Abstract
Transplantation of neural stem cells (NSCs) holds great potential for the treatment of spinal cord injury (SCI). However, transplanted NSCs poorly survive in the SCI environment. We injected NSCs into tibial nerve and transplanted tibial nerve into a hemisected spinal cord and investigated the effects of lithium chloride (LiCl) on the survival of spinal neurons, axonal regeneration, and functional recovery. Our results show that most of the transplanted NSCs expressed glial fibrillary acidic protein, while there was no obvious expression of nestin, neuronal nuclei, or acetyltransferase found in NSCs. LiCl treatment produced less macrosialin (ED1) expression and axonal degeneration in tibial nerve after NSC injection. Our results also show that a regimen of LiCl treatment promoted NSC differentiation into NF200-positive neurons with neurite extension into the host spinal cord. The combination of tibial nerve transplantation with NSCs and LiCl injection resulted in more host motoneurons surviving in the spinal cord, more regenerated axons in tibial nerve, less glial scar area, and decreased ED1 expression. We conclude that lithium may have therapeutic potential in cell replacement strategies for central nervous system injury due to its ability to promote survival and neuronal generation of grafted NSCs and reduced host immune reaction.
Keywords: spinal cord injury, neural stem cell, lithium chloride, tibial nerve, transplantation, neuron
Introduction
Neural stem cells (NSCs) can be isolated from both fetal central nervous system (CNS) tissue1–3 and adult CNS tissue4–6. Under certain conditions, NSCs can differentiate into neuron, astrocyte, and oligodendrocyte and migrate to damaged areas, replacing degenerated or dead neural cells. Because of this potential of multidifferentiation, NSCs play an important role in replacement therapy in CNS injury or disease. Recent studies found that NSCs have protective effects in the treatment of multiple sclerosis, amyotrophic lateral sclerosis, and stroke7–9. Similarly, NSCs can play several roles in the treatment of spinal cord injury (SCI)10 including replacement of damaged neuronal and glial cells, remyelination of spared axons, restoration of neuronal circuitry, and bridging of lesion cavities. They can also enhance production of neurotrophic factors, anti-inflammatory cytokines, and other molecules to promote tissue sparing and neovascularization. They may also contribute to establishing a permissive environment for neural plasticity and axonal regeneration. Since the local environment is not suitable for the survival of NSCs after acute SCI, more strategies should be considered to protect NSCs and to improve their survival after transplantation to promote functional recovery. Since peripheral nerve transplantation for the treatment of SCI had been studied by many authors, and since transplantation of NSCs has produced some interesting results in the treatment of peripheral nerve injury11–14, it is possible that peripheral nerve could serve as a vehicle to host NSCs and that transplantation of peripheral nerve with NSCs may have some effects in the repair of damaged spinal cord.
Lithium has been used to treat bipolar disease for over 60 years. Recently, lithium was found to have therapeutic effects in the treatment of CNS injury and some degenerative diseases such as stroke, brain and SCI, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease15. More recently, lithium has been found to upregulate the expression of neurotrophic factors, to promote CNS neurogenesis, to inhibit apoptosis promoter expression, and to protect brain tissue in ischemic and traumatic injuries16,17, indicating that lithium may offer similar protection in the setting of SCI. Some studies found that lithium promotes NSC proliferation, neural differentiation, and brain-derived neurotrophic factor (BDNF) production18. The effects of lithium on NSCs offered the potential that NSC transplantation plus lithium treatment may promote functional recovery after SCI. However, few studies reported on the use of the combination strategy of NSC transplantation plus lithium in the treatment of SCI.
Based on these theories, we sought to determine whether the combination strategy of transplantation of peripheral nerve with NSCs plus lithium treatment can improve functional recovery after SCI. To delineate this possibility, we employed an in vivo spinal cord hemisection model. Our findings show that the combination of lithium chloride (LiCl) and transplantation of tibial nerve with NSCs had better effects in promoting functional recovery after spinal cord hemisection injury.
Materials and Methods
Cell Isolation and Culture
Under sterile conditions, spinal cords from E13.5 transgenic Sprague-Dawley (SD) rats expressing green fluorescent protein (GFP; a gift from Professor Wutian Wu of University of Hong Kong) were dissected out and prepared for stem cell culture following procedures described previously with modifications1,19,20. Briefly, the spinal cord was separated from surrounding connective tissue. After peeling off the meninges and nerve roots, the cord was cut into small pieces and transferred into a 15 mL centrifuge tube containing Accutase (Invitrogen, Carlsbad, CA, USA) and culture medium (described below) and dissociated to a single-cell suspension by gentle mechanical trituration through a fire-polished Pasteur pipette. The dissociated cells were filtered through a cell strainer (BD Falcon, San Jose, CA, USA) and plated onto poly-l-lysine-/laminin-coated dishes. The culture medium consisted of Dulbecco's modified eagle medium (DMEM): nutrient mixture F- 12 (Invitrogen, Carlsbad, CA, USA), bovine serum albumin (1 mg/mL, Sigma-Aldrich, St. Louis, MO, USA), B27 (Invitrogen), N2 (Invitrogen), epidermal growth factor (20 ng/mL, Peprotech, Rocky Hill, NJ, USA), basic fibroblast growth factor (20 ng/mL, Peprotech), and Pen–Strep (100 IU/mL, Invitrogen).
Cells were maintained in an incubator with a humidified atmosphere containing 5% CO2 at 37 °C. The medium was changed every 2 d. At around 80% confluence, cells were passaged at the ratio of 1:4 to 1:6 after initial plating. These passaged cells were designated as “first passage” (P1). When P1 generation NSCs reached 80% confluence, cells were harvested and suspended in culture medium at a concentration of 1.0 × 105 cells/μL before transplantation.
Ligation of Tibial Nerve and Transplantation of NSCs
Animals were weighed and anesthetized by intraperitoneal injection of 10% chloral hydrate (0.4 mL/100 g). After an incision was made in the back of right thigh, the tibial nerve was exposed, clamped with microforceps, and ligated just beneath the separation from the common peroneal nerve. Then, 1 μL of cell suspension containing 1.0 × 105 NSCs was slowly injected over 5 min into the distal tibial nerve using microsyringe, and the microsyringe was left in place for 2 more minutes after injection. The injection site was closed with 11-0 suture, and the skin was closed with 1-0 suture. After NSC injection, animals were randomly divided into 2 groups (12 animals in each group). One group (NSC + LiCl group) received daily intraperitoneal injections of LiCl (85 mg/kg bodyweight, dissolved in distilled water) until euthanasia21. The other group (NSC + NS group) received daily intraperitoneal injections of saline as the control treatment.
Tissue Processing of Tibial Nerve and Immunohistochemistry
One week after NSC transplantation, animals were given an overdose of 10% chloral hydrate (0.5 mL/100 g) and were transcardially perfused and fixed with 4% paraformaldehyde. Tibial nerve with transplanted NSCs was removed and postfixed overnight and then transferred to 30% sucrose. Tibial nerve from 1 animal of each group was cut into 20-µm-thick cross sections, and other tibial nerves were cut into horizontal sections.
Tibial nerve sections were permeabilized and blocked with 0.3% Triton X-100/10% normal goat serum (NGS, Invitrogen) in 0.01 M phosphate buffered saline (PBS; pH 7.4) for 30 min. Primary antibodies were then applied to the sections overnight at 4 °C. The following day, sections were incubated with fluorescein-conjugated secondary antibodies (Alexa 568 goat antimouse [1:400] or Alexa 568 goat antirabbit [1:400], both from Invitrogen) for 1 h at room temperature before mounting with antifade mounting medium. The following primary antibodies were used: monoclonal mouse antinestin Ab (1:100, Millipore, Burlington, MA, USA) for identifying NSCs; monoclonal mouse anti-neuronal nuclei (NeuN) Ab (1:100, Millipore), mouse anti-acetyltransferase (ChAT) Ab (1:100, Millipore), and polyclonal rabbit anti-glial fibrillary acidic protein (GFAP) Ab (1:100, Sigma-Aldrich) for detecting differentiation of NSCs; and mouse anti-ED1 Ab (1:100, Serotec, Raleigh, NC, USA) for detecting inflammatory cell infiltration of tibial nerve.
After immunohistochemical staining, 5 sections were randomly selected from each sample for taking pictures. The size of GFAP-, ED1-, and GFP-positive staining area in each section was measured using ImageJ software (version 1.50, National Institutes of Health [NIH]). In brief, areas of positive staining were enhanced by adjusting brightness and contrast in ImageJ software. Then, images were converted to binary images and the sizes of positive areas were measured. The ratios of GFAP- or ED1-positive area to GFP-positive area were calculated.
Spinal Cord Hemisection and Transplantation of Tibial Nerve
Adult female SD rats (180 to 200 grams) were randomly divided into 2 groups, with 1 group receiving NSC injection into the ligated nerve after tibial nerve ligation (N = 24/group). Each group was divided into 2 subgroups that received either LiCl (85 mg/kg) or NS (2 mL/100 g) via intraperitoneal injection after surgery (n = 12/subgroup). One week after tibial nerve ligation, animals were weighed again and anesthetized by intraperitoneal injection of 10% chloral hydrate (0.4 mL/100 g). The ligated tibial nerve was exposed and excised for transplantation. The procedures used for spinal cord hemisection and for preoperative and postoperative animal care were described in detail in previous publications20,22. Briefly, after the removal of a 2.8 mm piece of hemicord at T10 on the right side, the prepared tibial nerve was cut into a 3-mm-long piece, with or without NSC injection, and implanted into the hemisection gap. The dura and wound were closed in layers. All animals continued to receive LiCl (85 mg/kg) or NS (2 mL/100 g) intraperitoneal injection after the second surgery. All animal handling, surgical procedures, and postoperative care were performed in accordance with the Guide for the Care and Use of Laboratory Animals23.
Evaluation of Rat Hind Limb Function
Two and four weeks after tibial transplantation, hind limb locomotor function of each animal was video recorded and evaluated by two other researchers using blood born barrier (BBB) scale24.
Tissue Processing of Spinal Cord and Immunohistochemistry
Four weeks after tibial nerve transplantation, animals were given an overdose of 10% chloral hydrate (0.5 mL/100 g) and were transcardially perfused and fixed with 4% paraformaldehyde. Spinal cord with transplanted tibial nerve was removed and postfixed overnight and then transferred to 30% sucrose. A 1-cm-long spinal cord segment of each animal was cut longitudinally at 20 µm thickness.
Spinal cord sections were permeabilized and blocked with 0.3% Triton X-100/10% normal goat serum (NGS, Invitrogen) in 0.01 M PBS (pH 7.4) for 30 min, and primary antibodies were then applied to the sections overnight at 4 °C. The following day, sections were incubated with fluorescein-conjugated secondary antibodies (Alexa 568 goat antimouse [1:400] or Alexa 488 goat antirabbit [1:400], both from Invitrogen) for 1 h at room temperature before mounting with antifade mounting medium. The following primary antibodies were used: monoclonal mouse anti-NeuN Ab (1:100, Millipore), monoclonal mouse anti-chondroitin sulfate proteoglycan (CSPG) Ab (1:200, Millipore), polyclonal rabbit anti-GFAP Ab (1:100, Sigma-Aldrich), mouse anti-NF200 Ab (1:100, Sigma-Aldrich), and mouse anti-ED1 Ab (1:100, Serotec).
After immunohistochemistry (IHC) staining, 5 sections were randomly selected from each sample for taking pictures. The size of areas taken by positive GFP, NeuN, NF200, GFAP, and ED1 staining and the size of whole picture were measured using ImageJ software (NIH) as mentioned above. When measuring the size of the spinal cord in the selected sections, the blank area around the spinal cord should be deleted before measuring. Then the ratios of these positive staining areas to the area of spinal cord were calculated.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6.01 software (GraphPad Software, La Jolla, CA, USA). All data were expressed as mean ± standard deviation. The difference between 2 groups was compared using t-test. One-way analysis of variance was used for the analysis of multiple groups, and q-test was used for pair comparison between groups. A P < 0.05 was considered statistically significant.
Results
Survival and Migration of Transplanted NSCs in Tibial Nerve
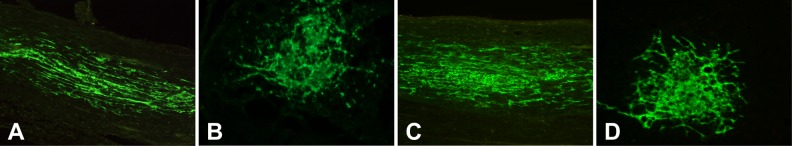
One week after injection, transplanted GFP-positive NSCs survived well in tibial nerve, which showed green color under fluorescence microscope (Fig. 1). On tibial nerve longitudinal section, NSCs migrated along the axons to both ends (Fig. 1A and C). On tibial nerve cross section, most transplanted NSCs located in the injection area, though some cells migrated through the small clearances among axons. Depending on the morphology, NSCs survived well inside tibial nerve in both NS and LiCl groups.
Fig. 1.
The survival of transplanted neural stem cells (NSCs) in tibial nerve. (A, B) Longitudinal and cross section of tibial nerve of control group; (C, D) longitudinal and cross section of tibial nerve of LiCl group. Transplanted NSCs survived well inside tibial nerve.
Differentiation of Transplanted NSCs in Tibial Nerve
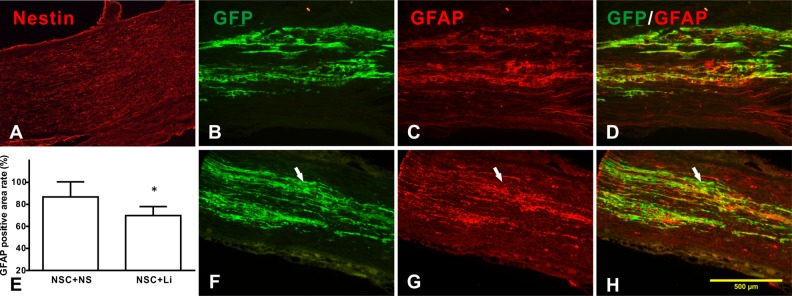
One week after transplanted into tibial nerve, transplanted NSCs showed strong GFP-positive expression. However, no obvious nestin-positive expression was seen in either control or LiCl group (Fig. 2A), as well as the NeuN or ChAT (data not shown), indicating that most NSCs differentiated after being transplanted into tibial nerve but not NeuN- or ChAT-positive neurons.
Fig. 2.
Differentiation of transplanted neural stem cells (NSCs) in tibial nerve. (A) No obvious nestin-positive expression was observed; (B–D) control group; (E) comparison of glial fibrillary acidic protein (GFAP)-positive areas in different groups; and (F–H) lithium chloride (LiCl) group. (B, F) NSCs that expressed green fluorescent protein (GFP) are shown in green color; (C, G) the GFAP-positive staining area of both control and LiCl group. The LiCl group had smaller GFAP-labeled area (E); (D, H) merged picture shows most GFAP-positive area of overlap with GFP-labeled area in control group. White arrow shows GFAP-negative area. *Compared with control group, P < 0.05.
One week after transplantation, after GFAP staining, both control and LiCl groups had massive positive GFAP staining. Almost all GFAP-positive area was confined inside GFP-labeled area. In the control group, most of the GFAP-positive area overlapped with GFP-positive area (Fig. 2B–D). However, in LiCl group, the GFAP-positive area is smaller and had lesser overlapped area with GFP-labeled area (Fig. 2F–H).
Measuring the size of GFAP-positive area and calculating the percentage of GFAP-positive area to the GFP-positive area, the percentages of control and LiCl groups were 86.7% ± 13.8% and 69.8% ± 8.2%, respectively. LiCl group is much smaller than the control group (P < 0.05, Fig. 2E).
Inflammatory Cell Infiltration in Tibial Nerve of Different Groups
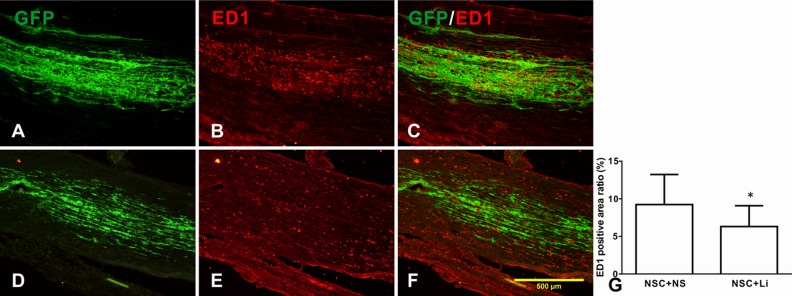
One week after NSC injection, populous ED1-positive macrophage infiltration was seen in both NSC + normal saline (NS) group (Fig. 3B) and NSC + LiCl group (Fig. 3E). Most of the macrophage infiltration occurred around the injected NSCs (NSC + NS group, Fig. 3A, C and NSC + LiCl group, Fig. 3D, F). The ED1-positive cover rates of NSC + NS and NSC + LiCl group were 8.97% ± 4.07% and 6.31% ± 2.78%, respectively. The ED1 expression of NSC + LiCl group was lower than NSC + NS group, showing a significant difference (P < 0.01; Fig. 3G), indicating that lithium may suppress inflammation caused by NSC injection.
Fig. 3.
The expression of ED1 in tibial nerve after neural stem cell (NSC) injection. (A–C) NSC + NS group; (D, E) NSC + lithium chloride group. (A, D) green fluorescent protein (GFP)-labeled NSCs; (B, E) ED1 expression in tibial nerve; (C, F) merged picture showing macrophages distributed around implanted NSCs; (G) the ED1-positive ratio in tibial nerve of different groups. *Compared with NSC + NS group, P < 0.05.
Outcome of Rat Hind Limb Function
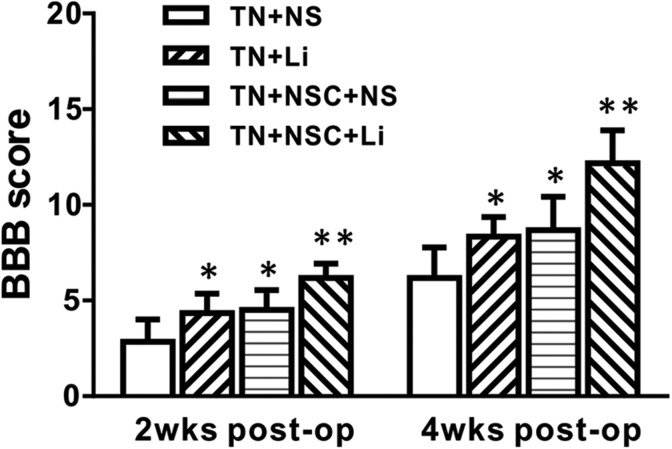
Two weeks after tibial nerve transplantation, only little functional recovery was seen in all groups. The animals in tibial nerve transplantation (TN) + NSC + LiCl group recovered best; recovery in both TN + NSC + NS and TN + LiCl groups was fair, while TN + NS group recovery was the worst. The BBB scores of different groups are listed in Table 1. The BBB score of TN + NSC + LiCl group was higher than other groups (P < 0.01); the BBB score of TN + NSC + NS group was a little higher than TN + LiCl group, but no difference was found after statistical analysis. The BBB score of TN + NS group was lower than other groups, showing a significant difference (P < 0.05; Table 1, Fig. 4).
Table 1.
Blood Born Barrier Score at 2 and 4 Wk after Transplantation of Different Groups.
| Groups | Two Week after Transplantation | Four Week after Transplantation |
|---|---|---|
| TN + NS group | 2.83 ± 1.17 | 6.17 ± 1.60 |
| TN + LiCl group | 4.33 ± 1.03* | 8.33 ± 1.03* |
| TN + NSC + NS group | 4.50 ± 1.05* | 8.67 ± 1.75* |
| TN + NSC + LiCl group | 6.17 ± 0.75** | 12.17 ± 1.72** |
Abbreviations: NSC = neural stem cell; LiCl = lithium chloride.
*Compared with TN + NS group, P < 0.05.
**Compared with other groups, P < 0.01.
Fig. 4.
Comparison of blood born barrier scores at different times after surgery of all groups *Compared with TN + NS group, P < 0.05.
Four weeks after tibial nerve transplantation, more right–hind limb functional recovery was seen in all groups. The animals in TN + NSC + LiCl group recovered best. Four-week recovery in both TN + NSC + NS and TN + LiCl groups was much better than at 2 wk. TN + NS group recovered to some extent as well, but still not as well as other groups. The BBB scores of different groups were as follows: TN + NS group: 6.17 ± 1.60; TN + LiCl group: 8.33 ± 1.03; TN + NSC + NS group: 8.67 ± 1.75; and TN + NSC + LiCl group: 12.17 ± 1.72. After statistical analysis, the BBB score of TN + NSC + LiCl group was higher than other groups (P < 0.01). The BBB scores of TN + NSC + NS group and TN + LiCl group were higher than TN + NS group (P < 0.05; Table 1, Fig. 4), but no significant difference was found between these 2 groups.
Differentiation of NSCs in Tibial Nerve after Transplantation
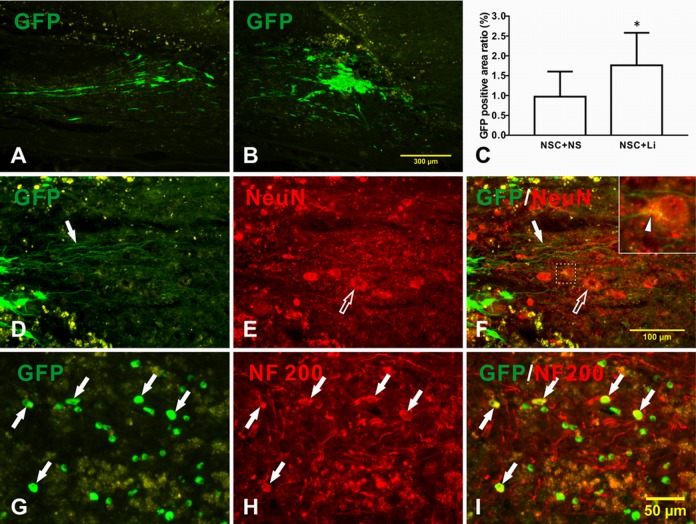
Four weeks after the transplantation of tibial nerve with NSCs, the transplanted NSCs survived well, expressing GFP protein with bright green color (Fig. 5A and B). There were more bright green labeled NSCs in TN + NSC + LiCl group (Fig. 5B) than in TN + NSC + NS group (Fig. 5A), with an average GFP positive area ratio of 1.762% ± 0.193% and 0.972% ± 0.182%, respectively (Fig. 5C, P < 0.01). In the lithium-treated group, transplanted NSCs sent out many long neurites, which grew into host parenchyma (Fig. 5D–F) and had direct contact with host neuron (Fig. 5F, upper-right box). The longest neurite measured was 0.4 mm (Fig. 5F, arrow). No neurite was found in TN + NSC + NS group.
Fig. 5.
Differentiation of transplanted neural stem cells (NSCs) in tibial nerve in host spinal cord after spinal cord injury. (A–C) Transplanted NSCs survived well; (D) NSCs in TN + NSC + lithium chloride (LiCl) group sent out long neurites (arrow) and grew into host parenchyma; (E) host motoneurons (hollow arrow); and (F) merged picture. Upper right is magnified picture of dotted box in the middle. A contact of regenerated axon with host neuron can be seen (arrow head); (G) green fluorescent protein (GFP)-positive NSCs; (H) NF200-positive expression in cell body and neurites; (I) merged picture indicates double-labeled cells showing yellow color (arrow).
Four weeks after the transplantation of tibial nerve with NSCs, no nestin expression was found in transplanted NSCs. Some NSCs in the LiCl-treated group express NF200 (Fig. 5G–I). However, no NeuN- or ChAT-positive differentiated neuron was found in transplanted NSCs. No neuron differentiation was found in the control group.
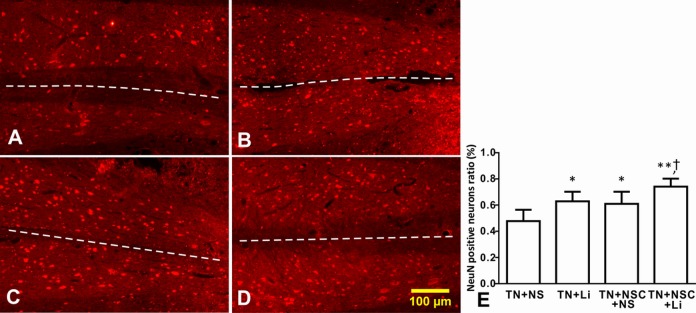
Survival of Host Spinal Cord Motoneurons after NSC Transplantation
Four weeks after tibial nerve transplantation, the numbers of host neurons in the left side of spinal cord and beyond the glial scar of all groups were close to normal. In TN + NS group, the number of NeuN-positive neurons in the injury side significantly decreased (Fig. 6A). The number of host neurons in the injury side decreased in TN + LiCl and TN + NSC + NS groups (Fig. 6B and C), while in TN + NSC + LiCl group, only a few host neurons died in the injured side beyond the glial scar (Fig. 6D).
Fig. 6.
The number of host neurons on both sides of spinal cord of different groups as injured side on the lower right. Asterisks indicate the injured side of spinal cord, while white dotted lines indicate the midline of spinal cord. (A) TN + NS group; (B) TN + lithium chloride (LiCl) group; (C) TN + neural stem cell (NSC) + NS group; (D) TN + NSC + LiCl group; and (E) the neuron number ratio of injured side in different groups. *Compared with TN + NS group, P < 0.05; **compared with TN + NS group, P < 0.01; †compared with TN + LiCl and TN + NSC + NS group, P < 0.05.
The numbers of host neurons of both sides were counted in coronal sections, and the ratios of neuron number on the injured side to the other side of different groups were as follows: TN + NS group: 47.8% ± 8.6%; TN + LiCl group: 62.9% ± 7.3%; TN + NSC + NS group: 61.0% ± 9.3%; and TN + NSC + LiCl group: 74.1% ± 6.1%. After statistical analysis, the ratio of neuron number on the injured side to the neuron number on the other side in TN + NS group was significantly lower than other groups (P < 0.01; Fig. 6E). The ratios in TN + LiCl group and TN + NSC + NS group were similar, higher than that of TN + NS group (P > 0.05; Fig. 6E). The neuron number ratio in injured side of TN + NSC + LiCl group is much higher than the 3 other groups (Fig. 6E).
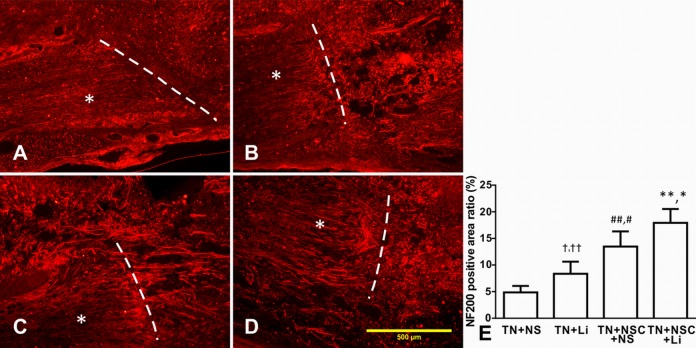
Axonal Regeneration after Tibial Nerve Transplantation in Different Groups
Four weeks after transplantation, transplanted tibial nerve integrated well into host spinal cord, and NF200-positive regenerated axons could be found in all groups. In TN + NS group, only a few NF200-positive axons were found (Fig. 7A), while more regenerated axons were found in TN + LiCl group (Fig. 7B), TN + NSC + NS group (Fig. 7C), and TN + NSC + LiCl group (Fig. 7D). Most of the regenerated axons were found in both ends of transplanted tibial nerve and in host spinal cord near the host–implant interface. In TN + NSC + LiCl group, intensive regenerated axons were found inside the implanted tibial nerve (Fig. 7D).
Fig. 7.
The expression of NF200 in implanted tibial nerve and host spinal cord 4 wk after transplantation. Asterisks indicate implanted tibial nerves, while white dotted lines indicate host-implant interfaces. (A) TN + NS group, only a few regenerated axons were found. (B) TN + lithium chloride (LiCl) group. (C) TN + neural stem cell (NSC) + NS group, more regenerated axons were found in tibial nerve and host spinal cord. (D) TN + NSC + LiCl group, numerous parallel regenerated axons were found in implanted tibial nerve and host-implant interface. (E) The NF200-covered area ratio of different groups 4 wk after transplantation. †Compared with TN + NS group, P < 0.05; ††compared with TN + NSC + LiCl group, P < 0.01; ##compared with TN + NS group, P < 0.01; #compared with TN + LiCl group, P < 0.05; **compared with TN + NS group, P < 0.01; *compared with TN + NSC + NS group, P < 0.05.
We measured the NF200-positive staining covered area using ImageJ software and calculated the ratio of NF200-positive area to whole visual field. The ratios in all groups were as follows: TN + NS group: 4.87% ± 1.21%; TN + LiCl group: 8.36% ± 2.27%; TN + NSC + NS group: 13.47% ± 2.85%; and TN + NSC + LiCl group: 17.93% ± 2.59%. After analysis, the NF20-covered ratio increased dramatically from TN + SC group to TN + NSC + LiCl group, and there were significant differences among all these groups (Fig. 7E).
Expression of CSPG in Different Groups
Four weeks after tibial nerve transplantation, numerous strong CSPG-positive expressions were found in the area of transplanted tibial nerve in host spinal cord in control group without NSCs and lithium injection (TN + NS group; Fig. 8A). The expression of CSPG significant decreased in animal with lithium injection (TN + LiCl group; Fig. 8B) or with NSCs transplantation (TN + NSC + NS group; Fig. 8C). Only few CSPG-positive staining was found scattered around the grafted area in animal with NSCs transplantation and lithium injection (TN + NSC + LiCl group; Fig. 8D).
Fig. 8.
Expression of chondroitin sulfate proteoglycan (CSPG) in different groups. (A) TN + NS group, strong CSPG-positive expression was found in the area of transplantation of tibial nerve in host spinal cord; (B) TN + lithium chloride (LiCl) group; (C) TN + neural stem cell (NSC) + NS group; and (D) TN + NSC + LiCl group.
Expression of GFAP and Tissue Reaction after SCI in Different Groups
Four weeks after tibial nerve transplantation, massive GFAP-positive expression was found in the glial scar that surrounded the reaction area in all groups. The GFAP-positive glial scar-surrounded tissue reaction area was most extended in TN + NS group (Fig. 9A), while the reaction area was reduced in TN + LiCl group and TN + NSC + NS group (Fig. 9B and C). The tissue reaction area in TN + NSC + LiCl group was the smallest of all groups (Fig. 9D).
Fig. 9.
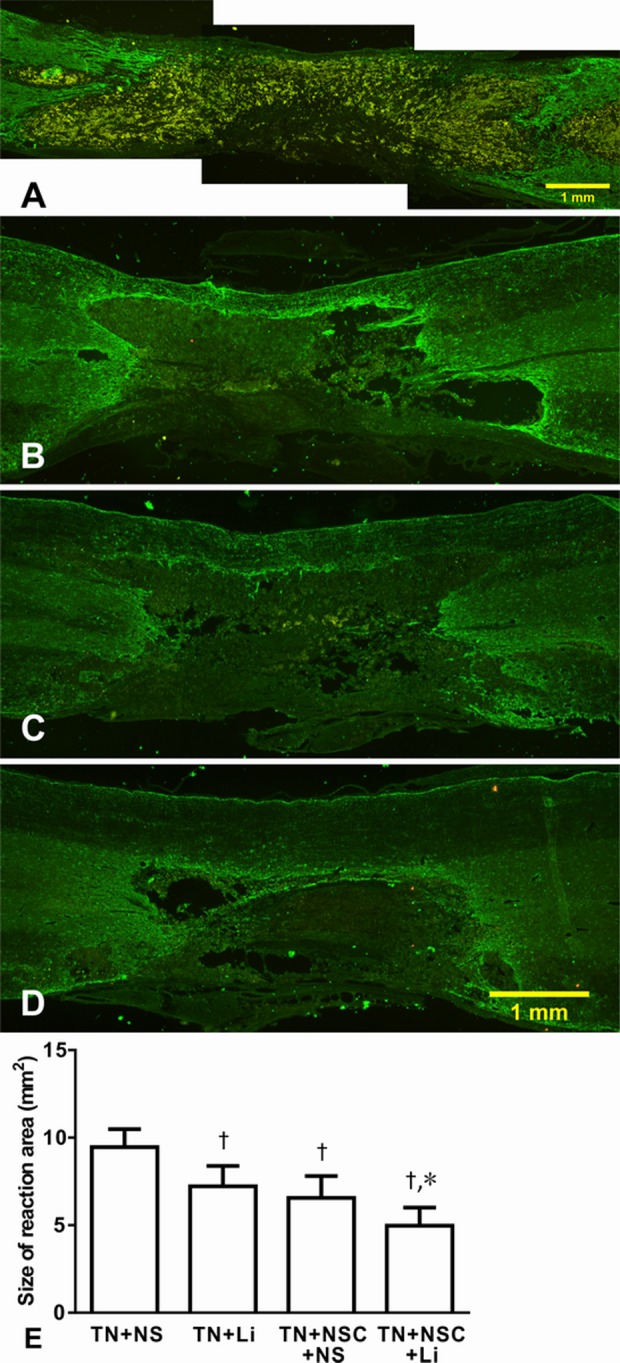
Tissue reaction area surrounded by glial fibrillary acidic protein-positive glial scar in different groups 4 wk after tibial transplantation. (A) TN + NS group; (B) TN + lithium chloride (LiCl) group; (C) TN + neural stem cell (NSC) + NS group; (D) TN + NSC + LiCl group; and (E) comparison of the reaction area of different groups. *Compared with TN + LiCl and TN + NSC + NS groups, P < 0.05; †compared with TN + NS group, P < 0.01.
We measured the tissue reaction area that surrounded GFAP-positive glial scar. The reaction areas of different groups were as follows: TN + NS group: 9.46 ± 1.03 mm2; TN + LiCl group: 7.22 ± 1.16 mm2; TN + NSC + NS group: 6.56 ± 1.25 mm2; and TN + NSC + LiCl group: 4.97 ± 1.03 mm2. After statistical analysis, TN + NS group had the largest reaction area (P < 0.01; Fig. 9E). Tissue reaction areas of TN + LiCl group and TN + NSC + NS group were smaller and had no difference between these 2 groups, while the reaction area of TN + NSC + LiCl group was significantly smaller than the other 3 groups (P < 0.05; Fig. 9E).
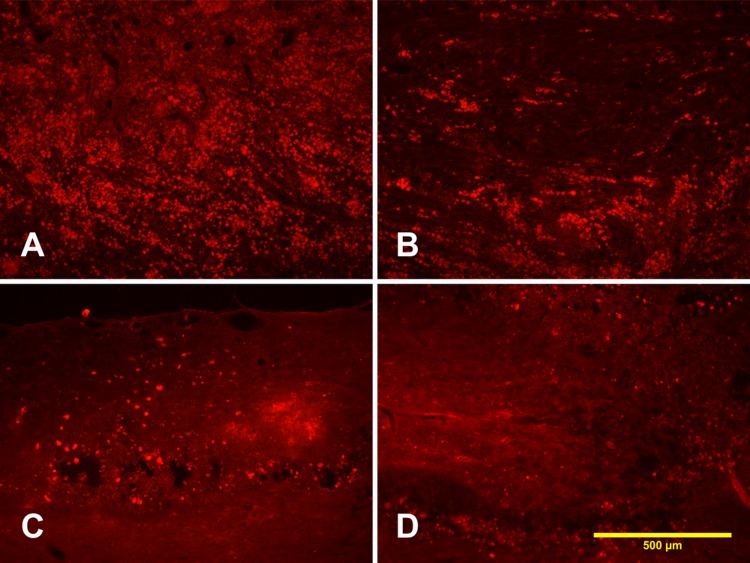
Expression of ED1 in Different Groups after SCI
Four weeks after tibial nerve transplantation, massive ED1-positive expression was found inside or outside the reaction area in all groups. In TN + NS group, massive macrophage infiltration was found in the injured spinal cord (Fig. 10A), while in TN + LiCl group and TN + NSC + NS group, ED1-positive macrophage-infiltrated areas were decreased (Fig. 10B and C). The ED1-positive area in TN + NSC + LiCl group was smaller than any other group (Fig. 10D).
Fig. 10.
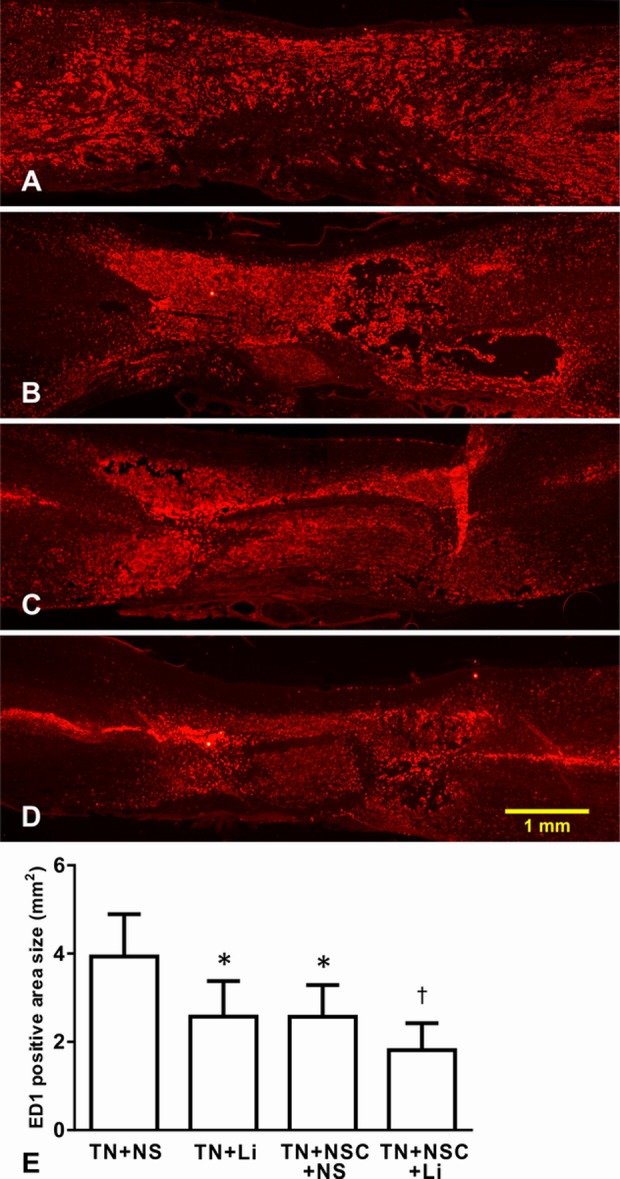
Expression of ED1 in different groups. (A) TN + NS group, massive macrophage infiltration was found in spinal cord; (B) TN + lithium chloride (LiCl) group; (C) TN + neural stem cell (NSC) + NS group; (D) TN + NSC + LiCl group; and (E) comparison of ED1-labeled area in different groups. *Compared with TN + NS group, P < 0.05; †compared with TN + NS group, P < 0.01.
We measured the ED1-positive area in coronal sections, and the ED1-covered areas of different groups were as follows: TN + NS group: 3.93 ± 0.96 mm2; TN + LiCl group: 2.77 ± 1.14 mm2; TN + NSC + NS group: 2.46 ± 0.71 mm2; and TN + NSC + LiCl group: 1.81 ± 0.61 mm2. After statistical analysis, TN + NS group had the largest macrophage-infiltrated area (Fig. 10E). ED1-covered areas in TN + LiCl group and TN + NSC + NS group were smaller (Fig. 10E). It looked like the TN + NSC + LiCl group had the smallest ED1-covered area of all the groups. However, statistical analysis revealed no significant differences among TN + LiCl group, TN + NSC + NS group, and TN + NSC + LiCl group (Fig. 10E).
Discussion
The goal of this study was to determine whether the transplantation of NSCs or LiCl injection or both would have any effect on the axonal regeneration and functional recovery after SCI. Our results demonstrate that both NSCs and LiCl significantly suppress traumatic inflammation, reduce host neuron death and tissue damage, and promote axonal regeneration and functional recovery. In addition, lithium enhances neuronal differentiation of transplanted NSCs. These 2 therapies in combination could, therefore, produce better results in the treatment of SCI.
Lithium Enhances Survival and Neuronal Differentiation of Transplanted NSCs
In our study, we first inject NSCs into the distal part of transected tibial nerve. One week after transplantation, no expression of nestin, NF200, NeuN, or ChAT was found in NSCs, while most of the NSCs were positive for GFAP expression. LiCl injection reduced GFAP expression but has no effect on other factors. As we know, peripheral nerve injury involves Wallerian degeneration, myelin collapse, and Schwann cell proliferation25. Schwann cells secrete large amounts of neurotrophic factors to promote repair of nerve injury, including BDNF, GDNF, and so on26. Although BDNF has a positive effect on neuronal differentiation of NSCs27, the effects of BDNF may be overtaken by other cells and cytokines inside damaged peripheral nerve, which could induce NSCs to differentiate into other cell types instead of neurons. It was reported that, under certain conditions, NSCs can differentiate into endothelial cells28 and myocytes29.
When NSCs were transplanted into an SCI gap via tibial nerve with 4 wk of LiCl intervention, more NSCs survived and some NSCs differentiated into neuron-like cells expressing NF200. It has been reported that transplanted NSCs can differentiate into astrocytes and neurons after SCI30–32. Kim’s study confirmed that LiCl promotes cultured hippocampal neural progenitor cells (NPCs) differentiation into a calbindin-positive neuronal cell type33. Su’s study showed LiCl contributes to transplanted NSCs differentiation into NeuN-positive neurons in normal spinal cord27. Our experiment showed that these differentiated neurons may come from GFAP-negative cells within transplanted NSCs. Since neuronal differentiation of NSCs takes time, the results of studies mentioned above were observed several months after transplantation. When LiCl intervention was extended; however, the local microenvironment could be improved and made suitable for the differentiation of NSCs into neurons. In addition, LiCl promotes the survival and neuronal differentiation of transplanted NSCs, which may be conducted through promoting NSCs’ secretion of BDNF34.
Lithium and NSC Suppress Trauma-related Inflammation and Preserve More Spared Tissue
In the setting of inflammatory cell infiltration that ensues after SCI, the expression of many inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1a, IL-1β, and IL-1, was significantly increased35. The cytotoxicity of these inflammatory cytokines can lead to apoptosis of neurons and oligodendrocytes. In acute SCI, macrophages and lymphocytes that infiltrate injured tissues secrete TNF and other neurotoxic factors, resulting in demyelination and axonal degeneration36. Astrocytes and microglial cells can inhibit anti-inflammatory cytokine IL-10 expression by synthesis of a variety of proinflammatory cytokines (for instance, IL-6 and TNF), inflammatory chemokines, and nitric oxide via the glycogen synthase kinase 3 (GSK-3) pathway37. Increased IL-6 expression can act on endogenous NSCs, leading to their differentiation into reactive astrocytes and expressed CSPG38, which, in turn, inhibit axonal regeneration39.
In our study, we found that there was still significant local macrophage infiltration even 4 wk after transplantation. Compared with the group with tibial nerve transplantation only, macrophage infiltration into the spinal cord in the other 3 groups was less, while animals treated with NSCs and LiCl had the least ED1 staining, indicating both NSCs and LiCl could inhibit inflammatory response after transplantation. Lithium is a direct inhibitor of GSK-3 by phosphorylation of serine, which inhibits the activity of GSK and, thus, suppresses inflammation40,41. Lithium also can reduce inflammation-induced neurotoxicity and reduce ischemia-related nerve damage42. A recent study has shown that long-term use of lithium can reduce p105 content in macrophages and inhibit lipopolysaccharide (LPS)-mediated NF-κB activation, which results in macrophage apoptosis43. De Meyer found that therapeutic doses of LiCl inhibit the enzyme inositol monophosphate and induce cultured macrophage apoptosis44. In addition, lithium’s ability to upregulate the expression of BDNF can also indirectly control the activity of GSK so as to suppress inflammation45. The coordinated effects of lithium and NSCs on the inhibition of inflammation and macrophage activity benefit both tissue repair and axon regeneration46.
It was reported that lithium inhibits macrophage activity by inhibiting LPS or inositol monophosphate43,44. Ben-Hur found that intraventricular transplantation of NPCs or intravenous NPC injection attenuated brain inflammation in acute and chronic encephalomyelitis by suppressing T-cell chemical substances46. Busch also found that multipotent progenitor cells significantly decrease matrix metalloproteinase-9 release from macrophages, effectively preventing induction of axonal dieback47.
Lithium and NSC Suppress Glial Scar Formation
Our results also suggest that decreased traumatic inflammation may be accompanied by reduced glial scar formation. In our study, tibial nerve transplantation with NSCs or LiCl injection after SCI results in smaller tissue reaction area than transplantation with tibial nerve only, and the combination of NSC transplantation and LiCl injection results in the smallest reaction area, indicating that either NSC transplantation or LiCl injection could inhibit glial scar formation. It’s well known that CSPG plays an important role in glial scar formation after SCI48 and accounts for inhibition of axonal regeneration49. Our results showed that both NSCs transplantation and lithium injection can reduce CSPG expression after SCI, which in accordance with the suppressed glial scar formation. Lithium has been reported to have neuroprotective effects in CNS injury and degenerative diseases. Yu applied LiCl in the treatment of brain injury, and there were significantly smaller damaged areas after LiCl treatment50. Hwang transplanted NSCs transfected with Olig2 gene after SCI, resulting in better white matter sparing and reduced cavity formation51. Gilad’s study showed that LiCl could reduce ornithine decarboxylase to enhance neuronal survival but inhibits astroglial growth, at the same time promoting transformation of astroglia from epithelioid (flat) to process-bearing morphology cells52. The results of our study support the idea that LiCl inhibits the functions of astrocytes and reduces the glial scar formation. However, the effect of NSC and lithium on the expression of CSPG after SCI needs further investigation.
Effect of Lithium and Transplanted NSCs on Host Neurons
In this study, we also investigate the effects of NSC transplantation and LiCl injection on host spinal neurons. Our results showed that, 4 wk after transplantation, there were more NeuN-expressing neurons spared both rostrally and caudally next to the site of injury of host spinal cord with NSC transplantation and/or LiCl injection compared with those without NSCs/LiCl intervention. This result indicates that the combination strategy of transplantation of NSCs and application of LiCl after SCI also have a protective effect on the host’s own motor neurons.
The possible mechanisms for the protection of host neurons by transplanted NSCs and LiCl include (1) LiCl directly inhibiting GSK-3 by phosphorylation of GSK-353, or indirect inhibition of GSK-3 by activating PI3K and Akt-1, thereby activating the β-catenin pathway15, reducing the death of neurons; (2) Our results showed the transplantation of NSCs and application of LiCl significantly inhibit macrophage infiltration, which also plays an important role in protecting host neurons; and (3) B cell lymphoma protein-2 (Bcl-2) pathway may also be involved in the survival of neurons. In CNS injury, Bcl-2 can protect neurons from apoptosis; and chronic administration of lithium dramatically increases the expression of Bcl-2 protein in nucleus magnocellularis neurons54,55.
Effect of Lithium and NSCs on Axonal Degeneration and Regeneration
In peripheral nerve injury, Wallerian degeneration occurs in the distal part of the affected nerve and proceeds in steps of axonal degeneration, myelin breakdown, macrophage infiltration, and removal of myelin and axon debris. In our study, when tibial nerve with NSCs was transplanted into the injured area of the spinal cord, the number of regenerated axons was higher in groups that received NSC transplantation and/or LiCl injection compared with the group with tibial nerve transplantation only at 4 wk after surgery. There were more regenerated axons in animals receiving NSC transplantation alone than receiving LiCl injection alone, and the combined application of NSCs and LiCl injection resulted in the best axonal regeneration. A recent study showed that transplantation of NSCs promotes axonal regeneration after SCI56, and the combination of neurotrophic factors, such as BDNF and GDNF, had better effects on axonal regeneration57–59.
It was reported that NSCs promote axonal regeneration by reducing CSPG immunoreactivity in the extracellular matrix in injured peripheral nerve12. The CSPG resulting from CNS injury can activate the GSK-3 signaling pathway to inhibit axonal regeneration60. LiCl can upregulate GSK-3 phosphorylation, inhibit GSK-3, and promote axonal growth61.
The increase in the number of axons spared by LiCl injection may also relate to its inhibition of inflammatory response. In our study, ED1 expression in tibial nerve was much less after LiCl intervention compared to the control group, indicating that both NSC transplantation and LiCl intervention inhibit the inflammatory response after nerve injury. In addition, there are more preserved host motoneurons after the application of NSCs and LiCl, and these neurons may go on to sprout more axons that are able to grow into transplanted tibial nerve.
Therefore, in this experiment, in addition to its known ability to inhibit GSK-3, lithium could promote the synthesis of BDNF in transplanted NSCs and promote axonal regeneration.
Effect of Lithium and NSCs on Functional Recovery after SCI
In our study, 2 and 4 wk after NSC transplantation, the BBB scores were higher in animals that received either NSC transplantation or LiCl injection than those that received tibial nerve transplantation only. These results show that after SCI, the transplantation of NSCs or injection of LiCl can significantly promote functional recovery of lower limb in rats, and treating with both could produce better results, in accordance with the enhancement of axonal regeneration explained above. Given together, NSC transplantation and LiCl injection significantly inhibit glial cell response after SCI, decrease macrophage infiltration, reduce glial scar formation, preserve more spared neural tissue and host neurons, promote axonal regeneration, and, finally, result in better recovery of motor function.
In summary, we provide evidence that lithium can enhance the NF200-positive neuron differentiation of transplanted NSCs in tibial nerve and that concomitant treatment with NSC transplantation and LiCl injection promotes axonal regeneration and functional recovery in spinal cord hemisectioned rats. The combination of NSC transplantation and LiCl injection may represent an effective strategy for promoting axonal regeneration in the injured spinal cord.
Acknowledgment
The authors would like to thank Clarity Manuscript Consultant LLC (Indianapolis) for their language editing assistance.
Authors’ Note: Li-Qun Zhang, Wen-Ming Zhang, and Jian-Hua Lin designed the experiments. Li-Qun Zhang, Zi-Xing Xu, and Wen-Bin Lan performed the experiments. Li-Qun Zhang and Lingxiao Deng wrote and revised this article.
Ethical Approval: This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Statement of Human and Animal Rights: Surgical procedures and postoperative care was performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by Natural Science Foundation of Fujian Province (grant no. 2015J01458), the National Natural Science Foundation of China (grant no. 81401008), and Key Clinical Specialty Discipline Construction Program of Fujian, China (grant no. 2012-149).
References
- 1. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. [DOI] [PubMed] [Google Scholar]
- 2. Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340(6233):471–473. [DOI] [PubMed] [Google Scholar]
- 3. Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994;372(6503):263–266. [DOI] [PubMed] [Google Scholar]
- 4. Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–189. [DOI] [PubMed] [Google Scholar]
- 6. Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288(5471):1660–1663. [DOI] [PubMed] [Google Scholar]
- 7. Liu YP, Seckin H, Izci Y, Du ZW, Yan YP, Baskaya MK. Neuroprotective effects of mesenchymal stem cells derived from human embryonic stem cells in transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2009;29(4):780–791. [DOI] [PubMed] [Google Scholar]
- 8. Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302(5642):113–117. [DOI] [PubMed] [Google Scholar]
- 9. Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422(6933):688–694. [DOI] [PubMed] [Google Scholar]
- 10. Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012;122(11):3824–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng LN, Duan XH, Zhong XM, Guo RM, Zhang F, Zhou CP, Shen J. Transplanted neural stem cells promote nerve regeneration in acute peripheral nerve traction injury: assessment using MRI. Am J Roentgenol. 2011;196(6):1381–1387. [DOI] [PubMed] [Google Scholar]
- 12. Heine W, Conant K, Griffin JW, Hoke A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004;189(2):231–240. [DOI] [PubMed] [Google Scholar]
- 13. Murakami T, Fujimoto Y, Yasunaga Y, Ishida O, Tanaka N, Ikuta Y, Ochi M. Transplanted neuronal progenitor cells in a peripheral nerve gap promote nerve repair. Brain Res. 2003;974(1–2):17–24. [DOI] [PubMed] [Google Scholar]
- 14. Baez JC, Gajavelli S, Thomas CK, Grumbles RM, Aparicio B, Byer D, Tsoulfas P. Embryonic cerebral cortex cells retain CNS phenotypes after transplantation into peripheral nerve. Exp Neurol. 2004;189(2):422–425. [DOI] [PubMed] [Google Scholar]
- 15. Young W. Review of lithium effects on brain and blood. Cell Transplant. 2009;18(9):951–975. [DOI] [PubMed] [Google Scholar]
- 16. Zhu ZF, Wang QG, Han BJ, William CP. Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull. 2010;83(5):272–277. [DOI] [PubMed] [Google Scholar]
- 17. Bian Q, Shi T, Chuang DM, Qian Y. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007;1184:270–276. [DOI] [PubMed] [Google Scholar]
- 18. Su H, Zhang W, Guo J, Guo A, Yuan Q, Wu W. Lithium enhances the neuronal differentiation of neural progenitor cells in vitro and after transplantation into the avulsed ventral horn of adult rats through the secretion of brain-derived neurotrophic factor. J Neurochem. 2009;108(6):1385–1398. [DOI] [PubMed] [Google Scholar]
- 19. Han SS, Kang DY, Mujtaba T, Rao MS, Fischer I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp Neurol. 2002;177(2):360–375. [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57(11):1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujimura A, Ohashi K, Ebihara A. Influence of chronic lithium treatment on urinary amount of furosemide in rats. Jpn J Pharmacol. 1991;56(4):421–426. [DOI] [PubMed] [Google Scholar]
- 22. Iannotti C, Li H, Yan P, Lu X, Wirthlin L, Xu XM. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol. 2003;183(2):379–393. [DOI] [PubMed] [Google Scholar]
- 23. National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press; 2011. p 246. [Google Scholar]
- 24. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. [DOI] [PubMed] [Google Scholar]
- 25. Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anatom. 1999;194(Pt 1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Su H, Chu TH, Wu W. Lithium enhances proliferation and neuronal differentiation of neural progenitor cells in vitro and after transplantation into the adult rat spinal cord. Exp Neurol. 2007;206(2):296–307. [DOI] [PubMed] [Google Scholar]
- 28. Sekiguchi H, Ii M, Jujo K, Thorne T, Ito A, Klyachko E, Hamada H, Kessler JA, Tabata Y, Kawana M, Asahi M, Hagiwara N, Losordo DW. Estradiol promotes neural stem cell differentiation into endothelial lineage and angiogenesis in injured peripheral nerve. Angiogenesis. 2013;16(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412(6848):736–739. [DOI] [PubMed] [Google Scholar]
- 30. Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA. 2005;102(39):14069–14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee KB, Choi JH, Byun K, Chung KH, Ahn JH, Jeong GB, Hwang IK, Kim S, Won MH, Lee B. Recovery of CNS pathway innervating the sciatic nerve following transplantation of human neural stem cells in rat spinal cord injury. Cell Mol Neurobiol. 2012;32(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Webber DJ, Bradbury EJ, McMahon SB, Minger SL. Transplanted neural progenitor cells survive and differentiate but achieve limited functional recovery in the lesioned adult rat spinal cord. Regen Med. 2007;2(6):929–945. [DOI] [PubMed] [Google Scholar]
- 33. Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, Son H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem. 2004;89(2):324–336. [DOI] [PubMed] [Google Scholar]
- 34. Chen K, Henry RA, Hughes SM, Connor B. Creating a neurogenic environment: the role of BDNF and FGF2. Mol Cell Neurosci. 2007;36(1):108–120. [DOI] [PubMed] [Google Scholar]
- 35. Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp Neurol. 2003;184(1):313–325. [DOI] [PubMed] [Google Scholar]
- 36. Emmetsberger J, Tsirka SE. Microglial inhibitory factor (MIF/TKP) mitigates secondary damage following spinal cord injury. Neurobiol Dis. 2012;47(3):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang WC, Lin YS, Wang CY, Tsai CC, Tseng HC, Chen CL, Lu PJ, Chen PS, Qian L, Hong JS, Lin CF. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology. 2009;128(Suppl 1): e275–e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okano H, Okada S, Nakamura M, Toyama Y. Neural stem cells and regeneration of injured spinal cord. Kidney Int. 2005;68(5):1927–1931. [DOI] [PubMed] [Google Scholar]
- 39. Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002;137:313–332. [DOI] [PubMed] [Google Scholar]
- 40. Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry. 1999;4(2):117–128. [DOI] [PubMed] [Google Scholar]
- 41. Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010;31(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21(2):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang M, Jin W, Zhou X, Yu J, Lee AJ, Sun SC. Deregulation of Tpl2 and NF-kappaB signaling and induction of macrophage apoptosis by the anti-depressant drug lithium. Cell Signal. 2009;21(4):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Meyer I, Martinet W, Van Hove CE, Schrijvers DM, Hoymans VY, Van Vaeck L, Fransen P, Bult H, De Meyer GR. Inhibition of inositol monophosphatase by lithium chloride induces selective macrophage apoptosis in atherosclerotic plaques. Br J Pharmacol. 2011;162(6):1410–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Phiel CJ, Klein PS. Molecular targets of lithium action. Annu Rev Pharmacol Toxicol. 2001;41:789–813. [DOI] [PubMed] [Google Scholar]
- 46. Ben-Hur T. Immunomodulation by neural stem cells. J Neurological Sci. 2008;265(1–2):102–104. [DOI] [PubMed] [Google Scholar]
- 47. Busch SA, Hamilton JA, Horn KP, Cuascut FX, Cutrone R, Lehman N, Deans RJ, Ting AE, Mays RW, Silver J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J Neurosci. 2011;31(3):944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siebert JR, Conta Steencken A, Osterhout DJ. Chondroitin sulfate proteoglycans in the nervous system: inhibitors to repair. Biomed Res Int. 2014;2014:845323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ohtake Y, Li S. Molecular mechanisms of scar-sourced axon growth inhibitors. Brain Res. 2015;1619:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, Chuang DM. Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J Neurotrauma. 2012;29(2):362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hwang DH, Kim BG, Kim EJ, Lee SI, Joo IS, Suh-Kim H, Sohn S, Kim SU. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gilad GM, Gilad VH. Astroglia growth retardation and increased microglia proliferation by lithium and ornithine decarboxylase inhibitor in rat cerebellar cultures: Cytotoxicity by combined lithium and polyamine inhibition. J Neurosci Res. 2007;85(3):594–601. [DOI] [PubMed] [Google Scholar]
- 53. Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93(16):8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bush AL, Hyson RL. Lithium increases bcl-2 expression in chick cochlear nucleus and protects against deafferentation-induced cell death. Neuroscience. 2006;138(4):1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schuettauf F, Rejdak R, Thaler S, Bolz S, Lehaci C, Mankowska A, Zarnowski T, Junemann A, Zagorski Z, Zrenner E, Grieb P. Citicoline and lithium rescue retinal ganglion cells following partial optic nerve crush in the rat. Exp Eye Res. 2006;83(5):1128–1134. [DOI] [PubMed] [Google Scholar]
- 56. Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150(6):1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88(6):1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deng LX, Hu J, Liu N, Wang X, Smith GM, Wen X, Xu XM. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp Neurol. 2011;229(2):238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deng LX, Deng P, Ruan Y, Xu ZC, Liu NK, Wen X, Smith GM, Xu XM. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci. 2013;33(13):5655–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28(36):8914–8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Gu Q, Dong Y, Zhou W, Song H, Liu Y, Liu M, Yuan Y, Ding F, Gu X, Wang Y. Inhibition of gecko GSK-3beta promotes elongation of neurites and oligodendrocyte processes but decreases the proliferation of blastemal cells. J Cell Biochem. 2012;113(6):1842–1851. [DOI] [PubMed] [Google Scholar]