Abstract
Permanent deficits that occur in memory, sensation, and cognition can result from central nervous system (CNS) trauma that causes dysfunction and/or unregulated CNS regeneration. Some therapeutic approaches are preferentially applied to the human body. Therefore, cell transplantation, one of the therapeutic strategies, may be used to benefit people. However, poor cell viability and low efficacy are the limitations to cell transplantation strategies. Biomaterials have been widely used in several fields (e.g., triggering cell differentiation, guiding cell migration, improving wound healing, and increasing tissue regeneration) by modulating their characteristics in chemistry, topography, and softness/stiffness for highly flexible application. We reviewed implanted biomaterials to investigate the roles and influences of physical/chemical properties on cell behaviors and applications. With their unique molecular features, biomaterials are delivered in several methods and mixed with transplanted cells, which assists in increasing postimplanted biological substance efficiency on cell survival, host responses, and functional recovery of animal models. Moreover, tracking the routes of these transplanted cells using biomaterials as labeling agents is crucial for addressing their location, distribution, activity, and viability. Here, we provide comprehensive comments and up-to-date research of the application of biomaterials.
Keywords: central nervous system, implantation, biomaterials, physical, chemical
Central nervous system (CNS) diseases include spinal cord injuries (SCIs) and traumatic brain injuries (TBIs). In general, loss of motor, sensory, and autonomic functions appear with SCIs, whereas symptoms of physical, sensory, cognitive, and swallowing deficits, as well as behavioral issues, are the consequences of TBIs. In the process of trauma, damage from a mechanical force is the first harm to the body. Then, inflammation emerges via 2 cell types, microglia and microphages, in the CNS, and this state inhibits myelination. Finally, astrocytes appear in a reactive state to form glial scar tissue that differs from native tissue due to a lack of nutrient supplement function1,2. CNS trauma may cause permanent deficits mainly due to an inability of CNS regeneration but also because of glial scar tissue formation. Several methods, such as endogenous cell therapy and exogenous cell therapy, are performed to treat CNS injuries. Cell transplantation is a more achievable therapeutic strategy for CNS injuries because cells are easily obtained compared to organs. However, several barriers to exogenous cell therapy exist, including a low viability of transplanted cells, dispersed cells distributed in the body, and uncontrolled cell differentiation, and these limit the therapeutic efficacy of cells3–5. Biomaterials that have flexibility in mimicking natural environments could overcome obstacles of cell transplantation and thereby improve cell transplantation issues for the therapy of CNS injuries. We review (1) the role of the physical/chemical property of biomaterials on cell behavior, (2) the influence of the physical/chemical property of biomaterials on implantation, and (3) the distribution of transplanted cells using a cell tracker employing biomaterials to provide a more comprehensive review of biomaterial application in CNS regeneration medicine.
Role of the Physical/Chemical Property of Biomaterials on Cell Behaviors
A cell’s fate can be manipulated by signaling through specific environmental physical/chemical factors, such as the chemistry, stiffness, or topography of a matrix. In this section, we describe the role of electric charges, stiffness, and topography of biomaterial on cellular behavior such as cell adhesion, cell proliferation, and cell differentiation.
Effects of Electric Charges on Cell Behaviors
The effects of electric charges on neural cell cultivation and differentiation have been investigated on carbon nanotubes (CNTs) exhibiting semiconductivity characteristics, which have potential in applying to neural electrodes. Those studies showed that formation of a functional synapse was observed, with evidence of spontaneous synaptic currents and spontaneous action potential frequencies when mature hippocampal neurons were cultured on CNTs6. CNT is a candidate material for cell cultivation. A CNT chemistry effect of electric charge (eg., positively, negatively, neutral charge) would affect cell behavior (eg., cell proliferation or differentiation). Hippocampal neuron cells were grown on a positively charged CNT grafted with ethylenediamine (EN), which revealed more outgrowth and branching activities than those of cells grown on negatively charged carboxylic functional groups or neutrally charged poly(m-aminobenzene sulfonic acid) (PABS)7. Moreover, a positive charge effect also has been applied in neuronal cell differentiation, such that neuronal stem cells (NSCs) differentiated into a neural lineage without induction factors under cultivation with CNTs. Single-walled CNTs (SWCNTs) and polyethyleneimine (PEI), forming multilayer thin films through a layer-by-layer (LBL) method, showed comparable results in biocompatibility, neurite outgrowth, and neural marker expressions to those of the widely used biopolymer, poly-L-ornithine (PLO)8. But a negative charge, such as poly(acrylic acid) (PAA) or poly(methacrylic acid) (PMAA), grafted on CNT also can increase higher neurite outgrowth and neuron differentiation of human embryonic stem cells (hESCs) than that with a conventional PLO substrate9,10. These results may be the reason why neural differentiation is preferable for the hESCs and NSCs, and thus neural differentiation is observed after replacement of an inhibition differentiation medium to a general cultural medium. Transdifferentiation was employed in a negative charge using a carboxylated multiwalled CNT (MWCNT) to promote neural differentiation of human bone marrow mesenchymal stem cells (hBMMSCs). One study provided two major roles of carboxylated MWCNTs that promoted hBMMSC neural differentiation by upregulating neural growth factors and the carboxylated MWCNTs that trapped these neural growth factors to create a suitable environment for long-term neural differentiation11.
Native hydrogels exhibit a property of low cell attachment. Therefore, an electric charge effect becomes a possibility for designing cell-repellent hydrogels. One method is to use a positive charge of material for improving cell cultivation due to the presence of a negative charge of a cellular plasma membrane (head group of phosphatidylserine and phosphatidylinositol) and a negative charge of carbohydrate portions of glycolipids and glycoproteins12. Poly(propylene fumarate-co-ethylene glycol) (p(PF-co-EG)) hydrogels have been incorporated with a positively charged arginine polymer to increase the cell density by evidence of an increment of vitronectin on the hydrogels13. Moreover, cell-repellent 2-hydroxyethyl methacrylate (HEMA) hydrogels showed the greatest cell attachment and spreading when the cells were cultured on positively charged 2-methacryloxy ethyltrimethyl ammonium chloride (MAETAC)–grafted HEMA hydrogels. The negatively charged sodium 2-sulfoethyl methacrylate (SEMA)–grafted HEMA hydrogels were ranked second, whereas neutrally charged HEMA hydrogels were the worst in terms of cell attachment and cultivation14.
In addition to synthetic polymer, the chemistry effects on cell behaviors of natural polymers were also investigated. Extracellular matrix, fibronectin (Fn), or hyaluronic acid (HA) was modified in negatively charged alginate, and their influence was compared to the neuronal differentiation. The data showed that mouse embryonic stem cells (mESCs) encapsulated in a group of alginate or alginate-HA exhibited increased differentiation of neurons according to evidence of synaptic and different neuronal subtype markers15. Except for differentiation of stem cells, maintaining stemness of a stem cell is an important issue for in vitro cultivation and proliferation. Maintaining stemness of mESCs using a collagen-based, poly(lactic-co-glycolic acid) (PLGA)– based, and positive chitosan-based 3-dimensional scaffold was investigated. Results indicated that all 3 scaffolds could maintain stemness compared with the traditional 2-dimensional dish with feeder cells. When comparing the cell proliferation of 3-dimensional scaffolds, chitosan-based 3D scaffolds had higher cell numbers than those of collagen-based and PLGA-based 3D scaffolds16.
The electric charge effect of materials on cells’ behavior is summarized in Table 1. Overall, it seems that positively charged materials assisted cell proliferation, whereas negatively charged biomaterials tended to promote the cells to differentiate.
Table 1.
Electric Charge Effect on Cells’ Behavior.
| Cell | Material | Result | Reference |
|---|---|---|---|
| Hippocampal neurons |
|
Positively charged EN-CNT revealed most cell outgrowth and branching activities | 7 |
| NSCs |
|
SWCNT/PEI showed comparable results with PLO | 8 |
| HASMC |
|
Agmatine-p(PF-co-EG) enhanced cell attachment | 13 |
|
|
Positively charged MAETAC-grafted HEMA hydrogels had best cell adhesion result | 14 |
| mESCs |
|
Chitosan-based scaffolds had higher cell numbers than those of collagen-based and PLGA-based 3-dimensional scaffolds | 16 |
| hESCs |
|
Both PAA-CNT and PMAA-CNT can increase neurite outgrowth and neuron differentiation of hESCs compared with PLO | 9, 10 |
| mESCs |
|
Alginate or alginate-HA exhibited increased differentiation of neurons | 15 |
Abbreviations: EN, ethylenediamine; Fn, fibronectin; HA, hyaluronic acid; HASMC, human aortic smooth muscle cell line; HEMA, hydroxyethyl methacrylate; hESCs, human embryonic stem cells; MAETAC, 2-methacryloxy ethyltrimethyl ammonium chloride; mESCs, mouse embryonic stem cells; NSCs, neuronal stem cells; (p(PF-co-EG), poly(propylene fumarate-co-ethylene glycol); (p(PF-co-EG), poly(propylene fumarate-co-ethylene glycol); PAA, poly(acrylic acid); PABS, poly-m-aminobenzene sulfonic acid; PEI, polyethyleneimine; PLGA, poly(lactic-co-glycolic acid); PLO, poly-L-ornithine; PMAA, poly(methacrylic acid); SEMA, sodium 2-sulfoethyl methacrylate; SWCNT, single-walled CNT.
Stiffness Effects on Cell Behaviors
Manipulating the stiffness of material is also a tool for regulating cell adhesion and differentiation. One obstacle of applying a hydrogel in cell cultivation is low cell attachment on it caused by low sliding friction on the surface. Therefore, controlling the stiffness or mechanical strength would be an alternative option. The attachment area of NIH3T3 on the surface of a polyacrylamide gel possessed a positive correlation to stiffness when designing materials in the range of 10 to 3000 Pa, but a decreased attachment area of the cells was observed in the range of 3000 to 10,000 Pa17. HA, a natural polysaccharide, was also investigated in the relationship between cell adhesion and the stiffness property of materials. A higher stiffness property (storage modules at 17,000 Pa) of HA hydrogels improved cell adhesion of epithelial HeLa cells, preosteoblast cells, and particularly NIH3T3 fibroblasts compared to those of storage modules at 600 and 2500 kPa18. In addition to a cross-linker, interpenetrating networks (IPNs) are an alternative method to manipulate stiffness. Arulmoli et al19. used IPNs, composed of fibrin-based gels (salmon fibrinogen and salmon thrombin) and thiolated HA, to simulate a brain tissue environment at 202.3 ± 17.33 Pa and reduced human neural stem cell death.
To mimic the environments of native cells, which are cultivated in a 3-dimensional structure in a live body, a series design of a 3-dimensional system of biomaterials was studied. Mechanical strength is also important to control cell behavior, such as survival, metabolite, or growth factor secretion, when cells are encapsulated in a material. Orive et al20. compared survival and antibody production of hybridoma cells within 2 mechanical strength–type gels: solid (main force of 11 g per bead for breakdown) and liquefied (main force of 4 g per bead for breakdown) core alginate-agarose beads. They found that the liquefied type can have a higher survival of cells and antibody production compared with the solid type. The team advanced to improve cell survival in solid-type alginate-agarose beads up to 70 days compared to 15 days for the liquefied type when adding another cell line, BHK fibroblasts, and C2C12 myoblasts.
The roles of mechanical strength in stem cell fate determination were studied. Different mechanical strengths mimicking native tissues of brain, muscles, and bone on hydrogels were reported. Naive MSCs are initially small and round but develop increasingly branched, spindle, or polygonal shapes for further differentiation into neuron, myogenic, and osteogenic lineages when cells are grown on matrices of ∼E (0.1-1 kPa), ∼E (8-17 kPa), and ∼E (25-40 kPa), respectively21. Importantly, stemness maintenance is an issue for the development of cellular biology. The mechanical strength of hydrogels controlled to 10 kPa was reported to maintain the stemness of hESCs for at least 60 days22.
Based on the stiffness effect on cells’ behavior (Table 2), the stiffness can control cell adhesion, growth, and differentiation. The higher stiffness of hydrogels is suitable for cell culture as they are cultivated in a 2-dimensional environment; in contrast, a softer hydrogel is preferred when cells are grown in a closed 3-dimensional construction. In addition, lower mechanical strength would be preferable for brain tissue cultivation.
Table 2.
Effect of Stiffness of Materials on Cells’ Behavior.
| Cell | Material | Result | Reference |
|---|---|---|---|
| NIH3T3 | PAA hydrogel (10–10,000 Pa) |
|
17 |
| HeLa cells, preosteoblast cells, NIH3T3 |
|
|
18 |
| hNSPCs, HECFC-ECs |
|
|
71 |
| MSCs |
|
|
21 |
| hESCs |
|
|
22 |
Abbreviations: HA, hyaluronic acid; HECFC-ECs, human cord blood–derived endothelial cells; hESCs, human embryonic stem cells; hNSPCs, human neural stem progenitor cells; MSCs, mesenchymal stem cells; PAA, polyacrylamide.
Topography Effects on Cell Behaviors
Topography affects cell adhesion, growth, and in particular differentiation through cellular morphology alternation. Sridharan and colleagues23 demonstrated that embryonic stem cells (ESCs) in structureless and soft gelatin matrix differentiated into all 3 lineages, but an elongated shape with long filaments and ectodermal lineage differentiation cells were observed when they had a fibril structure with collagen or a collagen-carbon nanotube (CNT) matrix. A micropattern with controlled width/spacing was modeled to examine the differentiation ability of stem cells. The micropatterned microenvironment, using a linear pattern with a width/spacing (W/S) of 40/30 μm, guided neuronal lineage growth, whereas a W/S pattern of 20/40 μm was reported to guide myogenic lineage differentiation24,25. The nanopatterned microenvironments also revealed the ability to control stem cell fates. A similar phenomenon was reported in studies of hMSCs that nanopatterning with 350-nm lines resulted in spontaneous neurogenic differentiation of hMSCs, while that of 250-nm-width lines directed hMSCs toward both neurogenic and myogenic differentiation26,27. These neurogenic differentiations were associated with the integrin-activated focal adhesion kinase (FAK)26. In addition to a line pattern, a square pattern also has been investigated on differentiation. Cells can proliferate and differentiate on the square pattern with 300-μm × 300-μm, 200-μm spacing and 5-μm × 5-μm, 5-μm spacing but not on a pattern with 50-μm × 50-μm, 50-μm spacing. This is because the largest square pattern has enough space for a lot of cells to adhere on a large pattern, and the smallest square pattern can allow cells to extend their filopodia on the one small square pattern, but the pattern with 50-μm × 50-μm, 50-μm spacing limited the cells in one square space. An advanced topographic pattern was built by designing in-line plus a square pattern using CNTs to construct a neural network environment28.
Poor cell adhesion on a cell-repellent hydrogel could be improved by using topography. Polyethylene glycol–based hydrogels are materials with low cell adhesion. To improve this issue, linear micropatterned Acr-sP(EO-stat-PO) hydrogels with a 10-µm groove were designed, and results showed an improvement in cell adhesion. Moreover, the spread of L292 cells increased on linear micropatterned hydrogels without a coating of the bioadhesive molecule, vitronectin, during the culture period, whereas the cells on nonmicropatterned smooth hydrogels were only adsorbed but did not spread on the hydrogel after 1 day29. In addition to cell adhesion and proliferation that can be manipulated through topography, cellular morphology can also be directed by topography for promoting cell differentiation. Surface wrinkles were designed on a poly(2-hydroxyethyl methacrylate) (PHEMA) hydrogel surface to verify their effect on hMSC morphology and differentiation. The hMSCs on lamellar wrinkles of the PHEMA hydrogel possessed higher aspect ratios of cell adhesion area and differentiated into an osteogenic lineage, whereas those on hexagonal wrinkles of the PHEMA hydrogel showed lower aspect ratios of cell adhesion area and differentiated into an adipocyte lineage30.
A 3-dimensional structure can also control topography through interior porous structures. Mouse induced pluripotent stem cells (miPSCs) cultured on a poly(lactic-co-glycolic acid) (PLGA) porous scaffold, through salt leaching/solvent evaporation, reached a maximum cell number after 10 days postseeding, which was 5 to 6 times higher than those on the nonporous controls31. Khayyatan et al32. used a freezing temperature to control the porosity of collagen scaffolds. The highest infiltration of human induced pluripotent stem cell–derived neural progenitors (hiPSC-NPs) was observed when cultured in a smaller porosity collagen scaffold (<50 μm) prepared at –196°C, suggesting that a smaller porosity has a higher surface area in the interior of the collagen scaffold to create more adhesion space for cells. However, homogeneous pore size could not be easily achieved by the above-described method. The inverted colloidal crystal (ICC) scaffold using polystyrene beads with a mean diameter of 158 μm could tightly control pore morphology and provide interior pores’ connection of the scaffold. Their results showed that ICC-made scaffolds increased the adhesion and viability of miPSCs compared to those of freeform ones33.
We summarize the effect of materials’ topography on cell behavior in Table 3. In general, the major application of topography control is in cell differentiation because the cells can be limited in the space of the topography pattern to achieve cell morphology manipulation. In addition, amounts of cell infiltration also can be controlled through porosity of the 3-dimensional scaffold.
Table 3.
Effect of Topography of Materials on Cells’ Behavior.
| Cell | Material | Result | Reference |
|---|---|---|---|
| hNSCs |
|
|
28 |
| hMSCs |
|
|
26, 27 |
| hMSCs |
|
|
24, 25 |
| hNSCs |
|
|
28 |
| L292 cells hMSCs |
|
|
29 |
|
|
30 | |
| miPSCs |
|
|
31 |
| hiPSC-NPs |
|
|
32 |
Abbreviations: hiPSC-NPs, human induced pluripotent stem cell–derived neural progenitors; hMSCs, human mesenchymal stem cells; hNSCs, human neural stem cells; l × w, s, length × width, space; miPSCs, mouse induced pluripotentstem cells; PHEMA, poly(2-hydroxyethyl methacrylate); W/S, width/spacing.
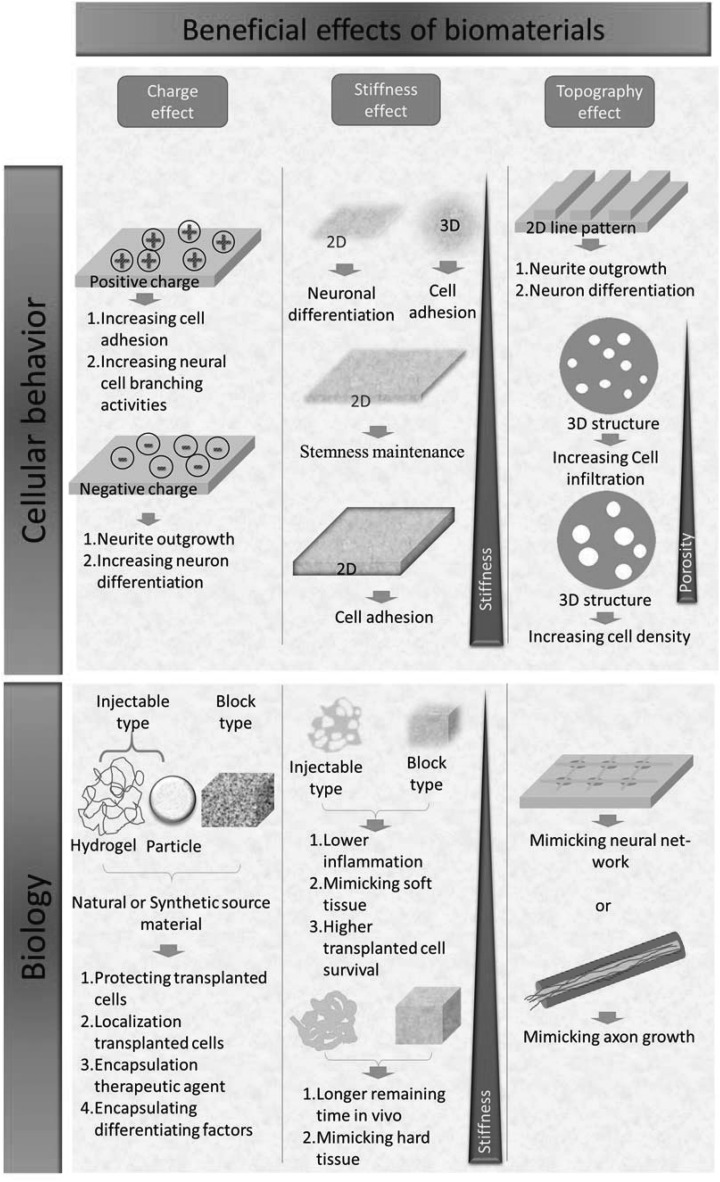
We summarize environments that are suitable to cell cultivation and differentiation through manipulating physical/chemical properties of the biomaterials in Figure 1. With these approaches, matrices can be designed to elicit enhanced cell attachment for a cell-repellent-based substrate, maintenance of stemness for cell banking, or directed cell differentiation into specific lineages for cell therapies.
Figure 1.
Beneficial effects of biomaterials on cellular behavior and biology through the physical/chemical properties of biomaterials.
Influence of Physical/Chemical Property of Biomaterials on Implantation
Cell transplantation appears to be a promising regenerative medicine, but the cells alone seem not fully satisfactory to the outcome of treatment. Biomaterials providing flexible characteristics in regulating cell behavior in the above description would be a possible solution to resolve the bottleneck. Designation of an implanted material would render them with biocompatibility preferentially and other tailored characteristics such as biodegradability, a niche-creatable environment, or a nonrestricted size or shape property3,34. To fully address biomaterials’ effect on implantation, we review the issue of biomaterial implantation based on the effect of its chemical properties, topography, and stiffness. The following contents describe the Chemical/ECM of Biomaterials on Implantation; the citation/reference of the issue has been cited in the sentence.
CNS Disease Treatment by Biomaterials Alone
To promote angiogenesis in the brain, basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) were adsorbed into a porous block scaffold hybrid of gelatin and 3-(glycidoxypropyl) trimethoxysilane (GPSM), and the gelatin/GPSM/bFGF/EGF scaffold was used to implant into a cavity in the cerebral cortex. The gelatin/GPSM/bFGF/EGF scaffold resolved the problem of low cell survival and remained for 60 days in the brains of animals. The scaffold formed an integrated connection with the host brain, and newborn cells represented by vascular endothelial (NAGO-positive), astroglial (GFAP-positive), and microglial (Iba1-positive) cells were increased to 2-fold in the presence of bFGF/EGF in the implanted scaffold35. However, the additives did not promote neuronal cell formation and migration and an unbiodegradable block used in vivo. Hydrogels are a cross-linked network within a porous structure with a dynamic hydration status, which is similar to the extracellular matrix (ECM) environment serving as a complicated microenvironment for supporting cell and tissue structures, regulating cellular behaviors, and promoting cell-to-cell communication. A neurite-promoting peptide sequence, IKVAV, was grafted to a biocompatible material, HA, to form an injectable material and then implanted into the lesion area of the cortex for 6 weeks. The results showed that invasion of host cells, especially nerve fibrils, was observed36. Collagen and other injectable biocompatible materials grafted with glycosaminoglycan were implanted into the cerebral cortex for neural regeneration in TBI. The results showed that more migratory cells (DCX-positive cells) and neural progenitor cells (NPCs) (NeuN-positive cells) were increased about 7-fold at 21 days. Importantly, fewer inflammatory cells (marker of ED1) in the implanted matrix and lesion boundary zone were observed compared to the nonimplanted group37.
How to control cell differentiation in vivo is also an issue in regeneration medicine. The other type of injectable biomaterial, not hydrogel based, was used for trophic factor delivery through the biodegradable property of materials to achieve controlled release. Biodegradable poly(ester-amide) microspheres, composed of adipic acid, L-phenyl-alanine, and 1,4-butanediol, were designed to load with differentiation factors, including Wnt3A, BMP4, and cyclopamine. After the microspheres had been incubated with hiPSC-derived neuroepithelial-like stem cells, cortical differentiation of the cells was observed in vitro. Moreover, the biodegradable poly(ester-amide) microspheres did not evoke a significant inflammatory response after transplantation into an intact rodent brain38.
Based on the delivery route, block biomaterials and injectable biomaterials are 2 major types with regard to implantation. The advantage of the block type is that it can last longer after implantation, whereas the injectable type can deliver the therapeutic agent more conveniently and with less of a surgical area requirement.
CNS Disease Therapy by Combinations of Cells and Biomaterials
Although biomaterials can act as scaffolds or trophic factor carriers for treating diseases of the CNS, less differentiated cells and a low amount of newborn endogenous cells (∼500 cells/ mm2) still need to be overcome in regeneration medicine35,39,40. Therefore, exogenous cells integrated into biomaterials become an alternative strategy in regeneration medicine.
Block Type Cells/Biomaterials
Natural materials are to be employed to bridge a spinal cord injury. Three materials—collagen, chitosan, and fibrin—were constructed as a block type and mixed with cells to compare their influence on transplanted cellular survival and the in vivo material degradation rate34. The authors used microfiber with a double-coaxial microfluidic device to embed neural stem/progenitor cells (NS/PCs) and basal materials and then used collagen (positive charge at pH 741), chitosan (positive charge at pH 742), or fibrin (negative net charge at pH 743) to assemble the construction. The results showed that microfibers with collagen successfully bridged host tissues and promoted the differentiation of 3 neural lineages. In addition, it was also found that the chitosan-coated microfibers also bridged transected spinal cord by a scar, but the highest cell proliferation and a more complete scar formation were observed as cells were engrafted in the collagen-coated microfibers during in vivo transplantation. The authors suggest that chitosan-coated microfibers exhibit too dense a structure in the interior compared with the others to inhibit host cell invasion. The lowest cell survival and scar formation were observed in the group of fibrin-coated microfibers, which might have been caused by the composition of fibrin, which is quickly digested in the microenvironment34.
Injectable-Type Cells/Biomaterials
The effects of ECM on cell survival and transplantation in injectable-type hydrogels were also investigated. He and colleagues44 demonstrated that cells can be protected when they are encapsulated in ECM materials before implantation. Studies showed that detached human embryonic stem cell–derived endothelial cells (hESC-ECs) kept in Matrigel, a complex extracellular environment secreted from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells, at 4°C could regain expression of cell adhesion and ECM molecules compared to those suspended in phosphate-buffered saline (PBS) as evidence of gene expression patterns. Moreover, engrafted Matrigel-encapsulated hESC-ECs showed more long-term survival of hESC-ECs than those of the cell-alone group postimplantation44. A series design based on ECM for neural regeneration has been reported. ECM types and their effects on neural stem cell (NSC) transplantation were investigated, and the results showed that a laminin-based collagen type I scaffold can enhance the transplanted cells’ survival compared with a fibronectin-based collagen type I scaffold. Furthermore, using a laminin-based collagen type I scaffold alone did not improve the damage to cognitive behavior45, suggesting that a scaffold effect in transplantation plays a role in protecting transplanted cells rather than repairing the damage site. Guan and colleagues46 further used collagen type I alone to mix human mesenchymal stem cells (hMSCs) for treatment of TBI. The highest biodistribution of transplanted hMSCs was observed when the cells were mixed with collagen scaffold, which was enhanced by 79% in the target site at 12 hours compared with that of the cell-alone group. The modified neurological severity score (mNSS) and Morris water maze evaluations all indicated significant improvement at day 28 after cell transplantation. Moreover, the researchers found that implanted hMSCs differentiated into neuronal cells (4.6%), oligodendrocytes (1.8%), and astrocytes (0.8%) in the presence of collagen more than those of the cell-alone treatment group46. Although transplanted hMSCs can transdifferentiate into neural-type cells after transplantation, major reasons for TBI improvement should be caused by other factors because of the few differentiated cells in the damage site. Qu et al47. had evaluated the expression levels of angiogenesis factors, neurogenesis factors, and tissue plasminogen activator (tPA) of the cells in the presence or absence of collagen type I scaffold. Both the cells alone and cells with collagen type I presented higher angiogenesis factors (NOTCH4, VEGFA, and TGFB) than neurogenesis factors (MDK, BCL2, and BIRC5). However, the cells cultured with the collagen type I scaffold showed more increased expression levels of factors in angiogenesis, neurogenesis, and tPA than that of the cell-alone group47. It revealed that the role of implanted scaffolds in the cell engraftment procedure is to provide an adhesion environment for transplanted cells and maintain their ECM molecular of transplanted cells and thus increase survival of engrafted cells, which possess neurotrophic factor release and assist the generation of newborn cells. This hypothesis was further explored by Ballios et al48. They demonstrated that HA-based injectable hydrogel increased survival of NSCs, the mechanism of which is through HA receptor CD44 on the cell. Based on the results, Führmann et al5. advanced to render material having the capability of controlling exogenous cell differentiation and maintenance of exogenous cell survival through Arg-Gly-Asp (RGD) peptide and PDGF-A when applied to spinal cord injury. Higher transplanted cell survival and migration were found in the hydrogel group compared to the media-only group. However, a teratoma with 3 germ layer cells was observed in the cell-only group and injectable hydrogel-containing cell group. In addition, an attenuated teratoma appeared in the hydrogel-containing cell group, suggesting that a grafted PDGF-A in the hydrogel enhanced more contact opportunity between human-induced pluripotent stem cell–derived oligodendrocyte progenitor cells (hiPSCs-OPCs) and PDGF-A.
The role of biomaterials can support not only cell replacement in regenerative medicine but also the cells that secrete the therapeutic agents to treat CNS disease49. It has been demonstrated that CNS disease can be improved after the treatment of trophic factors or therapeutics, such as dopamine required for Parkinson’s disease or anti–amyloid-β (Aβ) antibodies to target Alzheimer’s disease (AD). For this purpose, a long-term secreting device design would be a key issue. In general, injectable hydrophilic-type biomaterials are employed to achieve long-term therapeutic agent secretion through a cell-encapsulating platform. The most used hydrophilic types in the encapsulation system are alginate, agarose, chitosan, collagen, poly(ethyleneglycol), or polyvinylalcohol50. In addition, immune reactions of the host would involve encapsulated cell survival. Agarose/poly(styrene sulfonic acid) (agarose/PSSa) encapsulated with tyrosine hydroxylase-positive PC12 cells was investigated. Results showed a similar host reaction in the injection tracks and the place around the encapsulation cell gel, as evidenced by GFAP expression, meaning a lower host response was observed when using encapsulating system. Also, the agarose/poly(styrene sulfonic acid) (agarose/PSSa) encapsulated system extended the survival of encapsulated cells at least for 5 weeks51. To prolong the period of trophic factor secretion, researchers developed a device that integrated a membrane and a cell encapsulation system together. The integrated device can prolong cell survival at a high density for at least 1 year by managing the parameter of hydrogel stiffness and permeable membrane porosity50. The integrated device has been applied as passive immunization for Alzheimer’s disease to misfolded toxic proteins by continuous antibody delivery. Results showed that Aβ 40 level, Aβ 42 level, and amyloid plaque burden were decreased in the brain, and downregulated levels of phospho-tau pathology in the hippocampus were prevented52.
Most of injectable hydrogel is hydrophilic, which is easy to mix with cells. On the other hand, it requires a different procedure for delivering the cells when using a hydrophobic-based material such as PLGA. PLGA-based microparticles have been designed as a cell carrier to deliver NSCs into the stroke lesion cavity in the brain53. Researchers have used an oil-in-water (O/W) emulsion technique to prepare 100- to 200-μm microparticles and then coated fibronectin on the microparticles for cell attachment. The implantation results showed that primitive tissue formation was observed within 7 days after implantation into the lesion cavity. To provide a more adequate environment for de novo tissue formation, vascular endothelial growth factor (VEGF) was encapsulated into a PLGA-based cell carrier microparticle. It was observed that endothelial cells of the host integrated into this primitive tissue through released VEGF, attracting the formation of a neovasculature, and moreover, part of endothelial cells interspersed into grafted human neural stem cells (hNSCs)54.
Table 4 summarizes the effect of various sources of materials postimplantation. Choosing proper raw materials or using additive factors encapsulated in biomaterials would improve the outcome of cell therapy treatment, as well as achieve structure support and increase delivered cell survival in CNS therapy.
Table 4.
Effect of Chemistry of Materials Postimplantation.
| Purpose | Applied Site | Materials | Type | Cell | Evidence | Ref |
|---|---|---|---|---|---|---|
| Angiogenesis scaffold | Cerebral cortex | Gelatin/GPSM/bFGF/EGF | Block scaffold | — |
|
35 |
| CNS regeneration scaffold | Cerebrum | HA-IKVAV | Injectable hydrogel | — | Host cell invasion into scaffold | 36 |
| Brain trauma scaffold | Cerebral cortex | Collagen-GAGs | Injectable hydrogel | — |
|
37 |
| Generation of cortical neurons | Rodent brain | PEAs/Wnt3A/BM P4/cyclopam ine | Injectable particles | — |
|
38 |
| SCI scaffold | Spinal cord |
|
Block scaffold | Mice NS/PCs |
|
34 |
| TBI scaffold | Cortex | Collagen type I | Injectable hydrogel | hMSCs |
|
46 |
| TBI scaffold | Cortex |
|
Injectable hydrogel | NSCs |
|
45 |
| SCI scaffold | Spinal cord | HAMC-RGD/PDGF-A | Injectable hydrogel | hiPSCs-OPC |
|
5 |
| Stroke brain repair | Brain |
|
Injectable hydrogel | iPS-NPC |
|
56 |
| Dopamine secretion | Brain | Agarose/PSS a | Injectable particles | PC12 |
|
51 |
| Anti-Aβ antibody secretion | SC | PEG plus 0.45-μm porous membranes | Device implantation | Chimeri c-C2C12 |
|
52 |
| Neovascularization | Stroke brain | VEGF/PLG A | Injectable particles | hNSCs | More angiogenesis was found in microparticle-released VEGF than in microparticles without VEGF | 54 |
Abbreviations: Aβ, amyloid-β; BDNF, brain-derived neurotrophic factor; GAGs, glycosaminoglycans; GDNF, glial cell line–derived neurotrophic factor; GPSM, 3-(glycidoxypropyl) trimethoxysilane; HA, hyaluronic acid; HAMC, hyaluronan and methylcellulose; hiPSCs-OPCs, human induced pluripotent stem cells–derived oligodendrocyte progenitor cells; hMSCs, human mesenchymal stem cells; hNSCs, human neural stem cells; iPS-NPC, human induced pluripotent neural precursor; mNSS, modified neurological severity scores; NS/PCs, neural stem/progenitor cells; PEAs, poly(ester amides); PEG, polyethylene glycol; PLGA, poly(D,L-lactic acid-co-glycolic acid); PSSa, poly-(styrene sulfonic acid); SC, subcutaneous; SCI, spinal cord injury; TBI, traumatic brain injury; VEGF, vascular endothelial growth factor.
Stiffness Effect of Biomaterials on Implantation
CNS Disease Treatment by Biomaterials Alone
Mechanical strength affects the inflammation status of the tissue. The mechanical property of the brain in the body is the softest, but that of electrodes which may be implanted into the brain is several orders of magnitude rigid. Moshayedi and colleagues55 investigated the effect of matrix stiffness on inflammation when materials were implanted into a body. They rendered one with a softer mechanical property like brain (G′ = 100 Pa) and the other with a stiffer property like muscle (G′ = 30 Pa) through crosslinking agents in polyacrylamide. The results showed that softer implanted material showed less inflammatory gene expression postimplantation55. In addition to inflammation of body response, stiffness of materials would decide their standing time in the body. Higher mechanical strength of materials would deposit in the body longer than soft materials did56.
CNS Disease Therapy by Combinations of Cells and Biomaterials
Block-Type Cells/Biomaterials
Mechanical strength of implanted materials has been chosen according to the site of treatment. Researchers designed a block biodegradable scaffold using PLGA to deliver exogenous cells for a midline lateral hemisection in the spinal cord of an adult rat. They simulated spinal cord architecture that contained an inner structure of gray matter having neural stem cells and an outer structure with white matter exhibiting long and axially oriented pores for axonal guidance and radial porosity to allow fluid transport. Open-field locomotion results showed significant improvement in scaffold plus exogenous cells compared with the lesion-control groups. Moreover, GAP43 (axonal marker) and BDA tracking could be observed rostral and caudal to the injury when rats received scaffold plus exogenous cells therapy. Furthermore, both new neurofilament formation of the host and a reducing glial scar (GFAP-positive) formation were observed in the scaffold plus exogenous cells group. Although the scaffold plus exogenous cells group showed promise in functional recovery and reduced epidural and glial scar formation, the scaffold-alone group also received a significantly improved outcome57. This might be the reason why mechanical support is mandatory in the treatment of spinal cord injury. In contrast, in the brain, which is a softer tissue in the body, a polyglycolic acid (PGA) scaffold, a hydrophilic, and low mechanical strength materials have been employed to coimplant with NSCs for brain injury. Results confirmed that the PGA plus exogenous NSCs can fill the cavity of a hypoxic-ischemic brain with loss tissue and promote blood vessel formation. The authors also found that biobridges appeared between host tissue and donor cells58.
Injectable-Type Cells/Biomaterials
Different mechanical strength of materials would affect the cells in which the materials are delivered. Different types of ECM exhibit various mechanical properties. Tate and colleagues compared a laminin-based (∼G′ = 0.8 Pa) and fibronectin-based (∼G′ = 20 Pa) collagen type I scaffold in encapsulating NSCs to implant into the TBI site. The highest survival of implanted cells was observed in the group that received the laminin-based collagen type I scaffold, which had 2 times and 7 times as much as the fibronectin-based and cell-alone groups, respectively. Moreover, the laminin-based collagen type I scaffold showed better functional recovery in cognitive behavior than cells alone and fibronectin-based collagen type I45.
From previous studies (summarized in Table 5), it seems that choosing a mechanical strength of implanted materials would depend on the injured site and the implanted cells delivered by biomaterials. Stiffer materials would be required for injury sites that exhibit higher mechanical strength and vice versa.
Table 5.
Effect of Stiffness of Materials Postimplantation.
| Purpose | Applied site | Materials | Type | Cell | Evidence | Reference |
|---|---|---|---|---|---|---|
| Brain inflammation | Brain | PAA gel: - G′ = 30 kPa - G′ = 100 Pa | Block scaffold | —a | Softer material has less inflammatory gene expression postimplantation | 55 |
| Brain stroke repair | Brain stroke | HA gels: - G′ = 100 Pa - G′ = 350 Pa - G′ = 1000 Pa | Injectable scaffold | —a |
|
56 |
| SCI scaffold | Spinal cord | 50:50 PLGA/PLGA-PLS | Block scaffold | mNSCs |
|
57 |
| HI scaffold | HI brain injury | PGA scaffold | Block scaffold | mNSCs |
|
58 |
| Cell survival | TBI | G′ = 0.8 Pa (laminin based) G′ = 20 Pa (fibronectin based) | Injectable scaffold | NSCs | Highest survival of implanted cell in softer material | 45 |
Abbreviations: HA, hyaluronic acid; HI, hypoxic-ischemic; mNSCs, murine neural stem cells; NF, neurofilament; NSCs, neural stem cells; PAA, poly(acrylic acid); PLGA, poly(lactic-co-glycolic acid); PLS, poly-L-lysine; SCI, spinal cord injury.
aNot applied.
Topography Effect of Biomaterials on Implantation
Biomaterials not only play a role in supporting cell survival but also are tailorable for tissue morphology in regeneration medicine. Kato-Negishi and colleagues59 prepared a network structure of neurospheroids in vitro through topographic microchambers for precisely controlling the organization of transplanted cells. They constructed polydimethylsiloxane (PDMS) microchambers with various diameters (50, 100, 150, and 300 μm), depths (50, 100, 150, and 300 μm), and distances (100, 200, 300, and 600 μm). In the condition of 100, 100, and 200 μm (diameter, depth, and distance), more orderly network structures between neurospheroids were observed. The authors further stamped the neurospheroid network (NSN) onto the cerebral cortex by simply peeling off PDMS microchambers from the network neurospheroid after a 1-day in vitro cultivation. Their results demonstrated that the NSN stamped onto brain tissue showed spontaneous [Ca2+]i responses for more than 8 days and synaptic connections between the stamped NSN and host neurons in the cortical tissues. Although material-free cell transplantation can prepare an organizational structure of neural tissue, it will be hard to apply to surgical procedures in the future. Another type of scaffold was designed in the columnar shape, named micro–tissue-engineered neural networks (micro-TENNs), to form an axonal architecture to restore neural circuits for long-distance deficits. The researchers used an agarose-based micro-column structure, the interior of which was filled with ECM suitable for cerebral cortical neuron cell culture and the exterior of which was coated with carboxymethyl cellulose (CMC) for enhanced stiffness. The micro-TENNs minimized the invasively implanted area in rat brains through enhanced stiffness on the exterior of micro-TENNs39.
We summarize the topography effect of materials postimplantation in Table 6. Topography applied in the implantation has more limitations due to product realization in clinical use in recent years. The potential of topography is to simulate the architectural structure of tissues or organs in the body, and this characteristic may be more useful in the designation of organoid structure in vitro.
Table 6.
Effect of Topography of Materials Postimplantation.
| Purpose | Applied Site | Materials | Type | Cell | Evidence | Reference |
|---|---|---|---|---|---|---|
| Premade neural network transplantation | Cortical tissue | Premade neural cell network by PDMS | Block Scaffold free premade neural network | Rat cerebral cortices |
|
59 |
| Restore lost long-distance axonal pathways | Rat brain | CMC/agarose/ECM/cells | Block scaffold | Cerebral cortical neurons |
|
39 |
Abbreviations: CMC, carboxymethyl cellulose; ECM, extracellular matrix.
The chemistry, stiffness, or topography of biomaterials can be employed in cell transplantation to assist cell therapy, but cellular responses in the human body are not mediated by only one parameter. Moshayedi et al56. combined these 3 parameters in a hydrogel and mixed them with human NSCs for stroke brain therapy. They found the lowest remaining volume of infarcted tissue when the mechanical strength of hydrogel was at 350 Pa. In topography design, they created environmental spatial cues by grafting matrix metalloproteinase (MMP)–degradable peptide on the hydrogels for vascular cell invasion into the hydrogels. Moreover, the effects of chemistry, adhesion motifs, and growth factors on neural progenitor differentiation were explored by a statistical design-of-experiment (DOE) approach. The data showed that adhesive peptides played an important role in neuronal differentiation of human neural progenitor cells (hiPSC-NPCs) in vivo rather than those of growth factors. Moreover, the hydrogel containing both adhesion peptide motifs and growth factors (BMP4 or BDNF) promoted the differentiation of hiPSC-NPCs to astrocytes more than that of neuronal differentiation in vivo.
Overall, CNS injury can be treated by biomaterials alone or complex cell/biomaterial methods through maintaining the shape of the injured site; creating some space with a permissive interface for the invasion of glial cells, blood vessels, and axons cells; protecting transplanted cells; or delivering therapeutic agents. The effect of chemical properties of biomaterials would be a role in providing structural support to the injured site and protecting exogenous cell survival. The stiffness properties of biomaterials would affect host inflammation, exogenous cell survival, and therapeutic agent release; moreover, they determine the standing time of materials in the body. The topography effects of biomaterials are major in the environment, mimicking in particular the architectural design. The influence of 3 parameters of biomaterials on biology is summarized in Figure 1. In addition, delivery types of materials play an important role for the design of implantation materials. We summarize the advantages and disadvantages of various delivery types of biomaterials for further design of implantation materials in Table 7. Both block- and injectable-type biomaterials can act as a backstop to support the trauma site. However, the standing period in the body, surgical area, and delivery method of biomaterials are different. In general, block-type biomaterials are employed in SCI due to advantages of higher mechanical strength and long-term performance of block-type biomaterials. In contrast, it is better to use injectable-type biomaterials for TBI due to advantages of less surgical area required in the treatment and trophic factor delivery for promoting regeneration.
Table 7.
Advantages and Disadvantages of Various Delivery-Type Biomaterials.
| Advantages | Disadvantages | |
|---|---|---|
| Injectable hydrogels |
|
|
| Injectable particles |
|
|
| Block-type materials |
|
|
Cell Tracking Application of Biomaterials in the CNS
The application of biomaterials is used not only for assisting in cell therapy but also in cell labeling for tracing the distribution of implanted cells. A dual-functional probe has been developed with optical and magnetic properties for doubly confirming the location of implanted cells60. The researchers used a polystyrene magnetite nanocluster (PMNC) as the inside core, and the outside of PMNC was coated with 2 layers of silica sandwiched with a layer of rhodamine to form a fluorescent-magnetite nanocluster (FMNC). After synthesis of FMNC, mesenchymal stem cells (MSCs) were incubated with FMNC to examine the efficacy of cell tracking in the ischemic mouse brain. Iron payload for MSCs can achieve 18.42 ± 1.7 pg/cell and does not affect MSC functions. Moreover, small quantities of FMNC-labeled cells can be detected in magnetic resonance imaging (MRI) for more than 1 month60. One team further designed a cell-labeling agent with higher MRI sensitivity and efficiency with material composed of mesoporous-type silica and a small pore size of labeling agents, which avoided a too high uploading of the labeling agent in the cells to diminish the labeled cells’ function. Their results showed that a homing effect of labeled cells to the ischemic hemisphere was observed when the stroke animal received an intravenous injection of 1 × 106 cells, and those homing cells were functional with highly expressed nestin61. The cell tracking using MRI can observe its distribution with high-resolution images in the brain, but it is difficult to use the tracking agent in a whole-body scan. Tang et al62. developed a tracking agent combining probes of MRI, single-photon emission computed tomography (SPECT), and fluorescence to study the quantification of implanted cell distribution after intravenous or intracerebral injection into stroke rats. The percentage of implanted cells migrating to the lesion area was 35% after intracerebral injection, whereas 90% of implanted cells were trapped in the lung by intravenous injection. Nevertheless, less implanted cells were observed in the lesion area, and the delivery route showed significant improvement in neurobehavioral outcomes after 14 days of MSC treatment62. The transplanted cells could be observed by using a labeling agent, but implanted supportive materials could not easily distinguish them from the damaged cavity area with real-time visualization. To achieve visualization of the stroke pathology, tissue regeneration, and transplanted cells at the same time in an animal model, Bible and coworkers63 used a highly sensitive 19F-MRI contrast agent in a T2- and diffusion-weighted MRI session with multinuclear MRI. The cells were labeled with 19F-MRI contrast agent, mixed with extracellular matrix derived from decellularized matrix, and then implanted into a middle cerebral artery occlusion (MCAo) animal model. They found that the distribution of 19F-labeled implanted cells, implanted extracellular matrix, and stroke pathology can be clearly distinguished by T2- and diffusion-weighted MRI.
We summarize the development of a cell tracker in Table 8 to provide more comprehensive information for supporting the designation of biomaterials contained with exogenous cells when they are implanted into the body.
Table 8.
Development of Cell Labeling Tracker Applied in the Central Nervous System.
| Application | Labeling Agent | Cell No./Delivery Route | Instrument | Outcome | Reference |
|---|---|---|---|---|---|
| Stroke model | Fluorescent plus magnetite nanocluster | 5 × 105 mBM-MSCs/IC | T2-weighted FSE sequence MRI |
|
60 |
| Stroke model | Fluorescent plus mesoporous silica-coated SPIONs (fmSiO4@SPIONs) |
|
T2-weighted SE sequence MRI |
|
61 |
| Stroke model | MRI/SPECT/fluorescent tri-modal probe (125I-fSiO4@SPIOs) |
|
T2-weighted FSE sequence combined with SPECT | Quantifiable and real-time visualization of implanted cells:
|
62 |
| Stroke model | 19F-MRI contrast agent with fluorescence dye | 3.75×106 hNSCs/IC | T2-weighted MSME MRI combined with diffusion SMES sequence MRI |
|
63 |
Abbreviations: FSE, fast spin echo; hNSCs, human neural stem cells; IC, intracerebrally; IV, intravenously; mBM-MSCs, mouse bone morrow mesenchymal stem cells; MRI, magnetic resonance imaging; MSCs, mesenchymal stem cells; MSME, multislice multiecho sequence; SE, spin echo; SMES, stimulated multiecho trace; SPECT, single-photon emission computed tomography.
Conclusions and Future Perspective
Tissue engineering requires 3 elements—cells, signals, and a scaffold—to construct an organ, the goal of which is to regenerate or replace a damaged part. For many years, several efforts have been carried out by exogenous cell transplantation, growth factor treatments, scaffold implantation, or a complex of cell/biomaterial implantation in tissue engineering/regenerative medicine64. Moreover, tracking implanted therapeutic materials is involved in the trend of regenerative medicine. However, the goal of tissue or organ replacement is still unmet in this field. In one study, the organoids, which were produced in vitro and exhibited a 3-dimensional microanatomy structure, were found from a patient’s teratoma and studied to understand cancer formation65. In recent years, scientists have attempted to use stem cells or primary cells for constructing a therapeutic organoid, such as human intestinal organoids as the therapeutic organoid for inflammatory bowel disease66. Due to the complicated brain structure and microenvironment, most studies of brain organoids have focused on drug screening and explored the mechanisms of brain disease67–69. Few studies have applied a brain organoid to treat brain diseases70, indicating the complicated nature of the forebrain, midbrain, and hindbrain brain structures, functions, and cell types. Therefore, application to brain therapy through organoids may employ biomaterials to precisely manipulate the cell behavior, which has tissue specificity. In addition, in vitro cultivation system improvement (e.g., coupling to a perfusion system for dynamic control of nutrients and gas exchange to increase cell viability of interior organoids) may be an alternative method to speed up the developments of organ replacement67-69.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was funded by the Buddhist Tzu Chi Bioinnovation Center, Tzu Chi Foundation, Hualien, Taiwan.
References
- 1. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. [DOI] [PubMed] [Google Scholar]
- 2. Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38(12):1392–1400. [DOI] [PubMed] [Google Scholar]
- 3. Skop NB, Calderon F, Cho CH, Gandhi CD, Levison SW. Improvements in biomaterial matrices for neural precursor cell transplantation. Mol Cell Ther. 2014;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hou L, Coller J, Natu V, Hastie TJ, Huang NF. Combinatorial extracellular matrix microenvironments promote survival and phenotype of human induced pluripotent stem cell–derived endothelial cells in hypoxia. Acta Biomater. 2016;44:188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Führmann T, Tam RY, Ballarin B, Coles B, Elliott Donaghue I, van der Kooy D, Nagy A, Tator CH, Morshead CM, Shoichet MS. Injectable hydrogel promotes early survival of induced pluripotent stem cell–derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23–36. [DOI] [PubMed] [Google Scholar]
- 6. Lovat V, Pantarotto D, Lagostena L, Cacciari B, Grandolfo M, Righi M, Spalluto G, Prato M, Ballerini L. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005;5(6):1107–1110. [DOI] [PubMed] [Google Scholar]
- 7. Hu H, Ni YC, Montana V, Haddon RC, Parpura V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 2004;4(3):507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jan E, Kotov NA. Successful differentiation of mouse neural stem cells on layer-by-layer assembled single-walled carbon nanotube composite. Nano Lett. 2007;7(5):1123–1128. [DOI] [PubMed] [Google Scholar]
- 9. Chao TI, Xiang S, Chen CS, Chin WC, Nelson AJ, Wang C, Lu J. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2009;384(4):426–430. [DOI] [PubMed] [Google Scholar]
- 10. Chao TI, Xiang S, Lipstate JF, Wang C, Lu J. Poly(methacrylic acid)-grafted carbon nanotube scaffolds enhance differentiation of hESCs into neuronal cells. Adv Mater. 2010;22(32):3542–3547. [DOI] [PubMed] [Google Scholar]
- 11. Chen YS, Hsiue GH. Directing neural differentiation of mesenchymal stem cells by carboxylated multiwalled carbon nanotubes. Biomaterials. 2013;34(21):4936–4944. [DOI] [PubMed] [Google Scholar]
- 12. Cooper GM. Structure of the plasma membrane In: The cell: a molecular approach. 2nd ed Sunderland (MA): Sinauer Associates; 2000. [Google Scholar]
- 13. Tanahashi K, Mikos AG. Protein adsorption and smooth muscle cell adhesion on biodegradable agmatine-modified poly(propylene fumarate-co-ethylene glycol) hydrogels. J Biomed Mater Res A. 2003;67(2):448–457. [DOI] [PubMed] [Google Scholar]
- 14. Schneider GB, English A, Abraham M, Zaharias R, Stanford C, Keller J. The effect of hydrogel charge density on cell attachment. Biomaterials. 2004;25(15):3023–3028. [DOI] [PubMed] [Google Scholar]
- 15. Bozza A, Coates EE, Incitti T, Ferlin KM, Messina A, Menna E, Bozzi Y, Fisher JP, Casarosa S. Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials. 2014;35(16):4636–4645. [DOI] [PubMed] [Google Scholar]
- 16. Wei J, Han J, Zhao Y, Cui Y, Wang B, Xiao Z, Chen B, Dai J. The importance of three-dimensional scaffold structure on stemness maintenance of mouse embryonic stem cells. Biomaterials. 2014;35(27):7724–7733. [DOI] [PubMed] [Google Scholar]
- 17. Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60(1):24–34. [DOI] [PubMed] [Google Scholar]
- 18. Hachet E, Van Den Berghe H, Bayma E, Block MR, Auzely-Velty R. Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules. 2012;13(6):1818–1827. [DOI] [PubMed] [Google Scholar]
- 19. Arulmoli J, Wright HJ, Phan DT, Sheth U, Que RA, Botten GA, Keating M, Botvinick EL, Pathak MM, Zarembinski TI, et al. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016;43:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orive G, Hernandez RM, Gascon AR, Igartua M, Pedraz JL. Survival of different cell lines in alginate-agarose microcapsules. Eur J Pharm Sci. 2003;18(1):23–30. [DOI] [PubMed] [Google Scholar]
- 21. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. [DOI] [PubMed] [Google Scholar]
- 22. Musah S, Morin SA, Wrighton PJ, Zwick DB, Jin S, Kiessling LL. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano. 2012;6(11):10168–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sridharan I, Kim T, Wang R. Adapting collagen/CNT matrix in directing hESC differentiation. Biochem Biophys Res Commun. 2009;381(4):508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tay CY, Yu H, Pal M, Leong WS, Tan NS, Ng KW, Leong DT, Tan LP. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp Cell Res. 2010;316(7):1159–1168. [DOI] [PubMed] [Google Scholar]
- 25. D’Angelo F, Armentano I, Mattioli S, Crispoltoni L, Tiribuzi R, Cerulli GG, Palmerini CA, Kenny JM, Martino S, Orlacchio A. Micropatterned hydrogenated amorphous carbon guides mesenchymal stem cells towards neuronal differentiation. Eur Cell Mater. 2010;20:231–244. [DOI] [PubMed] [Google Scholar]
- 26. Teo BK, Wong ST, Lim CK, Kung TY, Yap CH, Ramagopal Y, Romer LH, Yim EK. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano. 2013;7(6):4785–4798. [DOI] [PubMed] [Google Scholar]
- 27. Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313(9):1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park SY, Choi DS, Jin HJ, Park J, Byun KE, Lee KB, Hong S. Polarization-controlled differentiation of human neural stem cells using synergistic cues from the patterns of carbon nanotube monolayer coating. ACS Nano. 2011;5(6):4704–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schulte VA, Diez M, Moller M, Lensen MC. Topography-induced cell adhesion to Acr-sP(EO-stat-PO) hydrogels: the role of protein adsorption. Macromol Biosci. 2011;11(10):1378–1386. [DOI] [PubMed] [Google Scholar]
- 30. Guvendiren M, Burdick JA. The control of stem cell morphology and differentiation by hydrogel surface wrinkles. Biomaterials. 2010;31(25):6511–6518. [DOI] [PubMed] [Google Scholar]
- 31. Worthington KS, Wiley LA, Guymon CA, Salem AK, Tucker BA. Differentiation of induced pluripotent stem cells to neural retinal precursor cells on porous poly-lactic-co-glycolic acid scaffolds. J Ocul Pharmacol Ther. 2016;32(5):310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khayyatan F, Nemati S, Kiani S, Hojjati Emami S, Baharvand H. Behaviour of human induced pluripotent stem cell–derived neural progenitors on collagen scaffolds varied in freezing temperature and laminin concentration. Cell J. 2014;16(1):53–62. [PMC free article] [PubMed] [Google Scholar]
- 33. Kuo YC, Chung CY. TATVHL peptide-grafted alginate/poly(gamma-glutamic acid) scaffolds with inverted colloidal crystal topology for neuronal differentiation of iPS cells. Biomaterials. 2012;33(35):8955–8966. [DOI] [PubMed] [Google Scholar]
- 34. Sugai K, Nishimura S, Kato-Negishi M, Onoe H, Iwanaga S, Toyama Y, Matsumoto M, Takeuchi S, Okano H, Nakamura M. Neural stem/progenitor cell-laden microfibers promote transplant survival in a mouse transected spinal cord injury model. J Neurosci Res. 2015;93(12):1826–1838. [DOI] [PubMed] [Google Scholar]
- 35. Deguchi K, Tsuru K, Hayashi T, Takaishi M, Nagahara M, Nagotani S, Sehara Y, Jin G, Zhang H, Hayakawa S, et al. Implantation of a new porous gelatin-siloxane hybrid into a brain lesion as a potential scaffold for tissue regeneration. J Cereb Blood Flow Metab. 2006;26(10):1263–1273. [DOI] [PubMed] [Google Scholar]
- 36. Wei YT, Tian WM, Yu X, Cui FZ, Hou SP, Xu QY, Lee IS. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed Mater. 2007;2(3):S142–S146. [DOI] [PubMed] [Google Scholar]
- 37. Huang K-F, Hsu W-C, Chiu W-T, Wang J-Y. Functional improvement and neurogenesis after collagen-GAG matrix implantation into surgical brain trauma. Biomaterials. 2012;33(7):2067–2075. [DOI] [PubMed] [Google Scholar]
- 38. Memanishvili T, Kupatadze N, Tugushi D, Katsarava R, Wattananit S, Hara N, Tornero D, Kokaia Z. Generation of cortical neurons from human induced-pluripotent stem cells by biodegradable polymeric microspheres loaded with priming factors. Biomed Mater. 2016;11(2):025011. [DOI] [PubMed] [Google Scholar]
- 39. Harris JP, Struzyna LA, Murphy PL, Adewole DO, Kuo E, Cullen DK. Advanced biomaterial strategies to transplant preformed micro-tissue engineered neural networks into the brain. J Neural Eng. 2016;13(1):016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang KF, Hsu WC, Chiu WT, Wang JY. Functional improvement and neurogenesis after collagen-GAG matrix implantation into surgical brain trauma. Biomaterials. 2012;33(7):2067–2075. [DOI] [PubMed] [Google Scholar]
- 41. Andrade ÂL, Ferreira JMF, Domingues RZ. Zeta potential measurement in bioactive collagen. Materials Research. 2004;7:631–634. [Google Scholar]
- 42. Swain SK, Dey RK, Islam M, Patel RK, Jha U, Patnaik T, Airoldi C. Removal of fluoride from aqueous solution using aluminum-impregnated chitosan biopolymer. Separ Sci Technol. 2009;44(9):2096–2116. [Google Scholar]
- 43. Arianna M, Ivana F, Francesco T, Bice F. Interaction of fibrinogen and albumin with titanium dioxide nanoparticles of different crystalline phases. J Phys Conf Ser. 2013;429(1):012014. [Google Scholar]
- 44. He N, Xu Y, Du W, Qi X, Liang L, Wang Y, Feng G, Fan Y, Han Z, Kong D, et al. Extracellular matrix can recover the downregulation of adhesion molecules after cell detachment and enhance endothelial cell engraftment. Sci Rep. 2015;5:10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J Tissue Eng Regen Med. 2009;3(3):208–217. [DOI] [PubMed] [Google Scholar]
- 46. Guan J, Zhu Z, Zhao RC, Xiao Z, Wu C, Han Q, Chen L, Tong W, Zhang J, Han Q, et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. 2013;34(24):5937–5946. [DOI] [PubMed] [Google Scholar]
- 47. Qu C, Mahmood A, Liu XS, Xiong Y, Wang L, Wu H, Li B, Zhang ZG, Kaplan DL, Chopp M. The treatment of TBI with human marrow stromal cells impregnated into collagen scaffold: functional outcome and gene expression profile. Brain Res. 2011;1371:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ballios BG, Cooke MJ, Donaldson L, Coles BL, Morshead CM, van der Kooy D, Shoichet MS. A hyaluronan-based injectable hydrogel improves the survival and integration of stem cell progeny following transplantation. Stem Cell Reports. 2015;4(6):1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elliott Donaghue I, Tam R, Sefton MV, Shoichet MS. Cell and biomolecule delivery for tissue repair and regeneration in the central nervous system. J Control Release. 2014;190:219–227. [DOI] [PubMed] [Google Scholar]
- 50. de Vos P, Lazarjani HA, Poncelet D, Faas MM. Polymers in cell encapsulation from an enveloped cell perspective. Adv Drug Deliv Rev. 2014;67–68:15–34. [DOI] [PubMed] [Google Scholar]
- 51. Miyoshi Y, Date I, Ohmoto T, Iwata H. Histological analysis of microencapsulated dopamine-secreting cells in agarose/poly(styrene sulfonic acid) mixed gel xenotransplanted into the brain. Exp Neurol. 1996;138(1):169–175. [DOI] [PubMed] [Google Scholar]
- 52. Lathuiliere A, Laversenne V, Astolfo A, Kopetzki E, Jacobsen H, Stampanoni M, Bohrmann B, Schneider BL, Aebischer P. A subcutaneous cellular implant for passive immunization against amyloid-beta reduces brain amyloid and tau pathologies. Brain. 2016;139(Pt 5):1587–1604. [DOI] [PubMed] [Google Scholar]
- 53. Bible E, Chau DYS, Alexander MR, Price J, Shakesheff KM, Modo M. The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials. 2009;30(16):2985–2994.19278723 [Google Scholar]
- 54. Bible E, Qutachi O, Chau DY, Alexander MR, Shakesheff KM, Modo M. Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles. Biomaterials. 2012;33(30):7435–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moshayedi P, Ng G, Kwok JCF, Yeo GSH, Bryant CE, Fawcett JW, Franze K, Guck J. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials. 2014;35(13):3919–3925. [DOI] [PubMed] [Google Scholar]
- 56. Moshayedi P, Nih LR, Llorente IL, Berg AR, Cinkornpumin J, Lowry WE, Segura T, Carmichael ST. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016;105:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen G, Hu YR, Wan H, Xia L, Li JH, Yang F, Qu X, Wang SG, Wang ZC. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells and Schwann cells. Chin Med J (Engl). 2010;123(17):2424–2431. [PubMed] [Google Scholar]
- 58. Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotech. 2002;20(11):1111–1117. [DOI] [PubMed] [Google Scholar]
- 59. Kato-Negishi M, Tsuda Y, Onoe H, Takeuchi S. A neurospheroid network-stamping method for neural transplantation to the brain. Biomaterials. 2010;31(34):8939–8945. [DOI] [PubMed] [Google Scholar]
- 60. Wang Y, Xu F, Zhang C, Lei D, Tang Y, Xu H, Zhang Z, Lu H, Du X, Yang GY. High MR sensitive fluorescent magnetite nanocluster for stem cell tracking in ischemic mouse brain. Nanomedicine. 2011;7(6):1009–1019. [DOI] [PubMed] [Google Scholar]
- 61. Zhang L, Wang Y, Tang Y, Jiao Z, Xie C, Zhang H, Gu P, Wei X, Yang GY, Gu H, et al. High MRI performance fluorescent mesoporous silica-coated magnetic nanoparticles for tracking neural progenitor cells in an ischemic mouse model. Nanoscale. 2013;5(10):4506–4516. [DOI] [PubMed] [Google Scholar]
- 62. Tang Y, Zhang C, Wang J, Lin X, Zhang L, Yang Y, Wang Y, Zhang Z, Bulte JW, Yang GY. MRI/SPECT/fluorescent tri-modal probe for evaluating the homing and therapeutic efficacy of transplanted mesenchymal stem cells in a rat ischemic stroke model. Adv Funct Mater. 2015;25(7):1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bible E, Dell’Acqua F, Solanky B, Balducci A, Crapo PM, Badylak SF, Ahrens ET, Modo M. Non-invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke-damaged rat brain by (19)F- and diffusion-MRI. Biomaterials. 2012;33(10):2858–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ajioka I. Biomaterial-engineering and neurobiological approaches for regenerating the injured cerebral cortex. Regen Ther. 2016;3:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith E, Cochrane WJ. Cystic organoid teratoma (report of a case). Can Med Assoc J. 1946;55(2):151–152. [PMC free article] [PubMed] [Google Scholar]
- 66. Holmberg FE, Seidelin JB, Yin X, Mead BE, Tong Z, Li Y, Karp JM, Nielsen OH. Culturing human intestinal stem cells for regenerative applications in the treatment of inflammatory bowel disease. EMBO Mol Med. 2017;9(5);558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydon PG, Kaplan DL. Bioengineered functional brain-like cortical tissue. Proc Natl Acad Sci U S A. 2014;111(38):13811–13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, Santos Costa V, Jiang P, Nguyen BK, Bolin JM, Daly W, et al. Human pluripotent stem cell–derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci U S A. 2015;112(40):12516–12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lindborg BA, Brekke JH, Vegoe AL, Ulrich CB, Haider KT, Subramaniam S, Venhuizen SL, Eide CR, Orchard PJ, Chen W, et al. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med. 2016;5(7):970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tieng V, Stoppini L, Villy S, Fathi M, Dubois-Dauphin M, Krause KH. Engineering of midbrain organoids containing long-lived dopaminergic neurons. Stem Cells Dev. 2014;23(13):1535–1547. [DOI] [PubMed] [Google Scholar]
- 71. Arulmoli J, Wright HJ, Phan DT, Sheth U, Que RA, Botten GA, Keating M, Botvinick EL, Pathak MM, Zarembinski TI, et al. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016;43:122–138. [DOI] [PMC free article] [PubMed] [Google Scholar]