Abstract
Parkinson’s disease (PD) causes motor dysfunction and dopaminergic cell death. Drug treatments can effectively reduce symptoms but often cause unwanted side effects. Stem cell therapies using cell replacement or indirect beneficial secretomes have recently emerged as potential therapeutic strategies. Although various types of stem cells have been proposed as possible candidates, adipose-derived stem cells (ADSCs) are easily obtainable, more abundant, less ethically disputed, and able to differentiate into multiple cell lineages. However, treatment of PD using adult stem cells is known to be less efficacious than neuron or embryonic stem cell transplantation. Therefore, improved therapies are urgently needed. n-Butylidenephthalide (BP), which is extracted from Angelica sinensis, has been shown to have anti-inflammatory and neuroprotective effects. Indeed, we previously demonstrated that BP treatment of ADSCs enhances the expression of neurogenesis and homing factors such as nuclear receptor related 1 protein, stromal-derived factor 1, and brain-derived neurotrophic factor. In the present study, we examined the ability of BP-pretreated ADSC transplantation to improve PD motor symptoms and protect dopamine neurons in a mouse model of PD. We evaluated the results using neuronal behavior tests such as beam walking, rotarod, and locomotor activity tests. ADSCs with or without BP pretreatment were transplanted into the striatum. Our findings demonstrated that ADSC transplantation improved motor abilities with varied efficacies and that BP stimulation improved the therapeutic effects of transplantation. Dopaminergic cell numbers returned to normal in ADSC-transplanted mice after 22 d. In summary, stimulating ADSCs with BP improved PD recovery efficiency. Thus, our results provide important new strategies to improve stem cell therapies for neurodegenerative diseases in future studies.
Keywords: Parkinson’s disease, adipose-derived stem cells, n-butylidenephthalide
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease that affects millions of people worldwide and is the second most common disease of its kind after Alzheimer’s disease1. PD has caused an increased economic burden and strain to health-care-related resources, particularly in countries with aging populations. While the cause of PD is still unknown, mutations in several specific genes, including α-synuclein (SNCA) and leucine-rich repeat kinase 2, have been implicated in increased PD susceptibility2. Major symptoms of PD occur in the motor system as a result of dopaminergic (DA) neuron cell loss. Although the underlying mechanisms of cell loss are still unclear, excessive oxidative stress, increased protein misfolding and aggregation, and impaired mitochondrial function have been shown to affect the pathogenesis of PD3. There is currently no cure for PD; however, several treatments, including L-3,4-dihydroxyphenylalanine (L-DOPA) and dopamine agonists, have been shown to be effective against movement disorder symptoms4. Although these treatments significantly alleviate some symptoms of PD, they are unable to stop disease progression or protect DA cells from cell loss during PD progression. Neural grafting of immature DA neurons in patients with PD has been studied since the late 1980s5. However, inconsistent results and side effects, such as graft-induced dyskinesias, have been observed, and the requirement for tissue collection from developing embryos has become a major ethical issue6. Recently, discoveries and developments in regenerative medicine and stem cell therapy have provided new approaches to the treatment of various diseases, and stem cell transplantation has emerged as a possible treatment for PD.
Although the study of stem cell therapy for the treatment of PD initially focused on transplantation of ventral mesencephalic tissue, human embryonic stem cells (ESCs) are an interesting alternative owing to the ability of these cells to differentiate into authentic DA neuron cells7,8. However, the use of embryo-derived stem cells as treatment materials has been the source of heated ethical debates6. In contrast, mesenchymal stem cells (MSCs) isolated from adult tissues are not controversial and therefore may be potential candidates for cell-based PD therapy. Indeed, several recent studies had investigated the ability of MSCs from different sources in treatment of several neurodegenerative diseases including PD9–11. It is suggested that MSCs are capable of inducing tyrosine hydroxylase (TH) and other neurotrophic factor expressions when cocultured with neuronal cells in vitro and improving behavioral performance in PD-affected animals. MSCs can be isolated from various tissues, including bone marrow (referred to as BM-MSCs) and adipose tissues (referred to as adipose-derived stem cells [ADSCs]). Although BM-MSCs have been studied extensively in the last few decades, the isolation process is painful, and the yield of BM-MSCs is low, particularly when compared to ADSCs, which are more abundant and easily isolated12–14. ADSCs are capable of differentiating into multiple cell lineages, including myocytes, chondrocytes, and neuronal cells such as dopaminergic cells15–17. The ability of ADSCs to treat brain damage resulting from several types of injuries and neural diseases has been investigated in numerous studies, and ADSCs have been found to reduce symptoms of brain disease directly by cell replacement or indirectly by releasing certain factors18. ADSCs also provide neuronal protection and reduce neuronal apoptosis13. The factors released by ADSCs are thought to decrease protein aggregation in patients with Alzheimer’s disease19. Furthermore, one study reported that ADSC transplantation improves behavioral performance in a drug-induced mouse model of PD20. However, while transplantation of these MSCs seems to be a promising approach to the treatment of neural diseases, the efficiency of this method is lower than that of ESC transplantation; thus, further improvements are required21–23.
The dry root of Angelica sinensis, also known as Danggui in Chinese medicine, has been shown to exert anti-inflammatory, antioxidant, and neuroprotective effects24–26. This plant contains several chemical compounds including organic acids, polysaccharide sulfate, and phthalides27. n-Butylidenephthalide (BP) is a naturally occurring compound derived from the herb’s chloroform extract. BP has been shown to possess anticancer, anti-inflammatory, and other protective effects28–30. Additionally, BP has been shown to significantly reduce amyloid-β deposition in an in vitro model of AD using induced pluripotent stem cell–derived neuronal cells31. In our previous study, we found that BP decreases SNCA accumulation and provides protection against DA neuron degeneration in a Caenorhabditis elegans model32. These findings suggest that BP may have the potential to prevent or remove pathological protein misfolding and aggregation and may possess neuroprotective effects. Moreover, because ligustilide, another phthalide compound derived from the same plant, also shows neuroprotective effects and promotes neurogenesis, we postulate that BP may have similar beneficial effects33,34.
In this study, we examined the therapeutic potential of ADSC transplantation in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced short-term mouse model of PD. PD was induced on day 2 after serial MPTP injections, killing DA neurons and causing symptoms of motor deficiency, similar to those observed in patients with PD. ADSC transplantation was performed on day 3 after MPTP administration. One previous study suggested that cells grafted in the substantia nigra (SN) have little effect, while those grafted in the striatum provide therapeutic effects for DA cell loss35. Based on this finding, we transplanted ADSCs into the mouse striatum as previously described36. We evaluated the possible neurogenic effects of BP by incubating ADSC cultures in BP-containing medium. Cell survival and gene expression patterns of neuronal and inflammatory genes were examined. We further examined the therapeutic effects of BP-pretreated ADSCs in our mouse model of PD and compared the observed effects with those of untreated ADSCs. Motor abilities, including coordination and balance, were monitored over a period of 23 d. Because cytotoxic dimethyl sulfoxide (DMSO) is used as a BP solvent, we also examined the effects of ethanol in the experiment as a possible alternative solvent.
Materials and Methods
Study Method
The study was performed with approval of the Institutional Review Board of China Medical University and Hospital Research Ethics Committee and the Taiwan Food and Drug Administration (TFDA), Ministry of Health and Welfare, Taiwan. The original protocol was approved by China Medical University and Hospital Research Ethics Committee (CMUH104-REC1-007).
Isolation and Characterization of ADSCs
Isolation and characterization of ADSCs were carried out as previously described37. During gynecological surgery, cells were harvested from 2 to 5 g of subcutaneous fat from the abdominal wall. Tissues collected in calcium-/magnesium-free phosphate-buffered saline (PBS) were dissected into 1 to 2 mm3 pieces and dissociated with 0.1% collagenase I (Invitrogen-Gibco, Waltham, MA, USA) for 60 min at 37 °C. The dissociated cells were transferred to ADSC culture medium as described below. Cells were incubated at 37 °C in a humidified incubator supplied with 5% CO2. After 2 d, cell debris and supernatant were removed, and the remaining cell culture was designated passage 0. In order to verify the cells were indeed ADSCs, cells were labeled with antibodies against the surface markers CD14, CD29, CD44, CD45 (human leukocyte antigen)-ABC (Dako, Glostrup, Denmark), CD34, CD49b, and human leukocyte antigen–antigen D related (BD Biosciences, San Diego, CA, USA). Labeled samples were analyzed using a flow cytometer (LSR II, BD Biosciences).
Culture of ADSCs
ADSCs were cultured in Keratinocyte-serum-free medium (SFM) containing bovine pituitary extract and epidermal growth factor (Gibco) at 37 °C in a humidified incubator supplied with 5% CO2. The culture medium was supplemented with N-acetyl-L-cysteine (2 × 10−3 M; Sigma-Aldrich, St. Louis, MO, USA), l-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (2 × 10−4 M; Sigma-Aldrich), and HyClone characterized fetal bovine serum (FBS; 10%; GE Healthcare, South Logan, UT, USA).
BP Treatment and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assays
The BP used in this study was synthesized by Alfa Aesar, USA. BP was diluted to stock concentration of 20 mg/mL in 100% DMSO (Sigma-Aldrich) or absolute ethanol (Sigma-Aldrich). Further dilutions to working concentration were prepared using culture medium or normal saline. To examine cell viability, ADSCs were first incubated in medium containing serial dilutions of BP for 24 or 48 h. The BP concentrations used in this study were 5, 10, 20, 40, 80, 160, and 320 μg/mL. MTT assays (Sigma-Aldrich) were then performed.
Real-time Polymerase Chain Reaction (PCR) Analysis of Gene Expression
Real-time PCR was performed using a StepOnePlus real-time PCR system (Applied Biosciences, Foster City, CA, USA). RNA was first extracted with TRIzol (Thermo Fisher Scientific, Carlsbad, CA, USA) from ADSCs after cells were treated with 1.25, 2.5, 5, or 20 μg/mL BP for 24 h. We performed reverse transcription of extracted RNA using a SuperScript III Reverse Transcriptase Kit (Invitrogen) to obtain cDNA. Real-time quantitative PCR (RT-qPCR) was then performed with FastStart Universal SYBR Green Master (Rox; Roche, Switzerland) to compare gene expression using specific primers as follows—brain-derived neurotrophic factor (BDNF): (forward) 5′-TCACACTCCACATCCCGTGAT-3′ and (reverse) 5′-TTACTCTGACCAACGCCCAAA-3′; nuclear receptor related 1 protein (Nurr1): (forward) 5′-GCTGAAGCCATGCCTTGTG-3′ and (reverse) 5′-GAAGAGTGGTAACTGTAGCTCTGAGAAG-3′; brain 4 (Brn4): (forward) 5′-TGCCGCGCAGGAGATC-3′ and (reverse) 5′-AGAACCAGACACGCACCACTT-3′; stromal-derived factor 1 αβ (SDF1αβ): (forward) 5′-CATGCCGATTCTTCGAAAGC-3′ and (reverse) 5′-TCAGCCGGGCTACAATCTG-3′; interleukin (IL) 6: (forward) 5′-AAAAAGGCAAAGAATCTAGATGCAA-3′ and (reverse) 5′-GTCAGCAGGCTGGCATTTGT-3′; IL8: (forward) 5′-ACCGGAAGGAACCATCTCACT-3′ and (reverse) 5′-ATCAGGAAGGCTGCCAAGAG-3′; and β-actin: (forward) 5′-GTGCGTGACATCAAAGAGAAGC-3′ and (reverse) 5′-TGGATGCCACAGGATTCCATAC-3′. The mRNA levels of each gene were normalized using the housekeeping gene β-actin.
Flow Cytometry Analysis
Flow cytometry analysis was performed on an LSR II flow cytometer (BD Biosciences). To characterize ADSCs, dissociated cells were incubated with phycoerythrin-conjugated anti-human CD44 antibody (1:50 dilution; BD Biosciences) or phycoerythrin-conjugated anti-human CD105 antibody (1:50 dilution; Invitrogen).
Animal Treatment and Anesthetization
C57BL/6 (B6) male mice (BioLASCO, Taipei City, Taiwan) were maintained in individually ventilated cages at the Laboratory Animal Service Center. The Institutional Animal Care and Use Committee of the China Medical University approved all animal treatments and experimental procedures (104-241). Mice were anesthetized 10 min before experiments by intraperitoneal administration of 10 mg/mL Zoletil in 0.04% Rompun at a dose of 0.08 mL per 10 g body weight.
PD Induction Using MPTP
PD was induced in mice at 8 w of age. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP-HCl) (Sigma-Aldrich) was dissolved in PBS at a stock concentration of 7 mg/mL. MPTP was administered in 4 intraperitoneal injections at a dose of 20 mg/kg. Injections were performed at 2-h intervals. Mice that were treated with MPTP were on randomization and blinding for each experimental group.
ADSC Transplantation
ADSCs were transplanted into the brains of mice 2 d after PD induction as described previously by Ding and colleagues36. Briefly, 1 × 106 cells were transplanted by intracerebral injection of 18 μL ADSCs at 3 different locations in the striatum. In pretreat intracerebral (i.c.) and intravenous (i.v.) group, additional i.v. injection of 1 × 106 ADSCs through tail vein was performed 2 d after i.c. injection.
Neuronal Behaviors of Mice before and after PD Induction
B6 mice were trained twice on each device before the experiment. Neuronal behaviors of target mice were examined using 3 separate apparatuses: overall activity was monitored with a locomotor device, balancing ability was monitored using beam walking, and coordination was monitored using a rotarod. Overall activity was monitored in an 8-channel locomotor activity box for 1 h. Horizontal and vertical movements in the chamber, resting time, and the distance traveled in the last 30 min were analyzed. Balancing ability of mice was recorded as the time the animals took to cross an 80-cm beam, and the rear foot slip frequency while crossing was determined. Coordination of the animals was recorded as the duration for which animals were able to remain stable on a rotating rod at a constant speed of 5 rpm during a 3-min period. Data from at least 3 independent experiments were used for statistical analysis. The data collection period started from day 0 and lasted until day 22 when MPTP-induced PD symptoms could not be detected from control mice. The mice were then sacrificed and had their brain tissue collected. Additionally, 3 mice brain samples from each group were collected on day 5 and striatum sections containing the injection site were examined to confirm the outcome of transplantation.
Brain Histology and Immunohistochemical (IHC) Staining of the SN
Mouse brains were harvested after anesthetization and sequential cardiac perfusion with PBS and 4% paraformaldehyde (PFA; Sigma-Aldrich). Whole brains were collected and incubated in 4% PFA for 4 h. Samples were then incubated for 16 h in 20% sucrose/PBS solution and then for an additional 16 h in 30% sucrose/PBS solution. Frozen sections were prepared from the collected brain samples. Selected sections were washed in Tris-base saline buffer containing 1% Tween 20 (TBST; Sigma-Aldrich) and then incubated at 93 °C in Trilogy pretreatment solution (Sigma-Aldrich) for 15 min. After cooling, the samples were permeabilized in 0.3% Triton X-100 (Sigma-Aldrich) for 30 min and then blocked in 0.5% FBS/PBS solution for 30 min. The sections were then incubated with anti-TH antibody (rabbit anti-TH antibody, diluted 1:200; AB152; Merck Millipore, Darmstadt, Germany) for 16 h at 4 °C. Additional sections were concurrently stained with anti-human mitochondria (anti-hMito) antibody (rabbit anti-human antibody, diluted 1:200; AB3598; Merck Millipore). The samples were then incubated for 1 h with fluorescence-conjugated goat anti-rabbit antibody (Alexa Fluor 594, diluted 1:200; A-11037; Invitrogen) and goat anti-mouse antibody (fluorescein isothiocyanate, diluted 1:200; 12-506; Merck Millipore). Finally, the samples were stained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the cell nuclei. Representative sections containing the same SN and striatum region from 3 individual mice of each group were observed using Zeiss Axio Imager A1 microscope with 10× and 40×objectives, respectively, and recorded with an AxioCam HRc camera. Colocalization of different antibodies was confirmed using the Leica TCS SP2 spectral confocal microscope (Leica Microsystems, Heidelberg, Mannheim, Germany). TH-expressing cells on the SN section were counted as TH-positive/DAPI-positive cells. The cell counts were averaged and compared to that of the control group.
Statistical Analysis
Results from all experiments were presented as means ± standard deviations. One-way analysis of variance (ANOVA) and Tukey’s post hoc test were performed to compare the means among different treatments using SigmaPlot. Differences with P values of less than 0.05 were considered statistically significant. Detailed group sizes in animal experiments are as follows: control and pretreat i.c. + i.v. group each contained 6 samples; saline group, ADSC i.c. group, and pretreat i.c. group each had 7 samples; and pretreat i.c. EtOH had 3 samples on day 22. Mice IHC sections was collected from additional experiments with 3 mice in each group on day 5. In the behavioral study, mean area under the curve (AUC) analysis was performed to detect intergroup difference between treatment groups and saline groups.
Results
ADSC Viability after BP Treatment
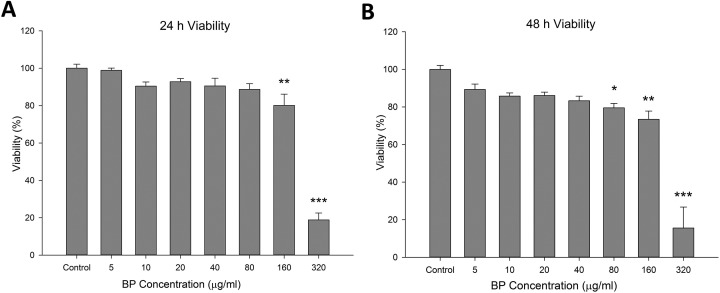
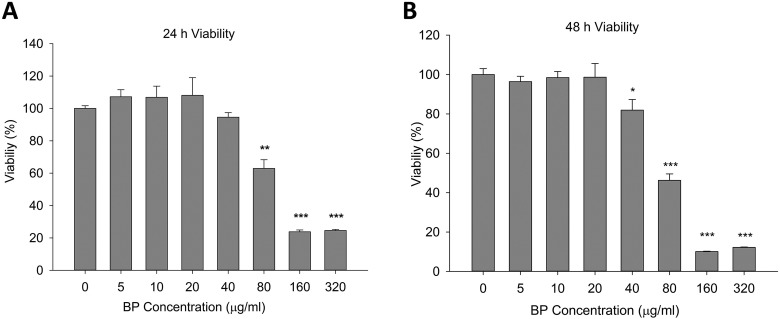
First, we investigated the therapeutic effects of BP-treated ADSCs in a MPTP-induced mouse model of PD. Analysis of cell viability in the presence of different concentrations of BP is important for determining the most suitable treatment conditions. ADSCs were cultured in serial-diluted BP for 24 or 48 h and were then analyzed to determine viability (Fig. 1A and B). ADSC viability decreased significantly when the BP concentration was 80 μg/mL or higher for the 24-h treatment period. Prolonged treatment (48 h) showed further reduction in viability in high concentrations and was stable at concentrations of 40 μg/mL or lower. These results indicated that high concentrations of BP had a negative impact on cell survival and growth, but that BP could be used at a concentration of 40 μg/mL or lower without affecting cell viability under our treatment conditions. In order to prevent possible long-term negative effects of higher concentration, we chose 20 μg/mL concentration as the upper limit for further gene expression tests.
Fig. 1.
Effects of n-butylidenephthalide (BP) pretreatment on adipose-derived stem cell viability. (A) The viability of cells was lower at high concentrations of BP. Viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays after the cells were incubated in medium containing BP at the indicated concentrations for 24 h. (B) Prolonged incubation with BP for 48 h further reduced cell survival at concentrations of 80 μg/mL and above. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control (0 μg/mL BP).
BP Pretreatment Enhanced the Expression of Homing Factors in ADSCs without Changing Cell Characteristics
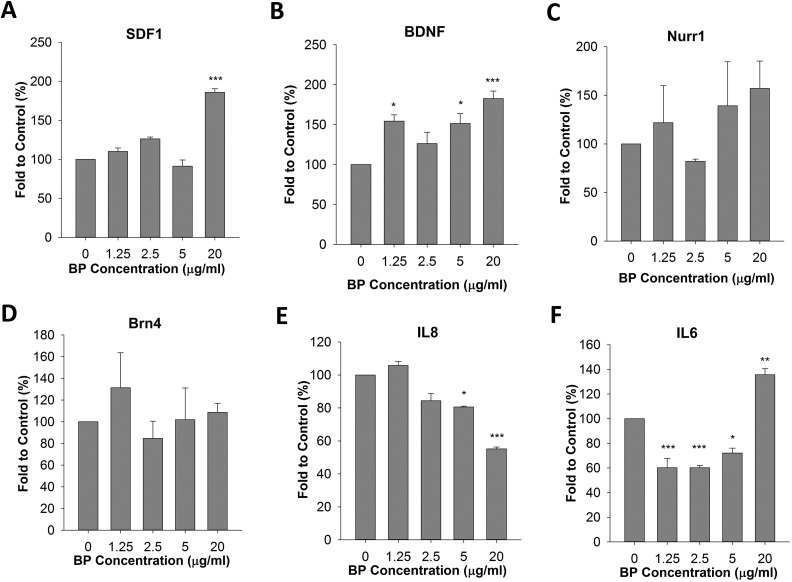
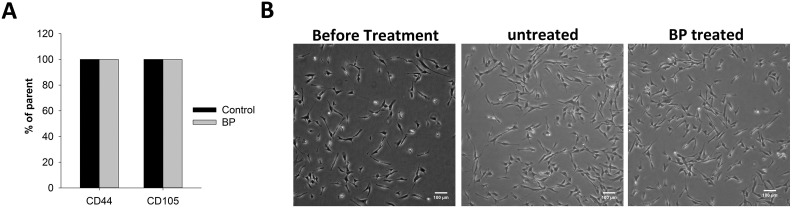
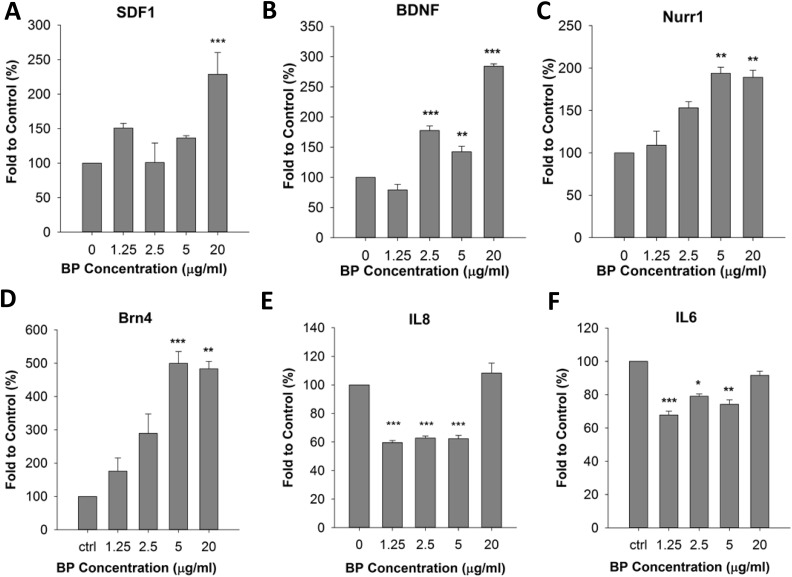
Because cell viability was significantly reduced in the presence of BP at concentrations above 40 μg/mL, we next examined changes in gene expression in ADSCs in the presence of 0 to 20 μg/mL BP. We evaluated the expression of several neuronal differentiation-promoting and homing genes including Nurr1, BDNF, SDF1αβ, and Brn4. Gene expression was measured by qPCR following reverse transcription of mRNA extracted from BP-treated cells. Our results showed that BDNF and SDF1αβ expression levels were increased by approximately 2-fold after treatment of the cells with 20 μg/mL BP for 24 h; in contrast, the Nurr1 and Brn4 genes showed only a slight increase in expression (Fig. 2A to D). We also examined immunodifferentiation and immunomodulatory genes, such as IL6 and IL8. We found that IL8 was significantly downregulated after treatment with 20 μg/mL BP (Fig. 2E). In contrast, IL6 expression increased by 35% in the presence of 20 μg/mL BP (Fig. 2F). The combined results of cell viability and gene expression analyses suggested that treatment with 20 μg/mL BP for 24 h could induce neuronal gene expression. This treatment also had complex effects on the expression of immunodifferentiation-related genes. To confirm the effects of treatment with 20 μg/mL BP on ADSC characteristics, we compared ADSCs with or without treatment using the cell surface markers CD44 and CD105. Our result showed that both BP-treated ADSCs and control ADSCs expressed CD44 and CD105 in all collected cells (Fig. 3A). Cell morphology before and after BP treatment did not vary significantly (Fig. 3B).
Fig. 2.
Effects of n-butylidenephthalide (BP) pretreatment on upregulation of neurogenesis-related genes and homing factors. (A to C) Expression levels of stromal cell-derived factor 1 (SDF1), brain-derived neurotrophic factor (BDNF), and nuclear receptor related 1 protein (Nurr1) were upregulated by treatment with 20 μg/mL BP for 24 h. (D) The expression of Brn4 was not significantly changed. (E) Interleukin 8 (IL8) expression was downregulated. (F) IL6 expression increased by 35%. *P < 0.05, **P < 0.01, and ***P < 0.001 versus the control (0 μg/mL BP).
Fig. 3.
Effect of n-butylidenephthalide (BP) pretreatment on cell morphology and characteristics. BP 19 pretreatment of adipose-derived stem cells (ADSCs) did not affect cell morphology and characteristics. (A) The surface markers CD44 and CD105 were analyzed in untreated and treated ADSCs. (B) The morphology of ADSCs was evaluated using light microscopy after incubation with or without BP for 24 h.
MPTP-induced Mouse Model of PD
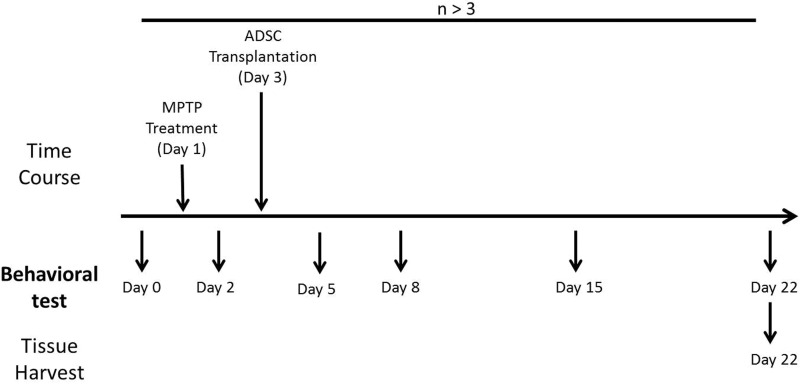
We adopted a previously established MPTP-induced short-term mouse model of PD to investigate the therapeutic effects of ADSCs and BP pretreatment on PD. The 12 neuronal behaviors of B6 mice, including motor skills, balance, and coordination, were evaluated on day 0 after 2 separate training sessions. On day 1, male B6 mice were intraperitoneally injected with MPTP at a dose of 20 mg per 10 kg body weight 4 times at 2-h intervals. The recovery of behavioral function for different cell/BP treatment groups was recorded on days 2, 5, 8, 15, and 22 after PD induction, and brains were then collected for histochemical analysis (Fig. 4).
Fig. 4.
Schematic representation of the experimental design. Neuronal behaviors of mice were recorded on days 0, 2, 5, 8, 15, and 22. Parkinson’s disease induction was performed by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration on day 1. Adipose-derived stem cell transplantation was performed on day 3. Brains of target mice were collected on day 22.
Transplantation of BP-pretreated ADSCs Improved Behavioral Recovery after PD Induction
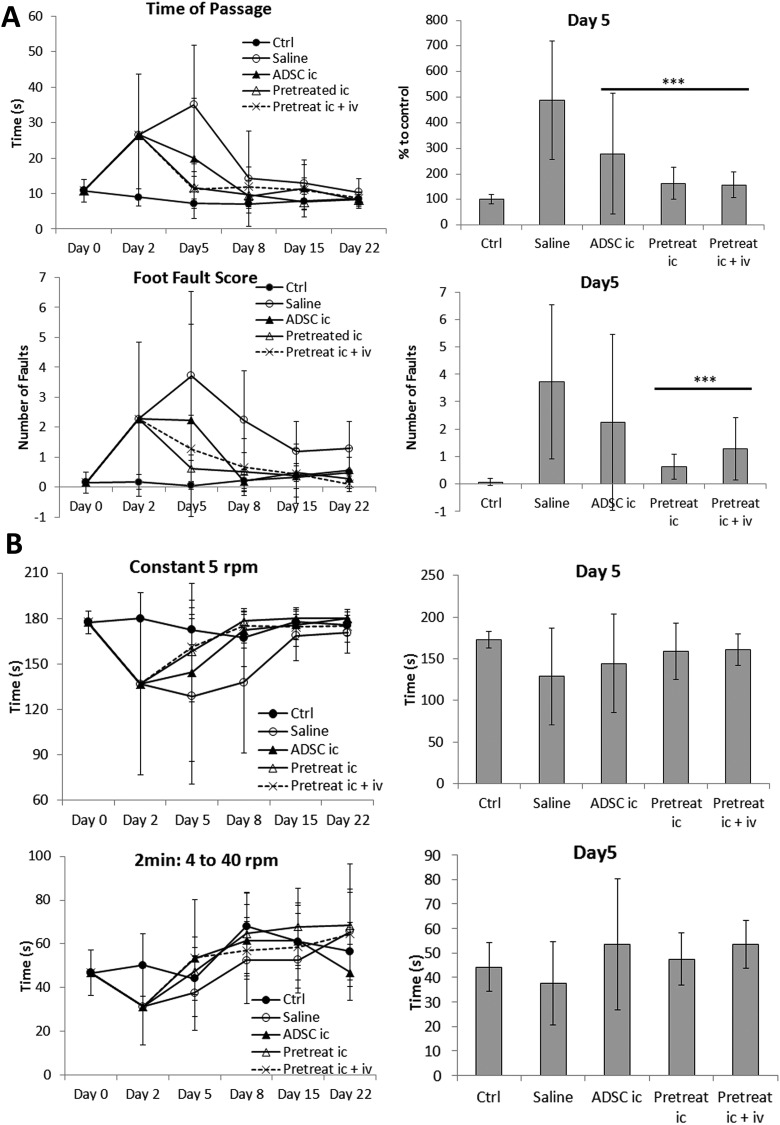
Because BP treatment of ADSCs altered cell expression patterns in favor of neuronal differentiation and homing, we examined whether ADSC transplantation may have therapeutic effects on the recovery of behavioral functions in MPTP-induced PD mice. ADSCs with or without BP treatment (20 μg/mL) were transplanted into PD mice via intracerebral injection on day 3. Induction of PD resulted in significant loss of motor skills, balance, and coordination in B6 mice, as was observed in beam walking (Fig. 5A), rotarod (Fig. 5B), and locomotor activity experiments (Fig. 5C to E), respectively. The loss of ability was most significant on day 2 or day 5, suggesting that the speed of recovery varied for the different neuronal behaviors. ADSC transplantation significantly improved the recovery of balancing ability on day 5 (beam walking and rotarod). Indeed, although PD mice treated with saline suffered further loss of time of passage, mice transplanted with ADSCs, or BP-pretreated ADSCs (both of the i.c. and i.c. + i.v. groups) exhibited significant improvement on day 5 compared to saline group (Fig. 5A). Loss of time of passage in mice transplanted with ADSCs, BP-pretreated ADSCs i.c. or BP-pretreated ADSCs i.c. + i.v. groups showed the predictive power compared with saline group (AUC: 0.774, 0.929, and 0.944, respectively). The foot fault score data also showed that mice transplanted with BP-pretreated ADSCs i.c. or BP-pretreated ADSCs i.c. + i.v. exhibited significant improvement on day 5 compared to saline group (Fig. 5A). The foot fault score data in mice transplanted with BP-pretreated ADSCs i.c. or BP-pretreated ADSCs i.c. + i.v. groups showed the highest predictive power compared with saline group (AUC: 0.964 and 0.861, respectively).
Fig. 5.
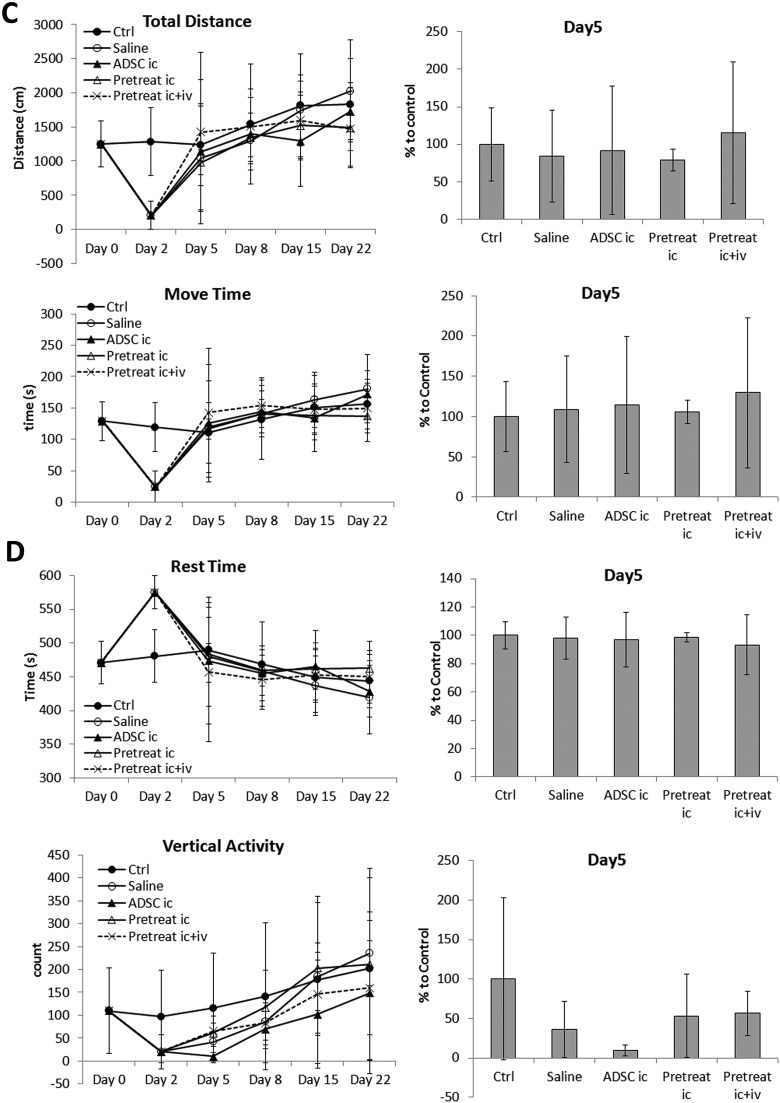
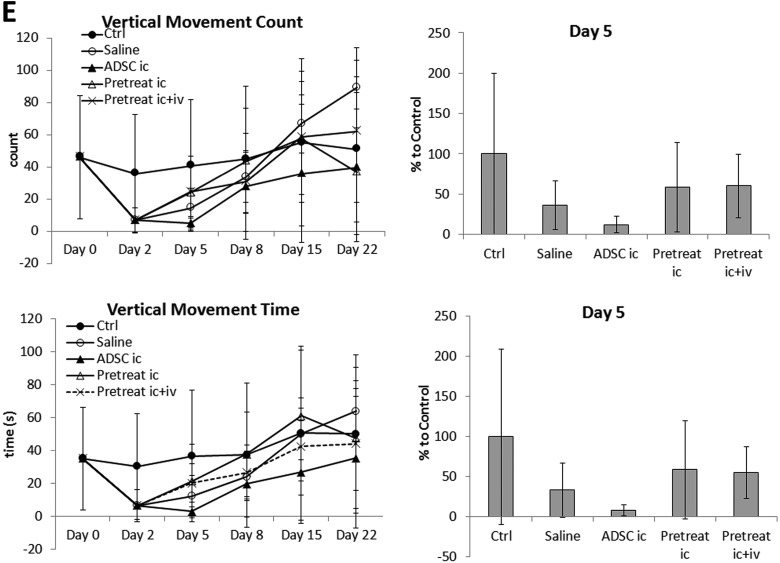
Effect of n-butylidenephthalide (BP) pretreatment on Parkinson’s disease (PD) recovery. (A) Transplantation with pretreated adipose-derived stem cells (ADSCs) significantly improved balancing ability on day 5 compared to that in mice transplanted with untreated ADSCs. (B) Coordination ability in PD mice improved slightly after transplantation with ADSCs or pretreated ADSCs. (C) All groups recovered similarly in terms of total distance traveled and total movement time; no significant improvement was observed compared to that in ADSC transplantation. (D) The total rest time of all groups did not differ significantly; however, slightly better recovery of vertical activity was observed in mice transplanted with BP-pretreated ADSCs. (E) Vertical movement and vertical moving times of mice transplanted with BP-pretreated ADSCs were improved slightly. The results at day 5 were quantified and compared in a bar graph. Error bars represent means ± SDs (N ≥ 6). ***P < 0.001 versus the saline group.
Repeated measures ANOVA was performed for mean different time point data with slope comparisons in all 5 groups. In Fig. 5A, time of passage data and foot fault score showed significant difference between 5 groups (P = 0.0001 and P = 0.003, respectively). The rotarod analysis of Constant 5 and 2 min: 4 to 40 rpm also showed significant difference between 5 groups (P = 0.044 and P = 0.024, respectively). These data showed the overall performance is different in 5 groups. However, there was no significance in all of the locomotor assays (Fig. 5C to E).
In addition, the results suggested that ADSCs pretreated with BP showed further behavioral improvement in post-PD induction recovery compared to untreated ADSCs. The difference between untreated and treated ADSCs was most significant in foot fault score (Fig. 5A). However, the difference was not as significant as shown in the rotarod test (Fig. 5B). Motor activity tests showed varying results for different parameters. There were no statistically significant differences in any of the parameters, although data from vertical activities suggested slightly better recovery in PD mice transplanted with BP-treated ADSCs (Fig. 5C to E).
We were also interested in determining whether combined intracerebral and i.v. transplantation provided additional therapeutic effects. ADSCs were transplanted into PD mice through intracerebral injection and then i.v. injection. However, we did not observe any further improvement during recovery in mice receiving double transplantations compared to that in mice receiving only intracerebral transplantation.
Changes in the Number of TH-expressing Cells after Transplantation of BP-treated ADSCs
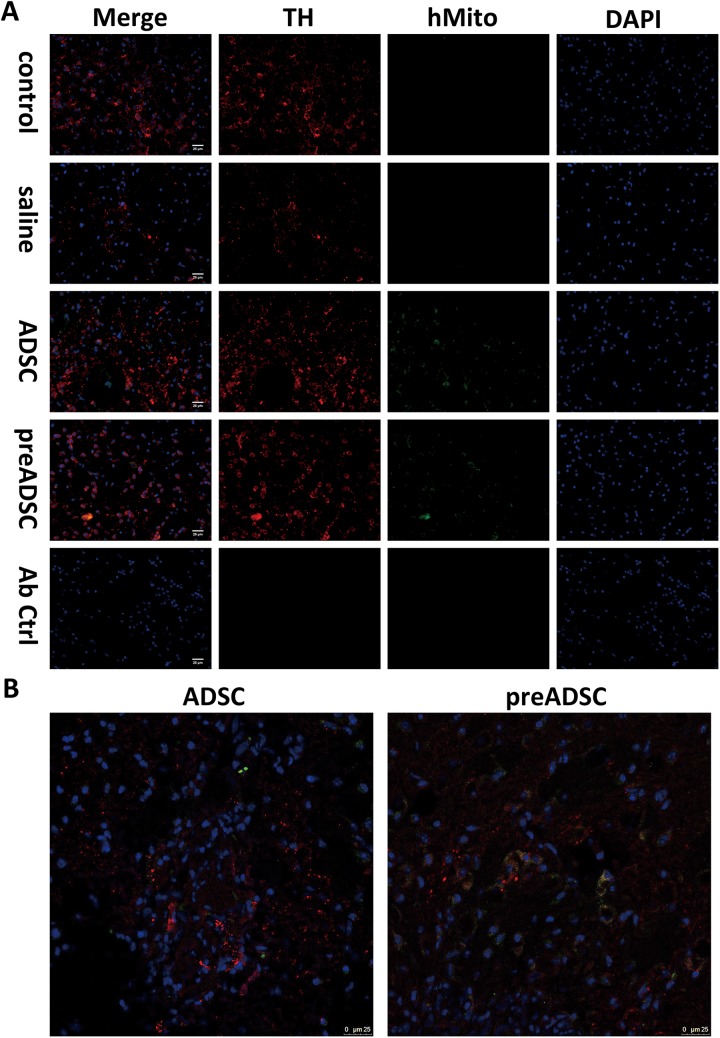
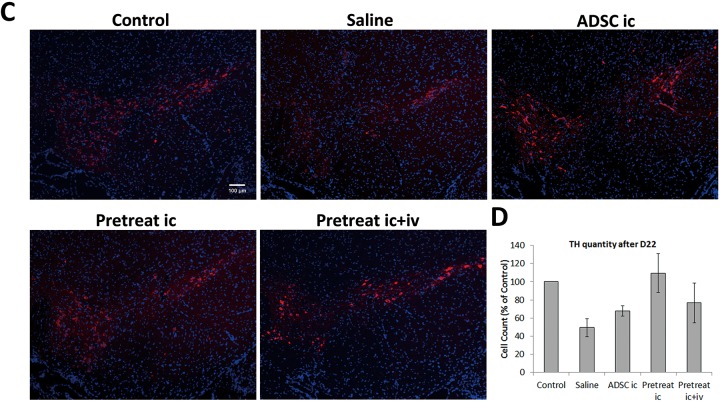
Our data in the behavioral study demonstrated that transplantation of BP-treated ADSCs improved post-PD recovery. To confirm successful transplantation, we collected mice on day 5 from each group except pretreat i.c. + i.v. The striatum sections from these mice were stained with both TH and human mitochondria-specific markers (Fig. 6A). Around our injection site, healthy control mice TH-positive staining can be seen to span across most striatum area, and small TH+ bright dots can be observed. In the striatum of mice injected with ADSCs or pretreated ADSCs, hMito-positive cells were presented around the injection scar. It is also worth noting that some of these cells were also TH positive. We further confirmed colocalization of TH and hMito signals in cells by confocal microscopy. This result indicated that the cells possibly differentiated into new DA expressing cells (Fig. 6B). We next investigated whether ADSC transplantation improved dopaminergic cell survival in mice. Brain samples of PD mice from the behavioral study were collected on day 23, and frozen sections were prepared. Sections of the SN region were stained with the dopaminergic cell marker TH (Fig. 6C). Although not statistically significant due to large variance among samples, the average number of TH-expressing cells dropped 25% after PD induction (Fig. 6D). Transplantation of untreated ADSCs did not significantly alter average cell numbers in the SN region on selected sections compared to that in untreated mice. However, cell numbers increased when PD mice underwent transplantation with BP-treated ADSCs and were comparable to those of healthy mice. Our results from the cell count, although not statistically significant, implicated that the increased numbers of TH-expressing cells in PD mice transplanted with BP-treated ADSCs possibly corroborated with the findings of our behavioral study, suggesting that BP-treated ADSCs exerted better therapeutic effects than untreated ADSCs and double transplantation treatments in behavioral recovery in mice with PD.
Fig. 6.
Number of tyrosine hydroxylase–expressing cells in mice transplanted with n-butylidenephthalide-pretreated adipose-derived stem cells (ADSCs). (A) Striatum of mice from control, saline, ADSC, preADSC groups were stained with tyrosine hydroxylase (red), human mitochondria marker (green), and 4′,6-diamidino-2-phenylindole (DAPI; blue), scale bar = 25 μm. (B) Colocalization of tyrosine hydroxylase (TH) and human mitochondria from (A) was confirmed by confocal microscopy. (C) The substantia nigra of mice from each group was stained for TH (red). DAPI (blue) was used for staining of nuclei. (D) Cells positive for both TH and DAPI were counted and presented as percentage versus control in the bar graph. Error bars represent means ± SDs (N = 3).
Changes in ADSC Viability and Gene Expression Following BP Pretreatment Using Ethanol as a Substitute Solvent for DMSO
The results from our behavioral study and tissue section analysis suggested that BP pretreatment of ADSCs improved the therapeutic effects of stem cell transplantation during PD recovery. In our experiments, BP was initially dissolved in DMSO, which exhibits neurotoxicity at high doses and is associated with safety concerns when used as solvent in the clinical setting. Therefore, to test whether ethanol could be used as a substitute solvent for BP, we examined ADSC viability when cells were treated with BP dissolved and diluted in 100% ethanol. After treatment of ADSCs with BP/ethanol for 24 or 48 h, we found that cell viability remained stable at concentrations of 20 μg/mL or lower (Fig. 7A and B). These results suggested that ADSCs had similar tolerance toward BP when DMSO or ethanol was used as a solvent. We also examined gene expression in ADSCs pretreated with BP/ethanol. BDNF, Nurr1, SDF1αβ, and Brn4 were significantly upregulated after 24 h of treatment with BP/ethanol, whereas IL8 and IL6 expression was not altered compared to that in untreated cells (Fig. 8A to F). This result clearly suggested that BP dissolved in ethanol was comparable to BP dissolved in DMSO with regard to maintenance of ADSC viability and increased neuronal gene expression.
Fig. 7.
ADSC viability after treatment with BP/ethanol. A. The viability of cells decreased at a BP concentration of 80 µg/mL or higher. Viability was evaluated by MTT assays after cells were incubated in medium containing BP at the indicated concentrations for 24 h. B. Prolonged incubation with BP for 48 h further reduced cell survival at concentrations of 40 µg/mL or higher. Error bars represent means ± SDs (N = 3). *p < 0.05; **p < 0.01; ***p < 0.001 versus control.
Fig. 8.
Effect of BP/ethanol pretreatment on upregulation of neurogenesis-related genes and homing factors. A–D. Expression levels of SDF1, BDNF, Nurr1, and Brn4 were upregulated following treatment with 20 µg/mL BP for 24 h. E–F. The expression of IL8 and IL6 was not significantly changed. *p < 0.05; **p < 0.01; ***p < 0.001 versus control (0 µg/mL BP).
We further examined these 2 pretreatment methods with DMSO or ethanol as an initial solvent in behavioral studies in our mouse model of PD (Online Supplementary Fig. 1). BP/ethanol pretreatment also significantly improved behavioral outcome during the 22-d recovery period of balancing ability after PD induction, and the level of improvement was similar to that observed for BP/DMSO pretreatment. Other neuronal behavior tests yielded less significant but similar results, with no difference between ethanol and DMSO as a solvent. Our data implied that both DMSO and ethanol could be used as initial solvents in ADSC transplantation therapy for treating PD and that ethanol was a better candidate owing to possible neurotoxicity induced by trace DMSO in the final solution.
Discussion
While there is no cure for slowing or stopping the progression of PD, drugs such as l-DOPA are effective in alleviating symptoms but not without undesired side effects. Cell replacement therapies have been evaluated since the 1970s, and therapies using stem cells of various origins have become a recent focus. Cell transplantations using ESCs or neural stem cells have been the center of many studies38,39. The outcomes of these studies provide insight into the therapeutic capacity of stem cells and encourage the investigation using other stem cell sources and different pretreatment methods. ADSCs become an interesting choice of cell source due to their availability, and several studies in mouse and rat models have shown that ADSCs are capable of providing neuronal regeneration and improve PD symptoms in these animal models40,41.
In our study, we investigated the therapeutic effects of BP treatment and ADSC transplantation on PD. BP-stimulated ADSCs remained viable up to a concentration of 20 to 40 μg/mL. Longer incubation of ADSCs with BP reduced cell viability at higher concentrations; thus, the optimal stable treatment conditions were chosen as 24 h incubation with 20 μg/mL BP.
We further investigated the potential effects of ADSC transplantation with or without BP stimulation on promotion of PD recovery. We showed that ADSCs improved recovery rate of balancing ability in mice with PD. Although the differences were less significant, other motor functions, such as coordination and overall motor activity, were also improved faster during the 21-d recovery period. At 22 d after induction of PD, we found that dopaminergic cells returned to numbers close to those in healthy mice. These results indicated that ADSCs indeed had neuroprotective and neurogenic effects.
Several compounds isolated from A. sinensis have been shown to exert anti-inflammatory and neuroprotective effects, and ligustilide has been shown to have neurogenic effects. In this study, we aimed to determine whether BP could also exert neurogenic effects in ADSCs. Our results of gene expression analysis showed that several neurogenesis-related genes were upregulated after incubation with BP. The concentration at which BP showed the largest neurogenic effects and minimal cell toxicity was 20 μg/mL. We demonstrated that BP-pretreated ADSCs increased behavioral improvements in mice with PD, suggesting that BP stimulation could enhance the therapeutic effects of ADSC transplantation in the treatment of PD. We also provided evidence suggesting that additional i.v. transplantation of ADSCs failed to further improve outcomes. Thus, in our model, intracerebral injection of ADSCs may have achieved the highest recovery possible.
Finally, we showed that 100% ethanol could be used as a less cytotoxic alternative to DMSO. We substituted DMSO with ethanol as initial solvent without affecting cell viability under our experimental conditions. The expression patterns of selected genes did not vary from those with DMSO as solvent. Our behavioral studies also provided similar results regardless of the solvent used.
In summary, we have shown that ADSC transplantation supported the recovery of motor function in our MPTP-induced mouse model of PD. We also demonstrated the potential of BP to stimulate neurogenesis in ADSCs. The therapeutic effects of this treatment could be improved by stimulating ADSCs with BP for 24 h before transplantation. Both ethanol and DMSO could be used as a solvent without affecting outcomes. Our findings provide insights into the establishment of stem cell therapies for brain diseases, such as PD, and may facilitate the development of methods to alleviate symptoms of motor dysfunction.
Supplementary Material
Footnotes
Ethical Approval: The study was performed with approval of the Institutional Review Board of China Medical University and Hospital Research Ethics Committee and the Taiwan Food and Drug Administration (TFDA), Ministry of Health and Welfare, Taiwan. The original protocol was approved by China Medical University and Hospital Research Ethics Committee (CMUH104-REC1-007).
Statement of Human and Animal Rights: This article does not contain any studies with humans; all animal subjects were treated and tested with maximum care and humanitarian effort under the supervision of IACUC, China Medical University, Taiwan.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Paul R. Sanberg (PRS) is the coeditor in chief of Cell Transplantation. Neither PRS nor any of his colleagues were involved in the peer-review process or decision for this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106-TDU-B-212-113004), China Medical University (102426CT), and China Medical University Hospital (DMR-106-075). Confocal microscopy in this study is fully supported by Mr. Ru-Chun Tai of the Instrument Center, Office of Research and Development, China Medical University.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. de Lau LML, Breteler MMB. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. [DOI] [PubMed] [Google Scholar]
- 2. Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18(R1):R48–R59. [DOI] [PubMed] [Google Scholar]
- 3. Greenamyre JT, Hastings TG. Parkinson’s—divergent causes, convergent mechanisms. Science. 2004;304(5674):1120–1122. [DOI] [PubMed] [Google Scholar]
- 4. Oertel W, Schulz JB. Current and experimental treatments of Parkinson disease: a guide for neuroscientists. J Neurochem. 2016:139(suppl 1)325–337. [DOI] [PubMed] [Google Scholar]
- 5. Madrazo I, León V, Torres C, Aguilera MC, Varela G, Alvarez F, Fraga A, Drucker-Colín R, Ostrosky F, Skurovich M. Transplantation of fetal substantia nigra and adrenal medulla to the caudate nucleus in two patients with Parkinson’s disease. N Engl J Med. 1988;318(1):51. [DOI] [PubMed] [Google Scholar]
- 6. Barker RA, de Beaufort I. Scientific and ethical issues related to stem cell research and interventions in neurodegenerative disorders of the brain. Prog Neurobiol. 2013;110:63–73. [DOI] [PubMed] [Google Scholar]
- 7. Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ganat YM, Calder EL, Kriks S, Nelander J, Tu EY, Jia F, Battista D, Harrison N, Parmar M, Tomishima MJ, Rutishauser U, Studer L. Identification of embryonic stem cell-derived midbrain dopaminergic neurons for engraftment. J Clin Invest. 2012;122(8):2928–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5(6):933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin G-Z, Cho S-J, Choi E-G, Lee Y-S, Yu X-F, Choi K-S, Yee S-T, Jeon J-T, Kim M-O, Kong I-K. Rat mesenchymal stem cells increase tyrosine hydroxylase expression and dopamine content in ventral mesencephalic cells in vitro. Cell Biol Int. 2008;32(11):1433–1438. [DOI] [PubMed] [Google Scholar]
- 11. Bouchez G, Sensebé L, Vourc’h P, Garreau L, Bodard S, Rico A, Guilloteau D, Charbord P, Besnard J-C, Chalon S. Partial recovery of dopaminergic pathway after graft of adult mesenchymal stem cells in a rat model of Parkinson’s disease. Neurochem Int. 2008;52(7):1332–1342. [DOI] [PubMed] [Google Scholar]
- 12. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 13. Reid AJ, Sun M, Wiberg M, Downes S, Terenghi G, Kingham PJ. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience. 2011;199:515–522. [DOI] [PubMed] [Google Scholar]
- 14. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trzaska KA, Rameshwar P. Dopaminergic neuronal differentiation protocol for human mesenchymal stem cells In: Vemuri M, Chase LG, Rao MS, eds. Mesenchymal stem cell assays and applications. Totowa, NJ: Humana Press; 2011:295–303. [DOI] [PubMed] [Google Scholar]
- 16. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human Adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galié M, Sbarbati A, Krampera M, Belluzzi O, Bonetti B. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008;17(5):909–916. [DOI] [PubMed] [Google Scholar]
- 18. Zavan B, Vindigni V, Gardin C, D’Avella D, Della Puppa A, Abatangelo G, Cortivo R. Neural potential of adipose stem cells. Discov Med. 2010;10(50):37–43. [PubMed] [Google Scholar]
- 19. Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi HS, Kim HJ, Oh J-H, Park H-G, Ra JC, Chang K-A, Suh Y-H. Therapeutic potentials of human adipose-derived stem cells on the mouse model of Parkinson’s disease. Neurobiol Aging. 2015;36(10):2885–2892. [DOI] [PubMed] [Google Scholar]
- 21. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83(5):723–730. [DOI] [PubMed] [Google Scholar]
- 22. Quinn N, Barker RA, Wenning GK. Are trials of intravascular infusions of autologous mesenchymal stem cells in patients with multiple system atrophy currently justified, and are they effective? Clin Pharmacol Ther. 2008;83(5):663–665. [DOI] [PubMed] [Google Scholar]
- 23. Lee PH, Lee JE, Kim H-S, Song SK, Lee HS, Nam HS, Cheong J-W, Jeong Y, Park H-J, Kim DJ, Nam CM, Lee JD, Kim HO, Sohn YH. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72(1):32–40. [DOI] [PubMed] [Google Scholar]
- 24. Huang SH, Lin CM, Chiang BH. Protective effects of Angelica sinensis extract on amyloid beta-peptide-induced neurotoxicity. Phytomedicine. 2008;15(9):710–721. [DOI] [PubMed] [Google Scholar]
- 25. Yang CL, Or TC, Ho MH, Lau AS. Scientific basis of botanical medicine as alternative remedies for rheumatoid arthritis. Clin Rev Allergy Immunol. 2013;44(3):284–300. [DOI] [PubMed] [Google Scholar]
- 26. Yu Y, Du JR, Wang CY, Qian ZM. Protection against hydrogen peroxide-induced injury by Z-ligustilide in PC12 cells. Exp Brain Res. 2008;184(3):307–312. [DOI] [PubMed] [Google Scholar]
- 27. Yi L, Liang Y, Wu H, Yuan D. The analysis of Radix Angelicae sinensis (Danggui). J Chromatogr A. 2009;1216(11):1991–2001. [DOI] [PubMed] [Google Scholar]
- 28. Chiu S-C, Chen S-P, Huang S-Y, Wang M-J, Lin S-Z, Harn H-J, Pang C-Y. Induction of apoptosis coupled to endoplasmic reticulum stress in human prostate cancer cells by n-butylidenephthalide. PLoS One. 2012;7(3):e33742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yeh JC, Cindrova-Davies T, Belleri M, Morbidelli L, Miller N, Cho CW, Chan K, Wang YT, Luo GA, Ziche M, Presta M, Charnock-Jones DS, Fan TP. The natural compound n-butylidenephthalide derived from the volatile oil of Radix Angelica sinensis inhibits angiogenesis in vitro and in vivo. Angiogenesis. 2011;14(2):187–197. [DOI] [PubMed] [Google Scholar]
- 30. Fu R-H, Hran H-J, Chu C-L, Huang C-M, Liu S-P, Wang Y-C, Lin Y-H, Shyu W-C, Lin S-Z. Lipopolysaccharide-stimulated activation of murine DC2.4 cells is attenuated by n-butylidenephthalide through suppression of the NF-κB pathway. Biotechnol Lett. 2011;33(5):903–910. [DOI] [PubMed] [Google Scholar]
- 31. Chang CY, Chen SM, Lu HE, Lai SM, Lai PS, Shen PW, Chen PY, Shen CI, Harn HJ, Lin SZ, Hwang SM, Su HL. N-butylidenephthalide attenuates Alzheimer’s disease-like cytopathy in down syndrome induced pluripotent stem cell-derived neurons. Sci Rep. 2015;5:8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu RH, Harn HJ, Liu SP, Chen CS, Chang WL, Chen YM, Huang JE, Li RJ, Tsai SY, Hung HS, Shyu WC, Lin SZ, Wang YC. n-Butylidenephthalide protects against dopaminergic neuron degeneration and α-synuclein accumulation in Caenorhabditis elegans models of Parkinson’s disease. PLoS One 2014;9(1):e85305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuang X, Wang L-F, Yu L, Li Y-J, Wang Y-N, He Q, Chen C, Du J-R. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic Biol Med. 2014;71(0):165–175. [DOI] [PubMed] [Google Scholar]
- 34. Xin J, Zhang J, Yang Y, Deng M, Xie X. Radix Angelica sinensis that contains the component Z-ligustilide promotes adult neurogenesis to mediate recovery from cognitive impairment. Curr Neurovasc Res. 2013;10(4):304–315. [DOI] [PubMed] [Google Scholar]
- 35. Bjorklund A, Stenevi U, Schmidt RH, Dunnett SB, Gage FH. Intracerebral grafting of neuronal cell suspensions. II. Survival and growth of nigral cell suspensions implanted in different brain sites. Acta Physiol Scand Suppl. 1983;522:9–18. [PubMed] [Google Scholar]
- 36. Ding D-C, Shyu W-C, Chiang M-F, Lin S-Z, Chang Y-C, Wang H-J, Su C-Y, Li H. Enhancement of neuroplasticity through upregulation of β1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27(3):339–353. [DOI] [PubMed] [Google Scholar]
- 37. Harn HJ, Lin SZ, Hung SH, Subeq YM, Li YS, Syu WS, Ding DC, Lee RP, Hsieh DK, Lin PC, Chiou TW. Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell Transplant. 2012;21(12):2753–2764. [DOI] [PubMed] [Google Scholar]
- 38. Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, Widner H, Rehncrona S, Brundin P, Björklund A, Lindvall O, Limousin P, Quinn N, Foltynie T. Long-term clinical outcome of fetal cell transplantation for Parkinson disease two case reports. JAMA Neurol. 2014;71(1):83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa S-I, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28(1):31–40. [DOI] [PubMed] [Google Scholar]
- 40. Masgutov RF, Masgutova GA, Zhuravleva MN, Salafutdinov II, Mukhametshina RT, Mukhamedshina YO, Lima LM, Reis HJ, Kiyasov AP, Palotás A, Rizvanov AA. Human adipose-derived stem cells stimulate neuroregeneration. Clin Exp Med. 2016;16(3):451–461. [DOI] [PubMed] [Google Scholar]
- 41. Schwerk A, Altschüler J, Roch M, Gossen M, Winter C, Berg J, Kurtz A, Akyüz L, Steiner B. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson’s disease. Regen Med. 2015;10(4):431–446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.