Abstract
Stroke can cause death and disability, resulting in a huge burden on society. Parkinson’s disease (PD) is a chronic neurodegenerative disorder characterized by motor dysfunction. Osteoarthritis (OA) is a progressive degenerative joint disease characterized by cartilage destruction and osteophyte formation in the joints. Stem cell therapy may provide a biological treatment alternative to traditional pharmacological therapy. Mesenchymal stem cells (MSCs) are preferred because of their differentiation ability and possible derivation from many adult tissues. In addition, the paracrine effects of MSCs play crucial anti-inflammatory and immunosuppressive roles in immune cells. Extracellular vesicles (EVs) are vital mediators of cell-to-cell communication. Exosomes contain various molecules such as microRNA (miRNA), which mediates biological functions through gene regulation. Therefore, exosomes carrying miRNA or other molecules can enhance the therapeutic effects of MSC transplantation. MSC-derived exosomes have been investigated in various animal models representing stroke, PD, and OA. Exosomes are a subtype of EVs. This review article focuses on the mechanism and therapeutic potential of MSC-derived exosomes in stroke, PD, and OA in basic and clinical aspects.
Keywords: stroke, Parkinson’s disease, osteoarthritis, mesenchymal stem cells, exosomes, miRNA
Introduction
Stroke, Parkinson’s disease (PD), and osteoarthritis (OA) are degenerative diseases associated with aging. Stroke is the leading cause of death and disability worldwide1. The standard treatment for stroke is tissue plasminogen activator (tPA) infusion within 4.5 h of onset2–4. Treatment with endovascular thrombectomy could extend the therapeutic window to 12 h after a stroke5–8. However, patients with stroke can develop long-term disability if cerebral blood flow is not recovered at a critical time point8. Therefore, the development of a novel therapy to restore brain function after an acute stroke is urgently necessary.
PD is the second most common neurodegenerative disease, with a prevalence of 1% to 2% among aging people9. The cause of PD is unknown but may involve genetic and environmental factors. Patients with PD have clinical features with progressive deterioration of motor functions, including bradykinesia, rigidity, resting tremors, and unstable gait. PD is associated with a pathological decrease in dopamine concentration, neuronal cell loss in the substantia nigra (SN), and Lewy body accumulation in other brain tissues10,11. A specific diagnostic test for PD is not available, and therefore its diagnosis mainly depends on clinical judgment. Functional connectivity measured through Positron emission tomography (PET) scan and functional MRI is helpful for making a clinical judgment9.
Pharmacological agents for dopamine replacement include L-3,4-dihydroxyphenylalanine (l-DOPA), carbidopa, and monoamine oxidase-B inhibitors. These agents are useful in the early stages of PD; however, their long-term use may reduce efficacy and cause side effects involving involuntary motor action that may have an impact on patients’ quality of life. Deep brain stimulation of the globus pallidus and subthalamic nuclei is another therapeutic modality. Although PD has several therapeutic modalities, no complete treatment can stop its degenerative process.
OA is a chronic degenerative joint disease occurring in older adults that is becoming a crucial health concern worldwide12,13. OA involves not only the knees but also the hands, hips, and spine and is characterized by the degeneration and destruction of the articular cartilage and changes in the subchondral bone with osteophyte formation14. Patients experience increasing pain and disability, resulting in decreased quality of life and a high economic burden15. OA is a multifactorial disease16. Its progression involves the interaction of personal factors (old age, female sex, obesity, genetics, and diet) and common factors (injury, misalignment, and abnormal loading of the joints), which increases the risk of comobility and mortality17. Current medical treatments for OA involve pain relief and joint mobility improvement. Acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, topical analgesics, corticosteroid injections, and hyaluronic acid injections are commonly prescribed pharmacological treatments. Physical therapy also results in functional improvement. However, these treatments cannot restore articular cartilage regeneration or modify degenerative processes18. By contrast, surgical arthroplasty is an optimal treatment for patients with symptomatic OA whose condition is not controlled by conservative therapies19. Surgical arthroplasty results in long-term functional improvement and improves quality of life. However, instability and infection are the most common limitations, necessitating further joint revision surgery, particularly in overweight patients20,21.
Stem cell therapy has been rapidly advancing in research and regenerative medicine for OA in recent years22. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) can differentiate into chondrocytes23–25. However, the clinical applications of ESCs or iPSCs have raised considerable concerns about the tumorigenicity, low efficiency, and genomic insertion of transgenic sequences26,27. By contrast, mesenchymal stem cells (MSCs) can be isolated from various adult tissues, including the bone marrow and adipose tissues, which can provide abundant stem cells for regenerative therapy. In addition to the ability to differentiate into chondrocytes, MSCs can modulate immune responses with immunosuppressive and anti-inflammatory properties through their paracrine effects. However, MSC therapy has a dose-dependent effect that requires many cells28.
Emerging evidence in recent years has shown that the paracrine effects of MSCs are mediated by the secretion of extracellular vesicles (EVs)29. Exosomes are a subtype of EVs, approximately 30 to 100 nm in diameter, and are released by cells in all living systems30,31. Exosomes are present in body fluids such as blood and cerebrospinal fluid31 and harbor proteins, lipids, microRNA (miRNA), and RNA. Intercellular communication has been observed in exosomes under various physiological and pathological conditions30,31. MSC exosomes have been studied in various disease models and have shown therapeutic potential in managing stroke, PD, and OA. This review article focuses on the therapeutic potential of MSC exosomes and future directions for their use in research on these degenerative diseases.
Pathophysiology of Stroke, PD, and OA
Pathophysiology of Stroke
A thromboembolic event of a major artery that supplies the brain causes ischemic stroke8. Platelets combined with fibrin and thrombin cause thrombus formation at the site of the occluded artery32,33. The occlusion of the main artery results in the obstruction of downstream small vessels and subsequently leads to the disruption of the blood–brain barrier (BBB) as a result of the dysfunction of endothelial cells, pericytes, and astrocytes34,35. The progression of ischemic neuronal death can be observed hours after the occlusion of an artery34,35. Therefore, thrombolytic treatment using tPA infusion for stroke involves the rapid recanalization of occluded blood vessels and minimization of neuronal death36. After a stroke, the ischemic brain proceeds with a series of remodeling events to enable limited spontaneous functional recovery37. According to past studies in experimental models and the human ischemic brain, endothelial cells residing in preexisting brain vessels are then activated and angiogenesis begins38–40. However, endothelial cells in the brain, which circulate endothelial progenitor cells, are also partially involved in angiogenesis41. Newly formed vessels are permeable in the early stages of recovery but become less leaky when they mature38,42. A past study found that improved neurological outcomes also accompanied increased angiogenesis43. Neural stem cells (NSCs) are harbored in the subventricular zone (SVZ) and subgranular zone of the brain36,44. These NSCs can generate new neurons throughout their lives44. Neurogenesis increased after stroke in experimental animals45,46 and has been found to couple with angiogenesis after stroke onset46,47. The newly generated neuroblasts in the SVZ migrate to the peri-infarct region along cerebral blood vessels46,47. Thus, neuroblasts have a vital functional role in brain repair after stroke48. NSC-derived oligodendrocyte progenitor cells (OPCs) can differentiate into mature oligodendrocytes through myelination49,50. Mature oligodendrocytes are vulnerable to cerebral ischemia. Therefore, OPCs generate new oligodendrocytes during brain repair processes, forming myelin sheaths around the newly generated axons in peri-infarct brain tissues51,52. After stroke, endothelial cells in the brain interact actively and mutually with oligodendrocytes to promote the growth of vessels and oligodendrocytes53.
Pathophysiology of PD
PD is a degenerative disease characterized by the progressive deterioration of motor function, affecting 0.3% of the entire population54. Abnormal accumulation of misfolded proteins in the brain, such as α-synuclein55, causes PD, PD dementia, dementia with Lewy bodies, and multiple system atrophy. Progressive degeneration and loss of dopamine neurons in the SN and nerve terminals in the striatum are the pathological mechanisms of PD56. α-synuclein acts in synaptic transmission and vesicle release57. Lewy bodies are the pathological aggregates of α-synuclein within neurons and glial cells55. The toxic conformations of α-synuclein, oligomers and protofibrils58, can propagate from cell to cell in a prion-like pattern59. This explains the progression of PD and its spread from the basal brain to neocortical areas60. In addition to the accumulation of α-synuclein, a co-aggregate of α-synuclein with amyloid β and τ has been found61–63. Furthermore, genome-wide association studies have found mitochondrial and lysosomal components including leucine-rich repeat kinase 2 (LRRK2)64, Parkin/PARK265, PTEN-induced putative kinase 1 (PINK1)66, and Parkinson disease protein 7 (DJ-1/PARK7)67 in PD and Coenzyme Q2 (COQ2) in MSA68. Cell metabolism and protein clearance together play a role in PD pathophysiology. Locus coeruleus noradrenergic neuron degeneration may result in dementia and depression69. Degeneration of serotonergic neurons in the raphe obscurus and medial raphe may likewise cause depression70. However, the cause of selective degeneration and the loss of specific neurons in PD remain elusive. Infectious agents71, pesticides72, heavy metals73, and living in rural environments74 have been identified as risk factors for PD.
Pathophysiology of OA
Inflammation plays a substantial role in the progression of OA. Advanced OA has shown considerable synovial histological reactions (proliferation or inflammation) and roentgenographic evidence of calcification75. Arthroscopy revealed changes in the cartilage with superficial fibrillation, deep fissures, erosions, and synovial inflammation76. Histologically, B lymphocytes, T lymphocytes, plasma cells, T-helper cells, and Human Leukocyte Antigen - antigen D Related (HLA-DR)-positive dendritic-like cell infiltrations can be found in the intensely inflamed synovium76. However, the severity of cartilage lesions is unrelated to the severity of synovitis in early OA77. Recent studies have reported that low-grade inflammatory processes can not only promote disease symptoms but also accelerate disease progression. Activated macrophages and other innate immune cells release inflammatory cytokines, which promote cartilage damage78. The synovial tissue obtained from a patient with OA showed an increased number of immune cells associated with pro-inflammatory cytokine expression, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, and IL-2279. Matrix metalloproteinase (MMP) 1, 3, and 13 are directly responsible for extracellular matrix remodeling80–82. Physicians prescribe either nonselective NSAIDs (ibuprofen, naproxen, and diclofenac), which act as cyclooxygenase (COX)-1 and -2 inhibitors, or selective NSAIDs (celecoxib and rofecoxib), which act as COX-2 inhibitors, for controlling pain in patients with OA. However, nonselective NSAIDs are associated with considerable gastrointestinal (GI) complications. Although selective NSAIDs cause substantially lower GI complications, they result in a considerably higher risk of cardiovascular events, including myocardial infarction and stroke83,84. New anti-inflammatory therapeutics for OA are under development, and some of these are being studied in randomized controlled trials. Successful treatment requires appropriate patient selection based on synovial inflammatory biomarker measurements85.
Stem Cell Therapy in Stroke, PD, and OA
In our previous article, we comprehensively reviewed the characteristics of MSCs86. In brief, stem cells can differentiate along different lineages and are capable of self-renewal. Adult MSCs are less problematic than ESCs in terms of tumorigenesis and ethical concerns. MSCs are stromal cells that can self-renew and exhibit multilineage differentiation. MSCs can be isolated from various tissues, such as the umbilical cord, endometrial polyps, menstrual blood, bone marrow, and adipose tissue. The ease of harvesting and the quantity of MSCs that can be obtained make them most practical for experimental and possible clinical applications. Other sources of MSCs may be discovered in the future. A major challenge is to elucidate the highly sophisticated mechanisms of differentiation, mobilization, and homing in MSCs. The multipotent properties of these cells make them an attractive choice for the development of clinical applications.
Stem Cell Therapy in Stroke
The aim of cell therapy is to replace, repair, or enhance the biological function of damaged cells and thereby restore brain integrity. Differentiated neuronal progenitors from stem cells can restore functional neuronal circuitry. We have previously reported that stem cell transplantation can repair the damage in animal87–90 and human91,92 stroke models. Moreover, stem cell therapy may secrete paracrine factors to promote the survival, migration, and differentiation of the endogenous precursor cells of the penumbra93. The clinical trials on stroke referred to in this study are drawn from 11 MSC records (searched on November 11, 2016, in clinicaltrials.gov, Table 1). Most relevant studies have used cultured and expanded autologous MSCs from bone marrow, adipose tissue, and umbilical cord. Technical approaches generally use an intravenous injection to deliver the cells directly into the vein without using a scaffold. Most studies are in stage I or II and have worldwide testing area distributions. Currently, the most common approach is intravenous (IV) injection, which is simpler than multicomponent interventions in terms of technical delivery and regulatory approval.
Table 1.
Clinical Trials of MSCs in Stroke.
| Year | Phase | Current Status | Area | MSCs | Trial | Intervention | Comparator |
|---|---|---|---|---|---|---|---|
| 2009 | 2 | Active, not recruiting | Europe | Autologous MSCs | Intravenous stem cells after ischemic stroke | Autologous mesenchymal stem cells | No intervention |
| 2012 | 1 | Recruiting | USA | BM-MSCs | Autologous bone marrow mesenchymal stem cell transplantation for chronic stroke | intracerebral stem cell transplantation | No |
| 2015 | 1 | Recruiting | China | BM-MSCs | Autologous bone marrow mesenchymal stem cell transplantation for chronic ischemic stroke | intracerebral stem cell transplantation | No |
| 2015 | 2 | Not yet recruiting | China | UC-MSCs | Umbilical cord–derived mesenchymal stem cells treatment in ischemic stroke | Human umbilical cord mesenchymal stem cells | No intervention |
| 2012 | 3 | Recruiting | Korea | Autologous MSCs | The stem cell application researches and trials In NeuroloGy-2 (STARTING-2) Study (STARTING-2) | MSC treatment | Standard treatment |
| 2010 | 2 | Recruiting | Spain | Adipose stem cell | Reparative therapy in acute ischemic stroke with allogenic mesenchymal stem cells from adipose tissue, safety assessment, a randomized, double blind placebo controlled single center pilot clinical trial (AMASCIS-01) | ASC treatment | Placebo: IV fluids |
| 2011 | 2 | Recruiting | Malaysia | BM-MSCs | Intravenous autologous mesenchymal stem cells transplantation to treat middle cerebral artery infarct | IV infusion of BM-MSC | Standard treatment |
| 2013 | 1/2 | Not yet recruiting | USA | Allogenic BM-MSCs | Mesenchymal stromal cells for ischemic stroke | IV infusion of BM-MSC | IV normal saline |
| 2013 | 1 | Unknown status | China | UC-MSCs | Umbilical cord–derived mesenchymal stem cells therapy in hypoxic ischemic encephalopathy | IV infusion of UC-MSC | No |
| 2011 | 1/2 | Recruiting | China | Autologous BM-MSCs, EPCs | Autologous bone marrow stromal cell and endothelial progenitor cell transplantation in ischemic stroke | IV infusion of BM-MSC | IV normal saline with 5% serum |
| 2016 | 2/3 | Recruiting | Europe | Adipose stem cell | Regenerative stem cell therapy for stroke in Europe | IV infusion of ASC | IV cell excipients |
Abbreviations: AMETIS, Autologous bone marrow stromal cell and endothelial progenitor cell transplantation in ischemic stroke; MSCs, mesenchymal stem cells; RESTORE, regenerative stem cell therapy for stroke in Europe; SAMCLS, mesenchymal stromal cells for ischemic stroke; UCMSC, umbilical cord mesenchymal stem cells; BMSCs, bone marrow stem cells; EPC, endothelial progenitor cells; ASC, adipose stem cells.
Stem Cell Therapy in PD
Bone marrow-derived MSCs (BM-MSCs) have been examined for their therapeutic effect in a PD model; these studies have demonstrated the survival of grafted cells, tyrosine hydroxylase (TH) expression, and behavioral improvement94–98. Other stem cells, such as adipose-derived and umbilical cord-derived (ADSCs and UC-MSCs, respectively) MSCs, also improve PD symptoms99,100. Moreover, genetically modified MSCs with neurotrophic proteins, such as glial cell line-derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF), or neurturin, have been indicated to have therapeutic potential in PD treatment. 101,102 In patients with PD, proliferation of activated microglia was noted in the SN103. TNF-α, IL-1β, and interferon-γ were elevated in the brains of patients with PD104. Immunosuppression therapy slowed PD progression105. Additionally, MSCs exhibited crucial anti-inflammatory and immunomodulatory effects on PD pathology. Only 3 clinical trials to date have adopted MSCs for PD therapy (Table 2). One trial is active but not yet recruiting, whereas the status of two other trials is unknown.
Table 2.
Clinical Trials of MSCs in Parkinson’s Disease.
| Year | Phase | Current Status | Area | MSCs | Trial | Intervention | Comparator |
|---|---|---|---|---|---|---|---|
| 2011 | 1/2 | Recruiting, unknown status | China | Autologous BM-MSCs | Mesenchymal stem cells transplantation to patients with Parkinson’s disease | IV BM-MSCs | No |
| 2015 | 1/2 | Active, not yet recruiting | USA | Allogenic BM-MSCs | Allogeneic bone marrow–derived mesenchymal stem cell therapy for idiopathic Parkinson’s disease | IV BM-MSCs | No |
| 2013 | 1/2 | Unknown status | Italy | Autologous BM-MSCs | Clinical trial to evaluate bone marrow stem cell therapy for PSP, a rare form of Parkinsonism | Intra-artery infusion of BM-MSCs | No |
Abbreviations: MSC, mesenchymal stem cells; PSP, progressive supranuclear palsy; BMSCs, bone marrow stem cells.
Stem Cell Therapy in OA
MSC therapy for OA may be a permanent biological treatment106,107. Stem cells from all sources, such as embryonic, induced pluripotent, fetal, and adult stem cells, can be used in this therapy. Among these, MSCs are the first choice because they can not only differentiate into a chondrogenic lineage under defined culture conditions but also modulate the immune responses of individuals through anti-inflammatory effects108,109. In addition to direct chondrocyte differentiation that repairs damaged OA joints, the paracrine effect of MSCs plays a crucial immunosuppressive and anti-inflammatory role in immune cells110. MSCs can inhibit the proliferation and differentiation of naive T lymphocytes into the T-helper type 1 (Th1) or IL-17-producing effector T (Th17) phenotype111. Increasing evidence has indicated that MSCs participate in tissue repair and regeneration through their secretome, which includes exosomes. The downregulation of inflammatory cytokines and the induction of chondrocyte regeneration are essential for repairing diseased joints112. Both soluble and contact-dependent signals from the environment trigger the therapeutic effect of MSCs. Therefore, various mediators and EVs secreted from MSCs in the surrounding extracellular environment play vital roles in achieving the therapeutic effect of MSCs for OA.
Exosome Introduction
In past decades, transplanted stem cells were believed to heal damaged tissue by directly differentiating into cells at the damaged site. However, recent evidence has attributed the beneficial effects of stem cell transplantation not to their direct differentiation abilities, but rather their ability to secrete bioactive molecules, which provide a regenerative microenvironment for various injured tissues to limit the area of damage and mount a self-regulated regenerative response113,114. EVs are crucial mediators of cell-to-cell communication, which is involved in normal physiological processes and additionally plays a role in the development and progression of diseases. Therefore, current studies are increasingly focusing on the role of EVs in MSC transplantation and their therapeutic potential (Fig. 1). The major subtypes of EVs are exosomes, microvesicles, and apoptotic bodies115. Exosomes are 40 to 100 nm in diameter and can be isolated from all bodily fluids including blood, urine, bronchoalveolar lavage fluid, breast milk, amniotic fluid, synovial fluid, pleural effusions, and ascites through centrifugation116. Exosomes are endocytic materials that contain a particular set of protein families from intracellular compartments including the plasma membrane, endocytic pathway, and cytosol117. Exosomes contain CD63 and CD81 (tetraspanin proteins), Alix (the regulator of endosomal trafficking), and HSP70 (the chaperone protein)31,118. Exosomes also include messenger RNA (mRNA) and miRNA, which can transfer genetic information to target cells119. These exosomes, which contain proteins, mRNA, and miRNA, function as messengers from donor cells to recipient cells and induce physiological changes in recipient cells. The mRNA packed within exosomes can be translated after entering into the recipient cells. By contrast, miRNA is involved in RNA silencing and posttranscriptional regulation of gene expression in recipient cells119.
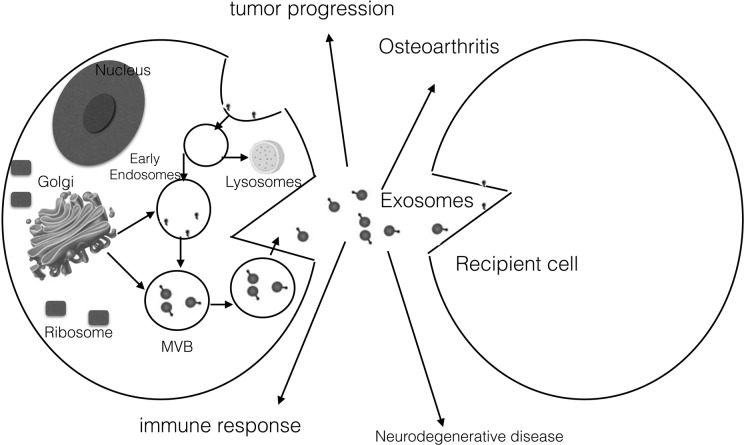
Fig. 1.
Exosome synthesis and action. A cell membrane is an inward budding and formed multivesicular body (MVB). Exosomes are released after the MVB fuses with the membrane. Exosomes can carry lipids, proteins, and nucleic acids to recipient cells; they act as intercellular communicators and play crucial roles in immune response, neurodegenerative disease, osteoarthritis, and tumor progression.
Stem Cells Actively Secrete Exosomes
MSC-derived exosomes can be steadily isolated from the MSC-conditioned medium. They are as effective as direct MSC transplantation, and their beneficial therapeutic effects have been demonstrated in various models, including those for cardiovascular disease, acute kidney injury, liver injury, lung injury, and cutaneous wound healing115,120. The protective effects are specific to MSC-derived exosomes and are not exhibited by fibroblast-derived exosomes121. MSCs can secrete a higher amount of exosomes than other types of cells122. The morphology, isolation, and storage conditions of MSC-derived exosomes are the same as those of exosomes derived from other cells122. MSCs can produce many more exosomes than other cells can122. In a myocardial infarction model, the use of exosomes derived from myc-transformed MSCs was found to reduce the infarction size123. The proposed mechanism was that the myc transformation of MSCs caused them to infinitely produce a large amount of exosomes, which would exert therapeutic effects. Moreover, the myc transformation of MSCs increased the proliferation rate, which reduced the time required for cell production123. Thus, this method can effectively enable MSCs to produce a substantial amount of exosomes.
Role of Exosomes in Immune Responses
Exosomes are considered carriers of immune responses124–128. Immunomodulation mediated by exosomes remains controversial. The promotion or suppression of immune responses depends on the characteristics of the parent cell129. Antigen-presenting cells (APCs), including dendritic cells (DCs) and B lymphocytes, secrete exosomes that carry immunostimulatory molecules. These molecules, which participate in the development of antigen-specific immune responses, include MHC-I, MHC-II, and CD80/CD86 DC exosomes–activated T cells130–135. In addition, B lymphocyte-derived exosomes can facilitate antigen presentation and stimulate T cells in vitro. These actions indicate a role in T cell memory and tolerance130,136. Moreover, exosomes derived from B lymphocytes could be delivered to follicular DCs in vitro, suggesting that follicular DCs might passively obtain peptide-loaded major histocompatibility complex II (MHC- II) molecules for stimulating CD4 T cells137. miRNA is involved in immune regulation138 and can be transferred by exosomes and affect immune activities139. Exosomes can be unidirectionally transferred between T cells and APCs139. Inhibition of exosome formation impaired APC exosome and miRNA transfer in T cells. However, the contribution of exosomes is difficult to determine because almost all cells can secrete exosomes, only one cell type can be studied in vitro, the in vivo setting is much more complicated, and exosome exchange may be bidirectional. Different organs may have different vesicle transfer mechanisms140,141. Therefore, exosomes can either activate or suppress the immune response depending on the donor cell type142–144. Exogenous miRNA delivery to target cells appears to be facilitated by exosomes. However, recipient uptake mechanisms should be explored further145.
Effect of Exosomes on the Brain
The regulation of immune function by exosomes has been reported for microglia or macrophages in the brain. The proteomics of exosomes secreted from microglia has identified several known vesicle proteins already present in B cells and DC-secreting exosomes; microglia-secreted exosomes also express MHC-II molecules146. Upon activation, the microglia release both membrane vesicles and soluble inflammatory cytokines including IL-1β, IL-6, and TNF-α147–149. During central nervous system (CNS) inflammation, the number of microglia-secreted exosomes increases, and they enter into cerebral spinal fluid (CSF) circulation150. Therefore, circulating exosomes can be regarded as the markers of inflammation that locally or systemically affect the CNS150. Endothelial cells in the brain can also release small membrane vesicles—endothelial microparticles (EMPs)—which are considered useful indicators of the status of the disordered endothelium151,152. After stroke, EMPs released from the injured endothelium are linked with microcirculatory injuries, capillary blockage, inflammatory processes, and BBB disruption. The amount of circulating EMPs has been correlated with the severity of stroke, volume of brain lesions, and outcome. When inflammatory cytokines (IFN-γ and TNF-α) are stimulated, endothelial cells secrete EMPs153.
The third exosome effect on the brain is derived from brain tumors. Tumor-derived exosomes can act like cancer vaccines due to their tumor-specific antigenicity and hereditary spastic paraplegia (HSP) that favor the activation of APCs154,155. Human gliomas can express a mutation of the epidermal growth factor receptor variant III (EGFRvIII). This variant can define clinically distinct glioblastoma subtypes156 and serve as a biomarker157. Glioma-secreted exosomes can also promote the oncogenic transformation of neighboring cells through the transfer of EGFRvIII158. Tumor-derived exosomes can additionally intervene in immune suppression by augmenting the activities of regulatory T cells and myeloid-derived suppressor cells; they suppress activated T cells and natural killer (NK) cells by inhibiting DC maturation159. Therefore, tumor-derived exosomes appear to harbor both immune-promoting and immune-supressing functions.
Potential of Stem Cell–Derived Exosomes in Stroke, PD, and OA Treatment
Exosome Therapy in Stroke
Neurons, astrocytes, and glia can release various membranous vesicles into the extracellular space. These EVs may act as carriers of proteins associated with neurodegenerative diseases. EVs may be involved in the spreading of these misfolded proteins in the brain. Therefore, only exosomes can be adopted as a treatment modality. Intravenous injection of exosomes has been demonstrated to be more efficient than the use of cells in treating stroke. Exosomes can transfer their cargo miRNA to recipient cells160,161. More than 700 miRNAs are bound to argonaute2, a component of the RNA-induced silencing complex in MSC-derived exosomes162. Engineered exosomes with elevated miRNA levels have a beneficial effect on brain remodeling after stroke163,164. Immunosuppression induced by stroke in peripheral blood can exacerbate stroke outcomes165,166. MSC-derived exosomes can communicate with NK cells and lymphocytes to attenuate postischemic immunosuppression167. Exosomes of miR133b-overexpressed MSCs have recently been reported to improve neural plasticity and functional recovery in a stroke model164,168. miR133b was downregulated in the rat brain after cerebral artery occlusion; however, the miR133b level increased after MSC administration164,168. The transfer of miR33b from MSCs to astrocytes through exosome-downregulated connected tissue growth factor expression can reduce glial scarring and promote neurite growth169. In a stroke model, miR-133b also inhibited Ras homolog gene family, member A (RhoA) expression in neurons, which promoted the regrowth of the corticospinal tract170. Exosomes of hASCs-mediated PKCδ splicing and increased neuronal survival171. Intravenous injection of Adipose derived stem cells (ADSCs)-derived exosomes could reduce the brain infarct zone and improve neurological function in a stroke model172. BM-MSCs derived from diabetic mice reduced miR-145 expression and aided recovery from stroke173. Intravenous injection of MSC-derived exosomes could improve functional recovery and neurite remodeling, neurogenesis, and angiogenesis163. Exosome miR-9 and miR-124, brain-specific miRNA, are promising biomarkers for diagnosing stroke severity and as alternatives to therapy174. The direct use of exosomes from specific cell sources has considerable potential in stroke treatment.
Potential Benefits of Exosomes in PD
No reliable diagnostic tool is currently available for PD. Exosomes have two roles in PD: as a diagnostic biomarkers and for therapy. For diagnosis, increased mutation in LRRK2 in urine was recently reported to be associated with idiopathic PD and the severity of cognitive impairment175–177. Another study found that the Neural cell adhesion molecule L1 (L1-CAM) exosome τ level was significantly higher in patients with PD than in controls and was correlated with the CSF tau levels178. The level of α-synuclein was also higher in L1-CAM-positive EV isolated from the plasma of patients with PD than in control patients179–181. The expression profiles of miRNA and mRNA in exosomes of PD also served as diagnostic tools for PD. Neurotrophin signaling, mechanistic target of rapamycin (mTOR), ubiquitin-mediated proteolysis, and dopaminergic and glutamatergic synapse were the most significant pathways in PD miRNA patterns182. For therapy, exosomes derived from human dental pulp have recently been found to reduce 80% of 6-hydroxydopamine (OHDA)-induced dopamine neuron apoptosis183. Exosomes carrying catalase exerted substantial neuroprotective effects on in vitro and in vivo models of PD184. In summary, the use of exosomes to treat PD is in its early stages, being mostly incorporated in diagnosis and rarely in treatment.
Potential Benefits of Stem Cell–Derived Exosomes in OA
Inflammation plays a vital role in the pathogenesis of OA. Catabolic factors, such as IL-1α or TNF-α, present in OA joints inhibit the differentiation of stem cells that impair chondrogenesis185. MSC-derived exosomes can suppress the secretion of the pro-inflammatory cytokines TNF-α and IL-1β and can also increase the secretion of anti-inflammatory cytokines, thus increasing the level of transformation growth factor-β. Exosomes may induce the conversion of Th1 cells into Th2 cells and reduce the differentiation of T cells into Th17 cells186. Therefore, MSC-derived exosomes can suppress the inflammation of OA joints and introduce a trophic effect that stimulates tissue-intrinsic stem cells to repair damaged tissues, similar to MSCs113. Although MSC-derived exosomes have exhibited considerable advances in many disease models, they have only now been incorporated into OA therapy. Zhang et al. reported that exosomes derived from human embryonic MSCs promoted osteochondral regeneration in a surgical rat model of osteochondral defects187. The model showed complete restoration of the cartilage and subchondral bone 12 wks after a single intra-articular exosome injection. By contrast, the contralateral phosphate buffered saline (PBS)-treated defects only formed fibrous repair tissues. miRNAs are also involved in chondrogenesis and cartilage degeneration in OA188. For instance, miR-140 is related to chondrocyte differentiation189. miR-320 directly targets MMP-13 and produces the IL-1β-stimulated catabolic effect190. Both miR-140 and miR-320 are significantly decreased in OA cartilage. By contrast, miR-455 overexpression during the aging process exacerbates OA progression191. MiR-181b is significantly downregulated during chondrogenic differentiation and significantly overexpressed in OA cartilage192. Therefore, MSC-derived exosomes likely attenuate OA progression through the delivery of miRNA. Various MSC-origin exosomes may function differently in OA. Clinical trials have demonstrated the therapeutic effects of BM-MSCs, adipose-derived MSCs (ADSCs), and human UC-MSCs in OA. Some clinical trials are ongoing22. However, the low RNA content in exosomes appears to be considerably influenced by donors, cell types, environments, and cell differentiation status. Baglio et al. concluded that adipose and bone marrow MSC subtypes secrete different transfer RNA species that may have clinical applications193. Furthermore, Salomon et al. demonstrated that under hypoxic conditions, placental MSCs released exosomes in a dose-dependent manner that stimulated placental microvascular endothelial cell migration and tube formation194.
Conclusion and Prospects
Stem cell–derived exosomes carried and transferred their cargo (similar to miRNA) to parenchymal cells in the brain or cartilage. Thus, exosomes mediate plasticity and functional recovery from stroke or OA. Because of the requirements of complex paracrine factors, exosomes may be used as a treatment modality for complicated diseases such as stroke and OA. Different miRNA contents of stem cell–derived exosomes can be used to modulate the therapeutic response to stroke and may increase their therapeutic potential. Moreover, exosomes can be used as a diagnostic marker for PD.
Exosomes have many benefits aside from the cell-based therapy reported in clinical trials for stroke195,196. In contrast to injecting cells into the vein systemically, exosomes, which have diameters measured in nanometers, may easily enter the brain by passing through the BBB197,198. Direct injection of MSCs may result in the obstruction of small vessels in organs199. Because of their small size, exosomes have no apparent obstructive effect on small vessels.
Research is ongoing on the benefits of the stem cell–derived exosome therapy for degenerative diseases such as stroke, PD, and OA. Stem cell–derived exosomes, whether naturally occurring or engineered, can provide therapeutic benefits. Although exosome therapies have shown positive results, most studies have focused on acute injury disease models. Stroke, PD, and OA are multifactorial chronic degenerative diseases with chronic inflammation. Additional studies are required to elucidate the pathogenesis of these degenerative diseases and the potential benefits of exosomes derived from different MSC sources, preconditioning statuses, doses, and therapeutic regimens.
The purity of exosomes should be further examined. Differential centrifugation and a sucrose gradient can yield a mixed gradient product200. Mass exosome production is expensive and time consuming. Thus, future studies should focus on reducing the cost and time required for exosome production. Regarding the modification of exosomes for therapy, exosome products should be thoroughly characterized to prevent adverse events.
Footnotes
Authors’ Note: This article was edited by Wallace Academic Editing.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Paul R. Sanberg (PRS) is the coeditor in chief of Cell Transplantation. Neither PRS nor any of his colleagues were involved in the peer-review process or decision for this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: The authors were supported by the following intramural grants from Buddhist Tzu Chi General Hospital: TCRD 104-07 (to Chang Y. H.) and TCRDI-104-01-03 (to Ding D. C.).
References
- 1. Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, et al. Factors influencing the decline in stroke mortality: a statement from the American heart association/American stroke association. Stroke. 2014;45(1):315–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. [DOI] [PubMed] [Google Scholar]
- 3. Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42(7):1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zivin JA. Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U.S. Food and Drug Administration (FDA). Ann Neurol. 2009;66(1):6–10. [DOI] [PubMed] [Google Scholar]
- 5. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. [DOI] [PubMed] [Google Scholar]
- 6. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. [DOI] [PubMed] [Google Scholar]
- 7. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. [DOI] [PubMed] [Google Scholar]
- 8. Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. [DOI] [PubMed] [Google Scholar]
- 9. Gao LL, Wu T. The study of brain functional connectivity in Parkinson’s disease. Transl Neurodegener. 2016;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14(5):518–531. [DOI] [PubMed] [Google Scholar]
- 11. Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373(9680):2055–2066. [DOI] [PubMed] [Google Scholar]
- 12. Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 suppl 1): S13–S19. [DOI] [PubMed] [Google Scholar]
- 13. Yoshimura N, Muraki S, Nakamura K, Tanaka S. Epidemiology of the locomotive syndrome: the research on osteoarthritis/osteoporosis against disability study 2005-2015. Mod Rheumatol. 2017;27(1):1–7 [DOI] [PubMed] [Google Scholar]
- 14. Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. [DOI] [PubMed] [Google Scholar]
- 15. Xie F, Kovic B, Jin X, He X, Wang M, Silvestre C. Economic and humanistic burden of osteoarthritis: a systematic review of large sample studies. Pharmacoeconomics. 2016;34(11):1087–1100. [DOI] [PubMed] [Google Scholar]
- 16. Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, et al. Osteoarthritis: new insights. part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. [DOI] [PubMed] [Google Scholar]
- 17. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138. [DOI] [PubMed] [Google Scholar]
- 18. Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85(1):49–56. [PubMed] [Google Scholar]
- 19. Vissers MM, Bussmann JB, Verhaar JA, Arends LR, Furlan AD, Reijman M. Recovery of physical functioning after total hip arthroplasty: systematic review and meta-analysis of the literature. Phys Ther. 2011;91(5):615–629. [DOI] [PubMed] [Google Scholar]
- 20. Liu XW, Zi Y, Xiang LB, Wang Y. Total hip arthroplasty: areview of advances, advantages and limitations. Int J Clin Exp Med. 2015;8(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- 21. Stiehler M, Goronzy J, Gunther KP. Total hip arthroplasty in overweight osteoarthritis patients. Orthopade. 2015;44(7):523–530. [DOI] [PubMed] [Google Scholar]
- 22. Chang YH, Liu HW, Wu KC, Ding DC. Mesenchymal stem cells and their clinical applications in osteoarthritis. Cell Transplant. 2016;25(5):937–950. [DOI] [PubMed] [Google Scholar]
- 23. Khillan JS. Generation of chondrocytes from embryonic stem cells. Methods Mol Biol. 2006;330:161–170. [DOI] [PubMed] [Google Scholar]
- 24. Singh Khillan J. Differentiation of embryonic stem cells into cartilage cells. Curr Protoc Stem Cell Biol. 2007;Chapter 1: Unit 1F.1. [DOI] [PubMed] [Google Scholar]
- 25. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 26. Illich DJ, Demir N, Stojkovic M, Scheer M, Rothamel D, Neugebauer J, Hescheler J, Zoller JE. Concise review: induced pluripotent stem cells and lineage reprogramming: prospects for bone regeneration. Stem Cells. 2011;29(4):555–563. [DOI] [PubMed] [Google Scholar]
- 27. Inui A, Iwakura T, Reddi AH. Human stem cells and articular cartilage regeneration. Cells. 2012;1(4):994–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. [DOI] [PubMed] [Google Scholar]
- 29. Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012;3:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gyorgy B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang ZG, Zhang L, Tsang W, Goussev A, Powers C, Ho KL, Morris D, Smyth SS, Coller BS, Chopp M. Dynamic platelet accumulation at the site of the occluded middle cerebral artery and in downstream microvessels is associated with loss of microvascular integrity after embolic middle cerebral artery occlusion. Brain Res. 2001;912(2):181–194. [DOI] [PubMed] [Google Scholar]
- 33. Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. [DOI] [PubMed] [Google Scholar]
- 34. Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;142(2):623–635. [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(7):1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ding DC, Lin CH, Shyu WC, Lin SZ. Neural stem cells and stroke. Cell Transplant. 2013;22(4):619–630. [DOI] [PubMed] [Google Scholar]
- 37. Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38(suppl 2):840–845. [DOI] [PubMed] [Google Scholar]
- 38. Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. [DOI] [PubMed] [Google Scholar]
- 40. Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6(10):1102–1103. [DOI] [PubMed] [Google Scholar]
- 41. Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90(3):284–288. [DOI] [PubMed] [Google Scholar]
- 42. Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58(4):313–320. [DOI] [PubMed] [Google Scholar]
- 43. Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller FD, Gauthier-Fisher A. Home at last: neural stem cell niches defined. Cell Stem Cell. 2009;4(6):507–510. [DOI] [PubMed] [Google Scholar]
- 45. Iwai M, Sato K, Kamada H, Omori N, Nagano I, Shoji M, Abe K. Temporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbils. J Cereb Blood Flow Metab. 2003;23(3):331–341. [DOI] [PubMed] [Google Scholar]
- 46. Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23(6):935–942. [DOI] [PubMed] [Google Scholar]
- 48. Wang X, Mao X, Xie L, Sun F, Greenberg DA, Jin K. Conditional depletion of neurogenesis inhibits long-term recovery after experimental stroke in mice. PLoS One. 2012;7(6): e38932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77(5):873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15(4):528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang RL, Chopp M, Roberts C, Jia L, Wei M, Lu M, Wang X, Pourabdollah S, Zhang ZG. Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligodendrogenesis. J Cereb Blood Flow Metab. 2011;31(2):614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang RL, Chopp M, Roberts C, Wei M, Wang X, Liu X, Lu M, Zhang ZG. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One. 2012;7(10): e48141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miyamoto N, Pham LD, Seo JH, Kim KW, Lo EH, Arai K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci. 2014;71(6):1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. [DOI] [PubMed] [Google Scholar]
- 55. Goedert M, Jakes R, Anthony Crowther R, Grazia Spillantini M. Parkinson’s disease, dementia with lewy bodies, and multiple system atrophy as alpha-Synucleinopathies. Methods Mol Med. 2001;62:33–59. [DOI] [PubMed] [Google Scholar]
- 56. Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334(6180):345–348. [DOI] [PubMed] [Google Scholar]
- 57. Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25(47):10913–10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, et al. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015;112(38): E5308–E5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 61. Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(21):12245–12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62(4):389–397. [DOI] [PubMed] [Google Scholar]
- 63. Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic interactions between abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30(21):7281–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. [DOI] [PubMed] [Google Scholar]
- 65. Matsumine H, Yamamura Y, Hattori N, Kobayashi T, Kitada T, Yoritaka A, Mizuno Y. A microdeletion of D6S305 in a family of autosomal recessive juvenile parkinsonism (PARK2). Genomics. 1998;49(1):143–146. [DOI] [PubMed] [Google Scholar]
- 66. Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. [DOI] [PubMed] [Google Scholar]
- 67. Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. [DOI] [PubMed] [Google Scholar]
- 68. Multiple-System Atrophy Research C. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369(3):233–244. [DOI] [PubMed] [Google Scholar]
- 69. German DC, Manaye KF, White CL, III, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32(5):667–676. [DOI] [PubMed] [Google Scholar]
- 70. Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14(3):153–197. [DOI] [PubMed] [Google Scholar]
- 71. Takahashi M, Yamada T. Viral etiology for Parkinson’s disease—a possible role of influenza A virus infection. Jpn J Infect Dis. 1999;52(3):89–98. [PubMed] [Google Scholar]
- 72. Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22(16):7006–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mizuno Y, Hattori N, Kitada T, Matsumine H, Mori H, Shimura H, Kubo S, Kobayashi H, Asakawa S, Minoshima S, et al. Familial Parkinson’s disease. Alpha-synuclein and parkin. Adv Neurol. 2001;86:13–21. [PubMed] [Google Scholar]
- 74. Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res. 2001;86(2):122–127. [DOI] [PubMed] [Google Scholar]
- 75. Gordon GV, Villanueva T, Schumacher HR, Gohel V. Autopsy study correlating degree of osteoarthritis, synovitis and evidence of articular calcification. J Rheumatol. 1984;11(5):681–686. [PubMed] [Google Scholar]
- 76. Lindblad S, Hedfors E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987;30(10):1081–1088. [DOI] [PubMed] [Google Scholar]
- 77. Myers SL, Brandt KD, Ehlich JW, Braunstein EM, Shelbourne KD, Heck DA, Kalasinski LA. Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol. 1990;17(12):1662–1669. [PubMed] [Google Scholar]
- 78. Liu-Bryan R. Inflammation and intracellular metabolism: new targets in OA. Osteoarthritis Cartilage. 2015;23(11):1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52(12):870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Martel-Pelletier J, McCollum R, Fujimoto N, Obata K, Cloutier JM, Pelletier JP. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994;70(6):807–815. [PubMed] [Google Scholar]
- 81. Chevalier X, Conrozier T, Gehrmann M, Claudepierre P, Mathieu P, Unger S, Vignon E. Tissue inhibitor of metalloprotease-1 (TIMP-1) serum level may predict progression of hip osteoarthritis. Osteoarthritis Cartilage. 2001;9(4):300–307. [DOI] [PubMed] [Google Scholar]
- 82. Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8(6): R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA, American Heart A. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American heart association. Circulation. 2007;115(12):1634–1642. [DOI] [PubMed] [Google Scholar]
- 84. Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332(7553):1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Philp AM, Davis ET, Jones SW. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology (Oxford), 2017;56(6):869–881. [DOI] [PubMed] [Google Scholar]
- 86. Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5–14. [DOI] [PubMed] [Google Scholar]
- 87. Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, Su CY, Li H. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27(3):339–353. [DOI] [PubMed] [Google Scholar]
- 88. Liu SP, Ding DC, Wang HJ, Su CY, Lin SZ, Li H, Shyu WC. Nonsenescent Hsp27-upregulated MSCs implantation promotes neuroplasticity in stroke model. Cell Transplant. 2010;19(10):1261–1279. [DOI] [PubMed] [Google Scholar]
- 89. Shyu WC, Lin SZ, Chiang MF, Su CY, Li H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26(13):3444–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shyu WC, Liu DD, Lin SZ, Li WW, Su CY, Chang YC, Wang HJ, Wang HW, Tsai CH, Li H. Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke. J Clin Invest. 2008;118(7):2482–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006;174(7):927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen DC, Lin SZ, Fan JR, Lin CH, Lee W, Lin CC, Liu YJ, Tsai CH, Chen JC, Cho DY, et al. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomized phase II study. Cell Transplant. 2014;23(12):1599–1612. [DOI] [PubMed] [Google Scholar]
- 93. Ding DC, Shyu WC, Lin SZ, Li H. Current concepts in adult stem cell therapy for stroke. Curr Med Chem. 2006;13(29):3565–3574. [DOI] [PubMed] [Google Scholar]
- 94. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–370. [DOI] [PubMed] [Google Scholar]
- 95. Offen D, Barhum Y, Levy YS, Burshtein A, Panet H, Cherlow T, Melamed E. Intrastriatal transplantation of mouse bone marrow-derived stem cells improves motor behavior in a mouse model of Parkinson’s disease. J Neural Transm Suppl 2007(72):133–143. [DOI] [PubMed] [Google Scholar]
- 96. Bouchez G, Sensebe L, Vourc’h P, Garreau L, Bodard S, Rico A, Guilloteau D, Charbord P, Besnard JC, Chalon S. Partial recovery of dopaminergic pathway after graft of adult mesenchymal stem cells in a rat model of Parkinson’s disease. Neurochem Int. 2008;52(7):1332–1342. [DOI] [PubMed] [Google Scholar]
- 97. Ye M, Wang XJ, Zhang YH, Lu GQ, Liang L, Xu JY, Sheng-Di C. Therapeutic effects of differentiated bone marrow stromal cell transplantation on rat models of Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(1):44–49. [DOI] [PubMed] [Google Scholar]
- 98. Delcroix GJ, Garbayo E, Sindji L, Thomas O, Vanpouille-Box C, Schiller PC, Montero-Menei CN. The therapeutic potential of human multipotent mesenchymal stromal cells combined with pharmacologically active microcarriers transplanted in hemi-parkinsonian rats. Biomaterials. 2011;32(6):1560–1573. [DOI] [PubMed] [Google Scholar]
- 99. McCoy MK, Martinez TN, Ruhn KA, Wrage PC, Keefer EW, Botterman BR, Tansey KE, Tansey MG. Autologous transplants of adipose-derived adult stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson’s disease. Exp Neurol. 2008;210(1):14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mathieu P, Roca V, Gamba C, Del Pozo A, Pitossi F. Neuroprotective effects of human umbilical cord mesenchymal stromal cells in an immunocompetent animal model of Parkinson’s disease. J Neuroimmunol. 2012;246(1-2):43–50. [DOI] [PubMed] [Google Scholar]
- 101. Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA. Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells. 2011;29(11):1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Eberling JL, Kells AP, Pivirotto P, Beyer J, Bringas J, Federoff HJ, Forsayeth J, Bankiewicz KS. Functional effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in parkinsonian rhesus monkeys. Hum Gene Ther. 2009;20(5):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–1291. [DOI] [PubMed] [Google Scholar]
- 104. Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm Suppl. 2000;58(7):143–151. [PubMed] [Google Scholar]
- 105. Kitamura Y, Itano Y, Kubo T, Nomura Y. Suppressive effect of FK-506, a novel immunosuppressant, against MPTP-induced dopamine depletion in the striatum of young C57BL/6 mice. J Neuroimmunol. 1994;50(2):221–224. [DOI] [PubMed] [Google Scholar]
- 106. Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, Fu FH, Huard J. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Toghraie FS, Chenari N, Gholipour MA, Faghih Z, Torabinejad S, Dehghani S, Ghaderi A. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee. 2011;18(2):71–75. [DOI] [PubMed] [Google Scholar]
- 108. Roberts S, Genever P, McCaskie A, De Bari C. Prospects of stem cell therapy in osteoarthritis. Regen Med. 2011;6(3):351–366. [DOI] [PubMed] [Google Scholar]
- 109. Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287(5457):1442–1446. [DOI] [PubMed] [Google Scholar]
- 110. Pers YM, Ruiz M, Noel D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23(11):2027–2035. [DOI] [PubMed] [Google Scholar]
- 111. Luz-Crawford P, Noel D, Fernandez X, Khoury M, Figueroa F, Carrion F, Jorgensen C, Djouad F. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One. 2012;7(9): e45272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jorgensen C, Noel D, Apparailly F, Sany J. Stem cells for repair of cartilage and bone: the next challenge in osteoarthritis and rheumatoid arthritis. Ann Rheum Dis. 2001;60(4):305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. [DOI] [PubMed] [Google Scholar]
- 114. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. [DOI] [PubMed] [Google Scholar]
- 115. Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8(19):4083–4099. [DOI] [PubMed] [Google Scholar]
- 117. Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fruhbeis C, Frohlich D, Kuo WP, Kramer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. [DOI] [PubMed] [Google Scholar]
- 120. Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol. 2016;7:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26(5):1474–1483. [DOI] [PubMed] [Google Scholar]
- 122. Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–341. [DOI] [PubMed] [Google Scholar]
- 123. Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, Padmanabhan J, Lee CN, de Kleijn DP, Lim SK. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Clayton A, Mason MD. Exosomes in tumour immunity. Curr Oncol. 2009;16(3):46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Anand PK. Exosomal membrane molecules are potent immune response modulators. Commun Integr Biol. 2010;3(5):405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. [DOI] [PubMed] [Google Scholar]
- 127. Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33(5):419–440. [DOI] [PubMed] [Google Scholar]
- 128. Cossetti C, Smith JA, Iraci N, Leonardi T, Alfaro-Cervello C, Pluchino S. Extracellular membrane vesicles and immune regulation in the brain. Front Physiol. 2012;3:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. [DOI] [PubMed] [Google Scholar]
- 130. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. [DOI] [PubMed] [Google Scholar]
- 132. Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147(3):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247(1-2):163–174. [DOI] [PubMed] [Google Scholar]
- 134. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110(9):3234–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35(2):89–93. [DOI] [PubMed] [Google Scholar]
- 136. Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26(19):4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165(3):1259–1265. [DOI] [PubMed] [Google Scholar]
- 138. O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10(2):111–122. [DOI] [PubMed] [Google Scholar]
- 139. Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Koppler B, Cohen C, Schlondorff D, Mack M. Differential mechanisms of microparticle transfer toB cells and monocytes: anti-inflammatory propertiesof microparticles. Eur J Immunol. 2006;36(3):648–660. [DOI] [PubMed] [Google Scholar]
- 141. Brown K, Sacks SH, Wong W. Extensive and bidirectional transfer of major histocompatibility complex class II molecules between donor and recipient cells in vivo following solid organ transplantation. FASEB J. 2008;22(11):3776–3784. [DOI] [PubMed] [Google Scholar]
- 142. Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66(18):9290–9298. [DOI] [PubMed] [Google Scholar]
- 143. Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36(1-3):247–254. [DOI] [PubMed] [Google Scholar]
- 144. Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–132. [DOI] [PubMed] [Google Scholar]
- 146. Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175(4):2237–2243. [DOI] [PubMed] [Google Scholar]
- 147. Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28(8):1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174(11):7268–7277. [DOI] [PubMed] [Google Scholar]
- 149. MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15(5):825–835. [DOI] [PubMed] [Google Scholar]
- 150. Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Novellino L, Clementi E, Giussani P, Viani P, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335(1):143–151. [DOI] [PubMed] [Google Scholar]
- 152. Morel O, Morel N, Jesel L, Freyssinet JM, Toti F. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin Immunopathol. 2011;33(5):469–486. [DOI] [PubMed] [Google Scholar]
- 153. Minagar A, Jy W, Jimenez JJ, Sheremata WA, Mauro LM, Mao WW, Horstman LL, Ahn YS. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology. 2001;56(10):1319–1324. [DOI] [PubMed] [Google Scholar]
- 154. Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23(5):1541–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bu N, Wu H, Sun B, Zhang G, Zhan S, Zhang R, Zhou L. Exosome-loaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with glioma. J Neurooncol. 2011;104(3):659–667. [DOI] [PubMed] [Google Scholar]
- 156. Pelloski CE, Ballman KV, Furth AF, Zhang L, Lin E, Sulman EP, Bhat K, McDonald JM, Yung WK, Colman H, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;25(16):2288–2294. [DOI] [PubMed] [Google Scholar]
- 157. Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. [DOI] [PubMed] [Google Scholar]
- 159. Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15(1):80–88. [DOI] [PubMed] [Google Scholar]
- 160. Penfornis P, Vallabhaneni KC, Whitt J, Pochampally R. Extracellular vesicles as carriers of microRNA, proteins and lipids in tumor microenvironment. Int J Cancer. 2016;138(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Vallabhaneni KC, Penfornis P, Dhule S, Guillonneau F, Adams KV, Mo YY, Xu R, Liu Y, Watabe K, Vemuri MC, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zhang ZG, Chopp M. Promoting brain remodeling to aid in stroke recovery. Trends Mol Med. 2015;21(9):543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31(12):2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334(6052):101–105. [DOI] [PubMed] [Google Scholar]
- 166. Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198(5):725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Doeppner TR, Herz J, Gorgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, Zhang ZG, Chopp M. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressed multipotent mesenchymal stromal cells. Cell Transplant. 2017;26(2):243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30(7):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Holtje M, Djalali S, Hofmann F, Munster-Wandowski A, Hendrix S, Boato F, Dreger SC, Grosse G, Henneberger C, Grantyn R, et al. A 29-amino acid fragment of Clostridium botulinum C3 protein enhances neuronal outgrowth, connectivity, and reinnervation. FASEB J. 2009;23(4):1115–1126. [DOI] [PubMed] [Google Scholar]
- 171. El Bassit G, Patel RS, Carter G, Shibu V, Patel A, Song S, Murr M, Cooper DR, Bickford PC, Patel NA. MALAT1 in human adipose stem cells modulates survival and alternative splicing of PKCdeltaII in HT22 cells. Endocrinology. 2017;158(1):183–195. doi:10.1210/en.2016-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, Shao PL, Chen YL, Chai HT, Lin KC, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7(46):74537–74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Cui C, Ye X, Chopp M, Venkat P, Zacharek A, Yan T, Ning R, Yu P, Cui G, Chen J. miR-145 Regulates diabetes-bone marrow stromal cell-induced neurorestorative effects in diabetes stroke rats. Stem Cells Transl Med. 2016;5(12):1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao H, Xu T, Chen L, Xu Y. Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute Ischemic stroke patients. PLoS One. 2016;11(9): e0163645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Fraser KB, Moehle MS, Alcalay RN, West AB, Consortium LC. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019 S LRRK2 carriers. Neurology. 2016;86(11):994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Fraser KB, Rawlins AB, Clark RG, Alcalay RN, Standaert DG, Liu N, Parkinson’s Disease Biomarker Program C, West AB. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov Disord. 2016;31(10):1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ho DH, Yi S, Seo H, Son I, Seol W. Increased DJ-1 in urine exosome of Korean males with Parkinson’s disease. Biomed Res Int. 2014;2014:704678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Shi M, Kovac A, Korff A, Cook TJ, Ginghina C, Bullock KM, Yang L, Stewart T, Zheng D, Aro P, et al. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement. 2016;12(11):1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S. Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem. 2015;290(5):2969–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, Mollenhauer B, Schneider A. Induction of alpha-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain. 2016;139(Pt 2):481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Bliederhaeuser C, Grozdanov V, Speidel A, Zondler L, Ruf WP, Bayer H, Kiechle M, Feiler MS, Freischmidt A, Brenner D, et al. Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol. 2016;131(3):379–391. [DOI] [PubMed] [Google Scholar]
- 182. Gui Y, Liu H, Zhang L, Lv W, Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6(35):37043–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Jarmalaviciute A, Tunaitis V, Pivoraite U, Venalis A, Pivoriunas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17(7):932–939. [DOI] [PubMed] [Google Scholar]
- 184. Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Heldens GT, Blaney Davidson EN, Vitters EL, Schreurs BW, Piek E, van den Berg WB, van der Kraan PM. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng Part A. 2012;18(1-2):45–54. [DOI] [PubMed] [Google Scholar]
- 186. Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, Li H, Liu Z, Shi C, Duan F, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831–840. [DOI] [PubMed] [Google Scholar]
- 187. Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. [DOI] [PubMed] [Google Scholar]
- 188. Le LT, Swingler TE, Clark IM. Review: the role of microRNAs in osteoarthritis and chondrogenesis. Arthritis Rheum. 2013;65(8):1963–1974. [DOI] [PubMed] [Google Scholar]
- 189. Miyaki S, Nakasa T, Otsuki S, Grogan SP, Higashiyama R, Inoue A, Kato Y, Sato T, Lotz MK, Asahara H. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60(9):2723–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Meng F, Zhang Z, Chen W, Huang G, He A, Hou C, Long Y, Yang Z, Zhang Z, Liao W. MicroRNA-320 regulates matrix metalloproteinase-13 expression in chondrogenesis and interleukin-1beta-induced chondrocyte responses. Osteoarthritis Cartilage. 2016;24(5):932–941. [DOI] [PubMed] [Google Scholar]
- 191. Swingler TE, Wheeler G, Carmont V, Elliott HR, Barter MJ, Abu-Elmagd M, Donell ST, Boot-Handford RP, Hajihosseini MK, Munsterberg A, et al. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 2012;64(6):1909–1919. [DOI] [PubMed] [Google Scholar]
- 192. Song J, Lee M, Kim D, Han J, Chun CH, Jin EJ. MicroRNA-181b regulates articular chondrocytes differentiation and cartilage integrity. Biochem Biophys Res Commun. 2013;431(2):210–214. [DOI] [PubMed] [Google Scholar]
- 193. Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, Rice GE. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8(7): e68451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8(8):1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Meckes DG, Jr, Gunawardena HP, Dekroon RM, Heaton PR, Edwards RH, Ozgur S, Griffith JD, Damania B, Raab-Traub N. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci U S A. 2013;110(31): E2925–E2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Gheldof D, Mullier F, Chatelain B, Dogne JM, Chatelain C. Inhibition of tissue factor pathway inhibitor increases the sensitivity of thrombin generation assay to procoagulant microvesicles. Blood Coagul Fibrinolysis. 2013;24(5):567–572. [DOI] [PubMed] [Google Scholar]
- 199. Chen K, Page JG, Schwartz AM, Lee TN, DeWall SL, Sikkema DJ, Wang C. False-positive immunogenicity responses are caused by CD20+ B cell membrane fragments in an anti-ofatumumab antibody bridging assay. J Immunol Methods. 2013;394(1-2):22–31. [DOI] [PubMed] [Google Scholar]
- 200. Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31(5):543–551. [DOI] [PubMed] [Google Scholar]