Abstract
Acute kidney injury (AKI) is a major clinical problem that still has no established treatment. We investigated the efficacy of cultured human peripheral blood mononuclear cells (PBMNCs) for AKI. Ischemia/reperfusion injury (IRI) was used to induce AKI in male nonobese diabetic (NOD/severe combined immunodeficiency) mice aged 7 to 8 wk. PBMNCs were isolated from healthy volunteers and were subjected to quality and quantity controlled (QQc) culture for 7 d in medium containing stem cell factor, thrombopoietin, Flt-3 ligand, vascular endothelial growth factor, and interleukin 6. IRI-induced mice were divided into 3 groups and administered (1) 1 × 106 PBMNCs after QQc culture (QQc PBMNCs group), (2) 1 × 106 PBMNCs without QQc culture (non-QQc PBMNCs group), or (3) vehicle without PBMNCs (IRI control group). PBMNCs were injected via the tail vein 24 h after induction of IRI, followed by assessment of renal function, histological changes, and homing of injected cells. Blood urea nitrogen and serum creatinine (Cr) 72 h after induction of IRI in the QQc PBMNCs group dramatically improved compared with those in the IRI control and the non-QQc PBMNCs groups, accompanied by the improvement of tubular damages. Interstitial fibrosis 14 d after induction of IRI was also significantly improved in the QQc PBMNCs group compared with the other groups. The renoprotective effect noted in the QQc PBMNCs group was accompanied by reduction of peritubular capillary loss. The change of PBMNCs’ population (increase of CD34+ cells, CD133+ cells, and CD206+ cells) and increased endothelial progenitor cell colony-forming potential by QQc culture might be one of the beneficial mechanisms for restoring AKI. In conclusion, an injection of human QQc PBMNCs 24 h after induction of IRI dramatically improved AKI in mice.
Keywords: acute kidney injury, ischemia/reperfusion, CD34, mononuclear cell, QQc culture
Introduction
The high prevalence of acute kidney injury (AKI) and its impact on the prognosis of critically ill patients in the intensive care unit (ICU) is one of the major problems in the field of critical care nephrology. AKI has been reported in 6% to 36% of all ICU patients, and blood purification therapy is required for 0.4% to 3.3% of ICU patients1,2. Regarding the prognosis of ICU patients with severe AKI, a multinational, multicenter study revealed a surprisingly high mortality rate (≥50%) of these patients in many countries all over the world2. However, there is currently no established treatment that promotes kidney repair in patients with severe AKI. Therefore, an effective therapy for AKI is urgently needed.
Cell-based regenerative therapy has been studied in animal models of AKI and there have been some reports of beneficial effects. The cells investigated so far include granulocyte colony-stimulating factor-mobilized peripheral blood CD34+ cells3 and mesenchymal stem cells (MSCs) derived from bone marrow4–8, adipose tissue9–12, umbilical cord blood13,14, or amniotic fluid15. In addition, renal progenitor cells generated from human-induced pluripotent stem (iPS) cells have been found to ameliorate AKI induced by ischemia/reperfusion injury (IRI) in mice16.
These cell therapies have been demonstrated to improve the time course of kidney function in animals after AKI. However, no clinical trials have successfully improved AKI with regenerative therapy in humans. When translational research is performed to apply such new clinical treatments, easy accessibility of the cell source, ease of preparing the cells, and cost should be considered. Autotransplantation avoids allosensitization and thus is the safest method of regenerative therapy at present.
Endothelial progenitor cells (EPCs) promote angiogenesis and can be easily collected from the peripheral blood. In the kidneys, blood supply to the nephrons and maintenance of kidney function are regulated by the peritubular capillary (PTC) network; and collapse of this network is thought to be important in the pathophysiology of IRI.
Short-term quality and quantity controlled (QQc) culture of peripheral blood mononuclear cells (PBMNCs) is a recently established method for enhancing the number and proliferative capacity of CD34+ EPCs17,18. In this study, we examined the effect of human PBMNCs incubated in QQc culture medium on AKI in mice by evaluating whether QQc-cultured PBMNCs could restore kidney function and reverse tubular/PTC damage in a mouse model of AKI. In most previous IRI studies in animals, cells were injected immediately or shortly after the induction of IRI4,10,16. Delayed cell administration studies are limited3. We conducted cell therapy 24 h after the release of clamped renal pedicles, at which time AKI was thoroughly induced, thereby providing useful implications for future clinical trials.
Materials and Methods
Animals
Male immune-deficient nonobese diabetic (NOD/severe combined immunodeficiency [SCID]) mice aged 7 to 8 wk and weighing 20 to 25 g (Jackson Lab, Kawasaki, Japan) were used for all experiments. The NOD mouse is the model for type 1 diabetes mellitus; however, onset of diabetes in male NOD mice occurs around 150 d after birth, and no mice used in this experiment were diabetic during the experimental period. NOD/SCID mice lack functional T and B cells and are immunodeficient; therefore, rejection of human cells could be neglected, and more pure insight into the protective potential of human cells could be obtained without immunological modulation. Animal care and treatment conformed to institutional guidelines and international laws and politics. The experimental protocol was approved by the Center for Animal Research at Juntendo University.
Human PBMNCs
Healthy male volunteers aged 20 to 30 y provided peripheral blood (70 mL) after giving oral and written informed consent. The procedure for obtaining informed consent was approved by the ethics committee of Juntendo University (2013086) and Shonan Kamakura General Hospital (TGE00352-024).
Serum-free QQc Culture
Human PBMNCs were isolated as described previously, and 1 × 103 PBMNCs were added to each well of a 24-well plate (BD Falcon, Bedford, MA, USA) and cultured in serum-free QQc culture medium for 7 d17–19. Briefly, QQc culture medium is an optimized combination of growth factors and cytokines (20 ng/mL thrombopoietin, 20 ng/mL interleukin (IL) 6, 100 ng/mL stem cell factor (SCF), 100 ng/mL Flt-3 ligand, and 50 ng/mL vascular endothelial growth factor; all from Peprotech, Rocky Hills, NJ, USA) in serum-free stem cell medium (Stemcell Technologies, Vancouver, Canada). QQc culture was performed in this medium for 7 d, which has been shown to dramatically and optimally expand and enhance the vasculogenic potential of EPCs.
EPC Colony-forming Assay and Cell Population Assay
The vasculogenic potential of human PBMNCs after QQc culture was assessed by the EPC colony-forming assay as described previously17–19. This colony-forming assay was designed to separate total colony-forming units (CFUs) into 2 different types of EPC-CFUs, which were primitive (small cells) and definitive (large cells). The definitive EPC-CFUs (dEPC-CFUs) are a predominantly vasculogenic cell population with greater differentiation potential. Briefly, 2 × 105 human PBMNCs were seeded into a 35-mm hydrophilic tissue culture dish. After 14 d, total EPC-CFUs, primitive EPC-CFUs, and dEPC-CFUs were counted in a blinded manner by 2 investigators. The experiments were performed in triplicate.
In the cell population assay, freshly isolated non-QQc PBMNCs and QQc PBMNCs were subjected to flow cytometry (FCM) to detect surface antigen positivity of hematopoietic stem or lineage-committed cells as well as endothelial lineage cells as previously reported17,18,20. The scatter diagram of each non-QQc PBMNC and QQc PBMNC population was gated into 3 cell size populations of lymphocytes, monocytes, and larger cells. The percent positivity of a hematopoietic cell population for each gate in non-QQc PBMNCs and QQc PBMNCs was evaluated and then calculated relative to that of the total cells in the 3 gates. The ratio of the percent positivity of the total cells of QQc PBMNCs to that of the total cells of non-QQc PBMNCs was further calculated for each cell population. FCM analysis was performed using the LSRFortessa cell analyzer (BD Biosciences, San Jose, CA, USA) and Flow Jo software, version 7.6.5 (Tomy Digital Biology Co. Ltd., Tokyo, Japan). Antibodies recognizing the cell populations were used as described previously18,19.
IRI Model and Cell Therapy
Mice were kept under a 12-h light–dark cycle at a temperature of 25 °C and received water and food ad libitum. IRI was inducted as reported previously21. Briefly, mice were anesthetized by intraperitoneal injection of pentobarbital and buprenorphine hydrochloride and placed on a heating pad (37 °C) for 30 min. The kidneys of the anesthetized mice were exposed through flank incisions, and nontraumatic clamps were placed across the bilateral renal pedicles. After confirming a dusky color of both kidneys, the kidneys were replaced in the retroperitoneum for 30 min. The clamps were then removed and reperfusion of the kidneys was confirmed visually. After skin closure, 0.5 mL saline was injected intraperitoneally and the mouse was kept on the heating pad until it recovered fully from anesthesia.
To test the effects of human PBMNCs, IRI mice (day 0) were randomly divided into 3 groups. The QQc PBMNCs group received 1 × 106 human QQc PBMNCs in 0.1 mL saline intravenously via the tail vein 24 h after IRI (day 1). The non-QQc PBMNCs group received 1 × 106 human PBMNCs without QQc culture at 24 h after IRI. Mice with injection of the vehicle after IRI were used as a control group (IRI control), whereas mice with sham operation plus the vehicle and sham operation plus 1 × 106 QQc PBMNCs were used as a normal control and homing control group, respectively.
Evaluation of Kidney Function
To assess the changes in kidney function, serial blood samples were withdrawn from the tail vein at baseline, 24 h, 48 h, 72 h, and 7 d after IRI, and serum was kept at −80 °C until analysis. Blood urea nitrogen (BUN) was measured using a BUN Test Wako kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and serum Cr was determined by HPLC as described previously22.
Kidney Tissue Preparation and Evaluation
Kidney tissues were obtained from mice anesthetized by intraperitoneal injection of pentobarbital and buprenorphine hydrochloride. For light microscopy, tissues were fixed in 10% neutral-buffered formalin, transferred to 70% ethanol, and then processed to yield paraffin sections (2 µm thick). Sections were stained with hematoxylin and eosin for analysis of tubular damage and with Masson’s trichrome stain for calculation of the interstitial fibrosis area.
Tubular damage, including epithelial necrosis, tubular dilatation, cast formation, and loss of the brush border, was evaluated as described previously23. Ten fields per mouse incorporating the cortex and outer medulla were captured by digital imaging (×200), and a semiquantitative evaluation was performed by scoring for the damaged area in each field as follows: 0: 0%; 1: <10%; 2: 10% to 25%; 3: 25% to 50%; 4: 50% to 75%% and 5: >75%. Scores were assessed on 3 mice in each group at each time point.
Interstitial fibrosis was evaluated quantitatively with imaging software (cellSens®, Olympus, Japan). Fourteen days after IRI, Masson’s trichrome-stained kidney sections were analyzed from 3 mice in the QQc PBMNCs, non-QQc PBMNCs, and vehicle control groups. At ×200 magnification, the area of blue Masson’s trichrome staining (fibrosis) was automatically captured and calculated by the software. The interstitial fibrosis area was expressed as the blue area/total tissue area in 10 fields per kidney in each mouse.
Homing of Injected Human Cells to the Kidney and Other Organs
To test the homing of injected human PBMNCs to the kidney, lung, spleen, and bone marrow, QQc or non-QQc PBMNCs were labeled with the cell tracker PKH67 before injection (Green Fluorescent Cell Linker Kit, Sigma-Aldrich, IL, USA). Cells were incubated with fluorescent PKH for 5 min at 25 °C, washed 3 times, and suspended in 0.1 mL of saline before injection.
The kidneys, lungs, and spleens harvested from the anesthetized mice were fixed overnight in 4% paraformaldehyde in a darkroom at 4 °C. Fixed tissues were processed in a graded sucrose series and preserved in optimum cutting temperature (−80 °C) until analysis. Four-µm-thick cryosections were then cut, and the number of PKH-positive cells per long axis slice was counted in each organ of 3 mice in each group (4 fields per mouse) at each time point.
To count human PKH-positive cells in bone marrow, femora harvested from the mice were immersed in 4% paraformaldehyde in a darkroom at 4 °C and cryosections were prepared for analysis using the Kawamoto method24.
PTC Loss
In 4-µm-thick cryosections of the kidneys, vessels were labeled by using a purified anti-mouse CD31 antibody (1:100, BD Pharmingen, San Diego, CA, USA) that did not cross-react with human antigens. Fifteen to 20 fields incorporating the cortex and outer medulla were captured by digital imaging (×400). Each image was then divided into 252 squares using a grid. Each square without a PTC (capillary loss) was scored and the final score was represented as a percentage PTC loss3.
Mouse Endothelial Antigen Expression in Homed Human PBMNCs
Expression of mouse vascular endothelial antigen in human PBMNCs was evaluated using an anti-mouse CD31antibody that did not cross-react with human antigens (BD Pharmingen). Four-µm-thick cryosections of kidneys from the QQc PBMNCs group taken 24 h and 14 d after induction of IRI were stained with anti-mouse CD31 antibody.
Statistical Analysis
All data are expressed as mean ± standard error. Comparison between 2 groups was made by Mann–Whitney U test, and comparison among 3 groups was made by analysis of variance followed by post hoc test. SPSS statistics version 11.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis on a personal computer, and P values < 0.05 was considered significant.
Results
QQc PBMNCs Dramatically Restored Kidney Function
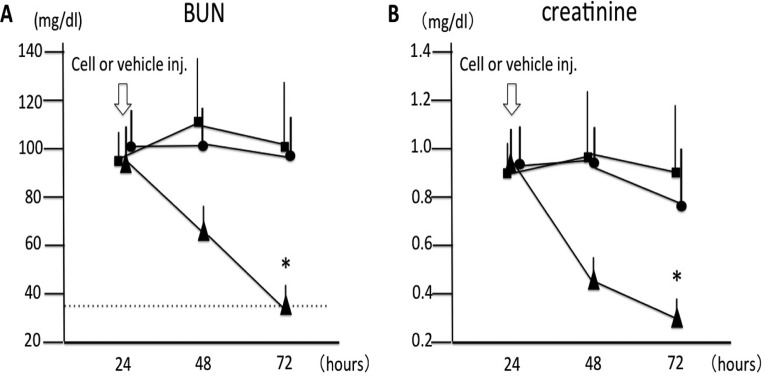
Changes in kidney function are shown in Fig. 1. Twenty-four hours after induction of IRI, the BUN levels did not differ among the IRI control (n = 13), non-QQc PBMNCs (n = 13), and QQc PBMNCs groups (n = 13). However, the QQc PBMNCs group showed dramatic improvement of BUN 48 h after injection of 1 × 106 cells compared with that in the IRI control group (99.5 ± 39.4 mg/dL in the IRI control group vs. 36.1 ± 4.3 mg/dL in the QQc PBMNCs group, P < 0.05; Fig. 1A). Serum Cr also showed significant improvement 48 h after cell injection in the QQc PBMNCs group compared with that in the IRI control group (0.89 ± 0.19 vs. 0.25 ± 0.06 mg/dL, respectively, P < 0.05; Fig. 1B). In contrast, non-QQc PBMNCs did not have any beneficial effect on BUN or Cr (Fig. 1A and 1B).
Fig. 1.
Changes in kidney function after cell therapy. (A) Blood urea nitrogen (BUN): BUN levels before ischemia/reperfusion injury (IRI) were below 35 mg/dL in all mice. BUN increased at 24 h after IRI induction and remained over 90 mg/dL in the IRI control group (n = 13). BUN in the quality and quantity control (QQc) peripheral blood mononuclear cells (PBMNCs) group (n = 13) significantly decreased 48 h after cell injection and improved to an almost normal range. (B) Creatinine: Serum creatinine (Cr) levels before IRI induction were below 0.1 mg/dL in all mice. Serum Cr also showed significant improvement by QQc PBMNC injection 48 h after cell injection compared with that in the IRI control group. A 1 × 106 injection with non-QQc PBMNCs (n = 13) did not show any beneficial effect on kidney function (on BUN or Cr levels). (•): IRI control, (▴): QQc PBMNCs group, and (▪): non-QQc PBMNCs group. *P < 0.05 versus IRI control group. Dotted line represents upper normal limit of BUN.
Effect of Cell Therapy on Kidney Damage
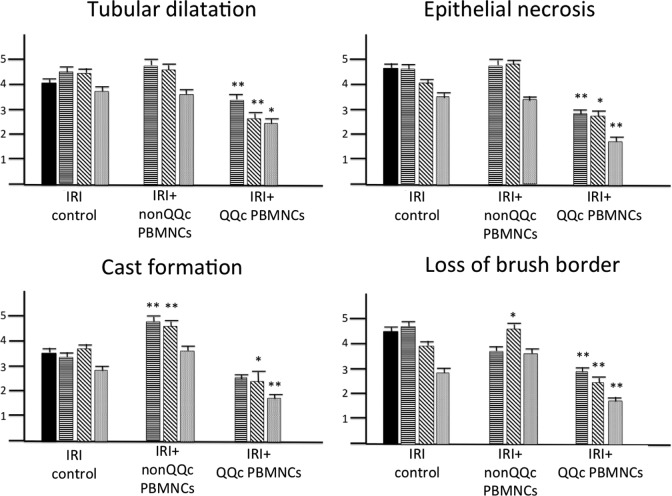
Tubular damage was evaluated semiquantitatively by the assessment of epithelial necrosis, tubular dilatation, cast formation, and loss of the brush border. As shown in Fig. 2, all of these tubular damage parameters were significantly improved in the QQc PBMNCs group compared with those in the IRI control group. In contrast, some parameters (cast formation and loss of the brush border) were worse in the non-QQc PBMNCs group compared with those in the IRI control group at 48 and/or 72 h after induction of IRI.
Fig. 2.
Changes of tubular damage after cell therapy. Tubular damage including tubular dilatation, epithelial necrosis, cast formation, and loss of brush border were semiquantitatively evaluated. ( ): 24 h, (
): 24 h, ( ): 48 h, (
): 48 h, ( ): 72 h, (
): 72 h, ( ): 7 d after ischemia/reperfusion injury (IRI) induction, respectively. *P < 0.05, **P < 0.01 versus IRI control at the same time point.
): 7 d after ischemia/reperfusion injury (IRI) induction, respectively. *P < 0.05, **P < 0.01 versus IRI control at the same time point.
QQc PBMNCs Improve Interstitial Fibrosis in the Recovery Phase of IRI
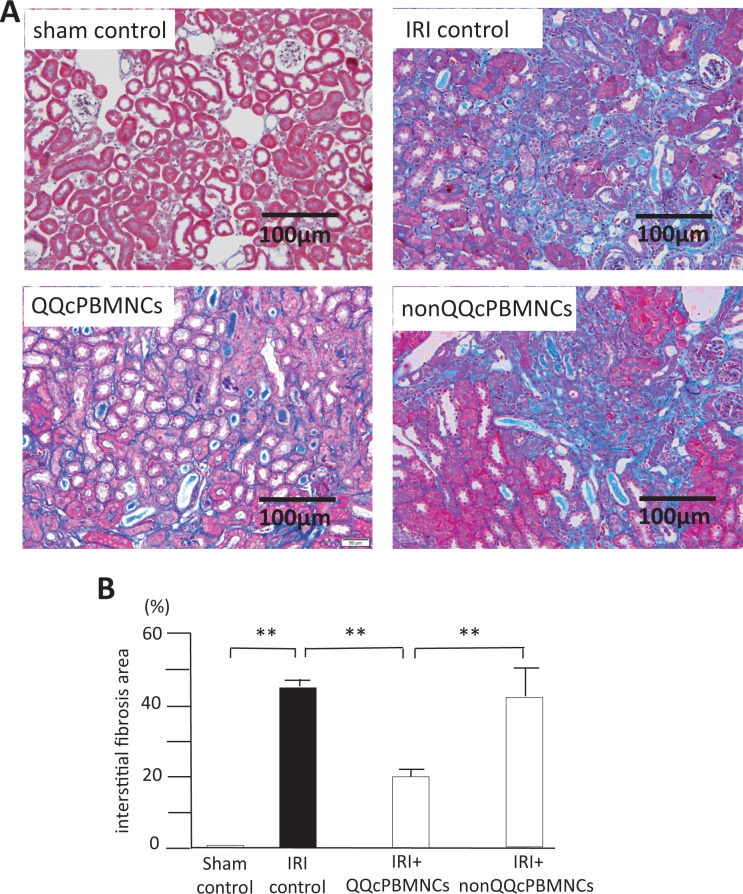
The extent of interstitial fibrosis was evaluated in the recovery phase of AKI by quantitative image analysis. The sham control group did not show interstitial fibrosis (0.02% ± 0.005%), whereas significant interstitial fibrosis was seen in IRI control group 14 d after IRI induction. As shown in Fig. 3A and B, there was a marked decrease in the interstitial fibrosis area in the QQc PBMNCs group compared with that in the IRI control group (45.2% ± 1.8% in the IRI control group vs. 21.9% ± 8.0% in the QQc PBMNCs group, P < 0.01). The interstitial fibrosis area did not differ between the non-QQc PBMNCs group and the IRI control group, but it was significantly larger than that in the QQc PBMNC group (P < 0.01).
Fig. 3.
Improvement of interstitial fibrosis by QQc PBMNCs. A: Masson-Trichrome stained sections of sham control (upper left), IRI control (upper right), QQc PBMNCs (lower left), and non-QQc PBMNCs (lower right) at 14 days after IRI induction. B: Percent of interstitial fibrosis area was quantitatively evaluated at 14 days after IRI induction. IRI control (black bar) showed high interstitial fibrosis area (45.2 ± 1.8%). QQc PBMNCs group (grey bar) showed significant improvement of interstitial fibrosis compared with IRI control (p < 0.01). Non-QQc PBMNCs 1 × 106 did not improve interstitial fibrosis. **p < 0.01.
Improvement of PTC Loss by QQc PBMNCs
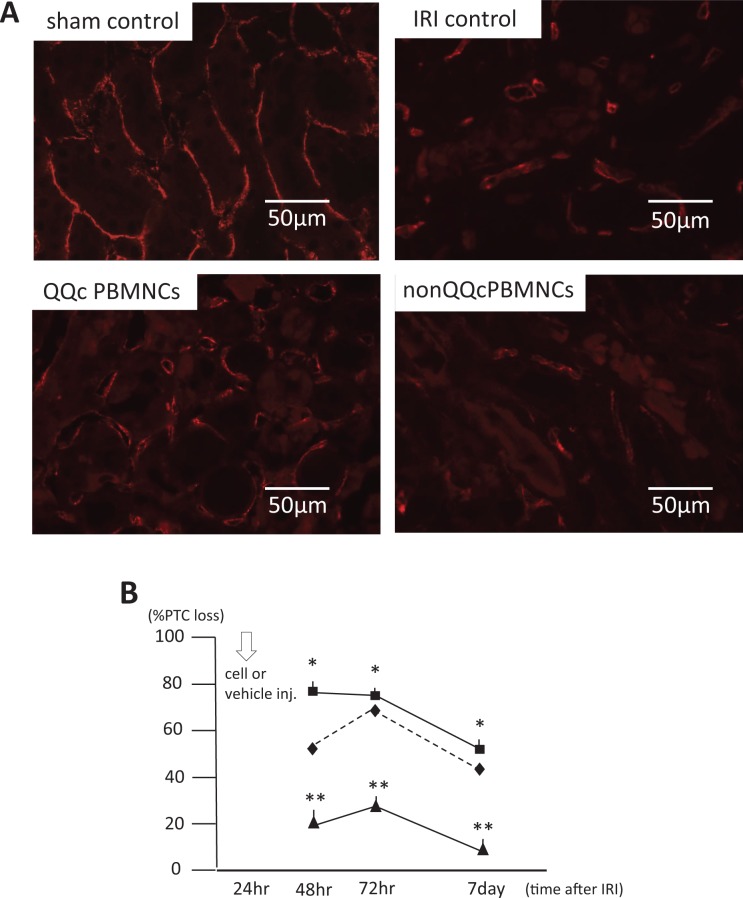
Figure 4A demonstrates the PTCs with anti-mouse CD31 antibody stains of kidney sections. Severe PTC loss was seen in the IRI control group, whereas there was less PTC loss in the QQc PBMNCs group. The time courses of PTC loss in the IRI control group, QQc PBMNCs group, and non-QQc PBMNCs group are shown in Fig. 4B. Forty-eight hours after induction of IRI, there was significantly less PTC loss in the QQc PBMNCs group than in the IRI control group or the non-QQc PBMNCs group (IRI control: 43.1% ± 2.0%; QQc PBMNCs: 21.5% ± 1.0%; and non-QQc PBMNCs: 78.4% ± 2.3%). Thus, injection of QQc PBMNCs had a protective effect against PTC loss in the early phase of IRI.
Fig. 4.
Peritubular capillary (PTC) loss and its time course after cell injection. (A) CD31 staining of sham control, IRI control, QQc PBMNCs, and non-QQc PBMNCs. (B) IRI control (⋄) showed severe PTC loss at 48 hours after IRI induction. 1 × 106 QQc PBMNCs (▴) significantly improved PTC loss at 24 hours after cell injection, and continued to significantly lower PTC loss levels than IRI control group and non-QQc PBMNCs group (▪). * p < 0.01 non-QQc PBMNCs vs. QQc PBMNCs, ** p < 0.01 IRI control vs. QQc PBMNCs.
Homed Human PBMNCs in Mouse Kidneys Express Mouse Endothelial Antigen
We evaluated whether human PBMNCs in mouse kidneys express vascular endothelial antigen. QQc PBMNCs did not express CD31 24 h after injection, but some QQc PBMNCs expressed CD31 14 d after induction of IRI (Fig. 5).
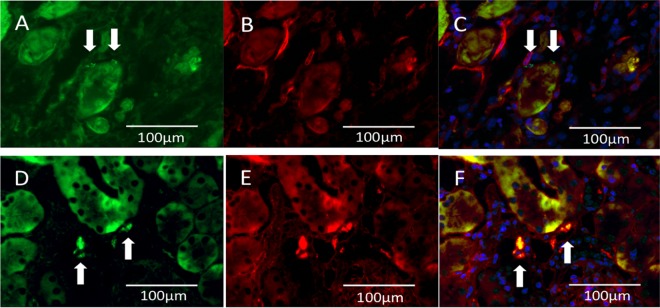
Fig. 5.
Human peripheral blood mononuclear cells (PBMNCs) express CD31 antigen at 14 d after injection. (A–C) Immunofluorescent imaging of the quality and quantity control (QQc) PBMNCs group 48 h after ischemia/reperfusion injury (IRI). (D–F) Immunofluorescent imaging of the QQc PBMNCs group 14 d after IRI. (A) and (D) show fluorescein isothiocyanate imaging, (B) and (E) show Texas-red imaging (anti-mouse CD31), and (C) and (F) show merged images. Arrows indicate PKH-labeled PBMNCs. The QQc PBMNCs did not express mouse CD31 antigen at 48 h (C). However, homed human QQc PBMNCs in the kidney 14 d after IRI (arrow) show anti-CD31 antigen (F).
Homing of Injected Human Cells to the Kidney and Other Organs
Recruitment of human PBMNCs to the kidney was investigated through tracking PKH-labeled cells by immunofluorescence microscopy.
In a preliminary examination, only 0.2 to 2 PBMNCs (per section) were found in IRI kidneys at 24 h after injection with 5 × 104 QQc PBMNCs. As for 1 × 106 QQc PBMNCs, only 2 to 3 QQc PBMNCs (per section) were found in the kidneys 24 h after cell injection in the sham operation control group. However, significant accumulation of PBMNCs in IRI kidneys was found after injection with 1 × 106 QQc PBMNCs as shown in Fig. 6. Injected QQc PBMNCs were present in the spaces between tubules including in the spaces around capillaries. Homing of QQc PBMNCs in the kidney peaked 24 h after injection and then gradually decreased, with a few PKH-labeled cells still present 14 d after induction of IRI. More PBMNCs accumulated in IRI kidneys after injection of the non-QQc PBMNCs group than did in those of the QQc PBMNCs group (Table 1). The number of non-QQc PBMNCs in IRI kidneys peaked 48 h after injection and then gradually decreased.
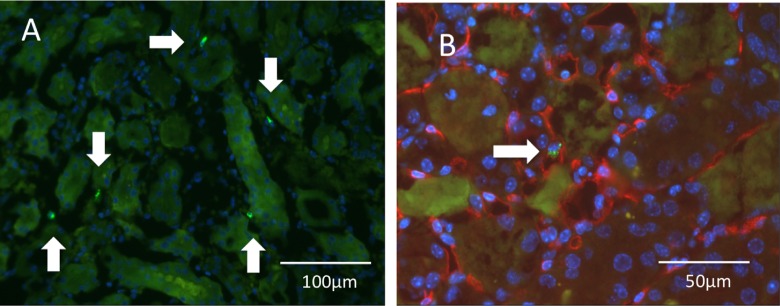
Fig. 6.
Homing of quality and quantity control (QQc) peripheral blood mononuclear cells (PBMNCs) into damaged kidney. PKH-labeled green-colored human QQc PBMNCs are seen in the spaces between tubules (arrow) 24 h after injection with 1 × 106 QQc PBMNCs. (A) Lower magnification without CD31 staining. (B) PKH-labeled cells were located in the inner space of PTCs adjacent to the capillary wall (arrow; CD31: red color).
Table 1.
Homing of Injected Human PBMNCs in the Kidney.
| IRI Model | Sham | ||||
|---|---|---|---|---|---|
| Time after Cell Injection | 24 h | 48 h | 7 d | 14 d | 24 h |
| QQc PBMNCs group | 86.3 ± 6.4 | 44.1 ± 3.4 | 40.9 ± 6.8 | 50.8 ± 6.3 | 1.2 ± 0.8 |
| Non-QQc PBMNCs group | 229.0 ± 16.0* | 350.3 ± 25.2* | 150.9 ± 35.2* | 40.9 ± 5.1 | ND |
Note: Data indicate PBMNCs’ number in each organ slice in 3 mice at indicated time point.
Abbreviation: PBMNC, peripheral blood mononuclear cell; IRI, ischemia reperfusion injury; QQc, quality and quantity control; ND, not done.
*P < 0.01 versus post-QQc group at same time point.
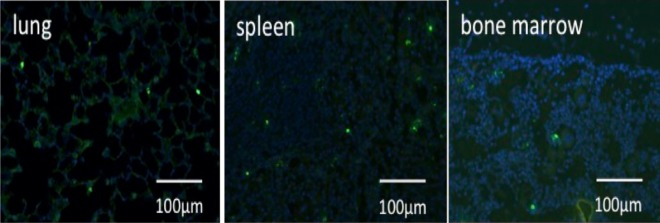
Recruitment of injected human PBMNCs to other organs was also observed (Fig. 7; Table 2), with PBMNCs being detected in the normal lung, spleen, and bone marrow of each group. In each of these organs, accumulation of non-QQc PBMNCs was greater than that of QQc PBMNCs.
Fig. 7.
Homing of quality and quantity control (QQc) peripheral blood mononuclear cells (PBMNCs) into lung, spleen, and bone marrow 24 h after injection of 1 × 106 QQc PBMNCs.
Table 2.
Homing of Injected Human PBMNCs in Lung, Spleen, and Bone Marrow.
| IRI Model | Sham | |||
|---|---|---|---|---|
| Time after Cell Injection | 24 h | 48 h | 7 d | 24 h |
| Lung | ||||
| QQc PBMNCs group | 38.9 ± 9.9 | 9.3 ± 1.3 | 13.4 ± 2.7 | 11.3 ± 2.3 |
| Non-QQc PBMNCs group | 140.5 ± 15.9* | 133.3 ± 15.9* | 14.8 ± 4.6* | ND |
| Spleen | ||||
| QQc PBMNCs group | 157.8 ± 29.5 | 74.6 ± 15.2 | 28.9 ± 6.0 | 29.3 ± 4.4 |
| Non-QQc PBMNCs group | 478.3 ±15.7* | 541.9 ± 20.6* | 379.6 ± 87.3* | ND |
| Bone marrow | ||||
| QQc PBMNCs group | 19.5 ± 3.1 | 2.5 ± 0.8 | 1.3 ± 0.5 | 3.3 ± 1.7 |
| Non-QQc PBMNCs group | 132.2 ± 3.8* | 140.1 ± 12.1* | 78.8 ± 32.3* | ND |
Abbreviations: PBMNC, peripheral blood mononuclear cell; IRI, ischemia reperfusion injury; QQc, quality and quantity control, ND, not done.
*p < 0.05 vs. QQc PBMNCs group at same time points.
Effect of QQc Culture on EPC-CFU and Cell Population
The effect of QQc culture on PBMNCs was evaluated by EPC-CFU assay and FCM analysis. QQc culture of PBMNCs from healthy volunteers significantly increased the number of definite and total EPC colonies compared with uncultured PBMNCs (P < 0.05; Fig. 8). Cell population analysis by FCM showed that 1 wk of QQc culture significantly increased the number of EPC marker-positive cells (CD34+ cells and CD133+cells) and anti-inflammatory M2 macrophage marker-positive cells (CD206+ cells). As shown in Fig. 9A, there were only a few cells in gate C among the non-QQc PBMNCs, but 1 wk of QQc culture significantly increased the cells in gate C (almost all of which were M2 macrophages). CD34+ cells initially existed in gate A (lymphocyte fraction) and increased in gate B (monocyte fraction) after QQc culture. The ratio of the percentage positive cell population between the total cells of QQc PBMNCs and non-QQc PBMNCs is shown in Fig. 9B. CD34+ cells, CD133+ cells, and CD206+ cells significantly increased almost 10, 6, and 40 times, respectively, compared with those of non-QQc PBMNCs. CCR2+ M1 macrophages almost disappeared after 1 wk of QQc culture.
Fig. 8.
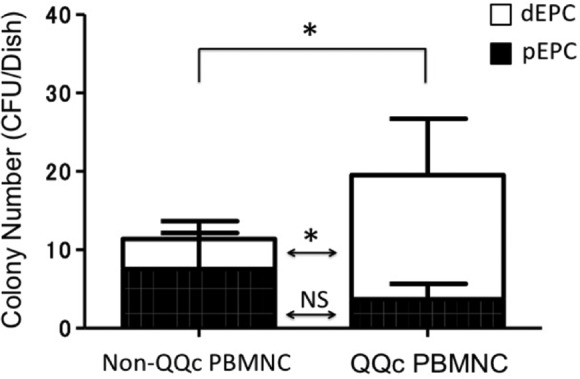
Endothelial progenitor cell (EPC) colony–forming assay. QQc culture significantly increased the number of definite EPC (dEPC) colonies and total EPC colonies. Open bar indicates dEPC and closed bar indicates primitive EPC (pEPC) colonies. Abbreviation: PBMNC, peripheral blood mononuclear cell; QQc, quality and quantity control; EPC, endothelial progenitor cell; CFU, colony-forming unit; ns, not significant. *P < 0.05 between groups.
Fig. 9.
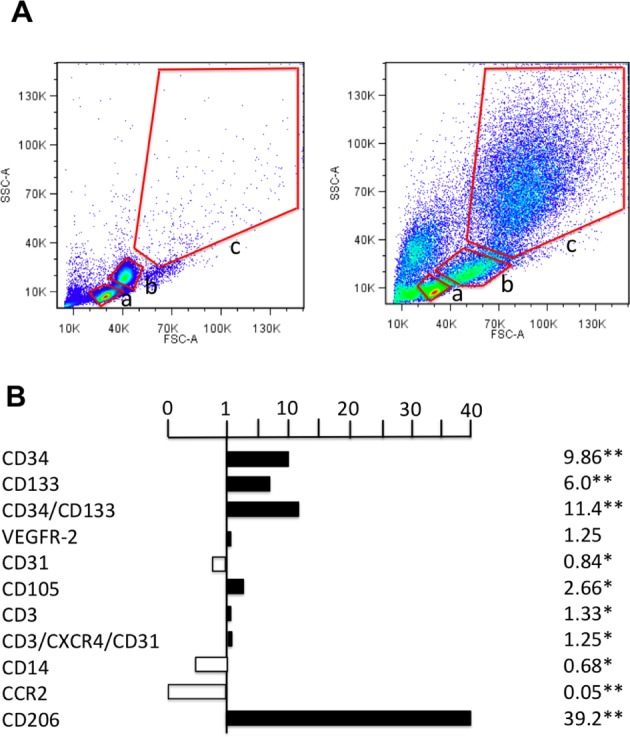
Flow cytometry (FCM) analysis of non-QQc PBMNCs and QQc PBMNCs. (A) Scatter diagrams of non-QQc PBMNCs and QQc PBMNCs by FCM analysis. The red lines indicate the cellular-sized gates of lymphocytes (a), monocytes (b), and the larger cells (c). (B) The graph shows the ratio of percent (%) cell positivity in QQc PBMNCs to that in non-QQc PBMNCs. n = 4 healthy volunteers. The investigated cell surface markers were as follows: hematopoietic stem cell (Cd34, CD133), endothelial cell (VEGFR-2, CD31, CD105), T cell (CD3, CD3/CXCR4/CD31), monocyte (CD14), M1 macrophage (CCR2), and M2 macrophage (CD206). Abbreviation: CCR2, CC chemokine receptor 2; FSC-A, forward scatter–area; SSC-A, side scatter–area; PBMNCs, peripheral blood mononuclear cells; QQc, quality and quantity control; VEGFR-2, vasular endotheilal growth factor recepor-2. *P < 0.05, **P < 0.01.
Discussion
We found that human PBMNCs cultured in a vasculogenic conditioning medium dramatically improved renal function and reduced histological damage in a mouse model of IRI-induced AKI, even when administered 24 h after induction of AKI. BUN and Cr levels were significantly reduced 48 h after injection of QQc PBMNCs and were significantly lower than those in the IRI control and non-QQc PBMNCs groups. Both acute PTC loss and interstitial fibrosis in the recovery phase of IRI were also significantly reduced in the QQc PBMNCs group compared with those in the IRI control and the non-QQc PBMNCs groups. Asahara et al. isolated CD34+ EPCs from peripheral blood MNCs, and CD34+ EPCs dramatically improved hind-limb ischemia in an animal model20. In this experiment, we proved that human QQc PBMNCs can ameliorate kidney organ damage due to IRI. These findings might encourage the clinical application of cell-based therapy for AKI, which still has no established treatment.
After injection with human QQc PBMNCs, some of these cells were localized in the space between the tubules in the early phase of IRI-induced AKI. Although many cells were trapped in the lungs, bone marrow, and spleen after injection via the tail vein, a sufficient number reached the kidney and influenced the progression of kidney damage. Although the same number of QQc PBMNCs (1 × 106 cells) was injected in the sham control group, almost none of these cells were found in the kidney. Our preliminary experiment revealed that the number of PBMNCs used for cell therapy had an important influence on the effect. When we injected 5 × 104 QQc PBMNCs 24 h after induction of IRI, almost none of the injected cells were recruited to the kidneys and neither renal function nor pathological findings improved compared with those in the IRI control group. Therefore, both a sufficient number of cells and ischemic signaling may be necessary for recruitment of MNCs to a damaged organ. In addition, more non-QQc PBMNCs than QQc PBMNCs were trapped in several organs including the lungs, spleen, and bone marrow. QQc PBMNCs might have more potential than non-QQc PBMNCs in targeting damaged organs in response to ischemic signals. However, this has not been determined, and further studies are necessary to evaluate whether selective targeting of damaged organs by human PBMNCs is influenced by QQc culture.
In the first 7 d after IRI, human QQc PBMNCs recruited to the kidneys did not express endothelial antigen markers. Therefore, it is conceivable that human PBMNCs mediated renal repair in the early phase of IRI by paracrine mechanisms rather than by replacement of damaged vessels. Local production of various cytokines by QQc PBMNCs, including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF) 1, and angiopoietins, might be involved in promoting cellular repair in this model. The therapeutic effect occurred despite a relatively small number of injected cells and their short-term residence in the target zone. Therefore, mechanisms other than transdifferentiation of injected cells delivered to the damaged organs into tissue-specific cells might play a significant role in the observed positive outcome. Normal human CD34+ cells not only express transcripts for but also secrete detectable amounts of VEGF, HGF, IGF-1, fibroblast growth factor 2, Flt-3 ligand, and IL 825. Sahoo et al. demonstrated that the exosomes secreted from mobilized human CD34+ cells had angiogenic paracrine activity in vitro and in vivo26. Masuda et al. demonstrated enhanced gene expression for vascular regeneration and anti-inflammation in QQc PBMNCs using quantitative real-time polymerase chain reaction (qRT-PCR)19. Gene expression of proangiogenic growth factors (VEGF, angiopoietins, and IGF-1) and proangiogenic cytokines (IL-8 and IL-10) was significantly higher, and inflammatory cytokines were significantly lower in QQc PBMNCs than in non-QQc PBMNCs. They expanded their in vitro findings to an in vivo qRT-PCR experiment (gene expression for tissue regeneration in murine ischemic muscle) and confirmed the mechanism by which the cell therapy ameliorated organ damage. Although we could not perform gene expression experiments to examine tissue regeneration in the damaged kidneys, the regenerative paracrine activity of QQc PBMNCs might be related to the mechanisms that reduced tissue damage and improved function in our study. Fourteen days after IRI, a small number of QQc PBMNCs showed expression of CD31 antigen. Therefore, a few of the injected human QQc PBMNCs might have collaborated with resident mouse endothelial cells to restore PTC damage in the recovery phase of IRI-induced AKI.
QQc culture influenced the effects of human PBMNCs; since non-QQc PBMNCs did not improve IRI as did QQc PBMNCs, despite the same number of cells being injected. In fact, some pathological parameters (including cast formation and loss of the brush border) were worse in the non-QQc PBMNCs group compared with the IRI control group. On the other hand, QQc PBMNCs dramatically improved IRI in the present study. The reasons for this difference in potential for ameliorating kidney damage between non-QQc PBMNCs and QQc PBMNCs should be examined.
We found a significant difference in EPC colony-forming potential (vasculogenic potential) between non-QQc PBMNCs and QQc PBMNCs, with the latter showing more potential to form EPC colonies. According to a previous report17, non-QQc PBMNCs and QQc PBMNCs had different regenerative potentials. The total cell count decreased during QQc culture, but there was a significant increase in CD34+ cells and CD133+ cells. Furthermore, QQc culture induced macrophages that were phenotypically polarized into angiogenic, anti-inflammatory subsets: classical M1 to alternative CD206+ M2. qRT-PCR revealed increased expression of proangiogenic genes in QQc-PBMNCs compared to that in non-QQc PBMNCs19. Expansion of vasculogenic CD34+ cells and phenotypic transition of MNCs with anti-inflammatory and angiogenic potential was also confirmed in this study and might be important for in vivo application of this cell therapy.
The decrease of BUN, serum Cr, and corresponding injury parameters in QQc PBMNCs groups was rapid and dramatic. These findings suggest that protection could have been largely due to rapid improvement in renal blood flow (RBF). RBF is the major factor that influences renal injury and function in the context of early AKI. In turn, improvement of RBF could be due to protection from vasoconstriction and/or protection from endothelial injury or loss. However, we could not determine whether the protective effect was due to the primary vasomotor effects or primary endothelial effects. Furthermore, rapid improvement in RBF and the consequent relief from hypoxia/ischemia will protect the endothelium from ongoing damage. We could not precisely determine the pathophysiological mechanisms; however, from the results in this study, we postulate that protection from continuing vasoconstriction and endothelial damage are possible mechanisms of cell therapy in the IRI-induced AKI model. Cytokines released by QQc PBMNCs might improve RBF or stimulate regeneration of surviving mouse endothelial cells. By relieving persistent ischemia, cell therapy could have decreased overall injury, thereby decreasing long-term injury by tubulointerstitial fibrosis. Even delayed administration of human QQc-cultured PBMNCs provided protection against continued acute injury.
In summary, human PBMNCs cultured in a vasculogenic conditioning medium dramatically improved IRI-induced AKI in mice, even when administered 24 h after induction of AKI. The amelioration of PTC damage by QQc PBMNCs resulted in significant improvement of interstitial fibrosis during the recovery phase of IRI. Microcirculatory improvement and anti-inflammatory mechanisms mediating significant changes of cell populations to cells that possess the nature of EPCs and anti-inflammatory macrophages might contribute to the improvement of IRI-induced AKI in this model. Non-QQc human PBMNCs did not improve IRI-induced AKI in this model. Therefore, whole PBMNCs might not be appropriate for cell therapy. The phenotypic transition of PBMNCs to cells with regenerative and anti-inflammatory potential might be required for effective cell therapy. More direct mechanisms why the injured kidney tissues and functions were improved by cell therapy should be clarified. However, the fact that human-cultured PBMNCs could improve severe AKI in mice might be an important step and open the next door of this cell therapy to clinical application.
Acknowledgments
We deeply thank Dr. Takao Suzuki, the president of Tokushukai Medical Group, for his continuous and unifying support for this project. We also thank Mrs. Kayo Arita and Mr. Satoshi Fujimura, Department of Plastic and Reconstructive Surgery, Juntendo University School of Medicine, Ochanomizu, Japan, for their technical assistance.
Authors’ Note: All authors gave consent for submission and publication.
Author Contribution: The authors T.O., S.K., and R.T. made the experimental protocol, and T.O. conducted the whole experiments. The authors Y.M., K.I., H.M., and S.H. supported the experiments; R.M., M.S., D.K., and E.N. supported to establish the IRI model and creatinine measurement; K.O. supported pathological experiments including tissue fixation, preparation of tissue specimen, and immunofluorescent staining; S.S. reviewed the manuscript and advised to make the final manuscript; and H.M. permitted the experiment.
Ethics Approval and Consent to Participate: Ethics approval was obtained before starting the study. The approval number of the ethical committee was written in the manuscript. The mouse experimental protocol was approved by the Center for Animal Research at Juntendo University.
Statement of Human and Animal Rights: Human rights and privacy were fully protected in this study. Animal care and treatment conformed to institutional guidelines and international laws and politics.
Statement of Informed Consent: Peripheral blood from healthy volunteers were obtained after giving oral and written informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All experiments were conducted using a research grant from Shonan Kamakura General Hospital.
References
- 1. Santos WJ, Zanetta DM, Pires AC, Lobo SM, Lima EQ, Burdmann EA. Patients with ischemic, mixed and nephrotoxic acute tubular necrosis in the intensive care unit—a homogenous population? Crit Care. 2006;10(2):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. [DOI] [PubMed] [Google Scholar]
- 3. Li B, Cohen A, Hudson EH, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010;121(20):2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. La Manna G, Bianchi F, Cappuccilli M, Cenacchi G, Tarantino L, Pasquinelli G, Valente S, Della Bella E, Cantoni S, Claudia C, Neri F, Tsivian M, Nardo B, Ventura C, Stefoni S. Mesenchymal stem cells in renal function recovery after acute kidney injury: use of a differentiating agent in a rat model. Cell Transplant. 2011;20(8):1193–1208. [DOI] [PubMed] [Google Scholar]
- 5. Eliopoulos N, Zhao J, Forner K, Birman E, Young YK, Bouchentour M. Erythropoietin gene-enhanced marrow mesenchymal stromal cells decrease cisplatin-induced kidney injury and improve survival of allogeneic mice. Mol Ther. 2011;19(11):2072–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Milwid JM, Ichimura T, Li M, Jiao Y, Lee J, Yarmush JS, Parekkadan B, Tilles AW, Bonventre JV, Yarmush ML. Secreted factors from bone marrow stromal cells upregulate IL-10 and reverse acute kidney injury. Stem cells Int. 2012;2012:392050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu P, Feng Y, Dong C, Yang D, Li B, Chen X, Zhang Z, Wang Y, Zhou Y, Zhao L. Administration of BMSCs with muscone in rats with gentamicin-induced ALI improves their therapeutic efficacy. PLoS One. 2014;9(5):e97123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moghadasali R, Azarnia M, Hajinasrollah M, Arghani H, Nassiri SM, Molazem M, Vosough A, Mohitmafi S, Naiarasi M, Ajdari Z, Yazdi RS, Bagheri M, Ghanaati H, Rafiei B, Gheisari Y, Baharvand H, Aghdami N. Intra-renal arterial injection of autologous bone marrow mesenchymal stromal cells ameliorates cisplatin-induced acute kidney injury in a rhesus macaque mulatta monkey model. Cytotherapy. 2014;16(6):734–749. [DOI] [PubMed] [Google Scholar]
- 9. Yasuda K, Ozaki T, Saka Y, Yamamoto T, Gotoh M, Ito Y, Yuzawa Y, Matsuo S, Maruyama S. Autologous cell therapy for cisplatin-induced acute kidney injury by using non-expanded adipose tissue-derived cells. Cytotherapy. 2012;14(9):1089–1100. [DOI] [PubMed] [Google Scholar]
- 10. Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, Huo HC, Pinkemell K. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion–induced acute kidney injury. Nephrol Dial Transplant. 2010;25(12):3874–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burgos-Silva M, Semedo-Kuriki P, Donizetti-Oliveira C, Costa PB, Cenedeze MA, Hiyane MI, Pacheco-Silva A, Camara NO. Adipose tissue-derived stem cells reduce acute and chronic kidney damage in mice. PLoS One. 2015;10(11):e0142183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Overath JM, Gauer S, Obermuller N, Schubert R, Schafer R, Gesiger H, Baer PC. Short-term preconditioning enhances the therapeutic potential of adipose-derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Exp Cell Res. 2016;342(2):175–183. [DOI] [PubMed] [Google Scholar]
- 13. Morigi M, Rota C, Montemurro T, Montelatici E, Lo Cicero V, Imberti B, Abbate M, Zoja C, Cassis P, Longaretti L, Rebulla P, Introna M, Capelli C, Benigni A, remuzzi G, Lazzari L. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28(3):513–522. [DOI] [PubMed] [Google Scholar]
- 14. Liu P, Feng Y, Dong D, Liu X, Chen Y, Wang Y, Zhou Y. Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury. Sci Rep. 2016;6:20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rota C, Imberti B, Pozzobon M, Piccoli M, De Coppi P, Atala A, Gagliardini E, Xinaris C, Benedetti V, Fabricio AS, Squarcina E, Abbate M, Benigni A, Remuzzi G, Morigi M. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012;21(11):1911–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toyohara T, Mae S, Sueta S, Inoue T, Yamagichi Y, Kawamoto T, Kasahara T, Hoshina A, Toyoda T, Tanaka H, Araoka T, Sato-Otsubo A, Takahashi K, Sato Y, Yamaji N, Ogawa S, Yamanaka S, Osafune K. Cell therapy using human induced pluripotent stem cell-derived renal progenitors ameliorates acute kidney injury in mice. Stem Cells Transl Med. 2015;4(9):980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Masuda H, Iwasaki H, Kawamoto A, Akimaru H, Ishikawa M, Li M, Shizuno T, Sato A, Ito R, Horii M, Ishida H, Kato S, Asahara T. Development of serum-free quality and quantity control culture of colony-forming endothelial progenitor cell for vasculogenesis. Stem Cells Transl Med. 2012;1(2):160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka R, Vaynrub M, Masuda H, Ito R, Kobori M, Miyasaka M, Mizuno H, Warren SM, Asahara T. Quality control culture system restores diabetic endothelial progenitor cell vasculogenesis and accelerates wound closure. Diabetes. 2013;62(9):3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masuda H, Tanaka R, Fujimura S, Ishikawa M, Akimaru H, Shizuno T, Sato A, Okada Y, Iida Y, Itoh J, Itoh Y, Kamiguchi H, Kawamoto A, Asahara T. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. J Am Heart Assoc. 2014;3(3):e000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. [DOI] [PubMed] [Google Scholar]
- 21. Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol. 2012;303(11):F1487–F1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuen PST, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol. 2004;286(6):F1116–F1119. [DOI] [PubMed] [Google Scholar]
- 23. Stockman G, Leemans JC, Claessen N, Weening JJ, Florguin S. Hematopoietic stem cell mobilization therapy accelerates recovery of renal function independent of stem cell contribution. J Am Soc Nephrol. 2005;16(6):1684–1692. [DOI] [PubMed] [Google Scholar]
- 24. Hosoya A, Hoshi K, Sahara N, Ninomiya T, Akahane S, Kawamoto T, Ozawa H. Effects of fixation and decalcification on the immunohistochemical localization of bone matrix proteins in fresh-frozen bone sections. Histochem Cell Biol. 2005;123(6):639–646. [DOI] [PubMed] [Google Scholar]
- 25. Majka M, Janowska-Wieczorek A, Ratajczak J, Ehrenman K, Piertzkowski Z, Kowalska MA, Gewirtz AM, Emerson SG, Ratajczak MZ. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97(10):3075–3085. [DOI] [PubMed] [Google Scholar]
- 26. Sahoo S, Klychko E, Thorne T, Misener S, Shinnick K, Millay M, Ito A, Liu T, Kamide C, Agarwal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109(7):724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]