Abstract
Psoriasis is a chronic skin condition whose pathogenesis is reported to be due to the activation of the interleukin-23/interleukin-17 (IL-23/IL-17) pathway. Here, we report that indoleamine 2,3-dioxygenase (IDO)-expressing fibroblasts reduce the activity of this pathway in activated immune cells. The findings showed that intralesional injection of IDO-expressing fibroblasts in imiquimod-induced psoriasis-like dermatitis on the back and ear (Pso. ear group) in mice significantly improves the clinical lesional appearance by reducing the number of skin-infiltrated IL-17+ CD4+ T cells (1.9% ± 0.3% vs. 6.9% ± 0.6%, n = 3, P value < 0.01), IL-17+ γδ+ T cells (2.8% ± 0.3% vs. 11.6% ± 1.2%, n = 3, P value < 0.01), IL-23+ activated dendritic cells (7.6% ± 0.9% vs. 14.0% ± 0.5%, n = 3, P < 0.01), macrophages (4.3% ± 0.1% vs. 11.3% ± 1.0%, n = 3, P value < 0.01), and granulocytes (2.5% ± 0.4% vs. 4.5% ± 0.3%, n = 3, P value < 0.01) as compared to untreated psoriatic mice. This finding suggests that IDO-expressing fibroblasts, and to a lesser extent, non-IDO primary fibroblasts suppress the psoriatic-like symptoms by inhibiting the infiltration of key immune cells involved in the development of psoriasis.
Keywords: IDO, psoriasis, IMQ, IL-17, IL-23, fibroblasts
Introduction
Psoriasis is one of the most common recurrent chronic inflammatory skin diseases, affecting 2% to 3% of the general population1. Recent studies demonstrated that interleukin-23 (IL-23) T helper 17 (Th17) pathway is linked to psoriasis2. It is reported that inflammatory myeloid dendritic cells expressing IL-23 initiate a set of signals that are crucial for the development and maintenance of Th173. The activated Th17 cells further secrete IL-17 and IL-22, which promote further recruitment of immune cells, keratinocyte proliferation, and sustained chronic inflammation3–5. There are several commercially available biological monoclonal antibodies (LY-2439821, AMG-827, and AZ17) at different phases of human clinicaltrials, which target the IL-23/IL-17 axis6,7. Although the use of these injectable biologics is far more effective than other treatments, the cost and high risk of side effects make them still less desirable. This is because patients treated with anti-IL-12 and/or IL-23 cytokine showed potential opportunistic infections such as salmonellosis and mycobacterial. These reports showed that immunity against these microorganisms depends on the expression of IL-12 and/or IL-238,9. As such, there is still a need to find a new strategy through which psoriasis can be treated without compromising the functionality of the immune system and any potential side effects.
Indoleamine 2,3-dioxygenase (IDO), which is a cytosolic rate-limiting enzyme and is mainly expressed by macrophages, dendritic cells, and trophoblasts, breaks down tryptophan (Trp)10. The expression of IDO by antigen-presenting cells (APCs) in vivo allows them to suppress unwanted T cell responses11. Our studies found that IDO-expressing primary dermal fibroblasts suppress inflammatory cell proliferation and induce apoptotic death in some subsets of T lymphocytes, but not skin cells12. These findings let us ask the question of whether intralesional injection of IDO-expressing fibroblasts in a previously used imiquimod (IMQ; Aldara cream) psoriatic mouse model13 improves the psoriatic clinical appearance by modulating the IL-23/Th17 pathway activity. Our findings revealed that the critical factors associated with psoriasis, such as clinical appearance, skin erythema and scaling score, skin thickness, the number of infiltrated IL-17-producing T cells, and IL-23-producing dendritic cells, are significantly improved upon injecting IDO-expressing fibroblasts.
Materials and Methods
Preparation of IDO-expressing Fibroblasts
As previously reported14, primary dermal fibroblasts from allogeneic B6 mice were isolated from skin biopsies and transduced with a lentiviral vector that contained IDO and Red Cherry gene. Following verification of IDO expression and enzyme activity, these cells were used for intralesional injection of psoriatic affected areas.
Induction of Psoriasis-like Condition
Eight- to 10-wk female BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice received a daily topical dose of 55 mg of IMQ cream (5%; Aldara; 3M Pharmaceuticals, London, Ontario, Canada) on the shaved areas of the mouse back and 5 mg on the right ear for 6 d to generate localized and generalized psoriasis. This dose was determined to induce psoriasis-like skin inflammation on day 3. On day 3, mice were divided into 5 groups, no IMQ treatment (normal), IMQ-treated (Pso.), IMQ plus either medium (Sham), 4 × 106 non-IDO fibroblast (Pso. + Fib), or 4 × 106 IDO-Red Cherry-expressing fibroblast injected (Pso. + IDOFib). Mice were intralesionally injected with either 4 × 106 cells or control fibroblasts/100 μL of medium/spot on dorsal areas (4 spots/mouse).
The lesions were photographed every other day. The presence of erythema and scaling on the back and ears were scored on a scale from 0 to 4 by an unbiased observer (0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked). Skin and ear thickness were measured every other day using the digital caliper. Upon euthanizing the mice on day 9, the size and weight of the auxiliary and inguinal lymph nodes and spleen were taken and evaluated.
Flow Cytometry
Mice were euthanized on day 9, and dorsal skins, right ears, and lymph nodes were collected and minced and digested with collagenase D (Sigma-Aldrich, Oakville, Ontario, Canada) (1 mg/mL) for 30 min. Samples were then gently pressed against 40-µm nylon mesh to pass through and release cells into a culture medium. Cell suspensions from the skin of the lesion, right ears, and lymph nodes were used, and the number of the granulocytes and macrophages were determined by their surface markers using fluorescein isothiocyanate (FITC)-conjugated anti-Gr-1 (eBiosciences, San Diego, CA, USA) for granulocytes, APC-conjugated anti-F4-80 (eBiosciences), and phycoerythrin (PE)-conjugated anti-CD11b for macrophages in 1% fetal bovine serum + phosphate buffered saline (FBS + PBS). In order to characterize IL-17-producing T cells and IL-23-producing Dendritic Cells (DCs), cell suspensions from the skin, right ears, and lymph nodes were cultured and stimulated with stimulating Cocktail (plus protein transport inhibitors; eBiosciences) overnight. To perform surface staining, 1 × 106 cells were stained for 30 min at room temperature in 1% FBS + PBS containing the PE-conjugated anti-CD4 (eBiosciences) for T cells and FITC-conjugated anti-γδ (eBiosciences) for T cells or FITC-conjugated anti-CD11c (eBiosciences) and PE-conjugated anti-CD86 (eBiosciences) for dendritic cells. In the next step, the cells were fixed and permeabilized using a fixation–permeabilization buffer (eBioscience) and incubated for 45 min at 4 °C. Cells were then washed once with permeabilization buffer (eBioscience) followed by intracellular staining using eFleur-conjugated anti-mouse IL-17 (0.5 µg per 100 µL) and eFleur-conjugated anti-mouse IL-23 (0.5 µg per 100 µL; eBioscience). Cells were analyzed using a BD Accuri™ C6 Flow Cytometer, gates on physical parameters. A total of 20,000 events were recorded for each assay.
Immunohistochemical Staining
Lesional areas were harvested at the end point of experiments, fixed in 10% buffered formalin solution, and embedded in paraffin. Tissue sections 5 μm thick were prepared and stained with hematoxylin and eosin (H&E) to evaluate the epidermal thickness. Sections were rehydrated, and nonspecific binding was blocked. In another set of experiments, upon blocking nonspecific binding, sections were incubated overnight at 4 °C with rabbit anti-CD3 primary antibodies (1:50 dilution; Abcam, Cambridge, MA, USA). Sections were then washed and incubated with rhodamine goat anti-rat immunoglobulin G secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 45 min. Finally, the slides were dehydrated and mounted in Permount (Fisherbrand, Pittsburgh, PA, USA).
Statistical Analysis
Data are reported as a mean ± standard deviation of 3 or more independent set of experiments. The statistical differences of mean value among treated and control groups were tested with one-way analysis of variance followed by post hoc comparisons using Bonferroni correction. A P value less than 0.05 was considered statistically significant.
Ethics Statement
All methods and procedures as well as the use of animals and tissue specimens derived from animals were approved by the Animal Ethics Committee of the University of British Columbia. Care and maintenance of all animals used in this study were in accordance with the principles of laboratory animal care and the guidelines of the institutional Animal Policy and Welfare Committee at The University of British Columbia.
Results
Intralesional Injection of IDO-expressing Fibroblasts Improves Clinical Appearance of Psoriatic-like Lesion
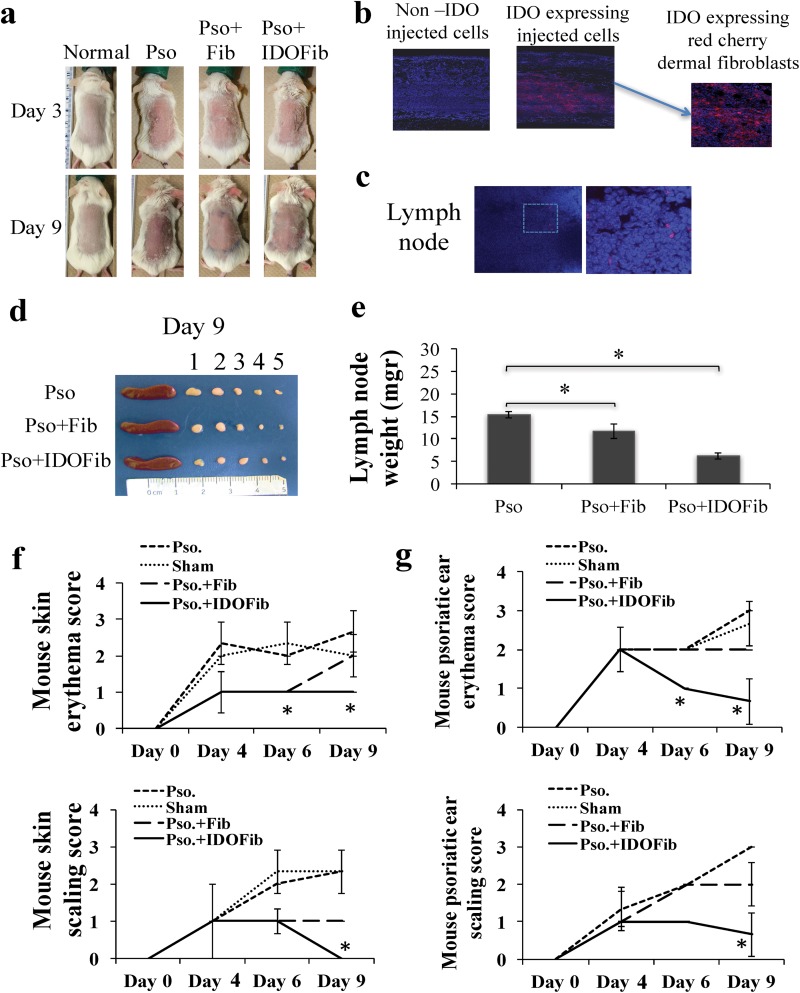
Daily topical application of IMQ caused skin erythema as early as day 3 and remained high up to day 9 tested (Fig. 1a). However, intralesional injection of IDO-expressing fibroblasts, and to a lesser extent, non-IDO-expressing fibroblast significantly improved psoriatic-induced erythema (Fig. 1a). Using fluorescence confocal microscopy, the IDO- and Red Cherry-expressing fibroblasts remained in the dermis (Fig. 1b) and some of them migrated to local auxiliary lymph nodes (Fig. 1c). The size and weight of the auxiliary lymph nodes (numbers 1 to 3, Fig. 1d), taken from psoriatic mice treated with intralesional injection of IDO (6.2 ± 0.8 vs. 15.3 ± 0.6 mg, n = 3, P value < 0.01) and, to a lesser extent, non-IDO-expressing cells (11.7 ± 1.6 vs. 15.3 ± 0.6 mg, n = 3, P value < 0.01), were markedly less than those taken from the psoriatic mice (Fig. 1d and e). No significant change has been detected in the weight of inguinal lymph nodes (data not shown). Our data revealed that significantly less erythema and silver scales scoring skin and ears of mice received intralesional injection of IDO-expressing fibroblasts than nontreated positive controls (Fig. 1f and g).
Fig. 1.
Psoriatic-like lesions before and after treatment. Psoriasis-like skin inflammation induced in BALB/c mice by daily topical application of 5% imiquimod (IMQ) cream. On day 3, mice were either untreated or treated with intralesionally-injecting medium, 4 × 106 fibroblasts or 4 × 106 indoleamine 2,3-dioxygenase (IDO)-expressing fibroblasts. The presence of erythema and scaling of the back skin and ear were scored on a scale from 0 to 4 (0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked). (a) Clinical appearance of IMQ-induced psoriatic-like lesions treated and untreated on days 3 and 9. (b) Presence of IDO-expressing fibroblasts in the dermis of injected lesions on day 9. (c) Presence of IDO-Red Cherry–expressing cells in regional lymph nodes. (d and e) The size and weight of auxiliary (numbers 1 to 3), inguinal lymph nodes (numbers 4 and 5) and spleen in treated and control mice. (f and g) Back skin and ear erythema and silver scale scoring in mice received different treatments as a function of time. The significant differences have been indicated by asterisks (*; P < 0.01; n = 3).
Skin Thickness Is Reduced in Psoriatic Animals that Received IDO-expressing Fibroblasts
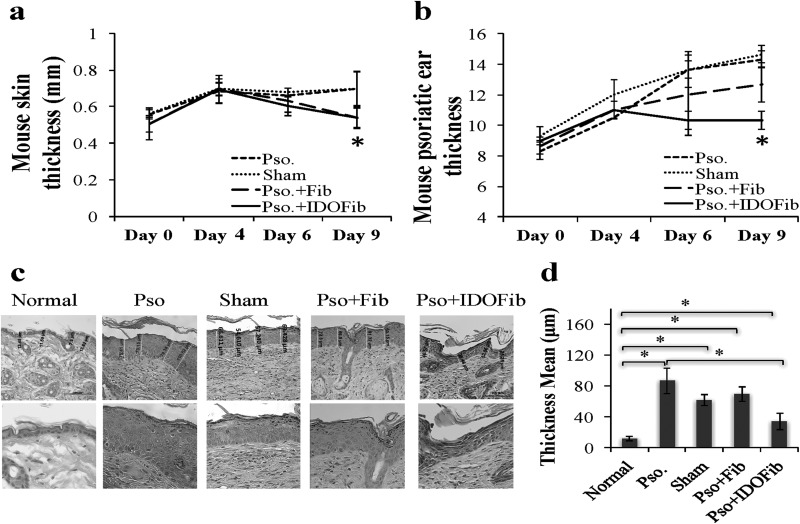
As shown in Fig. 2a, upon daily application of IMQ cream, skin thickness significantly increased on day 4 as compared to day 0 (0.69 ± 0.0 vs. 0.53 ± 0.03 mm, n = 12, P value < 0.01). This thickness remained the same for sham and Pso. groups on day 6 compared to day 4 (0.68 ± 0.02 vs. 0.70 ± 0.03 mm and 0.66 ± 0.0 mm vs. 0.68 ± 0.07, n = 3, P value > 0.05). However, this increase was abrogated upon injection of IDO-expressing fibroblasts (0.6 ± 0.05 vs. 0.69 ± 0.07 mm, n = 3, P value > 0.05). This further reduced up to day 9 (0.54 ± 0.05 vs. 0.69 ± 0.07 mm, n = 3, P value < 0.01) tested (Fig. 2a). Similarly, daily application of IMQ cream increased the ear thickness at day 4 as compared to day 0 (11.1 ± 0.63 vs. 8.8 ± 0.43 mm, n = 12, P value < 0.01; Fig. 2b). Our data showed no significant changes in ear thickness in mice with the injection of IDO-expressing fibroblasts on day 6 (10.30 ± 0.58 vs. 11.00 ± 0.58 mm, n = 3, P value > 0.05) and day 9 (10.30 ± 0.58 vs. 11.00 ± 0.58 mm, n = 3, P value > 0.05) as compared to that of day 4 (Fig. 2b). However, there was a marked increase in ear thickness in mice left untreated (Pso.; 14.33 ± 0.58 vs. 10.50 ± 0.00 mm, n = 3, P value < 0.01), treated with injecting medium (sham; 14.7 ± 0.58 vs. 12.0 ± 1.00 mm, n = 3, P value < 0.01), or non-IDO fibroblast (12.70 ± 1.1 vs. 11.00 ± 0.58 mm, n = 3, P value < 0.01) on day 9 compared to day 4 (Fig. 2b). The result of H&E staining of psoriatic lesion showed a significant increase in the thickness of epidermal layer of skin of Pso. group (86.7 ± 16.82 vs. 11.61 ± 2.84 μm, n = 3, P value < 0.01), sham (61.72 ± 6.82 vs. 11.61 ± 2.84 μm, n = 3, P value < 0.01), or non-IDO fibroblast (69.19 ± 9.65 vs. 11.61 ± 2.84 μm, n = 3, P value < 0.01) compared to that of normal mice and markedly reduced in injected IDO-expressing fibroblasts compared to that of normal mice (34.34 ± 10.42 vs. 11.61 ± 2.84 μm, n = 3, P value < 0.01; Fig. 2c and d).
Fig. 2.
Epidermal thickness following cell therapy. Skin and ear thickness were measured every other day using a digital caliper. On day 9 of the experiment, mice were sacrificed and back skin was harvested, fixed in 10% buffered formalin solution and embedded in paraffin. Tissue sections of 5 μm thickness were stained with hematoxylin and eosin (H&E). (a and b) Skin and ear thickness in control and treated psoriatic lesions were measured every other day using a digital caliper. (c and d) H&E-stained psoriatic skin lesion showing epidermal thickness and its quantitative analysis, respectively. The significant differences have been indicated by asterisks (*; P < 0.01; n = 3).
Intralesional Injection of IDO-expressing Fibroblasts Reduces Infiltration of Granulocytes and Macrophages
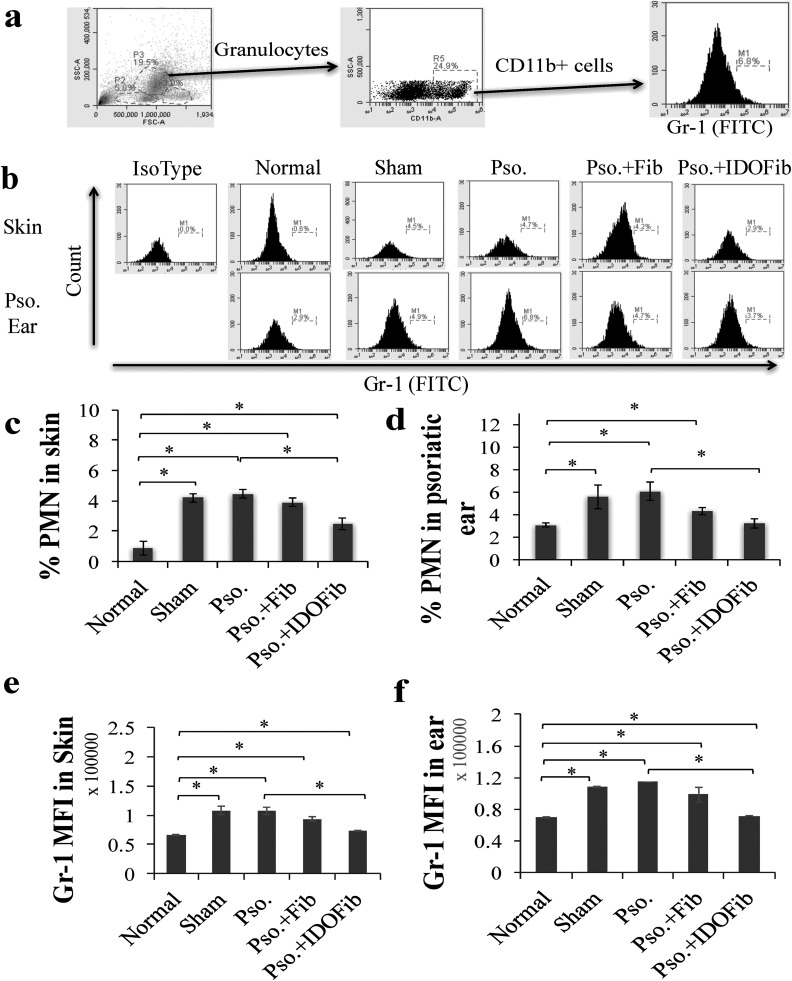
Granulocytes and macrophages in psoriatic lesions were identified as CD11b+ Gr-1+ and CD11b+ F4-80+ cells, respectively. Granulocytes were gated by their characteristic forward and side-scatter profiles that represent size and granularity of cells. The total population of cells within the CD11b gate was distributed as a scatterplot of fluorescence intensity versus side scatter. Gate for Gr-1 antigen was applied to CD11b+ granulocyte population to determine the mean fluorescence intensity (MFI) and relative expression of Gr-1 in skin and ear (Fig. 3a). The frequency of CD11b+ Gr-1+ cells in psoriatic-like lesions significantly increased in those of medium injected (sham; 4.2% ± 0.3% vs. 0.9% ± 0.5%, n = 3, P value < 0.01), Pso. group (4.5% ± 0.3% vs. 0.9% ± 0.5%, n = 3, P value < 0.01), and non-IDO fibroblasts injected (3.9% ± 0.3% vs. 0.9% ± 0.5%, n = 3, P value < 0.01) as compared to normal group (Fig. 3b and c). This increase significantly decreased in psoriatic IDO-expressing fibroblast-treated mice compared to Pso. group (2.5% ± 0.4% vs. 4.5% ± 0.3%, n = 3, P value < 0.01). Moreover, the MFI of Gr-1 molecule significantly increased in sham (107,857.2 ± 7,050.9435 vs. 65,780.9 ± 1,260.6, n = 3, P value < 0.01), Pso. (108,011.3 ± 6,288.48 vs. 65,780.9 ± 1,260.6, n = 3, P value < 0.01), and Pso. + Fib groups (93,587.9 ± 3,310.87 vs. 65,780.9 ± 1,260.6, n = 3, P value < 0.01) compared to normal mice. However, the levels of Gr-1 decreased in the IDO-expressing fibroblast-treated mice as compared to Pso. group (72,885.4 ± 716.06 vs. 108,011.3 ± 6,288.48, n = 3, P value < 0.01; Fig. 3e). Further, our results revealed that the number of granulocytes in IMQ-treated mouse ears significantly increased in Pso. group (6.1% ± 0.8% vs. 3.1% ± 0.2%, n = 3, P value < 0.01), sham (5.6% ± 1.0% vs. 3.1% ± 0.2%, n = 3, P value < 0.01), and non-IDO fibroblast-treated lesions (4.3% ± 0.3% vs. 3.1% ± 0.2%, n = 3, P value < 0.01) as compared to normal group. This increase significantly decreased upon injection of IDO-expressing fibroblasts (3.2% ± 0.4% vs. 6.1% ± 0.8%, n = 3, P value < 0.01; Fig. 3b and d). The MFI of Gr-1 was significantly decreased in the ear of IDO-expressing fibroblast-treated mice as compared to Pso. group (71,613.6 ± 327.4 vs. 114,562.1 ± 309.2, n = 3, P value < 0.01; Fig. 3f).
Fig. 3.
The frequency of CD11b+ Gr-1+ cells in the skin, ear, and lymph nodes after cell therapy. Upon euthanizing mice on day 9, the dorsal skins and right ears were collected. After obtaining single-cell suspensions, cells were stimulated in Cell Stimulation Cocktail overnight. Granulocytes were stained for specific surface markers for 30 min at room temperature in 1% fetal bovine serum + phosphate buffered saline–containing fluorescein isothiocyanate-conjugated anti-Gr-1 and phycoerythrin-conjugated anti-CD11b. (a) Representative flow cytometry plots of gating. (b) Representative flow cytometry plots of Gr-1+ CD11b+ cells in skin and ear. (c and d) Quantitative analysis of granulocytes in skin and ear, respectively. (e and f) Mean fluorescence intensity (MFI) of Gr-1 in skin and ear, respectively. Pso. ear, right ear which has received 5 mg imiquimod cream. The significant differences have been indicated by asterisks (*; P < 0.01; n = 3).
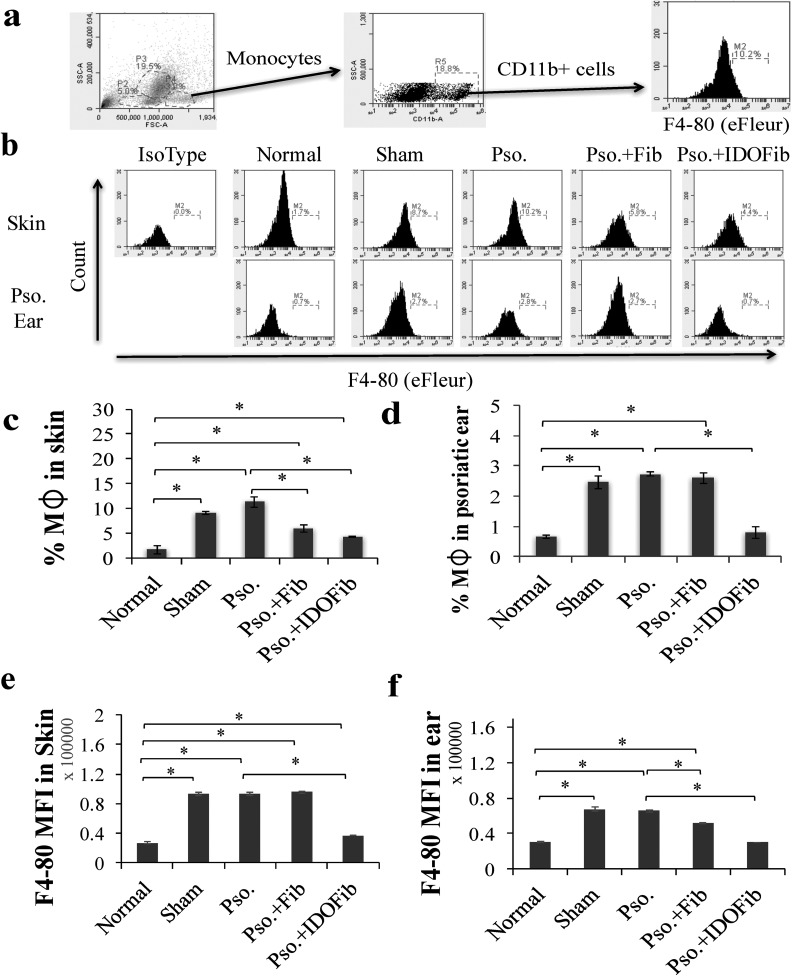
Monocytes were gated by their characteristic forward and side-scatter profiles that represent size and granularity of cells. The total population of cells within the CD11b gate was distributed as a scatterplot of fluorescence intensity versus side scatter. Gate for F4-80 antigen was applied to CD11b+ monocyte population to determine the MFI and relative expression of F4-80 in skin and ear (Fig. 4a). The number of CD11b+ F4-80+ macrophages is also increased in the skin of Pso. mice (11.3% ± 1.0% vs. 1.7% ± 0.8%, n = 3, P value < 0.01), sham (9.1% ± 0.4% vs. 1.7% ± 0.8%, n = 3, P value < 0.01), and non-IDO fibroblast injected groups (6.0% ± 0.8% vs. 1.7% ± 0.8%, n = 3, P value < 0.01) compared to normal mice (Fig. 4b and c). In contrast, there was a considerable reduction in the number of these cells in IDO-expressing fibroblast-treated mice compared to Pso. mice (4.3% ± 0.1% vs. 11.3% ± 1.0%, n = 3, P value < 0.01; Fig. 4b and c). The frequency of macrophages also significantly increased in the psoriatic ear lesions of Pso. (2.8% ± 0.1% vs. 0.7% ± 0.1%, n = 3, P value < 0.01), sham (2.5% ± 0.2% vs. 0.7% ± 0.1%, n = 3, P value < 0.01), and non-IDO fibroblast-treated lesions (2.6% ± 0.2% vs. 0.7% ± 0.1%, n = 3, P value < 0.01; Fig. 4b and d). IDO cell therapy reduced the number of these cells in the ear compared to Pso. mice (0.8% ± 0.2% vs. 2.7% ± 0.1%, n = 3, P value < 0.01; Fig. 4b and d). MFI of F4-80 was significantly decreased in the skin (37,254.4 ± 358.0 vs. 93,444.1 ± 1,732.1, n = 3, P value < 0.01) and ear (30,344.1 ± 176.4 vs. 65,677.8 ± 1,347.0, n = 3, P value < 0.01) of IDO-expressing fibroblast-treated mice as compared to the Pso. group (Fig. 4e and f).
Fig. 4.
The frequency of CD11b+ F4-80+ cells in the skin and ear after cell therapy. Upon euthanizing mice on day 9, the dorsal skins and right ears were collected. After obtaining single-cell suspensions, cells were stimulated in Cell Stimulation Cocktail overnight. Monocytes were stained for specific surface markers for 30 min at room temperature in 1% fetal bovine serum + phosphate buffered saline–containing adenomatous polyposis coli–conjugated anti-F4-80 and phycoerythrin-conjugated anti-CD11b. (a) Representative flow cytometry plots of gating. (b) Representative flow cytometry plots of F4-80+ CD11b+ cells in skin and ear. (c and d) Quantitative analysis of monocytes in skin and ear, respectively. (e and f) Mean fluorescence intensity (MFI) of F4-80 in skin and ear, respectively. Pso. ear, right ear which has received 5 mg imiquimod cream. The significant differences have been indicated by asterisks (*; P < 0.01; n = 3).
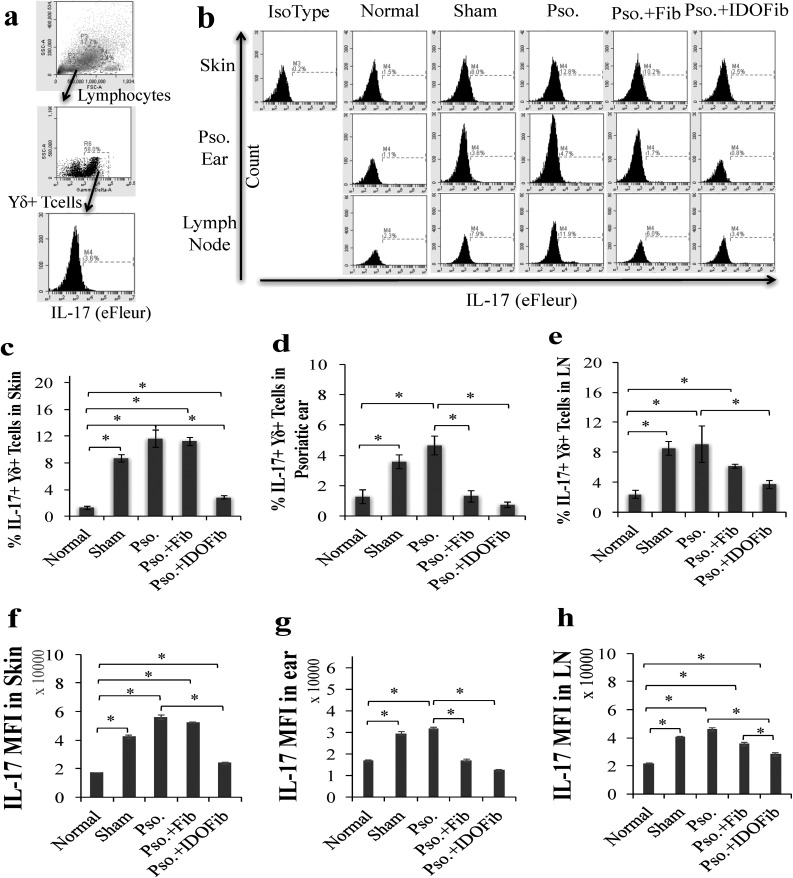
Intralesional Injection of IDO-expressing Fibroblasts Reduces Infiltration of Other Key Immune Cells
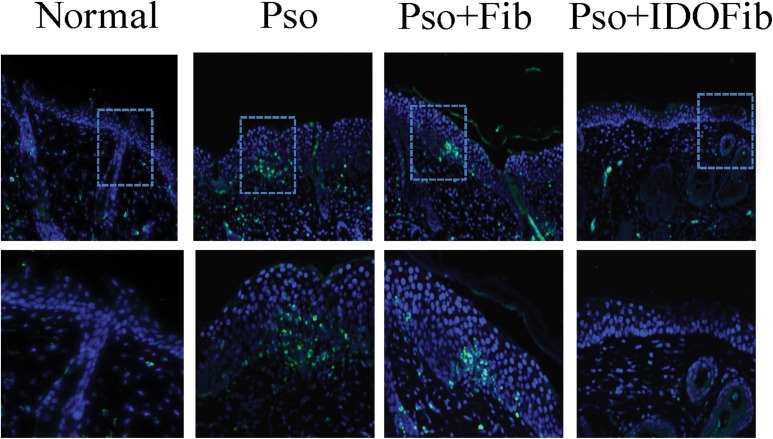
We also asked whether intralesional injection of IDO-expressing fibroblasts influences the number of the infiltrated CD3+ T cells. The results showed a marked reduction in the number of these cells in IDO-expressing fibroblasttreated lesions as compared to either none (Pso.) or non-IDO-expressing cell treated mice (Fig. 5). As IL-23/IL-17 axis is a key psoriasis treatment target and infiltrated IL-17+ γδ+ T cells, IL-17+ CD4+ T cells, and IL-23+ CD11c+ dendritic cells are associated with psoriatic development; here, we evaluated the presence of these cells in the psoriatic affected skin, ear, and lymph nodes. Lymphocytes were gated by their characteristic forward and side-scatter profiles that represent size and granularity of cells. The total population of cells within the γδ and CD4 gates were distributed as a scatterplot of fluorescence intensity versus side scatter. Gate for IL-17 antigen was applied to γδ+ and CD4+ populations to determine the MFI and relative expression of IL-17 in skin, ear, and lymph nodes (Figs. 6a and 7a). As shown in Figs. 6b to 4e, the number of γδ+ T cells–expressing IL-17 dramatically increased in Pso. mouse skin (11.6% ± 1.2% vs. 1.3% ± 0.3%, n = 3, P value < 0.01), ear (4.6% ± 0.6% vs. 1.3% ± 0.5%, n = 3, P value < 0.01), and lymph nodes (9.1% ± 2.4% vs. 2.4% ± 0.6%, n = 3, P value < 0.01) compared to normal mice. Intralesional injection of neither medium (8.7% ± 0.6% vs. 1.3% ± 0.3%, n = 3, P value < 0.01) nor non-IDO fibroblasts (11.2% ± 0.6% vs. 1.3% ± 0.3%, n = 3, P value < 0.01) reversed the frequency of these cells in psoriatic skin (Fig. 6b and c). However, intralesional injection of IDO-expressing fibroblasts significantly reduced the number of these inflammatory cells in skin compared to untreated psoriatic mice (2.8% ± 0.3% vs. 11.6% ± 1.2%, n = 3, P value < 0.01; Fig. 6b and c). No significant improvement has been detected in the number of IL-17-producing γδ+ T cells in psoriatic ear of medium treated (sham) mice compared to Pso. ears (3.6% ± 0.5% vs. 4.6% ± 0.6%, n = 3, P value > 0.05). However, intralesional injection of IDO-expressing fibroblasts (0.7% ± 0.2% vs. 4.6% ± 0.6%, n = 3, P value < 0.01) and, to a lesser extent, non-IDO fibroblast (1.3% ± 0.4% vs. 4.6% ± 0.6%, n = 3, P value < 0.01) significantly decreased the frequency of IL-17+ γδ+ T cells in psoriatic ear as compared to Pso. mice (Fig. 6b and d). We further found that injection of IDO-expressing fibroblasts (3.7% ± 0.6% vs. 9.1% ± 2.4%, n = 3, P value < 0.01), but neither sham (8.5% ± 0.9% vs. 9.1% ± 2.4%, n = 3, P value > 0.05) nor non-IDO fibroblasts (6.1% ± 0.2% vs. 9.1% ± 2.4%, n = 3, P value > 0.05) decreased the frequency of lymph node IL-17-producing γδ+ T cells compared to psoriatic untreated mice (Fig. 6b and e). MFI of IL-17 was significantly increased in Pso. mouse skin (55,974.2 ± 1,594.3 vs. 17,134.5 ± 126.6, n = 3, P value < 0.01), ear (31,639.9 ± 598.6 vs. 16,818.4 ± 416.6, n = 3, P value < 0.01), and lymph node (46,005.2 ± 1,325.3 vs. 21,454.0 ± 349.7, n = 3, P value < 0.01) as compared to the normal group. However, it was significantly decreased in IDO-expressing fibroblast-treated mice (skin: 23,804.9 ± 460.8 vs. 55,974.2 ± 1,594.3; ear: 12,438.3 ± 353.1 vs. 31,639.9 ± 598.6; and lymph nodes: 28,160.4 ± 1,120.7 vs. 46,005.2 ± 1,325.3, n = 3, P value < 0.01) as compared to the Pso. mice (Fig. 6f to h).
Fig. 5.
Indoleamine 2,3-dioxygenase–expressing fibroblasts reduce infiltration of T cells in the skin. The presence of CD3 T cells in skin sections. Single immunofluorescence staining for CD3 was done for 5-μm thick sections of skin taken on day 9 using rabbit anti-CD3 primary antibodies and rhodamine goat anti-rat immunoglobulin G as the secondary antibody.
Fig. 6.
Indoleamine 2,3-dioxygenase–expressing fibroblasts suppress infiltration of IL-17+ γδ+ T cells in skin, ear, and lymph node of psoriatic mice. Upon euthanizing mice on day 9, the dorsal skins, right ears, and lymph nodes were collected. After obtaining single-cell suspensions, cells were stimulated in Cell Stimulation Cocktail overnight. T cells were stained for their surface and intracellular markers in 1% fetal bovine serum + phosphate buffered saline–containing fluorescein isothiocyanate–conjugated anti-γδ and eFleur-conjugated anti-IL-17. (a) Representative flow cytometry plots of gating. (b) Representative flow cytometry plots of IL-17+ γ+ T cells in skin, ear, and lymph nodes. (c to e) Quantitative analysis of IL-17+ γδ+ T cells in skin, ear, and lymph nodes, respectively. (f to h) Mean fluorescence intensity (MFI) of IL-17 in skin, ear, and lymph nodes, respectively. Pso. ear, right ear which has received 5 mg imiquimod cream. The significant differences have been indicated by asterisks (*; P < 0.01; n = 3).
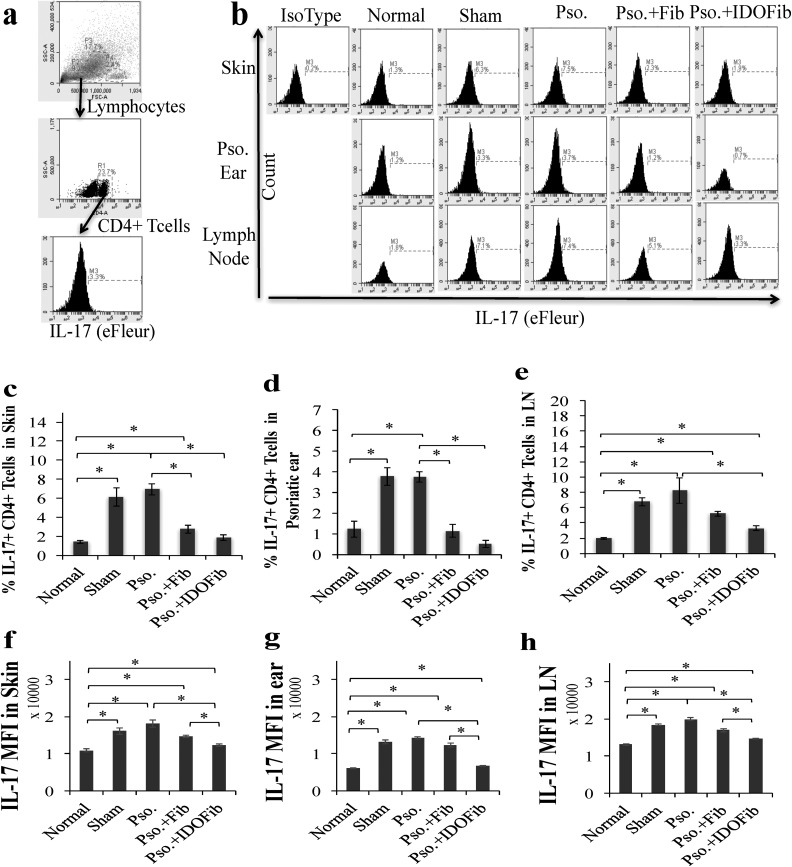
Fig. 7.
Indoleamine 2,3-dioxygenase–expressing fibroblasts suppress infiltration of IL-17+ CD4+ T cells in skin, ear, and lymph node of psoriatic mice. Upon euthanizing mice on day 9, the dorsal skins, right ears, and lymph nodes were collected. After obtaining single-cell suspensions, cells were stimulated in Cell Stimulation Cocktail overnight. T cells were stained for their surface and intracellular markers in 1% fetal bovine serum + phosphate buffered saline–containing phycoerythrin-conjugated anti-CD4 and eFleur-conjugated anti-IL-17. (a) Representative flow cytometry plots of gating. (b) Representative flow cytometry plots of IL-17+ CD4+ T cells in skin, ear, and lymph nodes. (c to e) Quantitative analysis of IL-17+ CD4+ T cells in skin, ear, and lymph nodes, respectively. (f to h) Mean fluorescence intensity (MFI) of IL-17 in skin, ear, and lymph nodes, respectively. Pso. ear, right ear which has received 5 mg imiquimod cream. The significant differences have been indicated by asterisks (*; P < 0.01; n = 3).
Our data in Fig. 7b to e revealed that daily application of IMQ cream markedly increased the number of IL-17-producing CD4+ T cells in the Pso. skin (6.9% ± 0.6% vs. 1.4% ± 0.2%, n = 3, P value < 0.01), ear (3.7% ± 0.3% vs. 1.2% ± 0.4%, n = 3, P value < 0.01), and lymph nodes (8.2% ± 1.7% vs. 2.0% ± 0.1%, n = 3, P value < 0.01) compared to normal mice. The results further showed no significant changes in the frequency of these cells in sham groups compared to Pso. skin (6.1% ± 1.0% vs. 6.9% ± 0.6%, n = 3, P value > 0.05), ear (3.8% ± 0.4% vs. 3.7% ± 0.3%, n = 3, P value > 0.05), and lymph nodes (8.2% ± 1.7% vs. 6.8% ± 0.5%, n = 3, P value > 0.05). The number of these cells significantly decreased in IDO-expressing fibroblast-treated groups compared to Pso. mice both in skin (1.9% ± 0.3% vs. 6.9% ± 0.6%, n = 3, P value < 0.01) and ear (0.5% ± 0.2% vs. 3.7% ± 0.3%, n = 3, P value < 0.01). Similarly, non-IDO fibroblast injection significantly decreased the frequency of IL-17+ CD4+ T cells in skin (2.8% ± 0.4% vs. 6.9% ± 0.6%, n = 3, P value < 0.01) and ear (1.1% ± 0.3% vs. 3.7% ± 0.3%, n = 3, P value < 0.01; Fig. 7b to d). While IL-17+ CD4+ T cells frequency in lymph nodes were decreased to 3.3% ± 0.3% following IDO-expressing fibroblasts injection compared to Pso. mice (8.2% ± 1.7%, n = 3, P < 0.01), no significant change was found in the number of these cells in non-IDO fibroblast injected mice compared to Pso. mice (5.2% ± 0.3% vs. 8.2% ± 1.7%, n = 3, P value > 0.05; Fig. 7b and e). IDO-expressing fibroblasts significantly decreased the MFI of IL-17 of mouse skin (12,364.8 ± 270.7 vs. 18,128.3 ± 932.3 n = 3, P value < 0.01), ear (6,717.3 ± 188.1 vs. 14,280.5 ± 268.6, n = 3, P value < 0.01), and lymph node (14,738.0 ± 84.2 vs. 21,454.0 ± 349.7, n = 3, P value < 0.01) compared to Pso. group (Fig. 7f to h).
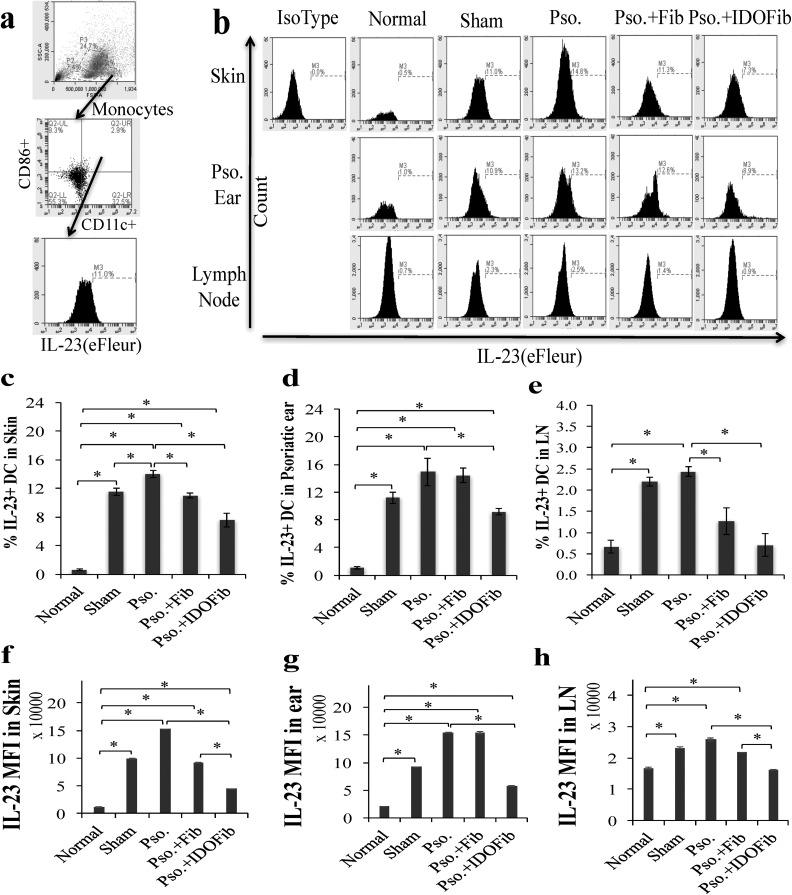
IDO Cell Therapy Suppressed Infiltration of IL-17+ T Cells and IL-23+ DCs
As IL-23 plays a central role in the Th17 cell expansion, we tested the effect of IDO cell therapy on the number of IL-23-producing DCs. Monocytes were gated by their characteristic forward and side-scatter profiles that represent size and granularity of cells. Gate for IL-23 antigen was applied to CD11c+ CD86+ populations to determine the MFI and relative expression of IL-23 in skin, ear, and lymph nodes (Fig. 8a). The frequency of IL-23+ CD11c+ DCs showed a dramatic increase in skin of the Pso. mice (14.0% ± 0.5% vs. 0.6% ± 0.1%, n = 3, P < 0.01), ear (14.9% ± 2.6% vs. 1.1% ± 0.2%, n = 3, P < 0.01), and lymph nodes (2.4% ± 0.1% vs. 0.7% ± 0.2%, n = 3, P < 0.01) compared to the normal values (Fig. 8b to e). While medium injection in psoriatic skin had no considerable effect on reduction of the frequency of IL-23+ CD11c+ DCs in skin (11.5% ± 0.5% vs. 14.0% ± 0.5%, n = 3, P < 0.01), the number of these cells significantly decreased in non-IDO (11.0% ± 0.4% vs. 14.0% ± 0.5%, n = 3, P value < 0.01) and IDO fibroblast-injected mice (7.6% ± 0.9% vs. 14.0% ± 0.5%, n = 3, P value < 0.01) compared to Pso. mice (Fig. 8b and c). Our data in Fig. 8b and d revealed that only IDO-expressing fibroblast injection markedly reduced the number of IL-23-producing DCs in ear compared to Pso. mice (9.2% ± 0.5% vs. 14.9% ± 2.6%, n = 3, P < 0.01). No significant changes were found in sham (11.2% ± 0.8% vs. 14.9% ± 2.6%, n = 3, P > 0.05) and non-IDO fibroblast-treated groups (14.4% ± 1.0% vs. 14.9% ± 2.6%, n = 3, P > 0.05). Further, injection of IDO-expressing fibroblasts (0.7% ± 0.3% vs. 2.4% ± 0.1%, n = 3, P < 0.01) and, to a lesser extent, non-IDO fibroblasts (1.3% ± 0.3% vs. 2.4% ± 0.1%, n = 3, P < 0.01) significantly decreased the frequency of IL-23+ CD11c+ DCs in lymph nodes compared to untreated mice (Fig. 8b and e). Moreover, the MFI of IL-23 molecule significantly increased in skin, ear, and lymph node of sham (skin: 98,842.3 ± 849.3 vs. 11,461.1 ± 1,243.5; ear: 92,239.0 ± 101.7 vs. 20,564.7 ± 406.0; and lymph nodes: 23,253.1 ± 281.7 vs. 16,667.1 ± 437.2, n = 3, P value < 0.01) and Pso. groups (skin: 152,781.3 ± 288.7 vs. 1,1461.1 ± 1,243.5; ear:154,110.0 ± 883.0 vs. 20,564.7 ± 406.0; and lymph nodes:25,926.6 ± 568.0 vs. 16,667.1 ± 437.2, n = 3, P value < 0.01) compared to normal mice. The levels of IL-23 decreased in the IDO-expressing fibroblast-treated mice (skin: 44,547.9 ± 647.6 vs. 152,781.3 ± 288.7; ear: 57,727.3 ± 1,226.8 vs. 154,110.0 ± 883.0; and lymph nodes: 16,225.0 ± 127.4 vs. 25,926.6 ± 568.0, n = 3, P value < 0.01), and to a lesser extent, in skin (91,466.3 ± 584.2 vs. 152,781.3 ± 288.7, n = 3, P value < 0.01) and lymph node (21,772.8 ± 40.3 vs. 25,926.6 ± 568.0, n = 3, P value < 0.01) of non-IDO fibroblast-injected mice (Fig. 8f to h).
Fig. 8.
Flow cytometry analysis of the percentage of IL-23+ dendritic cells in skin, ear, and lymph nodes. Upon euthanizing mice on day 9, the dorsal skins, right ears, and lymph nodes were collected. After obtaining single-cell suspensions, cells were stimulated in Cell Stimulation Cocktail overnight. Dendritic cells were stained for their surface and intracellular markers in 1% fetal bovine serum + phosphate buffered saline–containing fluorescein isothiocyanate-conjugated anti-CD11c, phycoerythrin-conjugated anti-CD86, and eFleur-conjugated anti-IL-23. (a) Representative flow cytometry plots of gating. (b) Representative flow cytometry plots of IL-23+ CD11c + DCs in skin, ear, and lymph nodes, respectively. (c to e) Quantitative analysis of IL-23+ CD11c + DCs in skin, ear, and lymph nodes, respectively. (f to h) Mean fluorescence intensity (MFI) of IL-23 in skin, ear, and lymph nodes, respectively. Pso. ear, right ear which has received 5 mg imiquimod cream. The significant differences have been indicated by asterisks (*; P < 0.01; n = 3).
Discussion
To study pathophysiology and therapeutic efficacy of psoriasis a relatively large number of psoriatic animal models have been developed, most of which are murine genetically engineered models. However, there are advantages and disadvantages for each of these models15,16. Recent studies showed that topical application of 5% IMQ, a TLR7 and TLR8 ligand, causes a psoriasis-like skin inflammation13. There is now compelling evidence that IMQ-induced psoriatic features are similar to those seen in humans including clinically hyperkeratosis, erythema, and scaling and immunologically neutrophil microabscesses and infiltration of γδ T cells and Th17 cells to the skin. In fact, not only the lesions phenotypically resemble those seen in humans, the histological characteristics and the mechanism of the IL-17/IL-23 axis of being involved are similar to that in psoriatic patients13,17. Here, we provided evidence that IDO cell therapy improves psoriasis condition as intralesional injection of IDO, and to a lesser extent, non-IDO-expressing cells significantly improve psoriatic-induced erythema and skin thickness scoring. The result of skin thickness measured by digital caliper and H&E staining of the psoriatic skin lesion showed a significant reduction in thickness of the epidermal layer in intralesional injected IDO-expressing cells. As lymph nodes become inflamed and enlarged in various infections and autoimmune diseases, we evaluated the size and weights of the lymph nodes taken from psoriatic and control mice and showed that intralesional injection of IDO markedly reduces the size of the regional lymph nodes taken from the psoriatic mice.
As interactions between T cells and antigen-presenting cells in lymph nodes are crucial for initiating cell-mediated adaptive immune responses18, many studies confirmed the capacity of fibroblasts as nonprofessional APCs when migrated to lymph node19. In our study, we found that IDO-Red Cherry-expressing cells are present in regional lymph nodes, though their interaction with naive T cells needs further study. Having said that we confirmed that the number of infiltrated CD3+ cells into psoriatic lesions that received the intralesional injection of IDO-expressing fibroblasts was significantly less than those that received either none or non-IDO fibroblasts. This might, at least in part, be due to the suppression of IL-23/IL-17. This is because the previous study20 showed a significant decrease in CD3+ T cells mainly in the epidermis of mice treated with anti-human IL-23 Monoclonal Antibody (mAB) in comparison to control mice.
In psoriatic patients, γδ T cells were greatly increased in affected skin and produced large amounts of IL-1721. Here, we found that the number of γδ+ T cells–expressing IL-17 in psoriatic affected skin, ear lesions, and lymph nodes significantly reduced upon intralesional injection of IDO-expressing cells. Under the same experimental condition, the number of CD4+ IL-17+ cells slightly increased in skin, ear, and regional lymph nodes in psoriatic lesions compared to untreated control and that moderately reduced upon IDO-expressing cell injection. Consistent with these findings, the number of CD11c+ DCs-expressing IL-23+ and APCs (F4-80+ CD11b+) within psoriatic skin samples significantly increased in IMQ-treated mice and that markedly suppressed in mice treated with IDO-expressing cells. Interestingly, the number of granulocytes that are normally high in psoriatic lesions significantly reduced in response to intralesional injection of IDO-expressing cells. We further showed that non-IDO fibroblasts also reduce the infiltration of macrophages, CD4+ IL-17+ cells, and IL-23+ DCs but to a lesser extent than IDO-expressing fibroblasts.
This can be due the fact that fibroblasts have weak antigen presenting properties in immunomodulation22,23. Our research group19 found that priming with fibroblasts has a tolerogenic impact on DCs. Fibroblast-primed DCs express coinhibitory molecules programmed cell death ligand 1 and 2 (PD-L1, PD-L1) that play a major role in suppressing the immune responses in cancer24, allotransplantation25, and autoimmune disease26. Our previous study has shown that upon coinhibitory activation, not only DCs lose their capacity to stimulate the proliferation of CD4 and CD8 T cells, the expression of IL-10 and IDO were increased that can suppresses T cell responses27. Further, it has been shown that Trp breakdown is necessary for inducing immune tolerance11. Two theories have been proposed to explain how Trp catabolism facilitates tolerance. One posits that Trp breakdown suppresses immune cell proliferation by dramatically reducing the supply of this critical essential amino acid. The other postulates that downstream metabolites of Trp catabolism (such as KynA) act to suppress certain immune cells, probably by proapoptotic mechanisms28. As such, one mechanism by which the IDO-expressing fibroblasts significantly improve the clinical appearance and immunological profile of IMQ-induced psoriasis is likely to be due to the release of Kynurenin (Kyn)/ Kynurenic acid (KynA). In fact, we have previously demonstrated that KynA suppresses the production and gene expression of IL-17 and IL-23 through GPCR35 activation (data not shown here).
Conclusion
Here, we report for the first time that IDO primary fibroblast therapy improves IMQ-induced psoriasis-like dermatitis in mice. Our data and those previously reported suggest that the immunosuppressive effects of fibroblasts plus Trp metabolites released from IDO-expressing cells are likely to be the mechanism by which IDO-expressing cells improve the clinical appearance of IMQ-induced psoriatic-like lesions through modulating the key immune cells seen in psoriatic lesions.
Footnotes
Ethical Approval: All methods and procedures as well as the use of animals and tissue specimens derived from animals were approved by the Animal Ethics Committee of the University of British Columbia.
Statement of Human and Animal Rights: This article does not contain any studies with humans. The care and maintenance of all animals was approved by the principles of laboratory animal care and the guidelines of the institutional Animal Policy and Welfare Committee at the University of British Columbia.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been supported by Canadian Institutes of Health Research (CIHR)/Operating Grant MOP-136945 grant as well as partially supported by the International Brotherhood of Electrical Workers. Sanam Salimi Elizei is supported by a WorkSafe BC Research Training Award.
References
- 1. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873. [DOI] [PubMed] [Google Scholar]
- 2. Nakajima K, Kanda T, Takaishi M, Shiga T, Miyoshi K, Nakajima H, Kamijima R, Tarutani M, Benson JM, Elloso MM, Gutshall LL, Naso MF, Iwakura Y, DiGiovanni J, Sano S. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J Immunol. 2011;186(7):4481–4489. [DOI] [PubMed] [Google Scholar]
- 3. Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Investig Dermatol. 2009;129(6):1339–1350. [DOI] [PubMed] [Google Scholar]
- 4. Lowes M, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Investig Dermatol. 2008;128(5):1207–1211. [DOI] [PubMed] [Google Scholar]
- 5. Malakouti M, Brown GE, Wang E, Koo J, Levin EC. The role of IL-17 in psoriasis. J Dermatolog Treat. 2014;6634(415):1–4. [DOI] [PubMed] [Google Scholar]
- 6. Genovese MC, Van Den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, Sloan-Lancaster J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62(4):929–939. [DOI] [PubMed] [Google Scholar]
- 7. Kurzeja M, Rudnicka L, Olszewska M. New interleukin-23 pathway inhibitors in dermatology: ustekinumab, briakinumab, and secukinumab. Am J Clin Dermatol. 2011;12(2):113–125. [DOI] [PubMed] [Google Scholar]
- 8. Maclennan C, Fieschi C, Lammas DA, Picard C, Susan E, Sanal O, Maclennan JM, Holland SM, Ottenhoff THM, Casanova J, Kumararatne DS. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against salmonella in humans. J Infect Dis. 2004;190(10):1755–1757. [DOI] [PubMed] [Google Scholar]
- 9. Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2013;27(5):622–627. [DOI] [PubMed] [Google Scholar]
- 10. Hayaishi O. Utilization of superoxide anion by indoleamine oxygenase-catalyzed tryptophan and indoleamine oxidation. Adv Exp Med Biol. 1996;398:285–289. [DOI] [PubMed] [Google Scholar]
- 11. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–774. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Tredget EE, Ghaffari A, Lin X, Kilani RT, Ghahary A. Local expression of indoleamine 2,3-dioxygenase protects engraftment of xenogeneic skin substitute. J Investig Dermatol. 2006;126(1):128–136. [DOI] [PubMed] [Google Scholar]
- 13. van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus A-M, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. [DOI] [PubMed] [Google Scholar]
- 14. Rezakhanlou AM, Habibi D, Lai A, Jalili RB, Ong CJ, Ghahary A. Highly efficient stable expression of indoleamine 2,3 dioxygenase gene in primary fibroblasts. Biol Proced Online. 2010;12(1):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danilenko DM. Review paper: preclinical models of psoriasis. Vet Pathol. 2008;45(4):563–575. [DOI] [PubMed] [Google Scholar]
- 16. Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Investig Dermatol. 2007;127(6):1292–308. [DOI] [PubMed] [Google Scholar]
- 17. Flutter B, Nestle FO. TLRs to cytokines: mechanistic insights from the imiquimod mouse model of psoriasis. Eur J Immunol. 2013;43(12):3138–3146. [DOI] [PubMed] [Google Scholar]
- 18. Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev. 2008;8(9):675–684. [DOI] [PubMed] [Google Scholar]
- 19. Jalili RB, Zhang Y, Hosseini-Tabatabaei A, Kilani RT, Khosravi Maharlooei M, Li Y, Salimi Elizei S, Warnock GL, Ghahary A. Fibroblast cell-based therapy for experimental autoimmune diabetes. PLoS One. 2016;11(1):e0146970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tonel G, Conrad C, Laggner U, Di Meglio P, Grys K, McClanahan TK, Blumenschein WM, Qin J-Z, Xin H, Oldham E, Kastelein R, Nickoloff BJ, Nestle FO. Cutting edge: a critical functional role for IL-23 in psoriasis. J Immunol. 2010;185(10):5688–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang H, Wang T, Zheng J, Jun Yan. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haniffa MA, Wang X, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CMU, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. [DOI] [PubMed] [Google Scholar]
- 23. Pinchuk I V, Beswick EJ, Saada JI, Boya G, Schmitt D, Raju GS, Brenmoehl J, Rogler G, Reyes VE, Powell DW. Human colonic myofibroblasts promote expansion of CD4+ CD25high Foxp3+ regulatory T cells. Gastroenterology. 2011;140(7):2019–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, Najafian N, Yagita H, Azuma M, Turka LA, Sayegh MH. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174(6):3408–3415. [DOI] [PubMed] [Google Scholar]
- 26. Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217(1):45–59. [DOI] [PubMed] [Google Scholar]
- 27. Khosravi-Maharlooei M, Pakyari M, Jalili RB, Salimi-Elizei S, Lai JCY, Poormasjedi-Meibod M, Kilani RT, Dutz J, Ghahary A. Tolerogenic effect of mouse fibroblasts on dendritic cells. Immunology. 2016;148(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81(4):247–265. [DOI] [PubMed] [Google Scholar]