Summary
Studies of cerebrospinal fluid (CSF) γδ T cells in children are limited, due especially to the lack of control data. In adults, gamma/delta T cells (TCR‐γδ) residing in the intrathecal space are sometimes involved in neuroinflammation. To evaluate the possible role of γδ T cells in paediatric neuroinflammation, we immunophenotyped cerebrospinal fluid (CSF) and blood lymphocytes using flow cytometry in a case–control study of 100 children with non‐inflammatory neurological disorders (NIND), 312 with opsoclonus–myoclonus (OMS) and 23 with other inflammatory neurological disorders (OIND). In NIND, the negative correlation between CSF γδ T cell frequency and patient age was striking: median frequency of 27% in infants and 3·3% in teens. Interindividual variations were largest in the youngest. There was no gender effect. In all OMS, after correcting for age, only a small effect of OMS severity remained. Measurement of markers for γδ T cell activation [human leucocyte antigen D‐related (HLA‐DR)], maturation (CD45RA, CD45RO) or intracellular cytokine staining [interleukin (IL)‐4, interferon (IFN)‐γ] failed to discriminate OMS and NIND groups. Of seven OMS immunotherapies/combinations, none altered the frequency of total CSF γδ T cells or subsets significantly. In OIND, the CSF γδ T cell frequency was < 10% for single samples of other paraneoplastic disorders [anti‐neuronal nuclear antibody (ANNA)‐1, PCA‐1, teratoma‐associated syndrome], cerebellar ataxia (post‐infectious, ataxia‐telangiectasia), acute disseminated encephalomyelitis, neuroborreliosis and encephalitis. This study provides new insights into CSF γδ T cells in the paediatric population. Although their role in CSF remains elusive, the negative age correlation, resistance to immunotherapy and our age cut‐off references for NIND are important findings for the design of future paediatric studies.
Keywords: double‐negative T cells, neuroblastoma, opsoclonus–myoclonus syndrome, paraneoplastic syndrome, paediatric neuroinflammation
Introduction
In the ontogeny of T lymphocytes, those committed to the expression of γ and δ chains are the first to develop, whereas those exclusively expressing α and β chains appear later and become more numerous 1. Gamma/delta (γδ) T cells [T cell receptor (TCR)‐γδ], also called ‘fetal‐type lymphocytes’ or CD4–CD8– ‘double‐negative T‐γ cells’, are unique and appreciated as a third arm of immune response 2. While these ‘non‐classical’ T cells possess inherent autoreactivity, their response may be driven more by imbalance, such as inflammation, tissue damage or cell transformation, than by specific pathogen challenge 3, 4. Other distinguishing features of γδ T cells include specialized anatomical distribution, a distinct pathway of developmental maturation, unique antigen specificities and capacity to protect the host against specific pathogens, and uniquely age‐dependent activities 5, 6. They may be evolutionarily ancient lymphocytes 7.
Although γδ T cells play a role in the pathogenesis of many chronic inflammatory diseases in humans 6, their involvement in chronic neuroinflammatory disorders is particularly germane. They are influential in regulating the extent and duration of central nervous system (CNS) inflammation 8, enhancing autoimmunity by restraining regulatory T cell responses 9. In adults with multiple sclerosis, they are recruited selectively to the cerebrospinal fluid (CSF) 10, clonally expanded 11, found in demyelinating lesions, increased in clinically isolated syndrome/multiple sclerosis (CIS/MS) relapse 12 and the TCR‐γδ repertoire is skewed 13. In Rasmussen encephalitis, an inflammatory refractory epilepsy of children, clonally restricted γδ T cells (Vδ1 chains) have been identified in surgically excised brain specimens along with activated αβ T cells 14. However, γδ T cells may play either a pro‐ or anti‐inflammatory role depending on the context, such as in experimental autoimmune encephalitis 15, 16, but they are not obligate participants 17, making them enigmatic. They are considered innate immune cells primarily, but can develop memory‐like adaptive responses, bridging the gap between adaptive and innate immunity 18.
In paediatric‐onset neuroinflammatory disorders, information on CSF γδ T cells is scant. Immune cell dysregulation has been implicated in the pathophysiology of opsoclonus–myoclonus syndrome (OMS) 19, a devastating autoimmune CNS complication of neuroblastic tumours of the body cavity 20, 21. γδ T cells are among the tumour‐infiltrating lymphocytes in neuroblastoma, which is detected in 50% of children with OMS 22, and display anti‐tumour activity in vitro 23, 24. Cancer immunotherapy with γδ T cells also has shown cytotoxic clinical activity 25. We have found immunophenotyping of lymphocytes, such as γδ T cells, in the CSF to be feasible in children and to yield valuable information on neuroinflammation that is not provided by blood 26.
The following data constitute a study of γδ T cells in OMS and a sampling of other paediatric‐onset neuroinflammatory disorders, such as other paraneoplastic, post‐infectious and demyelinating disorders and CNS infections, compared to non‐inflammatory paediatric neurological disorders. The aims were to (i) determine the phenotype and distribution of CSF γδ T cells in the paediatric population, (ii) provide ‘reference ranges’ and (iii) test for evidence of γδ T cell involvement in paediatric neuroinflammation.
Patients and methods
Study design
The case–control design of the study afforded cross‐sectional comparison of children with inflammatory (OMS, OIND) and non‐inflammatory neurological disorders (NIND). Because CSF from healthy children is not available for ethical reasons, the NIND act as a default passive ‘control’ group for OMS and the OIND as active controls in many neuroimmunological studies. All children were evaluated by the principal investigator (M.P.), and those with OMS were also evaluated by the co‐investigator (E.T.). In each group, total γδ T cell frequency was measured. Later, studies of markers for γδ T cell activation, maturation and intracellular staining were added in a subgroup of OMS and NIND.
Subjects
Three hundred and twelve children with OMS were recruited through the National Pediatric Myoclonus Center, the largest international centre for paediatric‐onset OMS, from 2002 to 2012. Their parents signed consent for this Institutional Review Board‐approved study (SIU School of Medicine, Springfield, IL, USA), and patients meeting appropriate criteria also signed assent. Additionally, Western IRB designated IRB exemption for retrospective analysis of demographic, clinical and laboratory data. Per protocol, additional CSF for flow cytometry was taken at the time of diagnostic lumbar puncture.
Clinical characteristics were recorded (Table 1). All patients with OMS had neurological symptoms at the time of evaluation. OMS duration category was acute (< 3 months) in 73 (23%), intermediate (3–12 months) in 140 (45%) and chronic (> 12 months) in 99 (32%). OMS severity category was mild in 116 (39%), moderate in 129 (44%) and severe in 49 (17%). There were 294 instead of 312 for the severity category because the categorization is based on the total score scale (explained in Methods), and some patients were unable to complete the total score evaluation. Treatment status was untreated in 50 (16%) treated previously, not currently, in 63 (20%) and treated currently in 199 (64%). CSF mononuclear cell count was ≤ 3 in all but three children (5, 6, 20) and CSF red blood cell count was ≤ 1. CSF protein and glucose were normal.
Table 1.
Clinical characteristics of groups
| Variable | NIND | OMS | OIND |
|---|---|---|---|
| n | 100 | 312 | 23 |
| Gender n (%) | |||
| Male | 51 (51) | 140 (45) | 9 (39) |
| Female | 49 (49) | 172 (55) | 14 (61) |
| Median age (years) (IQR) | 7·1 (2·6, 12) | 2·7 (1·9, 4·0) | 5·2 (3·2, 10) |
| Age range (years) | 0·47–18 | 0·9–7 | 0·2–17 |
| Age category n (%) | |||
| Infants | 14 (14) | 25 (8) | 2 (9) |
| Toddlers | 11 (11) | 150 (48) | 4 (17) |
| Preschool | 14 (14) | 79 (25) | 7 (30) |
| Older children | 61 (61) | 58 (17) | 10 (43) |
| Diagnosis n (%) | |||
| Ataxia | 20 (20) | ||
| Dev. delay | 12 (12) | ||
| Headache | 21 (21) | ||
| Movement dis. | 15 (15) | ||
| Seizures | 8 (8) | ||
| Eye mov. dis. | 4 (4) | ||
| Miscellaneous | 20 (20) | ||
| Inflam. ataxia | 3 (13) | ||
| CNS infection | 3 (13) | ||
| Demyelinating dis. | 3 (13) | ||
| Inflam. epilepsy | 1 (4) | ||
| Mixed mov. dis. | 1 (4) | ||
| Neurolupus | 1 (4) | ||
| Neurol. dis., other | 7 (13) | ||
| Paraneo. dis., other | 1 (4) | ||
| OMS aetiology n (%) | |||
| No tumour | 156 (50) | ||
| Tumour | 156 (50) |
Dev. delay = developmental delay; mov. = movement; dis. = disorder; NIND = non‐inflammatory neurological disorders; OMS = opsoclonus–myoclonus syndrome; OIND = other inflammatory neurological disorders.
Non‐inflammatory neurological disorders (NIND) included 100 children whose thorough diagnostic evaluation had not yielded an aetiology. Routine CSF studies, such as cell count, protein and glucose, were normal.
Other inflammatory neurological disorders (OIND) included 23 children without OMS. CSF showed elevated leucocyte count, elevated protein, markers of infection, or blood was positive for serological or infection testing.
Scoring of neurological status
In OMS, all patients were videotaped with written parental consent. For the purpose of score derivation, a trained observer blinded to treatment status rated motor impairment using the 12‐item Opsoclonus–Myoclonus Evaluation Scale 27. Each item was scored from 0 to 3, ranging from normal to severely abnormal. Total score was calculated as the sum of scores for individual items.
Sample procurement
To prevent contamination of CSF with blood due to trauma, minimize sedation risks during lumbar puncture, standardize the degree of stress on immune function and provide compassionate care, a controlled lumbar puncture was performed as described previously 26. In brief, the sterile procedure was performed during the mid‐morning after overnight fasting with the patient in the left lateral decubitus position using a #22‐gauge Quincke spinal needle. Propofol sedation after brief sevoflurane induction was required because children with OMS have paradoxical reactions to other sedatives. The quantity of CSF collected into polypropylene tubes was ≤ 15% of the calculated total CSF volume for age. The first 3 ml of CSF were sent for routine studies. The next 10–14 ml were collected on ice for flow cytometry and taken promptly by an assistant to the flow cytometry laboratory, where the cells were stained with monoclonal antibodies (mAbs) in < 1 h after collection (usually 15–20 min). In older infants, only 7 ml were collected based on our calculation of reduced total CSF volume. CSF testing was not performed in infants ≤ 6 months of age because monocytes predominate if CSF, impeding lymphocyte gating, and the allowable CSF volume would be insufficient. For parallel studies, blood was drawn by venipuncture and placed into a sodium heparin (2 ml) and an ethylenediamine tetraacetic acid (EDTA) tube (2 ml). The patients tolerated the procedures well.
Flow cytometric analysis
CSF and blood lymphocyte subset analysis was performed using published methods 26, 27. The key procedural points in the CSF lymphocyte assay were avoidance of loss of lymphocytes due to handling, the 50‐fold concentration step (resuspending lymphocytes in 200 µl in phosphate‐buffered saline containing 0·5% bovine serum albumin after final incubations), and no use of Optilyse B (only for blood lymphocyte processing). Cells were stained using a panel of directly conjugated monoclonal antibodies (mAbs) labelled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC) or PE‐cyanin 5.5 (PC5). The mAbs included CD3, CD4, CD8, CD45, CD16/56, CD25 (Beckman‐Coulter, Miami, FL, USA) and TCR‐γδ, HLA‐DR, CD45RO and CD45RA (Immunotech, Marseille, France). The γδ T cell assay tubes contained CD4/TCR‐γδ/CD8/CD3, CD45RA/TCR‐γδ/3/45, CD45RO/TCR/CD3/CD45 or IL‐4/IFN‐γ/TCR‐γδ/HLA‐DR. All samples were acquired and analysed by flow cytometry on a FACSCalibur cytometer equipped with a 488‐nm argon/633‐nm HeNe laser (Becton‐Dickinson, San Jose, CA, USA), using published quality control measures 26.
Statistics
Data were analysed statistically using GraphPad Prism version 7.02 (GraphPad Software, San Diego, CA, USA) and the Statistical Analysis System (SAS). The principal dependent variable was the percentage of CSF γδ T cells, and the principal independent variables were group (NIND, OMS, OIND), patient age and gender. In secondary analysis of OMS, tumour, neurological severity, chemotherapy and current treatment were included. To test for an apparent effect of patient age, data were analysed using analysis of covariance (ANCOVA) with age and OMS severity category or age and OMS duration category. Using the general linear model (GLM) procedure on SAS, the degrees of freedom were as follows: 3 for model, 290 for error and 293 for corrected total. Type 1 sums of squares (SS) were used. Data are reported as medians with interquartile range (IQR), unless specified otherwise. Correlation analysis utilized Spearman's correlations. The threshold for statistical significance was set at α = 0·05. Concentrations of inflammatory mediators measured in the principal investigator's laboratory, as referenced in a recent review 21, also were used for correlations with γδ T cells in OMS.
Results
NIND
CSF γδ T cells
The CSF γδ T cell frequency declined dramatically with patient age in the paediatric age range (Fig. 1). Expressed as a percentage of CD3+ T cells, the median CSF γδ T cell frequency was twice as high in infants than toddlers, three times higher than in 2–5‐year‐olds and eight times higher than in teens. There was no gender effect. The median (IQR) γδ T cell frequency was 7·3% (4.0, 12), constituting a small percentage of CSF lymphocytes. Variations between individuals also were noted.
Figure 1.
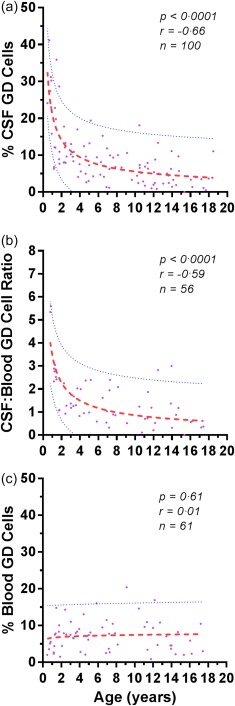
Effect of patient age on (a) the frequency of cerebrospinal fluid (CSF) γδ T cells, (b) the CSF/blood γδ T cell ratio, and (c) the frequency of peripheral blood γδ T cells in non‐inflammatory neurological disorders (NIND). Dotted lines indicate 5th and 95 percentiles. Dashes represent median curves from best‐fitting statistical models (log–log). The correlation coefficients are Spearman.
For clinical convenience, ‘reference ranges’ were computed based on common paediatric age group designations: infants, toddlers, pre‐school, school‐age, pre‐teens and teens (Table 2).
Table 2.
Convenient age cut‐offs for comparison of median CSF γδ T cell frequency in NIND, OMS and OIND*
| NIND | OMS | OIND | ||||
|---|---|---|---|---|---|---|
| Age range (years) | n | % (IQR) | n | % (IQR) | n | % (IQR) |
| 0–1 | 7 | 27 (23, 31) | – | – | 2 | (8·3, 8·5) |
| 1–2 | 11 | 12 (7·7, 23) | 78 | 9·9 (7·1, 15) | – | |
| 2–3 | 7 | 9·6 (8·0, 13) | 90 | 9·9 (6·4, 13) | 2 | (8·9, 6·7) |
| 3–5 | 14 | 9·0 (4·7, 11) | 79 | 9·4 (5·2, 14) | 5 | 7·9 (3·8, 12) |
| 5–10 | 21 | 7·3 (4·5, 10) | 40 | 6·0 (3·4, 8·6) | 8 | 4·8 (1·8, 9·2) |
| 10–15 | 31 | 5·0 (2·4, 6·9) | 11 | 4·0 (2·1, 8·1) | 3 | 4·7 (2·4, 7·5) |
| 15–18 | 9 | 3·3 (0·8, 3·8) | 7 | 3·3 (1·4, 6) | 3 | 1·1 (·87, 5·6) |
*Expressed as percentage of total events. Age cut‐offs provide quasi ‘reference ranges’. Total n was 100 for NIND, 312 for OMS and 23 for OIND. For comparison, corresponding means ± standard deviation (s.d.) for OMS (top to bottom) were 11·9 ± 6·9, 11·5 ± 6·4, 10·6 ± 6·6, 6·5 ± 6·6, 5·3 ± 4·1, and 4·8 ± 4·6. For OIND (top to bottom from 3–5‐year range, they were 0·3). NIND = non‐inflammatory neurological disorders; OMS = opsoclonus–myoclonus syndrome; OIND = other inflammatory neurological disorders; IQR = interquartile range; CSF = cerebrospinal fluid.
Cut‐points were correspondingly more frequent during the most changeable periods of CSF γδ T cell frequency and less frequent during the less changeable period. There was good agreement between median and mean frequencies.
Immunological intercorrelations between the frequency of CSF γδ T cells and that of certain other immune cells was found (Fig. 2). There were negative correlations with the frequency of CD+ T cells (and the CD4/CD8 ratio), and positive correlations with the frequency of CD3–CD16/56+ NK cells and CD25+CD4+ T cells. The percentage of γδ T cells correlated with that of CD3+ T cells. The percentage of γδ T cells was correlated negatively with that of CD3+ T cells (P < 0·0001, r = –0·62, n = 100). Thus, expressed as the percentage of CSF CD3+ cells, the greater the frequency of the CD4–CD8– T cells (γδ cells), the lower the frequency of the CD4+ CD8+ T cells (αβ cells).
Figure 2.
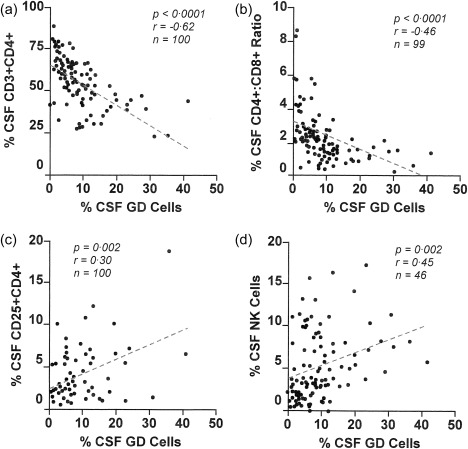
Scatter‐plots with correlations for cerebrospinal fluid (CSF) γδ T cell frequency and other CSF immune cell types in non‐inflammatory neurological disorders (NIND): (a) % CD4+ T cells, (b) CD4/CD8 T cell ratio, (c) % CD25+CD4+ T cells and (d) % natural killer (NK) cells. The correlation coefficients are Spearman.
The frequency of γδ T cell activation, maturation and intracellularly stained subsets was analysed, although sample sizes were smaller. The median percentage of HLA‐DR+ activated γδ T cells was 2·1% (IQR = 0·74, 3·9). CD45RA+ naive γδ T cells were 1·4% (IQR = 0, 2·7%) and CD45RO+ memory cells were 4·8% (IQR = 1·7, 7·5%). IL‐4+ γδ T cells were 23% (IQR = 9·7, 41%) and IFN‐γ+ γδ T cells were 16%.
The relation of patient age and CSF γδ T cell frequency was evaluated in the same subsets. CD45RA+ γδ T cell percentage perhaps trended downwards with age (P = 0·07, r = –0·30, n = 39). The percentage of CD45RO+ γδ T cells may have shown a similar trend (P = 0·09, r = –0·27, n = 39). However, the CSF CD45RA : CD45RO ratio did not correlate with patient age (median = 0·28; 0·15, 0·66 IQR) (n = 27). There were no significant age correlations for HLA‐DR+ (n = 37), IL‐4+ (n = 30) or IFN‐γ+ (n = 30) γδ T cells.
NIND data were analysed secondarily by diagnostic entity for the larger subgroups. The median CSF γδ T cell frequency for ‘ataxia’ (n = 20) was 8·7% (5·8, 12); median age 4·7 (2·6, 6·9) years. For ‘myoclonus’ (n = 11), it was 5.0% (1·6, 6·8); age, 4·9 (2·2, 12) years. For ‘headache’ (n = 21), it was 5.0% (1.6, 6.8); age, 12 (10, 15) years.
Blood γδ T cells
In peripheral blood, the median % γδ T cells was 7.0 (4.0, 8.9). For HLA‐DR+ γδ T cells it was 0·37 (0·45, 0·85); for CD45RA+ γδ T cells, 2·7 (1·3, 3·5); for CD45RO+ γδ T cells, 3·3 (2·5, 5·3).
OMS
CSF in ‘All OMS’
The GLM procedure of SAS was used to determine the statistical main effects of age and OMS severity category, P < 0·0001. Statistically removing the significant age effect (F = 13·26, Pr > F < 0·0001) dropped the significance of OMS severity category to P = 0·012, indicating that they were covariates. Significant differences between mild and severe cases were due to different age structures of the two groups: the acute patients and the severe patients were younger. When secondary analysis utilized only children aged ≤ 5 years, there was no age effect and no significant difference in the median CSF T cell frequency between OMS severity categories remained.
In the least‐square means follow‐up test for OMS severity category (adjusted means), mild and moderate severity categories were significantly different (0·0037), but moderate and severe categories did not differ from each other, nor did mild versus severe (0.068). The median CSF γδ T cell frequency was 6·9% (4·5, 10) (n = 116) for the mild severity category; 9·8% (6·4, 14) (n = 129) for moderate; and 9·7% (6·1, 14) (n = 49) for severe.
Similarly, there was a statistically significant main effect of age and OMS duration category, P < 0·0001. When corrected statistically for age, the effect of OMS duration category on CSF γδ T cells was no longer significant, so it was dropped from further analysis. Because there was no significant effect of gender on OMS severity or duration categories, data from males and females were combined in subsequent analyses.
OMS severity categories were not related to the frequency of γδ T cells positive for activation markers (HLA‐DR), maturation markers (CD45RA, CD45RO) or intracellular staining for cytokines IL‐4 or IFN‐γ (Supporting information, Table S1). The median CD45RA : CD45RO γδ T cell ratio was higher in OMS (0·48; 0·31, 1·0 IQR) than in NIND (0·28; 0·15, 0·66) (M‐W, P = 0.0043). CSF CD45RA+ γδ T cell frequency decreased with age (P = 0·002, r = –0·29, n = 117); for CD45RO+ γδ T cell frequency versus age, P = 0·04, r = –0·18. There were no significant age correlations for HLA‐DR+ (n = 96), IL‐4+ (n = 87) or IFN‐γ+ (n = 30) γδ T cells.
In ‘all OMS’, the median frequency (IQR) of CSF γδ T cells in the OMS data set was 9·7% (6·1, 15). The frequency of tumour versus no‐tumour‐found cases was identical (each 50%). The CSF γδ T cell frequency was 8·6% (5·1, 13) for tumour and 9·2% (6, 13) for no‐tumour‐found. Patient age was not a factor: 2·9 years (2·0, 3·8) in the tumour group and 2·7 (1·9, 4·2) in the other group. Neither comparison was statistically significant. Therefore, the data were combined in subsequent analyses.
Each of the median γδ T subset frequencies were % of median γδ T cell frequency (instead of CD3+ cells). Comparing the severe to mild OMS categories, there was a 41% increase and it was non‐linear (moderate OMS was the same as severe OMS). However, the HLA‐DR+ γδ T frequency had increased linearly by 69%. There was a 23% increase in CD45RA+ γδ T cell frequency and a 25% decrease in CD45RO+ γδ T cell frequency; both were non‐linear. We then focused our attention on the HLA‐DR+ γδ T cells in OMS, but also made a comparison with NIND. Although the total CSF γδ T cell frequency rises somewhat in OMS, the CSF HLA‐DR+ γδ T cell population becomes increasingly activated with increasing OMS severity.
When CSF γδ T cell frequency data were plotted on the curve fit derived from NIND (Fig. 3), 31 of 310 (10%) of the infants and toddlers exhibited values lying outside the CI, whether above the 95% CI (n = 18) or below the 5% CI (n = 13). Total score was comparable in OMS, falling within the CI (15 ± 8·6) and outside the CI (19 ± 9), whether above (15 ± 9) or below it (20 ± 9). The frequency of patients with OMS relapse was 67% above the CI and 54% below it. Both groups included untreated (17 versus 38%) and treated OMS. The frequency of CSF oligoclonal bands was 75% in the upper group and 25% in the lower group. The frequency of CSF B cell‐positive (> 2%) patients was comparable (72 versus 92%).
Figure 3.
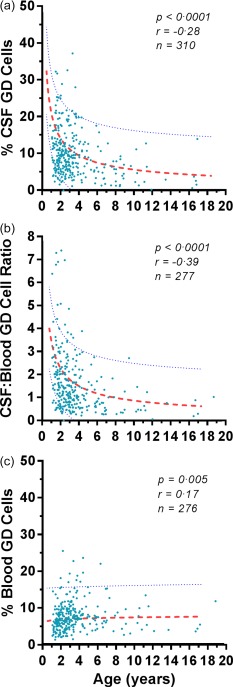
Effect of patient age on (a) the frequency of cerebrospinal fluid (CSF) γδ T cells, (b) the CSF/blood γδ T cell ratio, and (c) the frequency of peripheral blood γδ T cells in opsoclonus–myoclonus syndrome (OMS). Dotted lines indicate 5th and 95th percentiles derived from non‐inflammatory neurological disorders (NIND). Dashes represent median curves. The correlation coefficients are Spearman.
CSF in untreated OMS
In untreated OMS (n = 50), there was no significant effect of patient age on total score. The γδ T cell frequency was 9·0% (3·7, 14) for mild, 9·6% (7·0, 14) for moderate and 10·1% (5·0, 24) for severe categories. The frequency of CSF γδ T cells correlated negatively with that of CD4+ T cells (P < 0·0001, r = –0·56) and the CD4/CD8 ratio (P = 0·008, r = –0·37). It did not correlate with the percentage of CSF CD3+ or CD8+ T cells, CD25+CD4+ T cells, NK cells, CD19+ B cells or the CSF leucocyte count, or with CSF neurofilament light chain, B cell‐activating factor (BAFF), C‐X‐C motif chemokine ligand 1 (CXCL10), C‐C motif ligand 12 (CCL12) or CXCL13 concentrations or oligoclonal bands measured previously in these patients. In blood, there was a significant correlation with the concentration of CCL17 (P = 0·0007, r = 0·56), but not BAFF or CCL21.
CSF in treated OMS
There was no statistically significant difference in median (IQR) CSF γδ T cell frequency between NIND (2·3; 0·09, 5·0), untreated OMS (2·1; 2·2, 3·5) and treated OMS (1·95; 0·71, 17). Immunotherapy with adrenocorticotrophic hormone (ACTH), steroids, intravenous immunoglobulin (IVIg) as monotherapy; ACTH and IVIg or steroids and IVIg as dual therapy; or combined with cyclophosphamide, rituximab or steroid sparers had little impact on CSF γδ T cell frequency (Supporting information, Table S2). Therefore, data from these various subgroups were combined in subsequent analyses.
Blood γδ T cells in ‘all OMS’
In peripheral blood, the % γδ T cells was 5·7 (3·9, 8·0), which was not significantly different from NIND. The patient age of 2.7 (1·9, 5·9) years was lower than in NIND (P = 0·02, Mann–Whitney U‐test), but age was not correlated with the frequency of blood γδ T cells. The percentage of blood γδ T cells was 6·9 (5·2, 9·5) in the tumour group and 6·7 (5·0, 9·8) in the no‐tumour‐found group. The blood γδ T cell percentage did not correlate with OMS severity or duration.
OIND
CSF
The frequency of CSF γδ T cells in various other paediatric neuroinflammatory disorders was plotted versus patient age (Fig. 4). Each disorder is identified in the plot because many are orphan diseases, hence rare and hard to come by, and there are no other published data, so the individual's data may be important to researchers of particular diseases. In all but two patients it was < 10%. Disease subgroups were too small for reliable statistics, but no particular subgroup visual patterns emerged for patients with CSF γδ T cell frequencies above versus below the median. The frequency of CSF γδ T cells in the combined OIND group was 7·1% (3·0, 8·9), which was not significantly different from the NIND group.
Figure 4.
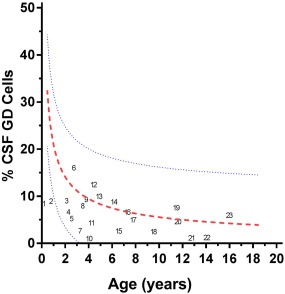
Cerebrospinal fluid (CSF) γδ T cell frequencies in other inflammatory neurological disorders (OIND) plotted against patient age. The outer blue lines indicate the 95%/5% confidence intervals CI) for non‐inflammatory neurological disorders (NIND); the red line represents the NIND mean for comparison. Individuals with various neuroinflammatory disorders are indicated by numbers as follows: other paraneoplastic syndromes, such as anti‐neuronal nuclear antibody (ANNA)‐1/anti‐Hu (4, 14), ANNA‐2/anti‐Yo (15), and teratoma‐associated neurological syndrome (23); demyelinating disorders, such as acute disseminated encephalomyelitis (3, 9) and multiple sclerosis (22); neuropsychiatric lupus (18); inflammatory cerebellar ataxias, such as post‐infectious acute cerebellar ataxia (20), ataxia‐telangiectasia (10) and unspecified ataxia (6); central nervous system infections, such as encephalitis (2, 8, 16) and Lyme neuroborreliosis (17); epilepsy (5); involuntary eye movements (1); developmental delay (11, 12); mixed movement disorder (13); and other neurological disorders unspecified (7, 19, 21).
Blood
In peripheral blood (n = 15), the median frequency of γδ T cells was 6·3% (3·4, 10).
Discussion
The principal novel observation was that CSF γδ T lymphocyte frequencies differed substantially between paediatric age groups. The significant negative correlation of patient age with the frequency of CSF γδ T cells and the CSF/blood γδ T cell ratio, not blood γδ T cells, suggests a greater percentage of γδ T cells in the intrathecal space of the youngest children. A CSF gradient does not provide an explanation, because other cell types would be affected and CSF CD19+ B cell frequency does not correlate with age 27. In interpreting these data, it should be noted that cell frequency is relative, not absolute; it also reflects changes in other cell populations.
When we previously reported an elevated mean frequency of CSF γδ T cells in OMS that correlated with neurological severity 26, we did not have enough samples (18 NIND, 36 OMS) to be aware of an age correlation and there were no published data. The most severe cases were also the youngest; the mildest were older. In the present study, after correcting for age, the effect size is now minimal. As a result, total CSF γδ T cell frequency is not a useful biomarker of disease activity in OMS as a group. Also, single‐sample representations of other paediatric inflammatory neurological disorders did not exceed the CSF γδ T cell frequencies found in non‐inflammatory neurological disorders. In the present study, however, we were able to confirm that the similar CSF γδ T cell frequencies we reported in tumour and non‐tumour OMS groups were not influenced by patient age. In our previous cross‐sectional study of blood in paraneoplastic OMS 28, we found that the absolute, but not relative, size of blood γδ T cell subsets was reduced (–44%, P = 0.02), as found for neuroblastoma without OMS 29, but there were not enough patients for analysis versus age (17 NIND, 17 OMS).
We compared our CSF γδ T cell frequencies with those reported in the literature. CSF double‐negative T cells, which include but are not identical with the γδ T cell population, were found to be higher in paediatric NIND (n = 12) compared to adult NIND both in frequency (at 7–8% by graphic estimate) and absolute counts (at 500–600 cells) 30. The range of values was large. Such was also the case for paediatric OIND (n = 17) compared with adult OIND 30. In children, γδ T cells were increased in the CSF of children with meningitis caused by mumps, Coxsackie B5, Echo 17, Epstein–Barr virus (EBV) and other viruses. They were also predominant in the CSF of children with tuberculosis meningitis (Vγ9Vδ2 TCR) and decreased with anti‐microbial therapy 31. In adults, Correale et al. 32 reported a mean γδ T cell percentage in CSF of approximately 2% for neurological controls and 3–5% for various inflammatory disorders; none had a value higher than 11%. Perrella et al. 33 found < 2% γδ T cells in the CSF of their neurological controls. The increased percentage of CSF γδ T cells with the vδ1+ phenotype reported in mumps meningitis by Bertotto et al. 34 did not exceed 14%. In adults with multiple sclerosis, γδ T cells (expressed as % of CD3+) were decreased in CSF (3·4% ± 0·5%) compared to NIND CSF (7·3 ± 0·5%); peripheral blood frequency was equal in both groups 35. By comparison, in healthy adults γδ T cells account for 1–5% of circulating T cells and are primarily Vγ9Vδ2 TCR 1. Peripheral blood γδ T cell abnormalities have been discussed elsewhere 36, and are outside the scope of the present discussion.
We do not currently have a ready explanation for why CSF γδ T cell frequency correlated with that of CSF NK cells and CD4+CD25+ T cells [presumptive regulatory T cells (Tregs)], not other αβ T cells, in NIND. In our search of the literature, we did not often see correlations tested between γδ T cells with other CSF cell types. As to whether the correlations could reflect some regulatory effect, studies of developmental frequency and distribution CSF of these other two cell types may provide an explanation later.
The potential effect of immunotherapy on γδ T cells, particularly in CSF, has received little attention. In peripheral blood, methotrexate treatment was associated with a decrease in disease‐elevated double‐negative and γδ T cell levels in patients with juvenile rheumatoid arthritis 37. Of note, cancer protection mediated by the immunotherapeutic agent rapamycin depends on γδ T cell stimulation 38. In our cross‐sectional study, commonly prescribed steroids, IVIG, rituximab, cyclophosphamide and other steroid sparers did not alter γδ T cell frequency significantly, at least in OMS.
The strengths of the study include the very large sample size, inclusion of samples from rare and understudied disorders, blinded‐scoring of videotapes for OMS severity and the use of the same experienced scorer. The methodology was consistent and CSF was obtained without contamination by blood and screened carefully for exclusion criteria. None of the controls showed evidence of neuroinflammation.
There were also study limitations. The powerful effect of patient age on the CSF γδ T cell frequency was a potential confounding variable in the youngest children, which had to be addressed statistically. The heterogeneous differences between patients could obscure detection of small effect sizes or contrary effects in patient subgroups and prevent rigorous subgroup analyses. We present cell frequency data, not counts, which, however, would be useful in further studies. Testing for chain‐specific γδ T cell subsets, which may play opposing roles, would have added to the phenotypical profile, but much novel information was collected all the same.
In summary, this first large paediatric age‐ranging study of the CSF γδ T cell population reveals that the γδ T cell frequency declines sharply with age from infancy and demonstrates marked heterogeneity in the youngest. Age is a potential confounding variable for infants and toddlers, but not for older children. CSF and blood γδ T cell frequencies were not well correlated, so assessment in blood did not offer a reliable prediction of the CSF frequency, emphasizing the importance of CSF immunophenotyping for assessment of immune cell populations in neuroinflammatory conditions, which has potential clinical utility. In the absence of CSF studies in healthy children for ethical reasons, our data from non‐inflammatory paediatric neurological disorders may be the closest approximation of paediatric ‘reference ranges’ for now and of value to other investigators studying paediatric neuroinflammatory disorders. Use of CSF γδ T cell absolute counts, in addition to frequencies, and focus on chain‐specific subsets (Vδ1+, Vγ1+, Vγ4+), which may have opposing actions, should be considered in future studies of CSF γδ T cells in the paediatric population.
Author contributions
M. P. designed the study and wrote the paper; N. M. and T. A. provided data entry, statistical analysis, and graphics; N. M. and T. A. collected the flow data; M. P. and E. T. performed the clinical evaluations and lumbar punctures and E. T. scored the videotapes; all authors vetted and approved the manuscript for submission.
Disclosure
The authors declare no commercial or financial disclosures.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Median frequency of cerebrospinal fluid (CSF) γδ T cell subsets by opsoclonus–myoclonus syndrome (OMS) severity category.
Table S2. Effect of immunotherapy on cerebrospinal fluid (CSF) γδ T cell frequency in opsoclonus–myoclonus syndrome (OMS).
Acknowledgements
M. R. P. is a clinician–scientist, founder and president of the National Pediatric Neuroinflammation Organization, Inc., and Adjoint Professor of Neurology at the University of Colorado School of Medicine. The National Pediatric Myoclonus Center is a specialty centre for children with opsoclonus–myoclonus syndrome and has evaluated more than 420 children worldwide. In 2015, it incorporated in the State of Florida as the National Pediatric Neuroinflammation Organization, Inc., and received 501(c)(3) non‐profit designation. The authors thank flow cytometrist Anna L. Travelstead BS, MT (ASCP), immunologist Edward J. Moticka PhD, and all the participating patients, families and referring or treating physicians.
References
- 1. De Rosa SC, Andrus JP, Perfetto SP et al Ontogeny of gamma delta T cells in humans. J Immunol 2004; 172:1637–45. [DOI] [PubMed] [Google Scholar]
- 2. Hayday AC. γδ Cells: a right time and right place for a conserved third way of protection. Annu Rev Immunol 2000; 18:975–1026. [DOI] [PubMed] [Google Scholar]
- 3. Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol 1993; 11:637–85. [DOI] [PubMed] [Google Scholar]
- 4. Born W, Cady C, Jones‐Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of γδ T‐cells. Adv Immunol 1999; 71:77–144. [PubMed] [Google Scholar]
- 5. Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev 2007; 215:46–58. [DOI] [PubMed] [Google Scholar]
- 6. Fay NS, Larson EC, Jameson JM. Chronic inflammation and γδ T cells. Front Immunol 2016; 7:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Y, Wu W, Wong WN et al Human gamma delta T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol 2009; 183:5622–9. [DOI] [PubMed] [Google Scholar]
- 8. Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand‐dependent mechanism. J Immunol 2005; 174:4678–87. [DOI] [PubMed] [Google Scholar]
- 9. Peterman F, Rothhammer V, Claussen MC et al Gamma delta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin‐23‐dependent mechanism. Immunity 2010; 33:351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mix E, Olsson T, Correale J, Kostulas V, Link H. CD4+, CD8+, and CD4– CD8– T cells in CSF and blood of patients with multiple sclerosis and tension headache. Scand J Immunol 1990; 31:493–501. [DOI] [PubMed] [Google Scholar]
- 11. Bieganowski P, Bieganowski K, Zaborski J, Czlonkowska A. Oligoclonal expansion of gamma delta T‐cells in cerebrospinal fluid of multiple sclerosis patients. Mult Scler 1996; 2:78–82. [DOI] [PubMed] [Google Scholar]
- 12. Schirmer L, Rothhammer V, Hemmer B, Korn T. Enriched CD161high CCR6+γδ T cells in the cerebrospinal fluid of patients with multiple sclerosis. JAMA Neurol 2013; 70:345–51. [DOI] [PubMed] [Google Scholar]
- 13. Nick S, Pileri P, Tongiani S, Uematsu Y, Kappos L, De Libero G. T‐cell receptor γδ repertoire is skewed in cerebrospinal fluid of multiple sclerosis patients: molecular and functional analyses of antigen‐reactive γδ clones. Eur J Immunol 1995; 25:355–63. [DOI] [PubMed] [Google Scholar]
- 14. Owens GC, Erickson KL, Malone CC et al Evidence for involvement of gamma delta T cells in the immune response to Rasmussen encephalitis. J Neuroinflammation 2015; 12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blink SE, Caldis MW, Goings GE et al γδ T cell subsets play opposing roles in regulating experimental autoimmune encephalitis. Cell Immunol 2014; 290:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajan AJ, Gao YL, Raine CS, Brosnan CF. A pathogenic role for gamma delta cells in relapsing–remitting experimental allergic encephalomyelitis in the SJL mouse. J Immunol 1996; 157:941–9. [PubMed] [Google Scholar]
- 17. Clark RB, Lingenheld EG. Adoptively transferred EAE in gamma delta T cell‐knockout mice. J Autoimmun 1998; 11:105–10. [DOI] [PubMed] [Google Scholar]
- 18. Lalor SJ, McLoughlin RM. Memory γδ T cells – newly appreciated protagonists in infection and immunity. Trends Immunol 2016; 37:690–702. [DOI] [PubMed] [Google Scholar]
- 19. Pranzatelli MR. The immunopharmacology of the opsoclonus–myoclonus syndrome. Clin Neuropharmacol 1996; 19:1–47. [DOI] [PubMed] [Google Scholar]
- 20. Kinsbourne M. Myoclonic encephalopathy of infants. J Neurol Neurosurg Psychiatry 1962; 25:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solomon GE, Chutorian AM. Opsoclonus and occult neuroblastoma. N Engl J Med 1968; 279:475–7. [DOI] [PubMed] [Google Scholar]
- 22. Pranzatelli MR, Tate ED. Trends and tenets in relapsing and progressive pediatric opsoclonus‐myoclonus. Brain Dev 2016; 38:439–48. [DOI] [PubMed] [Google Scholar]
- 23. Di Carlo E, Bocca P, Emionite L et al Mechanisms of the antitumor activity of human Vγ9Vδ2 T cells in combination with zoledronic acid in a preclinical model of neuroblastoma. Mol Ther 2013; 21:1034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schilbach KE, Geiselhart A, Wessels JT, Niethammer D, Handgretinger R. Human gammadelta T lymphocytes exert natural and IL‐2‐induced cytotoxicity to neuroblastoma cells. J Immunother 2000; 23:536–48. [DOI] [PubMed] [Google Scholar]
- 25. Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology 2014; 3:e27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pranzatelli MR, Travelstead AL, Tate ED et al B‐ and T‐cell markers in opsoclonus–myoclonus syndrome: immunophenotyping of CSF lymphocytes. Neurology 2004; 62:1526–32. [DOI] [PubMed] [Google Scholar]
- 27. Pranzatelli MR, Travelstead AL, Tate ED, Allison TJ, Verhulst SJ. CSF B‐cell expansion in opsoclonus–myoclonus syndrome: a biomarker of disease activity. Mov Disord 2004; 19:770–7. [DOI] [PubMed] [Google Scholar]
- 28. Pranzatelli MR, Travelstead AL, Tate ED et al Immunophenotype of blood lymphocytes in neuroblastoma‐associated opsoclonus–myoclonus. J Pediatr Hematol Oncol 2004; 26:718–23. [DOI] [PubMed] [Google Scholar]
- 29. Pressey JG, Adams J, Harkins L, Kelly D, You Z, Lamb LS Jr. In vivo expansion and activation of γδ T cells as immunotherapy for refractory neuroblastoma: a phase 1 study. Medicine (Baltimore) 2016; 95:e4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han S, Lin YC, Wu T et al Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol 2014; 192:2551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dieli F, Sireci G, Di Sano C. Predominance of Vgamma 9/V delta 2 T lymphocytes in the cerebrospinal fluid of children with tuberculosis meningitis: reversal after chemotherapy. Mol Med 1999; 5:301–12. [PMC free article] [PubMed] [Google Scholar]
- 32. Correale J, Mix E, Olsson T et al CD5+ B cells and CD4–8‐T cells in neuroimmunological diseases. J Neuroimmunol 1991; 32:123–32. [DOI] [PubMed] [Google Scholar]
- 33. Perrella O, Soscia M, Iaccarino C, Carrieri PB. Cerebrospinal fluid T‐cell receptor gamma/delta T lymphocyte subsets in patient with AIDS–dementia complex. J Biol Regul Homeost Agents 1992; 6:53–6. [PubMed] [Google Scholar]
- 34. Bertotto A, Spinozzi F, Gerli R et al γδ T lymphocytes in mumps meningitis patients. Acta Paediatr 1995; 84:1268–70. [DOI] [PubMed] [Google Scholar]
- 35. Droogan AG, Crockard AD, Hawkins SA, McNeill TA. Gamma delta T cell distribution in cerebrospinal fluid and peripheral blood of patients with multiple sclerosis. J Neurol Sci 1994; 126:172–7. [DOI] [PubMed] [Google Scholar]
- 36. Roden AC, Morice WG, Hanson CA. Immunophenotypic attributes of benign peripheral gammadelta T cells and conditions associated with their increase. Arch Pathol Lab Med 2008; 132:1774–80. [DOI] [PubMed] [Google Scholar]
- 37. Massa M, de Benedetti F, Robbioni P, Ramaenhi B, Albani S, Martini A. Association of methotrexate treatment with a decrease of double negative (CD4–CD8–) and gammadelta T cell levels in patients with juvenile rheumatoid arthritis. J Rheumatol 1993; 20:1944–8. [PubMed] [Google Scholar]
- 38. Dao V, Liu Y, Pandeswara S et al Immune stimulatory effects of rapamycin are mediated by stimulation of antitumor γδ T cells. Cancer Res 2016; 76:5970–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Median frequency of cerebrospinal fluid (CSF) γδ T cell subsets by opsoclonus–myoclonus syndrome (OMS) severity category.
Table S2. Effect of immunotherapy on cerebrospinal fluid (CSF) γδ T cell frequency in opsoclonus–myoclonus syndrome (OMS).


