Abstract
Background
High maternal weight is known to associate with both low free thyroxine and gestational diabetes mellitus. We explore a deiodinase-related mechanism that may help explain these associations.
Methods
Among 108 women receiving routine oral glucose tolerance testing for gestational diabetes mellitus, we collected biophysical data and measured free thyroxine and total triiodothyronine, using residual plasma samples.
Results
Fasting triiodothyronine/free thyroxine ratio and triiodothyronine were higher among women with gestational diabetes mellitus (p = 0.02; p = 0.04). The triiodothyronine/free thyroxine ratio and triiodothyronine measurements at 2 h were associated with weight (r = 0.20, p = 0.04; r = 0.22, p = 0.02); free thyroxine showed a non-significant inverse weight relationship (r = −0.06, p = 0.55). Glucose at all four intervals was associated with triiodothyronine/free thyroxine ratios, and triiodothyronine at 2 h. In stepwise regression, triiodothyronine/free thyroxine ratio predicted glucose more strongly than did weight.
Conclusion
These relationships may be explained by higher maternal weight inducing peripheral deiodinase activity, resulting in higher plasma glucose (via triiodothyronine stimulation) and thereby increasing gestational diabetes mellitus risk.
Keywords: Endocrinology, metabolism, obesity, diabetes
Introduction
Between 2013 and 2016, we reported negative associations between free thyroxine (fT4) and both maternal weight and gestational diabetes mellitus (GDM) among euthyroid women and speculated that conversion of fT4 to triiodothyronine (T3) via peripheral deiodinase activity might explain the lower fT4 levels.1–4 Recently, Yang et al. confirmed the inverse relationship between fT4 and GDM and identified a GDM prevalence of 17.25% in the lowest fT4 quintile, decreasing to 11.62% in the highest fT4 quintile.5 Given its low hormonal activity, fT4 is unlikely to influence plasma glucose directly. T3, the active metabolic form of thyroid hormone, is a more likely candidate, as it induces endogenous glycemic activity.6 The present study focuses on a cohort of pregnancies undergoing oral glucose tolerance testing (OGTT) at 24–28 weeks’ gestation, aimed at further exploring the above-described thyroid hormone/glucose relationships. Our data confirm earlier speculation that the T3/fT4 ratio (a measure of deiodinase activity) is directly related to both maternal weight and glucose. We hypothesize that weight-driven deiodinase activity may contribute to glycemic activity via T3 stimulation.
Materials and methods
Study population
At Women and Infants Hospital (WIH), routine screening for gestational diabetes is initiated by measuring glucose in a non-fasting (random) plasma sample between 24 and 28 weeks’ gestation. Women with glucose levels ≥7.17 mmol/L are recommended for OGTT. To qualify for inclusion in the present study, each woman’s serum screening records for Down syndrome needed to be available within the Division of Medical Screening and Special Testing, as this was to be the source for demographic data obtained at 11–14 weeks’ gestation (e.g. maternal ages, weights, gestational ages, singleton vs. multiple gestations, and other demographic data). Exclusion criteria included multiple pregnancies, pre-existing insulin dependent diabetes, or a family history of aneuploidy. Pregnancy completion dates were verified from existing hospital medical records.
Among 517 women meeting inclusion/exclusion criteria between June and August 2013, 148 women with glucose concentrations at 7.17 mmol/L or higher were identified as candidates for diagnostic testing via OGTT (29%); 117 of these appeared for further testing at WIH (79%). The OGTT protocol calls for measuring glucose in a fasting plasma sample, followed by a 100 g oral glucose challenge and then measuring glucose in samples obtained at 1, 2, and 3 h post-challenge. Plasma glucose levels are considered within the normal range at <5.28, <10, <8.61 and <7.78 mmol/L, respectively. Sufficient residual plasma was available for measuring T3, fT4, thyroid stimulating hormone (TSH), and insulin at all four intervals in 108 of the 117 women (92%). Residual plasma samples were promptly retrieved and stored at −20℃ until all pregnancies had been delivered.
The Institutional Review Board (IRB) for Human Studies at WIH waived the requirement for informed consent due to the study’s protocol being limited to use of residual plasma samples, in combination with existing demographic and related data previously recorded in conjunction with interpretations of prenatal screening measurements. The IRB required that all testing of residual samples for thyroid hormones be delayed until all pregnancies had been completed and that all data be de-identified.
Assay measurements
Glucose was measured using the hexokinase method on the Architect automated Aeroset immunoassay (Abbott Laboratory, Chicago, IL). Levels of fT4, total T3, TSH, and insulin were measured on the automated Dxl instrument (Beckman Coulter, Brea, CA) Total T3 was recommended as more reliable than free T3 for the current study (personal communication: Dr Francesco Celi), due to fT3 being an analog assay which provides an estimate of the fT3. As an additional limitation, fT3 is in lower concentration (2 orders of magnitude) than fT4, hence more prone to imprecision. Commercial materials were used for quality control of each immunoassay (BioRad, Hercules, CA). Coefficients of variation (CV) at all control levels were less than 6.5% for total T3, fT4, and TSH and less than 3.7% for insulin. Testing was completed in 2015.
Statistical analyses
Analyses included descriptive statistics, analysis of variance (ANOVA), Pearson correlation coefficients, t-tests for continuous variables, and chi square tests for categorical variables. The T3/fT4 ratio and weight underwent logarithmic transformation prior to analysis. The T3 and fT4 measurements did not change over the four time periods, differing only on the basis of assay variability. We arbitrarily selected the T3 and fT4 measurements at 2 h for comparison with maternal weight, and also for comparison with glucose over all four time periods. Linear stepwise regression was used to establish the relationships between thyroid hormones and glucose, and also between thyroid hormones and maternal weight. Statistical analyses were performed using SAS 9.4 (Cary, NC).
Results
Demographic and biochemical features among 108 women with and without GDM
Table 1 shows that the median maternal age of women having OGTT was 31 years; 91% were Caucasian, and average weight was 68.9 kg. These demographics did not differ significantly between the 79 women without GDM and 29 with GDM. Neither clinically significant hypothyroidism nor thyrotoxicosis was present in this cohort. Median fasting glucose was significantly higher among women with GDM. The T3/fT4 ratio and T3 were significantly higher among women with GDM. Thyroid hormone comparisons (no GDM vs. GDM) were consistent when measured at all four time intervals (data not shown).
Table 1.
Oral glucose tolerance testing (OGTT) following random glucose screening for gestational diabetes (GDM): demographic characteristics and thyroid relationships.
| OGTT recommended |
||||
|---|---|---|---|---|
| Characteristic | Yesb | GDM—no | GDM—yes | p a |
| Number of women | 108 | 79 | 29 | |
| Age (years (SD)) | 31 (5.5) | 30 (5.5) | 33 (5.0) | 0.07 |
| Race (% Caucasian) | 91 | 91 | 90 | 0.74 |
| Weight (kg) (SD)) | 68.9 (19) | 68.2 (19) | 71.8 (20) | 0.15 |
| Glucose (mmol/L)c | 4.40 (0.55) | 4.29 (0.43) | 4.84 (0.63) | <0.001 |
| Insulin (pmol/L)c | 25.0 (0.33) | 23.6 (0.32) | 34.7 (0.35) | 0.10 |
| T3 (nmol/L)c | 2.19 (0.37) | 2.15 (0.35) | 2.30 (0.41) | 0.04 |
| fT4 (pmol/L)c | 7.98 (1.34) | 8.11 (1.38) | 7.72 (1.16) | 0.11 |
| T3/fT4 ratioc | 0.28 (0.11) | 0.27 (0.11) | 0.29 (0.11) | 0.02 |
| TSH (mIU/L)c | 1.40 (0.29) | 1.40 (0.25) | 1.84 (0.39) | 0.76 |
fT4: free thyroxine; T3: triiodothyronine; TSH: thyroid stimulating hormone.
Comparisons are for GDM no vs. GDM yes.
Not shown—31 women who did not appear for oral glucose tolerance testing (OGTT).
Median (SD); measurements are fasting; insulin, T3/fT4 ratio, and TSH are log SD.
Thyroid hormone measurements in the 108 women before and after a 100 g glucose load
Table 2 shows that median TSH levels become significantly lower at 1, 2, and 3 h in comparison to 0 h, following oral administration of 100 g glucose. In contrast, median T3, fT4, and T3/fT4 ratios did not differ significantly at any of the four time periods. CV for T3 and fT4 were <10% for 95% and 93% of the women, respectively, indicating both satisfactory assay performance and absence of an effect after a glucose load. In subsequent analyses (shown below), only results from the 2 h samples are used for these hormone measurements. Plasma glucose and insulin levels increase significantly after the glucose load, showing the expected rapid rise and slow descent.
Table 2.
Thyroid levels in 108 women undergoing glucose tolerance testing (OGTT) with associated glucose and insulin measurements.
| Time (h) since administration of 100 g of glucose |
|||||
|---|---|---|---|---|---|
| Measurement | Fasting (0) | 1 | 2 | 3 | p a |
| T3 (nmol/L)b | 2.19 (0.37) | 2.16 (0.38) | 2.14 (0.39) | 2.18 (0.38) | 0.97 |
| (25th–75th) | (1.90–2.41) | (1.95–2.43) | (1.92–2.46) | (1.95–2.41) | |
| fT4 (pmol/L)b | 7.98 (1.34) | 8.11 (1.29) | 8.24 (1.24) | 8.11 (1.23) | 0.87 |
| (25th–75th) | (7.21–8.88) | (7.21–8.75) | (7.21–8.75) | (7.34–8.75) | |
| T3/fT4 ratioc | 0.28 (0.11) | 0.27 (0.11) | 0.27 (0.11) | 0.27 (0.11) | 0.98 |
| (25th–75th) | (0.23–0.32) | (0.23–0.32) | (0.23–0.32) | (0.22–0.32) | |
| TSH (mIU/L)c | 1.40 (0.29) | 1.13 (0.30) | 1.09 (0.29) | 1.04 (0.29) | <0.001 |
| (25th–75th) | (0.93–2.04) | (0.75–1.80) | (0.67–1.56) | (0.66–1.59) | |
| Glucose (mmol/L)b | 4.40 (0.55) | 8.36 (1.71) | 7.86 (1.76) | 5.94 (1.59) | <0.001 |
| (25th–75th) | (4.01–4.73) | (7.37–9.73) | (6.43–9.24) | (5.22–7.20) | |
| Insulin (pmol/L)c | 25.0 (0.33) | 227.5 (0.40) | 250.5 (0.39) | 197.2 (0.42) | <0.001 |
| (25th–75th) | (14.8–39.8) | (131.1–350.3) | (169.0–476.4) | (114.7–335.8) | |
fT4: free thyroxine; T3: triiodothyronine; TSH: thyroid stimulating hormone.
Analysis of variance (ANOVA).
Median (standard deviation).
Median (log standard deviation).
Maternal weight: Relationships with T3, fT4, and T3/fT4 ratio
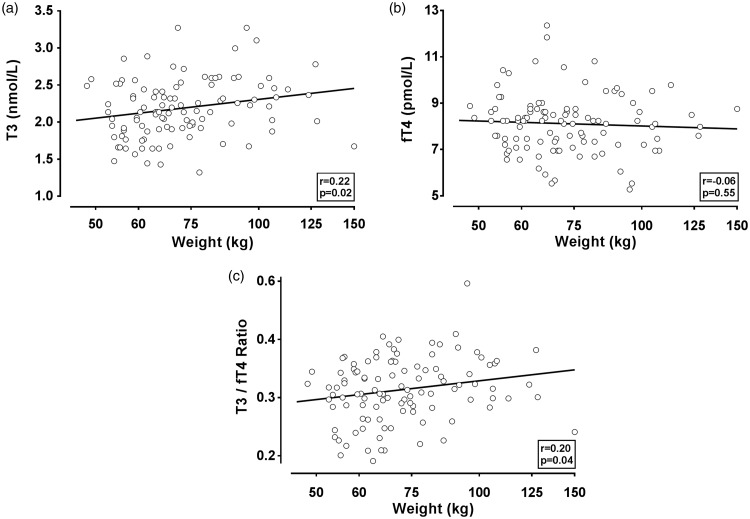
Figure 1 displays relationships between maternal weight and 2 h T3 and fT4 measurements, as well as the T3/fT4 ratio. T3 and the T3/fT4 ratio are positively associated with weight (r = 0.22, p = 0.02; r = 0.20, p = 0.04, respectively), while fT4 has no association (r = −0.06, p = 0.55).
Figure 1.
Scatterplots of T3 and fT4 measurements versus maternal weight in 108 women. T3 (a), fT4 (b), and T3/fT4 ratio (c) are shown on the vertical axis and compared with maternal weight. T3 and fT4 levels for individual women are measured at 2 h, as described in “Methods” section. Correlation coefficients and associated p values are shown in each figure. The line indicates results of a linear regression analysis. fT4: free thyroxine; T3: triiodothyronine.
Plasma glucose: Relationships with T3, fT4, and T3/fT4 ratio among the 108 women
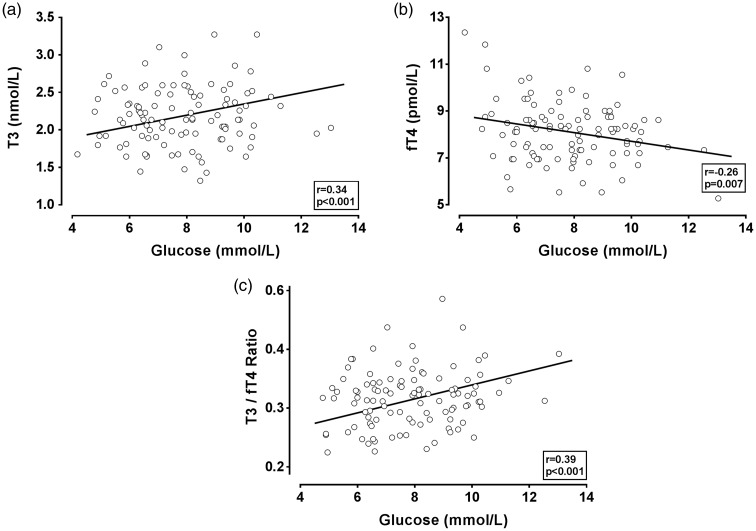
Figure 2 displays relationships between glucose and T3, fT4, and the T3/fT4 ratio (all measured at 2 h). T3 and the T3/fT4 ratio are positively associated with glucose (r = 0.34, p < 0.001; r = 0.39, p < 0.001, respectively), while fT4 has a negative association with glucose (r = −0.26, p = 0.007). Table 3 compares correlations between glucose at the four time periods with thyroid hormone measurements at 2 h. Differences in these correlation coefficients are attributable to glucose changes, as glucose measurements at 0, 1, 2, and 3 h are being compared with hormone measurements at 2 h. For T3 and the T3/fT4 ratio, all correlations with glucose are positive and highly significant, with the strongest found at 1 and 2 h. For fT4, all associations with glucose are negative, with the 2 h fT4 association with glucose reaching significance (r = −0.26, p < 0.007).
Figure 2.
Scatterplots of T3 and fT4 measurements versus glucose in 108 women. T3 (a), fT4 (b), and T3/fT4 ratio (c) are shown on the vertical axis and compared with glucose. T3, fT4, and glucose are measured at 2 h after OGTT. Correlation coefficients and associated p values are shown in each figure. The line indicates results of linear regression analysis. fT4: free thyroxine; OGTT: oral glucose tolerance testing; T3: triiodothyronine.
Table 3.
Correlations between 2 h triiodothyronine (T3) and thyroxine (fT4) measurements and glucose at four time points.
| T3 |
fT4 |
T3/fT4 |
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Glucose: | ||||||
| 0 h | 0.27 | 0.005 | −0.15 | 0.13 | 0.27 | 0.005 |
| 1 h | 0.40 | <0.001 | −0.17 | 0.07 | 0.38 | <0.001 |
| 2 h | 0.33 | <0.001 | −0.26 | 0.006 | 0.38 | <0.001 |
| 3 h | 0.26 | 0.007 | −0.18 | 0.07 | 0.27 | 0.005 |
In Table 4, we model the ability of maternal weight and the T3/fT4 ratio to predict the 2 h glucose level among the 108 pregnant women. Stepwise linear regression shows that maternal weight (p = 0.04) and the T3/fT4 ratio (p < 0.001) are independently associated with glucose measurements. However, the T3/fT4 ratio is a stronger predictor of glucose than weight, as shown by its higher partial r2 value (15%) in comparison to weight and glucose (4%).
Table 4.
Comparative strength of associations: glucose and T3/fT4 ratio vs. glucose and weight among 108 pregnant women.
| Univariate linear regression (glucose = b0 + b1
x) | |||||
|---|---|---|---|---|---|
| x | Step | b 0 | b 1 | p value | r 2 |
| log (T3/fT4) | 1 | 11.4378 | 6.2946 | <0.001 | 0.15 |
| log (weight) | 1 | 1.4428 | 3.4326 | 0.04 | 0.04 |
| Multivariate stepwise regression (glucose = b0 + b1
x1 + b2
x2) | |||||
|
xi
|
Step |
b
0
|
b
1
|
b
2
|
Cum r2 |
| log (T3/fT4) | 1 | 11.4378 | 6.2946 | 0.150 | |
| log (weight) | 2 | 7.4079 | 5.8976 | 2.0436 | 0.163 |
Glucose was measured in the sample taken 2 h after the oral glucose tolerance test.
Discussion
In our study, measurements of T3 and the T3/fT4 ratio but not fT4 are significantly associated with GDM, maternal weight, and glucose. The higher T3 and T3/fT4 ratio serve as indirect measures of increased peripheral deiodinase activity. T3 is known to induce endogenous glucose production.6 This relationship is usually characterized as a threshold effect, as when excess T3 attributable to thyrotoxicosis exacerbates pre-existing diabetes but subsides when euthyroidism is achieved.6–9 By contrast, the present data suggest a less extreme but continuous relationship between T3 and glucose that might be explained by normal variations in deiodinase activity, attributable to a weight-associated influence.
Overfeeding and calorie restriction studies that have been carried out by other investigators in non-pregnant adults demonstrate that T3 levels respond to variations in caloric intake, with caloric excess inducing higher T3 levels and caloric reduction resulting in lower T3 levels.10–13 While it is not feasible to measure deiodinase activity directly in humans via biopsies in liver or other organs, an overfeeding (high fat) study in wild-type mice induced not only higher T3 and lower T4 levels in serum, but also higher hepatic deiodinase-1 activity and mRNA.14 Overfeeding and calorie restriction studies to date have not included the T3/glucose relationship explored in our study.
Several authors have presented cross-sectional data similar to ours, including negative fT4 and positive fT3 correlations with weight15 and higher fT3/fT4 ratios with maternal obesity.16 Similar relationships that include not only fT4 and fT3/fT4 ratios with weight, but glucose, as well, have been reported during the past several years.17,18 In these studies, the highest fT3/fT4 ratio quintile and the lowest fT4 quintile were associated with the highest BMI, highest 1 h post-load glucose, highest hemoglobin A1c (HbA1c), and highest fasting plasma insulin. Similarities between thyroid hormone relationships with glucose and type 2 diabetes have also been reported recently in an ongoing study involving healthy middle aged subjects.19
Our data suggest that higher T3 levels associated with higher weight (as a measure of fat) may be responsible for a modest upward shift in glucose levels. We hypothesize that this occurs as a result of increased deiodinase activity during pregnancy. Such a shift could contribute independently to the best understood causal pathway: ectopic accumulation of intracellular lipid in muscle or liver, resulting in activation of protein kinase C by diacylglycerols.20–22
In the present study, median plasma glucose levels at 1, 2, and 3 h follow anticipated patterns after administration of a 100 g glucose load. When compared with the fasting state, median TSH levels are lower at all three time periods, a pattern variously attributed to either a glucose load23,24 or diurnal variability.25–27 Unlike TSH, T3, and fT4 do not vary significantly among the four OGTT time periods, again supporting observations by others.28,29 While serving to verify the historical integrity of these measurements, the analyses of greatest interest involve relationships between glucose, weight, T3, and fT4, thereby offering the prospect of gaining insight into another facet of glucose metabolism.
Our opportunistic study design has both advantages and shortcomings. In addition to evaluating categorical T3 and fT4 relationships based on the presence or absence of GDM, it is also an advantage to be able to compare T3 and fT4 relationships with maternal weight and glucose measurements, irrespective of GDM classification. As a shortcoming, OGTT measurements are available only from a subset of screened women, precluding determination of thyroid hormone relationships with weight and glucose in the population as a whole. Due to the cross-sectional nature of the study design, we were not able to evaluate trends in weight gain and in potential changes in thyroid hormone measurements over time which might alter relationships with glucose. Our protocol did not include rT3, thyroid antibodies, or thyroxine binding globulin, which could provide further insights.
In summary, we conclude that higher maternal weight induces deiodinase activity (as indicated by higher T3 and a higher T3/fT4 ratio). Higher deiodinase activity, in turn, is associated with higher plasma glucose. We propose that the known association between higher maternal weight and gestational hyperglycemia (or GDM) can be explained by enhanced endogenous glucose activity attributable to T3.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The Institutional Review Board (IRB) for Human Studies at Women and Infants Hospital waived the requirement for informed consent due to the nature of the study protocol.
Guarantor
JEH
Contributorship
JEH designed the analyses, developed the hypothesis, reviewed the literature, wrote the initial manuscript draft, established and maintained contact with the other authors, circulated manuscript drafts for editing and comment. LMN served as mathematician and data analyst and worked closely with JEH and GEP. He reviewed manuscript for correctness of numbers. GEP, as biostatistician, worked with LN on all aspects of the current data analysis and reviewed content of manuscript. GLM oversaw laboratory measurements (fT4, T3, TSH, insulin) used for the present manuscript. She participated in the development and review of the present manuscript. EE, as research technician, managed collection of samples, performed laboratory testing, and collected data on OGTT measurements and outcomes.
References
- 1.Haddow JE, Craig WY, Neveux LM, et al. Free thyroxine during early pregnancy and risk for gestational diabetes. PLoS ONE 2016; 11: e0149065–e0149065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddow JE, Craig WY, Palomaki GE, et al. Impact of adjusting for the reciprocal relationship between maternal weight and free thyroxine during early pregnancy. Thyroid 2013; 23: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddow JE, Craig WY, Neveux LM, et al. Implications of high free thyroxine (FT4) concentrations in euthyroid pregnancies: the FaSTER trial. J Clin Endocrinol Metab 2014; 99: 2038–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddow JE, Neveux LM, Palomaki GE, et al. An inverse relationship between weight and free thyroxine during early gestation among women treated for hypothyroidism. Thyroid 2015; 25: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang S, Shi FT, Leung PC, et al. Low thyroid hormone in early pregnancy is associated with an increased risk of gestational diabetes mellitus. J Clin Endocrinol Metab 2016; 101: 4237–4243. [DOI] [PubMed] [Google Scholar]
- 6.Potenza M, Via MA, Yanagisawa RT. Excess thyroid hormone and carbohydrate metabolism. Endocr Pract 2009; 15: 254–262. [DOI] [PubMed] [Google Scholar]
- 7.Sola E, Morillas C, Garzon S, et al. Association between diabetic ketoacidosis and thyrotoxicosis. Acta Diabetol 2002; 39: 235–237. [DOI] [PubMed] [Google Scholar]
- 8.Kreines K, Jett M, Knowles HC., Jr Observations in hyperthyroidism of abnormal glucose tolerance and other traits related to diabetes mellitus. Diabetes 1965; 14: 740–744. [DOI] [PubMed] [Google Scholar]
- 9.Pisarev MA. Interrelationships between the pancreas and the thyroid. Curr Opin Endocrinol Diabetes Obes 2010; 17: 437–439. [DOI] [PubMed] [Google Scholar]
- 10.Danforth E, Jr, Horton ES, O’Connell M, et al. Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J Clin Invest 1979; 64: 1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suda AK, Pittman CS, Shimizu T, et al. The production and metabolism of 3,5,3′-triiodothyronine and 3,3′,5-triiodothyronine in normal and fasting subjects. J Clin Endocrinol Metab 1978; 47: 1311–1319. [DOI] [PubMed] [Google Scholar]
- 12.Vagenakis AG, Burger A, Portnay GI, et al. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab 1975; 41: 191–194. [DOI] [PubMed] [Google Scholar]
- 13.Agnihothri RV, Courville AB, Linderman JD, et al. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid 2014; 24: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelletier P, Gauthier K, Sideleva O, et al. Mice lacking the thyroid hormone receptor-alpha gene spend more energy in thermogenesis, burn more fat, and are less sensitive to high-fat diet-induced obesity. Endocrinology 2008; 149: 6471–6486. [DOI] [PubMed] [Google Scholar]
- 15.Mannisto T, Surcel HM, Ruokonen A, et al. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid 2011; 21: 291–298. [DOI] [PubMed] [Google Scholar]
- 16.Kahr MK, Antony KM, DelBeccaro M, et al. Increasing maternal obesity is associated with alterations in both maternal and neonatal thyroid hormone levels. Clin Endocrinol 2016; 84: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassols J, Prats-Puig A, Soriano-Rodriguez P, et al. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab 2011; 96: 3717–3723. [DOI] [PubMed] [Google Scholar]
- 18.Knight BA, Shields BM, Hattersley AT, et al. Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur J Endocrinol 2016; 174: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roef GL, Rietzschel ER, Van Daele CM, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid 2014; 24: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014; 371: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 21.Petersen KF, Dufour S, Morino K, et al. Reversal of muscle insulin resistance by weight reduction in young, lean, insulin-resistant offspring of parents with type 2 diabetes. Proc Natl Acad Sci U S A 2012; 109: 8236–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011; 54: 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aksoy DY, Cinar N, Ucar F, et al. TSH response to oral glucose tolerance test in patients with obesity. In: Autoimmune thyroid disease & thyroid hormone action (translational). Houston, TX: The Endocrine Society, 2012 (Abstract MON-433).
- 24.Kamat V, Hecht WL, Rubin RT. Influence of meal composition on the postprandial response of the pituitary-thyroid axis. Eur J Endocrinol 1995; 133: 75–79. [DOI] [PubMed] [Google Scholar]
- 25.Patel YC, Alford FP, Burger HG. The 24-hour plasma thyrotrophin profile. Clin Sci 1972; 43: 71–77. [DOI] [PubMed] [Google Scholar]
- 26.Brabant G, Prank K, Ranft U, et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J Clin Endocrinol Metab 1990; 70: 403–409. [DOI] [PubMed] [Google Scholar]
- 27.Kok P, Roelfsema F, Frolich M, et al. Spontaneous diurnal thyrotropin secretion is enhanced in proportion to circulating leptin in obese premenopausal women. J Clin Endocrinol Metab 2005; 90: 6185–6191. [DOI] [PubMed] [Google Scholar]
- 28.Premachandra BN, Gossain VV, Perlstein IB. Thyroid hormone levels during glucose tolerance test in euthyroid subjects. Clin Endocrinol 1979; 10: 207–211. [DOI] [PubMed] [Google Scholar]
- 29.Nair R, Mahadevan S, Muralidharan RS, et al. Does fasting or postprandial state affect thyroid function testing? Indian J Endocrinol Metab 2014; 18: 705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]