Summary
Human chorionic gonadotrophin (hCG) and its β‐subunit (hCGβ) are tumour autocrine growth factors whose presence in the serum of cancer patients has been linked to poorer prognosis. Previous studies have shown that vaccines which target these molecules and/or the 37 amino acid C‐terminal hCGβ peptide (hCGβCTP) induce antibody responses in a majority of human recipients. Here we explored whether the immunogenicity of vaccines containing an hCGβ mutant (hCGβR68E, designed to eliminate cross‐reactivity with luteinizing hormone) or hCGβCTP could be enhanced by coupling the immunogen to different carriers [keyhole limpet haemocyanin (KLH) or heat shock protein 70 (Hsp70)] using different cross‐linkers [1‐ethyl‐3(3‐dimethylaminopropyl)carboiimide (EDC) or glutaraldehyde (GAD)] and formulated with different adjuvants (RIBI or Montanide ISA720). While there was little to choose between KLH and Hsp70 as carriers, their influence on the effectiveness of a vaccine containing the BAChCGβR68E mutant was less marked, presumably because, being a foreign species, this mutant protein itself might provide T helper epitopes. The mutant provided a significantly better vaccine than the hCGβCTP peptide irrespective of the carrier used, how it was cross‐linked to the carrier or which adjuvant was used when hCG was the target. Nonetheless, for use in humans where hCG is a tolerated self‐protein, the need for a carrier is of fundamental importance. Highest antibody titres were obtained by linking the BAChCGβR68E to Hsp70 as a carrier by GAD and using RIBI as the adjuvant, which also resulted in antibodies with significantly higher affinity than those elicited by hCGβCTP peptide vaccine. This makes this mutant vaccine a promising candidate for therapeutic studies in hCGβ‐positive cancer patients.
Keywords: adjuvant, B cell response, cancer vaccine, Hsp70, human chorionic gonadotrophin
Introduction
The pregnancy hormone human chorionic gonadotrophin (hCG) is a member of the glycoprotein hormone family. Like the other members of this family, luteinizing hormone (LH), follicle‐stimulating hormone (FSH) and thyroid‐stimulating hormone (TSH), hCG is a heterodimeric molecule consisting of a common α‐chain associated non‐covalently with a hormone‐specific β‐chain. Initially, hCG is expressed in the early embryo and is required for implantation into the uterus 1. Subsequently, synthesis shifts to the placental trophoblast where it stimulates the corpus luteum to produce progesterone and estrogen to ensure its maintenance for the duration of the pregnancy. The pioneering studies of Talwar and colleagues have shown that antibody‐mediated bioneutralization of hCG in women indeed prevents pregnancy 2, 3.
Highly sensitive assays have identified very low levels of hCG or hCGβ expression in normal tissues of both men and non‐pregnant women, but the function of these hormones in this context has still to be elucidated 4. hCG is also a biomarker for the detection of patients with placental and trophoblast‐derived cancers and patients with germ‐cell derived tumours. Importantly, the hormone‐specific β‐subunit hCGβ has been associated with a wide range of epithelial tumours ranging from bladder, lung, oral/facial, breast, cervical, ovarian, vaginal, prostate, renal and pancreatic carcinomas 5, 6, 7. Although the full biological role of hCGβ in these cancers is still being elucidated, model systems have shown that hCGβ is necessary for survival of the bladder cancer SCaBER 7 and the cervical cancer HeLa 8 cell lines. In these systems hCGβ may be functioning as an anti‐apoptotic growth factor 8, 9, 10. Furthermore, high titres of hCG‐specific antibody prevented the growth of an hCGβ‐expressing hepatoma H22 cell line xenografted into mice 11. This latter study also showed that the induced anti‐hCGβ antibodies reduced angiogenesis significantly in the H22 grafts. There is also evidence to implicate hCGβ in metastasis and invasion of cancer cells through down‐regulation of E‐cadherin 12, which normally prevents invasiveness of carcinoma cells 13. In 2010, a review of the 43 papers that listed hCGβ as a cancer biomarker identified 20 (47%) where the expression of hCGβ was associated with poor prognosis and accelerated death 6. It would seem logical, therefore, that bioneutralization of hCGβ in these cancers could improve the survival of cancer patients, thus identifying the subunit as an important target for anti‐cancer therapy.hCG is a structurally and immunologically well‐characterized molecule. The crystal structure has shown it to be a member of the cysteine‐knot superfamily of growth factors 14, 15. The use of competitive immunoassays 16, 17, 18, 19, 20, 21 and amino acid substitutions 22, 23 have identified 16 immunological regions on hCG, five epitopes of which have been mapped onto the α‐subunit, seven identified on the β‐subunit and four epitope clusters located on the interface between the α‐ and β‐chains. The hormone‐specific β‐subunit of hLH shares 85% of the amino acid sequence with the first 110 amino acid residues in hCGβ, which accounts for the dominant immune epitopes on hCG being shared with LH so that hCG‐induced antibodies may cross‐react with LH 23; it is also likely that many of the T cell epitopes will be shared with LH.
With the aim of neutralizing the role of hCG in pregnancy, Talwar and his group developed a heterospecies hCG anti‐fertility vaccine consisting of an ovine α‐ and human β‐subunit conjugated to tetanus or diphtheria toxoid. In a ground‐breaking human Phase II trial with this vaccine they found only one pregnancy in 1224 cycles in the immunized women who produced anti‐hCG antibodies levels above 50 ng/ml 3. The effect of the vaccine was reversible, because pregnancies were detected whenever the hCG antibody levels fell below the protective threshold 2, 3. Although, to our knowledge, the use of this vaccine has not been pursued further, it nevertheless demonstrates that it is possible to develop bioneutralizing hCG vaccines in humans and, indeed, in the last two decades hCGβ has been examined as a target for anti‐cancer vaccines. However, the heterospecies vaccine protected only 80% of the immunized women in this trial producing the bioneutralizing levels of anti‐hCG antibodies. The need for enhanced immunogenicity was recognized by Talwar, leading to his development of new formulations, including the use of Escherichia coli endotoxin and killed mycobacteria to boost the immune response 24. We have shown previously that immunization with a gonadotrophin‐releasing hormone (GnRH) analogue conjugated to mycobacterial Hsp70 as a carrier reduced the fertility of male mice 25.
Stevens promoted the use of the 37 amino acid C‐terminal segment of hCGβ (hCGβCTP), not present in LHβ, as a possible hCG‐specific vaccine candidate 26. Indeed, in a human Phase I trial involving 37 patients with recurrent or metastatic tumours, Triozzi et al. showed that synthetic hCGβCTP attached covalently to diphtheria toxoid induced hCG‐specific antibodies at levels between 0·1 and 2 μg immunoglobulin (Ig)G per ml (1–20 nM) in a dose‐dependent manner 27, 28. The effect of the vaccine on the tumours was not evaluated, although Triozzi et al. noted that two patients with colorectal cancers showed tumour regression 28. They did not, however, assess whether this was due to the induced hCG‐binding antibodies or because of their observation that the carrier and adjuvant induced strong T helper type 1 (Th1) and Th2 cytokine responses in all patients. However, we consider that the C‐terminal segment is not an ideal immunological target for two reasons. First, it contains four 0‐linked glycosylation sites, which are occupied in native hCG and its free hCGβ subunit, so that the carbohydrate chains could physically block or mask some of the potential B cell epitopes in the C‐terminal segment. Secondly, it is a highly flexible molecule with no fixed structure and is thus entropy‐rich, making it a poor immunogen favouring the production of antibodies with low affinity for the hCGβ target. In another approach, to overcome the poor immunogenicity of hCGβCTP, Xiangbing et al. constructed a fusion protein consisting of heat shock protein 65 (Hsp65) with 10 tandem repeats of hCGβ109–118 and a copy of hCGβ109–145 peptide. This vaccine was able to suppress the growth of mouse hepatoma H22 cells in mice 11, but it remains to be seen whether it will be able to induce bioneutralizing responses in outbred populations such as humans with their diverse human leucocyte antigen (HLA) haplotypes.
We have reported previously an alternative hCG vaccine candidate consisting of hCGβ with a single amino residue substitution (R68E) and which has minimal LH cross‐reactivity 23, 29, 30. We showed that the entropy‐rich C‐terminal segment becomes electrostatically fixed through the interaction between the Glu68 residue and the lysine and arginine residues in the C‐terminal segments. This directs the immune response towards hCGβ‐specific epitopes, including those in the C‐terminus of the β‐subunit, in both rabbits and mice using both conventional protein and DNA immunization 23, 29, 30, 31.
The present study evaluates whether our mutant is a more potent hCG‐specific vaccine candidate than hCGβCTP, while at the same time addressing the concerns regarding LH‐cross‐reactivity raised in the Talwar hCG trials 3. We have also sought to improve the immunogenicity of hCGβCTP and hCGβR68E by covalent coupling to either Hsp70 or keyhole limpet haemocyanin (KLH) and evaluated two oil‐in‐water adjuvant systems, RIBI and Montanide ISA72. We report here that our mutant hCGβR68E is superior to CTP as a vaccine candidate.
Material and methods
Reagents
Recombinant hCGβ produced in Chinese hamster ovary (CHO) cells was purchased from Sigma‐Aldrich (St Louis, Mo, USA); recombinant BAChCGβR68E was purified from baculovirus‐infected HiFive insect cells (see below). The C‐terminal peptide (hCGβCTP) representing the amino acid residues 108–145 of hCGβ was synthesized in vitro and kindly provided by Professor Vernon C. Stevens (Ohio State University, Columbus, OH, USA) or synthesized in house. Recombinant endotoxin‐free Hsp70 was a gift from Professor Theo Verrips (Utrecht University, Utrecht, the Netherlands) and KLH was purchased from Sigma‐Aldrich. The CTP‐specific monoclonal antibodies (mAbs) used in the study were OT3A2 (kindly provided by Dr E. Bos, NV Organon, Oss, the Netherlands) and 2F4/3 (Sigma‐Aldrich). The carrier‐specific antibodies used were rabbit anti‐KLH IgG (Sigma‐Aldrich) and rabbit anti‐Hsp70 anti‐serum kindly provided by Professor Theo Verrips.
Production and purification of baculovirus‐produced hCGβ‐R68E
The pBAC2hCGβR68E baculovirus expression plasmid for production of recombinant hCGβR68E with a C‐terminal His6‐tag 29 was introduced transiently into HiFive insect cells (Invitrogen, Carlsbad, CA, USA) and a single recombinant virus expressing BAChCGβR68E was isolated and expanded. For large‐scale production of the recombinant protein, the insect cells were grown in roller flasks in Express Five medium (Invitrogen) supplemented with 1% penicillin/streptomycin and 16 mM L‐glutamine to a density of 1·5 × 105 cells per ml at 28°C. The cells were infected with the recombinant baculovirus using a multiplicity of infection (MOI) of 10 and the supernatant harvested 72 h post‐infection, centrifuged and stored at immediately −70°C. Recombinant BAChCGβR68E was affinity purified in batches of 50–200 ml insect cell supernatants after dilution with an equal volume of 20 mM Na2HPO4 and 0·5 M NaCl pH 7.3 containing protease inhibitors (Sigma‐Aldrich). The BAChCGβR68E was then centrifuged at 4000 g, filtered through a 0·45‐μ filter and loaded onto HiTrap columns according to the manufacturer's instructions (GE Healthcare Life Sciences, Pittsburgh, PA, USA) using a high‐performance liquid chromatography (HPLC) system using a flow rate of 1 ml/min. After extensive washing with 20 mM Na2HPO4, 0·5 M NaCl pH 7.3, followed by 20 mM Na2HPO4, 0·5 M NaCl, 25 mM imidazole pH 7.3, the recombinant protein was eluted with 20 mM Na2HPO4, 0·5 M NaCl, 400 mM imidazole pH 7.3 and concentrated to 0·65–1·0 ml using Centricon YM‐10 columns (Millipore, Burlington, MA, USA) centrifuged at 4000 g. One μl of the initial supernatant and purified samples were separated on a 12·5% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) using the Phast System (GE Healthcare Life Sciences), silver‐stained and transferred to nitrocellulose membrane. Immunoblot analysis was carried out using hCGβ‐specific monoclonal antibodies and a 3,3'‐diaminobenzidine (DAB) enhanced liquid substrate system tetrahydrochloride for chromogenic detection (Sigma, St Louis, MO, USA).
Coupling of CTP and hCGβ‐R68E to carrier proteins
The recombinant proteins were conjugated to Hsp70 and KLH using either glutaraldehyde (GAD) (Sigma‐Aldrich) or 1‐ethyl‐3(3‐dimethylaminopropyl)carboiimide (EDC) (Pierce, Denbigh, UK) using a two‐step coupling procedure. For coupling with GAD, synthetic hCGβCTP (0·75 mg) or BAChCGβR68E (1 mg) was incubated with 0·075% GAD for 2 h at 4°C with gentle rotation followed by desalting using a PD10 column (Pharmacia, Uppsala, Sweden). For cross‐linking with EDC, the two‐step protocol recommended by the manufacturer was followed. In brief, hCGβCTP (0·75 mg) or hCGβ‐R68E (1 mg) was dialyzed into 0·1 M 2‐(N‐morpholino)ethanesulphonic acid (MES), 0·5 M NaCl, pH 6.0, incubated with 2 mg of EDC for 15 min at room temperature, de‐salted using a PD10 column and then added to an equal volume of Hsp70 or KLH in phosphate‐buffered saline (PBS) and incubated at room temperature for 2 h with gentle rotation.
The success of conjugation to Hsp70 was examined using analytical HPLC gel filtration, SDS gel electrophoresis and Western blotting using the PhastSystem and a highly sensitive sandwich enzyme‐linked immunosorbent assay (ELISA) using antibodies to the carriers, and a monoclonal CTP‐specific OT3A2 mAb which recognizes the amino acids 133–139. The molar coupling efficiency (number of antigen molecules per mole of carrier) was estimated by determining the amino acid composition of the final Hsp70–hCGβCTP and Hsp70–BAChCGβR68E conjugates and calculating the molar concentration of the antigens using selected amino acid residues. The KLH‐conjugate was too large for this analysis.
Immunization of mice
Six‐week old female BALB/c mice (Harlan Olac, Bicester, UK) were kept according to UK Home Office guidelines and the experimental procedures were covered by Home Office Animal Project guidelines. The animals used were primed with a 10‐μg aliquot of the Hsp70‐ or KLH‐conjugate containing hCGβCTP or BAChCGβR68E in RIBI (Sigma‐Aldrich) or Montanide ISA720 (Seppic, Paris, France) followed by a boost 21 days later. Two weeks after the boost, the animals were exsanguinated and the serum antibodies titred using direct‐binding ELISA. For this, Nunc MaxisorpC 96‐well flat‐bottomed microtitre plates were coated at 4°C overnight with 50 μl recombinant hCGβ (Sigma‐Aldrich), hCG or ovalbumin at 1 μg/ml or hCGβCTP peptide at 5 μg/ml in 50 mM carbonate‐bicarbonate buffer (CBB) pH 9.6. After washing the plates extensively with PBS, they were blocked with 2% w/v bovine serum albumin (BSA) in PBS for 30 min at room temperature followed by washes with PBS. The sera were serially diluted in PBS, 0·05% Tween 20 and 1% bovine serum albumin (BSA) and 50 μl was added to each well and incubated for 2 h at 37°C. The plates were washed extensively with PBS, incubated with horseradish peroxidase‐conjugated goat anti‐mouse IgG or subclass‐specific IgG (Sigma‐Aldrich) for 1 h at 37°C, washed and developed with 50 μl tetramethylbenzidine (TMB) and read at A630 using an ELISA plate reader. The avidity was determined using ELISA essentially as described above, using anti‐serum at a concentration of 80% of the plateau binding followed by incubation of the antibody–antigen complexes with increasing concentrations of (0·031–8 M) ammonium thiocyanate for 15 min at room temperature 32. The plates were subsequently washed and developed using horseradish peroxidase (HRP)‐conjugated goat anti‐mouse IgG, as described above; 50% inhibitory concentration of the ammonium thiocyanate was determined as the avidity index.
Statistical analysis
A 10‐point standard curve of anti‐serum dilution against signal (absorbance) was constructed for each anti‐serum produced from each mouse using a four‐parameter logistic curve fitting (http://elisaanalysis.com). The highest dilution that could be distinguished from the blank [mean absorbance + 2 standard deviations (s.d.) from ovalbumin‐immunized mice] was recorded as an index of immunogenic vaccine potency. The independent effects of different carriers, linkers and adjuvants on the titre were analysed using general linear model multivariate analysis of variance with a hierarchical design and Tukey's honest significant difference (HSD) post‐hoc analysis. Student's t‐test was used for the isotype and avidity analysis.
Results
Characterization of conjugates
Affinity‐purified BAChCGβR68E with a molecular weight of 25 kDa is smaller than the 45‐kDa CHO‐produced hCGβ (Fig. 1a) due to differences in the structural complexity of the carbohydrate chains, but not the diminished degree of glycosylation 33. As reported previously, baculovirus produced recombinant wild‐type and mutant hCGβ subunit folds correctly, as judged by their full recognition of a panel of conformation‐dependent monoclonal antibodies 31. Once purified, BAChCGβR68E and synthetic hCGβCTP were coupled chemically to Hsp70 and to KLH using GAD, which we had used previously to attach GnRH chemically to Hsp70 25, as well as the zero‐length cross‐linker, EDC. Western blot analysis of the Hsp70‐based conjugates shows covalent attachment of the immunogens to the carrier (Fig. 1b). We estimated the relative molar conjugation ratio of hCGβR68E : Hsp70 and hCGβCTP : Hsp70 as 4·7 : 1 and 31 : 1, respectively, by determining the increase in the molar content of tyrosine and valine, respectively, in a full amino acid quantification of conjugates relative to the native Hsp70 (Fig. 1c). It was not possible to gain a meaningful estimate of the coupling efficiency of BAChCGβR68E and hCGβCTP to KLH due to its very large molecular weight of 7·8 × 103 kDa.
Figure 1.
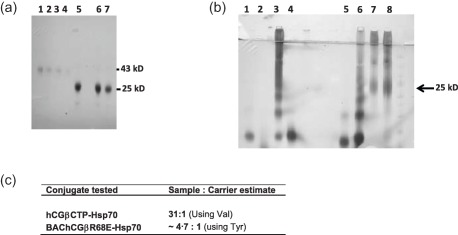
Purification of BAChCGβR68E and coupling of the immunogens to carrier proteins. (a) One μl of affinity‐purified recombinant BAChCGβR68E was separated by 12% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) followed by Western blotting and compared to Chinese hamster ovary (CHO)‐produced human chorionic gonadotrophin β subunit (hCGβ): lanes 1–4 show rhCGβ at 1·0, 0·5, 0·25 and 0·125 mg/ml and lanes 5–7 show three batch batches of purified BAChCGβR68E preparations. (b) Western blot analysis using the OT3A2 monoclonal antibody (mAb) showing the coupling of hCGβC‐terminal peptide (CTP) and BAChCGβR68E to heat shock protein 70 (Hsp70); lane 1, hCGβCTP; lane 2, Hsp70; lane 3, hCGβCTP‐Hsp70 conjugated with 1‐ethyl‐3(3‐dimethylaminopropyl)carbodiimide hydrochloride (EDC); lane 4, hCGβCTP mixed with Hsp70; lane 5, hCGβCTP; lane 6, hCGβCTP‐Hsp70 conjugated with glutaraldehyde (GAD); lane 7, BAChCGβR68E‐Hsp70 conjugated with EDC; and lane 8, BAChCGβR68E‐Hsp70 conjugated with GAD. (c) Evaluation of ratio of hCGβCTP : Hsp70 and BAChCGβR68E : Hsp70 calculated from total amino acid quantification of the conjugates and Hsp70.
Immunogenicity of hCBβR68E versus hCGβCTP
The immunogen–carrier complexes were used to immunize groups of female BALB/c mice with two different oil‐in‐water adjuvants, RIBI and Montanide ISA720, chosen because they have both been approved for human use (Table 1). The specificity of the elicited antibodies was characterized using end‐point titration ELISAs against the target antigens hCG, hCGβ and hCGβCTP and using ovalbumin as the negative control. The dilutions representing the highest dilution that could be distinguished from the mean absorbance plus 2 s.d. of ovalbumin were recorded (Fig. 2). There was no significant difference between the results obtained with intact hCG and the recombinant hCGβ when used as target antigens. We therefore combined the results obtained with these two antigens in our statistical analysis. Given that both BAChCGβR68E and hCGβCTP produced immune responses that are likely to be effectively devoid of LH cross‐reactivity, our first question was: which is the better immunogen? When targeting hCG/hCGβ, the BAChCGβR68E anti‐sera showed better binding to the antigens than the anti‐sera elicited with hCGβCTP (for BAChCGβR68E the mean titre was 1 : 26500; for hCGβCTP mean titre was 1 : 12600, P < 0·0001) (Table 2, Fig. 3). Even when titred against the synthetic hCGβCTP peptide as the target antigen, we found that the baculovirus‐derived recombinant protein elicited a more potent immune response than that observed with hCGβCTP conjugates as immunogens (BAChCGβR68E mean titre 1 in 210 800, hCGβCTP mean titre 1 in 54 500, P = 0·039; Fig. 4, Table 2).
Table 1.
Composition of the vaccines used to immunize the BALB/C mice
| Peptide/protein | Carrier protein | Cross‐linker | Adjuvant |
|---|---|---|---|
| hCGβCTP | Hsp70 | GAD | Ribi |
| hCGβCTP | Hsp70 | GAD | Montanide ISA720 |
| hCGβCTP | Hsp70 | EDC | Ribi |
| hCGβCTP | HSP70 | EDC | Montanide ISA720 |
| hCGβCTP | KLH | EDC | Ribi |
| hCGβCTP | KLH | EDC | Montanide ISA720 |
| BAChCGβR68E | Ribi | ||
| BAChCGβR68E | Montanide ISA720 | ||
| BAChCGβR68E | Hsp70 | GAD | Ribi |
| BAChCGβR68E | Hsp70 | GAD | Montanide ISA720 |
| BAChCGβR68E | Hsp70 | EDC | Ribi |
| BAChCGβR68E | Hsp70 | EDC | Montanide ISA720 |
| BAChCGβR68E | KLH | EDC | RIBI |
| BAChCGβR68E | KLH | EDC | Montanide ISA720 |
hCGβCTP = human chorionic gonadotrophin β subunit C‐terminal peptide; EDC = 1‐ethyl‐3(3‐dimethylaminopropyl)carboiimide; Hsp70 = heat shock protein 70; KLH = keyhole limpet haemocyanin; GAD = glutaraldehyde. We explored whether the immunogenicity of vaccines containing an hCGβ mutant (hCGbR68E) or hCGbCTP could be enhanced by coupling the immunogen to different carriers (KLH or Hsp70) using different cross‐linkers (EDC or GAD) and formulated with different adjuvants (RIBI or Montanide ISA720). The mutant provided a significantly better vaccine with significantly higher avidity than the hCGbCTP peptide. The highest antibody titres were obtained by linking it to Hsp70 carrier using GAD as cross‐linker and RIBI as adjuvant.
Figure 2.
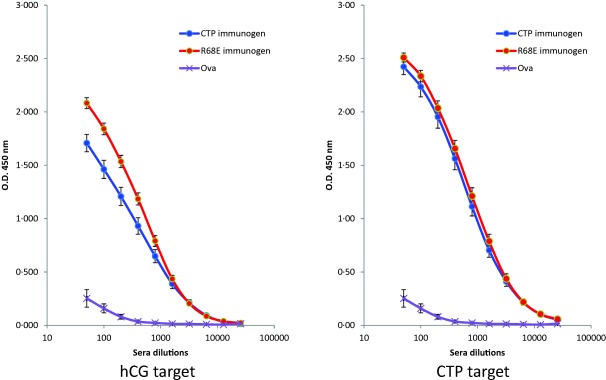
Titration of mouse immune sera. The sera from mice immunized with either human chorionic gonadotrophin β subunit C‐terminal peptide (hCGβCTP)‐ or BAChCGβR68E‐conjugate were end‐point titred using direct enzyme‐linked immunosorbent assays (ELISAs) on plates coated with human chorionic gonadotrophin (hCG) (left) and the synthetic hCGβCTP peptide (right). The graphs used data that include both linkers, both carriers and both adjuvants. They show the mean absorbance and ± 2 standard deviations (s.d.) indicated as bars through each data point. The non‐specific binding of the sera was determined using plates coated with ovalbumin (Ova).
Table 2.
Statistical analysis of the end‐point titres on enzyme‐linked immunosorbent assay (ELISA) on the antigens as indicated
| Immunogen mean (s.e.m.) | Linker mean (s.e.m.) | Adjuvant mean (s.e.m.) |
|---|---|---|
| CTP target | ||
|
hCGβCTP54500 (14200) n = 42 |
EDC 62 700 (20 900) n = 28 |
Montanide 35 300 (10 700) n = 14 |
|
RIBI 92 175 (41 200) n = 14 | ||
|
GAD 38 700 (10 200) n = 14 |
Montanide 35 500 (16700) n = 7 |
|
|
RIBI 41 800 (7800) n = 7 | ||
|
BAChCGβR68E 210800 (137300) n = 42 |
EDC 255 500 (205 700) n = 28 |
Montanide 22 900 (7700) n = 14 |
|
RIBI 482 000 (409 700) n = 14 | ||
|
GAD 121 300 (40 900) n = 14 |
Montanide 56 800 (10 900) n = 7 |
|
|
RIBI 185 800 (75700) n = 7 | ||
| hCG/hCGβ target | ||
|
hCGβCTP 12600 (3300) n = 80 |
EDC 14 800 (4900) n = 52 |
Montanide 17 500 (8500) n = 26 |
|
RIBI 12 100 (4900) n = 26 | ||
|
GAD 8500 (2000) n = 28 |
Montanide 2000 (400) n = 14 |
|
|
RIBI 15 000(3100) n = 14 | ||
|
BAChCGβR68E 26500 (3600) n = 84 |
EDC 24 900 (4700) n = 56 |
Montanide 25 300 (4600) n = 28 |
|
RIBI 24 400 (8300) n = 28 | ||
|
GAD 29 900 (2000) n = 28 |
Montanide 24 900 (6300) n = 14 |
|
|
RIBI 34 900 (9300) n = 14 | ||
Data represent mean [standard error of the mean (s.e.m.)] of raw data.
Figure 3.
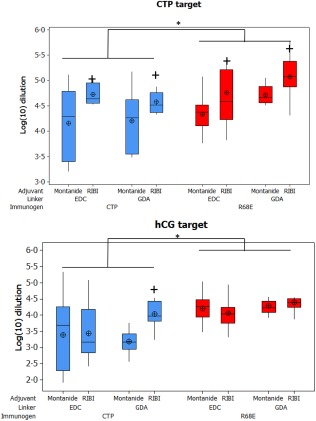
Statistical analysis of the end‐point titration of the sera from BALB/c mice immunized with human chorionic gonadotrophin β subunit C‐terminal peptide (hCGβCTP)‐ or BAChCGβR68E‐conjugates titred on hCGβCTP (upper diagram) or hCG/hCGβ (lower diagram). The log10 dilution of the end‐points for the relevant groups are shown using box‐and‐whisker diagrams where the median is indicated with a horizontal bar, the interquartile range (IQR) by a box; the whisker represents the range of data and the mean ± standard deviation (s.d.) of the log‐transformed data. Dilution end‐points were defined as the highest dilution that could be distinguished from the blank (mean absorbance ± s.d. from ovalbumin‐immunized mice). *Significance between BAChCGβR68E immunogen compared to the hCGβCTP immunogen (P < 0·05); + indicates the significant difference in titres between the adjuvant RIBI and Montenide ISA720 (P < 0·05). *Significant differences compared to hCGβCTP immunogen, with all other conditions the same.
Figure 4.
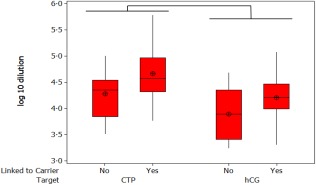
Statistical analysis of the antibody response to BAChCGβR68E immunogen generated as a free subunit or used when conjugated to heat shock protein 70 (Hsp70) or keyhole limpet haemocyanin (KLH) all combined with the adjuvant.
Enhancing the immunogenicity
In the reported Phase II trial with the hetero‐CG vaccine, a substantial fraction (∼20%) of the immunized women failed to develop protective immunity 24. One probable explanation could be that the vaccine formulation used was suboptimal for this group of recipients for genetic and/or immunological reasons. It is therefore possible that the number of poor responders could be reduced by using a vaccine with greater immunological potency. We therefore decided to explore the effect of different immunological carriers, chemical linkers and adjuvant systems on immunogenicity. No statistical differences were observed between Hsp70 and KLH as carriers, irrespective of the immunogen (BAChCGβR68E versus hCGβCTP) or linker (GAD versus EDC) (Fig. 3).
When considering the adjuvant system (RIBI versus Montanide ISA720), the BAChCGβR68E immunogen elicited no statistical difference in the antibody titres irrespective of the adjuvant, linkers or antigen targets used, the one exception of RIBI being the superior adjuvant with the hCGβCTP target (Fig. 3). However, the CTP vaccine revealed differences. When titred on its biological target hCG/hCGβ, the CTP immunogen formulated with RIBI produced significantly higher titre antibodies than those obtained using the Montanide ISA720 formulation, but only when the synthetic peptide had been cross‐linked to its carrier with GAD (mean titre RIBI 1 : 15 000, Montanide ISA720 1 : 2000, t‐test P < 0·05). When titred against the synthetic hCGβCTP peptide itself, the anti‐sera generated with RIBI elicited significantly higher antibody responses than immunogen adjuvanted with Montanide ISA720, irrespective of the linker (P < 0·001) (Fig. 3).
Combining all the antibody responses to the mutant recombinant BAChCGβR68E revealed no differences in the overall potency of the two adjuvants with respect to affinity (Fig. 5a). However, as shown in Fig. 5b,c the BAChCGβR68E elicit antibodies binding to hCGβCTP with lower avidity than to hCG, but with the same avidity as hCGβCTP‐induced antibodies independent of the carrier. For the analysis we used an avidity index defined as the concentration of ammonium thiocyanate required to dissociate 50% of the antigen–antibody complexes, as indicated in Fig. 5b. Using this index it can be seen that there were no statistical differences in the affinity of the specific antibodies produced by the hCGβCTP immunogens and independent of the carrier when binding to hCGβCTP. In contrast, hCGβCTP‐specific antibodies from hCGβCTP–Hsp70‐immunized mice bound to the synthetic peptide with significantly lower affinities (P = 0·007) (Fig. 5c). We have shown previously that the amino acid substitution in BAChCGβR68E fixed the C‐terminal part of hCGβ through electrostatic interaction, thus masking the immunodominant LH‐cross‐reactive epitope on hCGβ but enhancing an hCGβCTP‐specific epitope 29, 30, 31. It is therefore not surprising that the avidity of the hCGβCTP‐specific antibodies were comparable to that induced by hCGβCTP immunogens and higher than hCGβCTP antibodies induced by hCGβ immunogen. In addition, the antibodies induced with at the BAChCGβR68E immunogen had a significantly greater affinity overall than antibodies induced by hCGβ. Surprisingly, IgG2a and 2b titres were significantly lower with Montanide ISA720 than with the RIBI formulations (Fig. 6).
Figure 5.
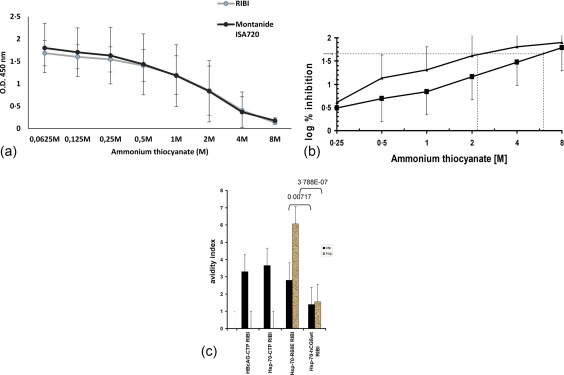
(a) Avidity of sera from the mice immunized with a BAChCGβR68E immunogen using the adjuvant RIBI and Montenide ISA720 adjuvant formulations produce antibodies with an identical ammonium thiocyanate dissociation when titred on human chorionic gonadotrophin β subunit (hCGβ) (*P < 0·0021 and **P < 0·0019, Student's t‐test). (b) However, the dissociation of the antigen–antibody complexes in sera from mice immunized with BAChCGβR68E‐heat shock protein 70 (HSP70) immunogen using the adjuvant RIBI titred on hCG (squares) and C‐terminal peptide (CTP) (triangles) was different. We define an avidity index as the concentration of ammonium thiocyanate that results in dissociation of 50% of the antibody‐antigen complex (indicated by the stippled lines). (c) The relative avidity indexes represented by 50% inhibitory concentrations of ammonium thiocyanate for antibodies rose to constructs for CTP (dark) and hCGβ (light). (Student's t‐test was used to determine the statistical significance as indicated).
Figure 6.
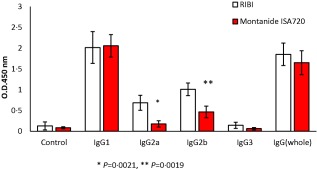
IgG subclass responses in mice immunized with the BAChCGβR68E immunogen using the RIBI and Montenide ISA720 adjuvant formulations and titred on human chorionic gonadotrophin β subunit (hCGβ).
Our results revealed a clear difference between the immunogens. Collectively, the BAChCGβR68E vaccine formulation gave significantly greater responses against both targets than did the hCGβCTP‐based vaccines (Fig. 3). Using hCG as target, the hCGβCTP linked to the carrier by GAD with the RIBI adjuvant gave the best antibody response of the hCGβCTP immunogen group, but this was significantly lower than the corresponding result with the BAChCGβR68E mutant (P < 0·014).
Discussion
Human CG has been associated traditionally with pregnancy, but recent decades have revealed that hCG and hCGβ are also biomarkers for trophoblastic and epithelial cancers, and the presence of hCGβ is predictive for poor survival of patients (recently reviewed in 6), possibly because it prevents apoptosis or functions as a cancer growth factor. Phases I and II trials of an anti‐fertility vaccine, based on a heterodimeric CG molecule, by Talwar and his group showed that it is possible to break immunological tolerance to hCGβ and thereby elicit sufficient levels of antibodies to prevent pregnancy in immunized women 3. hCGβ has therefore been considered subsequently as a potential immunotherapeutic anti‐cancer vaccine candidate 10, 11.
Morse et al. 34, 35 and Celldex Therapeutics Inc. (Hampton, NJ, USA) have recently explored an hCGβ‐targeting bladder carcinoma vaccine with a formulation that induced T and B cell‐mediated immune responses. It consisted of a fusion protein where the human monoclonal anti‐mannose receptor antibody B11 was extended with hCGβ at the C‐terminus (CDX‐1307). In a Phase II trial, CDX‐1307 was given with granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and Toll‐like receptors 3 and 7/8 agonists known to enhance the adaptive immune response, as well as cisplatin and gemcitabine for broader cancer cell targeting. The Phase II trial was discontinued after 14 months due to difficulties in recruiting a sufficient number of patients (R. K. Iles, personal communication). We have argued here that an hCGβ–based vaccine will produce predominantly LH cross‐reactive antibodies due to the immune dominance of the shared epitopes. Furthermore, we presume that the 85% sequence homology between the hormone‐specific subunit of LH and the first 110 amino acids of hCGβ indicates that the two hormones also share most of the major histocompatibility complex (MHC) class I epitopes. Although it is possible that such LH cross‐reactivity in both arms of the adaptive immune system may not be of immediate concern for cancer patients, we argue for hCGβ‐specific vaccines that predominantly target the antibody‐mediated arm of the immune system to avoid undesirable long‐term complications. Most efforts have been focused on the unique C‐terminal peptide of hCGβ. AVI BioPharma/Sarepta, Inc. (Cambridge, MA, USA) has taken a vaccine consisting of hCGβCTP37 coupled to diphtheria toxoid (CTP37‐DT) through Phase I with patients with a number of different epithelial cancers followed by a Phase II trial in 77 patients of metastasizing colorectal carcinomas. However, the vaccine‐induced hCGβCTP antibodies were not able to neutralize the tumour‐derived hCGβ due either to the high entropy of C‐terminal segment or because the hCGβCTP antibodies were of low affinity. It is therefore not clear whether the effect in the high responders was related to induction of hCG‐specific antibodies or to general stimulation of the immune system by the DT carrier, which elicited a systemic cytokine response 27. It is possible, furthermore, that better protection could be achieved in patients with hCGβ‐producing cancers.
We show here that our hCGβR68E mutant may be a more suitable immunogen than either hCGβ or hCGβCTP. The Glu68 mutation fixes the CTP via salt bridges to its positive amino acids, thereby not only blocking the immunodominant LH cross‐reactive epitopes but also creating a novel dominant CTP B cell epitope located possibly at the novel loop and including the amino acid residues 105–120 28, 29, 31. BAChCGβR68E conjugated to either Hsp70 or KLH produced significantly higher levels of immunoreactive hCG antibodies than hCGβCTP‐Hsp70 or hCGβCTP‐KLH, irrespective of whether they were titred against hCG, hCGβ or CTP. However, the difference in the antibody levels was not as pronounced when titred against CTP. There may be several reasons for this. The molar level of CTP per Hsp70 molecule was 6·6 times higher than for BAChCGβR68E per Hsp70. In addition, the CTP, was a synthetic peptide with at least four known B cell epitopes, some of which may be masked by the four 0‐linked carbohydrate residues present in the C‐terminal part of BAChCGβR68E. One would therefore expect that not all the antibody specificities elicited with hCGβCTP formulation would recognize hCG/hCGβ.
As with the anti‐fertility trial by Talwar and colleagues 3, the CTP37‐DT vaccine identified a significant group of non‐responders 27. The molecular basis for the inability of 20% of the individuals participating in two trials who failed to respond to the vaccines remains to be elucidated. It is possible that there are genetic reasons for this, as the two trials included diverse ethnic patients. However, all patients included in the two trials responded normally to the carrier, demonstrating a functional immune response. Because Moulton et al. reported that detectable levels of anti‐hCG antibodies were only seen after the second boosting 27, it is possible that enhancing the immunogenicity of the immunogen or vaccine formulation or repeated boosting may reduce the number of non‐responders. We explored whether we could enhance the immunogenicity of BAChCGβR68E or hCGβCTP by coupling the vaccine candidate to different carriers, using different cross‐linkers or formulating them with different adjuvants. While these different constructs induced a modest but statistically significant increase in the immunogenicity of hCGβCTP, these improvements were less pronounced with BAChCGβR68E. Nonetheless, even by enhancing the immunogenicity, the hCGβCTP vaccine formulation was not as potent as our mutant molecule. Differences in ability of the anti‐sera to neutralize circulating hCG may be even greater if, as we expect, the high entropy unconstrained CTP immunogen produces a low‐affinity response. While, as mentioned, the effect of conjugation with carrier was relatively modest, perhaps because hCG is a foreign molecule for mice, the involvement of carrier protein would be essential for human use as hCG is a tolerated self‐protein. Although the two adjuvants did not induce antibodies with overall differences in avidity, as revealed by ammonium thiocyanate dissociation, the superiority of RIBI with respect to the IgG subclass response and induction of the highest antibody titres emphasize the need for careful attention that needs to be paid to the choice of adjuvant for a vaccine intended for human use. The avidity analysis revealed that when tested on hCGβ CTP peptide‐coated plates the antibodies elicited by hCGβCTP and BAChCGβR68E immunogens had the same avidity, which was significant (P < 0·007 using Student's t‐test) rather than hCGβCTP‐specific antibodies induced by hCGβ conjugated to the same carrier. However, the affinities of the antibodies produced in hCGβCTP‐immunized mice were significantly lower when assayed on plates coated with hCG. This probably more reflects an assay artefact, because coating of the CTP peptide will anchor it in a fixed low‐entropy conformation. When hCG is coated to the plastic of the 96‐well plates the CTP will not all be immobilized, the plastic thus having no fixed conformation, and be very entropy‐rich, which will reduce the availability of the right binding conformation for the induced antibodies. In addition, perhaps the molar concentration of hCGβCTP peptide is higher in the peptide‐coated plates. What the avidity data demonstrated clearly is that the avidity of hCG‐specific antibodies produced by our mutant immunogen were significantly higher than the antigen‐specific antibodies produced by either hCGβCTP or hCGβ immunogens. This makes BAChCG βR68E a much better vaccine candidate.
In conclusion, we have compared two hCGβ‐specific vaccine candidates, hCGβCTP and BAChCGβR68E, delivered using different formulations and report here that the hCGβ mutant BAChCGβR68E is a significantly more potent (or effective) vaccine than hCGβCTP irrespective of the carrier used, how it was cross‐linked to the carrier or which adjuvant system used. The highest antibody titres were obtained by linking the ΒAChCGβR68E to Hsp70 as a carrier by GAD and using RIBI as the adjuvant, and although we do not know whether it will be a superior vaccine that can reduce the fraction of non‐responders identified in the Phase II trials of hCG vaccines so far, the increased immunogenicity relative to hCGβCTP appears promising.
Author contributions
P. J. D., T. L. and I. M. R. conceived the study. N. K., N. C., J. M., J. D. M., N. P., P. M. M. and J. J. carried out the experiments. F. H. and N. P. performed the statistical analysis. T. L., F. H. and I. M. R. wrote the manuscript. All authors have seen and approved the final version of the manuscript.
Disclosure
None; although Professor Roitt received generous support from Igeneon, GmBH Austria, the intervening bankruptcy of the company eliminated any possibility of a conflict of interest.
Acknowledgements
We thank Professor Theo Verrips, Department of Biomolecular Imaging, Utrecht University, the Netherlands for the gift of Hsp70 and anti‐Hsp70 anti‐serum, Professor Dr Vernon C Stephens, Ohio State University, Columbus, OH for the synthetic hCGβCTP and Dr E Bos, NV Organon, the Netherlands for the OT3A2 monoclonal antibody. We are grateful for the technical assistance of Ms Inger Bjorndahl in preparing the recombinant baculovirus supernatants and for the infections of the insect cells.
References
- 1. Fishel SB, Edwards RG, Evans CJ. Human chorionic gonadotropin secreted by preimplantation embryos cultured in vitro . Science 1984; 223:816–8. [DOI] [PubMed] [Google Scholar]
- 2. Talwar GP, Singh OM, Gupta SK et al The HSD‐hCG vaccine prevents pregnancy in women: feasibility study of a reversible safe contraceptive vaccine. Am J Reprod Immunol 1997; 37:153–60. [DOI] [PubMed] [Google Scholar]
- 3. Talwar GP, Singh O, Pal R et al A vaccine that prevents pregnancy in women. Proc Natl Acad Sci USA 1994; 91:8532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clin Biochem 2004; 37:549–61. [DOI] [PubMed] [Google Scholar]
- 5. Iles RK. Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol 2007; 260–262:264–70. [DOI] [PubMed] [Google Scholar]
- 6. Iles RK, Delves PJ, Butler SA. Does hCG or hCGbeta play a role in cancer cell biology? Mol Cell Endocrinol 2010; 329:62–70. [DOI] [PubMed] [Google Scholar]
- 7. Burczynska B, Booth MJ, Iles RK, Shah A, Shiled A, Butler SA. Stable knockdown of hCGbeta mRNA expression in bladder cancer cells results in significant growth inhibition. Anticancer Res 2013; 33:3611–4. [PubMed] [Google Scholar]
- 8. Jankowska A, Gunderson SI, Andrusiewicz M et al Reduction of human chorionic gonadotropin beta subunit expression by modified U1 snRNA caused apoptosis in cervical cancer cells. Mol Cancer 2008; 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti‐apoptosis effect and not cell proliferation. Br J Cancer 2000; 82:1553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butler SA, Iles RK. Ectopic human chorionic gonadotropin beta secretion by epithelial tumors and human chorionic gonadotropin beta‐induced apoptosis in Kaposi's sarcoma: is there a connection? Clin Cancer Res 2003; 9:4666–73. [PubMed] [Google Scholar]
- 11. Xiangbing H, Yankai Z, Ming L et al The fusion protein of HSP65 with tandem repeats of beta‐hCG acting as a potent tumor vaccine in suppressing hepatocarcinoma. Int Immunopharmacol 2010; 10:230–8. [DOI] [PubMed] [Google Scholar]
- 12. Wu W, Walker AM. Human chorionic gonadotropin beta (HCGbeta) down‐regulates E‐cadherin and promotes human prostate carcinoma cell migration and invasion. Cancer 2006; 106:68–78. [DOI] [PubMed] [Google Scholar]
- 13. Frixen UH, Behrens J, Sachs M et al E‐cadherin‐mediated cell‐cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991; 113:173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun PD, Davies DR. The cystine‐knot growth‐factor superfamily. Annu Rev Biophys Biomol Struct 1995; 24:269–91. [DOI] [PubMed] [Google Scholar]
- 15. Lapthorn AJ, Harris DC, Littlejohn A et al Crystal structure of human chorionic gonadotropin. Nature 1994; 369:455–61. [DOI] [PubMed] [Google Scholar]
- 16. Berger P, Bidart JM, Delves PS et al Immunochemical mapping of gonadotropins. Mol Cell Endocrinol 1996; 125:33–43. [DOI] [PubMed] [Google Scholar]
- 17. Berger P, Klieber R, Panmoung W, Madersbacher S, Wolf H, Wick G. Monoclonal antibodies against the free subunits of human chorionic gonadotrophin. J Endocrinol 1990; 125:301–9. [DOI] [PubMed] [Google Scholar]
- 18. Berger P, Kofler R, Wick G. Monoclonal antibodies against human chorionic gonadotropin (hCG): II. Affinity and ability to neutralize the biological activity of hCG. Am J Reprod Immunol 1984; 5:157–60. [DOI] [PubMed] [Google Scholar]
- 19. Bidart JM, Ozturk M, Bellet DH et al Identification of epitopes associated with hCG and the beta hCG carboxyl terminus by monoclonal antibodies produced against a synthetic peptide. J Immunol 1985; 134:457–64. [PubMed] [Google Scholar]
- 20. Bidart JM, Troalen F, Bohuon CJ, Hennen G, Bellet DH. Immunochemical mapping of a specific domain on human choriogonadotropin using anti‐protein and anti‐peptide monoclonal antibodies. J Biol Chem 1987; 262:15483–9. [PubMed] [Google Scholar]
- 21. Kofler R, Berger P, Wick G. Monoclonal antibodies against human chorionic gonadotropin (hCG): I. production, specificity, and intramolecular binding sites. Am J Reprod Immunol 1982; 2:212–6. [DOI] [PubMed] [Google Scholar]
- 22. Charrel‐Dennis M, Jackson AM, Lund T et al The major hormone‐specific discontinuous epitopes on human chorionic gonadotrophin. J Mol Endocrinol 2004; 32:571–81. [DOI] [PubMed] [Google Scholar]
- 23. Jackson AM, Klonisch T, Lapthorn AJ et al Identification and selective destruction of shared epitopes in human chorionic gonadotropin beta subunit. J Reprod Immunol 1996; 31:21–36. [DOI] [PubMed] [Google Scholar]
- 24. Talwar GP, Gupta JC, Shankar NV. Immunological approaches against human chorionic gonadotropin for control of fertility and therapy of advanced‐stage cancers expressing hCG/subunits. Am J Reprod Immunol 2011; 66:26–39. [DOI] [PubMed] [Google Scholar]
- 25. Hannesdottir SG, Han X, Lund T et al Changes in the reproductive system of male mice immunized with a GnRH‐analogue conjugated to mycobacterial hsp70. Reproduction 2004; 128:365–71. [DOI] [PubMed] [Google Scholar]
- 26. Stevens VC. Antifertility vaccine In: Perlmann P, Wigzell H, eds. Handbook of experimental pharmacology. Berlin, Germany: Spriger‐Verlag, 1999:443–61. [Google Scholar]
- 27. Moulton HM, Yoshihara PH, Mason DH, Iversen PL, Triozzi PL. Active specific immunotherapy with a beta‐human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res 2002; 8:2044–51. [PubMed] [Google Scholar]
- 28. Triozzi PL, Stevens VC, Aldrich W, Powell J, Todd CW, Newman MJ. Effects of a beta‐human chorionic gonadotropin subunit immunogen administered in aqueous solution with a novel nonionic block copolymer adjuvant in patients with advanced cancer. Clin Cancer Res 1997; 3:2355–62. [PubMed] [Google Scholar]
- 29. Charrel‐Dennis M, Terrazzini N, McBride JD et al The human chorionic gonadotropin‐beta arginine68 to glutamic acid substitution fixes the conformation of the C‐terminal peptide. Mol Endocrinol 2005; 19:1803–11. [DOI] [PubMed] [Google Scholar]
- 30. Porakishvili N, Chiesa MD, Chikadze N et al Elimination of luteinizing hormone cross‐reactive epitopes from human chorionic gonadotropin. Vaccine 2002; 20:2053–9. [DOI] [PubMed] [Google Scholar]
- 31. Chiesa MD, Martensen PM, Simmons C et al Refocusing of B‐cell responses following a single amino acid substitution in an antigen. Immunology 2001; 103:172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferreira MU, Katzin AM. The assessment of antibody affinity distribution by thiocyanate elution: a simple dose–response approach. J Immunol Methods 1995; 187:297–305. [DOI] [PubMed] [Google Scholar]
- 33. Chen W, Shen Q‐X, Bahl OP. Carbohydrate variants of the recombinant β‐subunit of the human choriogonadotropin expressed in baculovirus expression systems. J Biol Chem 1991; 266:4081–7. [PubMed] [Google Scholar]
- 34. Morse MA, Bradley DA, Keler T et al CDX‐1307: a novel vaccine under study as treatment for muscle‐invasive bladder cancer. Expert Rev Vaccines 2011; 10:733–42. [DOI] [PubMed] [Google Scholar]
- 35. Morse MA, Chapman R, Powderly J et al Phase I study utilizing a novel antigen‐presenting cell‐targeted vaccine with Toll‐like receptor stimulation to induce immunity to self‐antigens in cancer patients. Clin Cancer Res 2011; 17:4844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


