. L.), the University of Pécs Medical School Research Fund (AOK‐KA‐2013/16) (L. L.) and the Hungarian Scientific Research Fund (OTKA K‐105962) (T. B.) and by GINOP‐232‐15‐2016‐00050 and EFOP 3.6.1–16‐2016‐0004 (T. B.). The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pécs, Hungary.
References
- 1. Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med 2013; 11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 2015; 15:117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meng X, Yang J, Dong M et al Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol 2016; 13:167–79. [DOI] [PubMed] [Google Scholar]
- 4. Ketelhuth DF, Hansson GK. Adaptive response of T and B cells in atherosclerosis. Circ Res 2016; 118:668–78. [DOI] [PubMed] [Google Scholar]
- 5. Murphy KP. Janeway's immunobiology, 8th edn London and New York: Garland Science, 2012. [Google Scholar]
- 6. Kopcinovic LM, Culej J. Pleural, peritoneal and pericardial effusions – a biochemical approach. Biochem Med (Zagreb) 2014; 24:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montecino‐Rodriguez E, Dorshkind K. New perspectives in B‐1 B cell development and function. Trends Immunol 2006; 27:428–33. [DOI] [PubMed] [Google Scholar]
- 8. Ben‐Horin S, Bank I, Shinfeld A, Kachel E, Guetta V, Livneh A. Diagnostic value of the biochemical composition of pericardial effusions in patients undergoing pericardiocentesis. Am J Cardiol 2007; 99:1294–7. [DOI] [PubMed] [Google Scholar]
- 9. Ben‐Horin S, Shinfeld A, Kachel E, Chetrit A, Livneh A. The composition of normal pericardial fluid and its implications for diagnosing pericardial effusions. Am J Med 2005; 118:636–40. [DOI] [PubMed] [Google Scholar]
- 10. Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 2010; 10:778–86. [DOI] [PubMed] [Google Scholar]
- 11. McLendon PM, Robbins J. Proteotoxicity and cardiac dysfunction. Circ Res 2015; 116:1863–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zouali M. Natural Antibodies. In eLS, (Ed.): Wiley, 2009. doi:10.1002/9780470015902.a0001213.pub2 [Google Scholar]
- 13. Siloşi I, Siloşi CA, Boldeanu MV et al The role of autoantibodies in health and disease. Rom J Morphol Embryol 2016; 57:633–8. [PubMed] [Google Scholar]
- 14. Kohler H, Bayry J, Nicoletti A, Kaveri SV. Natural autoantibodies as tools to predict the outcome of immune response? Scand J Immunol 2003; 58:285–9. [DOI] [PubMed] [Google Scholar]
- 15. Baumgarth N. The double life of a B‐1 cell: self‐reactivity selects for protective effector functions. Nat Rev Immunol 2011; 11:34–46. [DOI] [PubMed] [Google Scholar]
- 16. Lobo PI. Role of natural autoantibodies and natural IgM anti‐leucocyte autoantibodies in health and disease. Front Immunol 2016; 7:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLOS ONE 2013; 8:e60726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mannoor K, Xu Y, Chen C. Natural autoantibodies and associated B cells in immunity and autoimmunity. Autoimmunity 2013; 46:138–47. [DOI] [PubMed] [Google Scholar]
- 19. Grönwall C, Silverman GJ. Natural IgM: beneficial autoantibodies for the control of inflammatory and autoimmune disease. J Clin Immunol 2014; 34: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grönwall C, Vas J, Silverman GJ. Protective roles of natural IgM antibodies. Front Immunol 2012; 3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gunti S, Notkins AL. Polyreactive antibodies: function and quantification. J Infect Dis 2015; 212 (Suppl 1):S42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J Immunol 2015; 194:13–20. [DOI] [PubMed] [Google Scholar]
- 23. Riemann D, Wollert HG, Menschikowski J, Mittenzwei S, Langner J. Immunophenotype of lymphocytes in pericardial fluid from patients with different forms of heart disease. Int Arch Allergy Immunol 1994; 104:48–56. [DOI] [PubMed] [Google Scholar]
- 24. Alter GM, Casazza JP, Zhi W, Nemeth P, Srere PA, Evans CT. Mutation of essential catalytic residues in pig citrate synthase. Biochemistry 1990; 29:7557–63. [DOI] [PubMed] [Google Scholar]
- 25. Czömpöly T, Olasz K, Nyárády Z, Simon D, Bovári J, Németh P. Detailed analyses of antibodies recognizing mitochondrial antigens suggest similar or identical mechanism for production of natural antibodies and natural autoantibodies. Autoimmun Rev 2008; 7:463–7. [DOI] [PubMed] [Google Scholar]
- 26. Czömpöly T, Olasz K, Simon D et al A possible new bridge between innate and adaptive immunity: are the anti‐mitochondrial citrate synthase autoantibodies components of the natural antibody network? Mol Immunol 2006; 43:1761–8. [DOI] [PubMed] [Google Scholar]
- 27. Morgunov I, Srere PA. Interaction between citrate synthase and malate dehydrogenase. Substrate channeling of oxaloacetate. J Biol Chem 1998; 273:29540–4. [DOI] [PubMed] [Google Scholar]
- 28. Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet 1997; 350:430–6. [DOI] [PubMed] [Google Scholar]
- 29. Danesh J, Youngman L, Clark S, Parish S, Peto R, Collins R. Helicobacter pylori infection and early onset myocardial infarction: case–control and sibling pairs study. BMJ 1999; 319:1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ridker PM, Danesh J, Youngman L et al A prospective study of Helicobacter pylori seropositivity and the risk for future myocardial infarction among socioeconomically similar U.S. men. Ann Intern Med 2001; 135:184–8. [DOI] [PubMed] [Google Scholar]
- 31. Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost 2011; 106:858–67. [DOI] [PubMed] [Google Scholar]
- 32. Rugonfalvi‐Kiss S, Endrész V, Madsen HO et al Association of Chlamydia pneumoniae with coronary artery disease and its progression is dependent on the modifying effect of mannose‐binding lectin. Circulation 2002; 106:1071–6. [DOI] [PubMed] [Google Scholar]
- 33. Chhibber‐Goel J, Singhal V, Bhowmik D et al Linkages between oral commensal bacteria and atherosclerotic plaques in coronary artery disease patients. NPJ Biofilms Microbiomes 2016; 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nemeth P, Small WC, Evans CT, Zhi W, Persson LO, Srere PA. Immunological mapping of fine molecular surface structures of citrate synthase enzymes from different cell types. J Mol Recognit 1991; 4:77–83. [DOI] [PubMed] [Google Scholar]
- 35. Petrohai A, Nagy G, Bosze S et al Detection of citrate synthase‐reacting autoantibodies after heart transplantation: an epitope mapping study. Transpl Int 2004; 17:834–40. [DOI] [PubMed] [Google Scholar]
- 36. Cornfield DB, Gheith SM. Flow cytometric quantitation of natural killer cells and T lymphocytes expressing T‐cell receptors alpha/beta and gamma/delta is not helpful in distinguishing benign from malignant body cavity effusions. Cytometry B Clin Cytom 2009; 76:213–7. [DOI] [PubMed] [Google Scholar]
- 37. Baumgarth N. B‐1 cell heterogeneity and the regulation of natural and antigen‐induced IgM production. Front Immunol 2016; 7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell 2010; 140:619–30. [DOI] [PubMed] [Google Scholar]
- 39. Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008; 8:34–47. [DOI] [PubMed] [Google Scholar]
- 40. Kyaw T, Tipping P, Bobik A, Toh BH. Protective role of natural IgM‐producing B1a cells in atherosclerosis. Trends Cardiovasc Med 2012; 22:48–53. [DOI] [PubMed] [Google Scholar]
- 41. Grönwall C, Reynolds H, Kim JK et al Relation of carotid plaque with natural IgM antibodies in patients with systemic lupus erythematosus. Clin Immunol 2014; 153:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol 2002; 3:944–55. [DOI] [PubMed] [Google Scholar]
- 43. Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation 2002; 106:184–90. [DOI] [PubMed] [Google Scholar]
- 44. Koren O, Spor A, Felin J et al Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA 2011; 108: 4592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krishnan M, Kannan TR, Baseman JB. Mycoplasma pneumoniae CARDS toxin is internalized via clathrin‐mediated endocytosis. PLoS One 2013; 8:e62706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 2004; 17:697–728, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Himmelreich R, Hilbert H, Plagens H, Pirkl E, Li BC, Herrmann R. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae . Nucleic Acids Res 1996; 24:4420–49. [DOI] [PMC free article] [PubMed] [Google Scholar]


