Abstract
Neurogenesis in the adult hippocampus is a unique process in neurobiology that requires functional integration of newly generated neurons, which may disrupt existing hippocampal network connections and consequently loss of established memories. As neurodegenerative diseases characterized by abnormal neurogenesis and memory dysfunctions are increasing, the identification of new anti-aging drugs is required. In adult mice, we found that melatonin, a well-established neurogenic hormone, and the melatonin analog 2-(2-(5-methoxy-1H-indol-3-yl)ethyl)-5-methyl-1,3,4-oxadiazole (IQM316) were able to induce hippocampal neurogenesis, measured by neuronal nuclei (NeuN) and 5-bromo-2′-deoxyuridine (BrdU) labeling. More importantly, only IQM316 administration was able to induce hippocampal neurogenesis while preserving previously acquired memories, assessed with object recognition tests. In vitro studies with embryonic neural stem cells replicated the finding that both melatonin and IQM316 induce direct differentiation of neural precursors without altering their proliferative activity. Furthermore, IQM316 induces differentiation through a mechanism that is not dependent of melatonergic receptors (MTRs), since the MTR antagonist luzindole could not block the IQM316-induced effects. We also found that IQM316 and melatonin modulate mitochondrial DNA copy number and oxidative phosphorylation proteins, while maintaining mitochondrial function as measured by respiratory assays and enzymatic activity. These results uncover a novel pharmacological agent that may be capable of inducing adult hippocampal neurogenesis at a healthy and sustainable rate that preserves recognition memories.
Keywords: adult neurogenesis, long-term memory, melatonin, neural stem cells
Introduction
The brain is capable of generating new neurons every day and throughout lifespan in the process of adult neurogenesis.1 The main neurogenic regions of the brain are the subventricular zone (SVZ) of the lateral ventricles, where new neurons are generated and then migrate to the olfactory bulb to become interneurons; and the subgranular zone (SGZ) in the dentate gyrus (DG) of the hippocampus, where new neurons differentiate and integrate into the local network as dentate granule cells.2,3 Since the hippocampus is a key structure for memory formation, many studies have focused on how new neurons contribute to memory. Hippocampal neurogenesis has been suggested to enhance memory formation by improving pattern separation4,5 and completion6 as well as memory resolution.7 Yet, recent studies found evidence that adult neurogenesis interferes with existing memories, leading to forgetting,8,9 suggesting that adult hippocampal neurogenesis impacts old and new memories differently. Adult hippocampal neurogenesis can be modulated by a variety of physiological and pathological factors.10 An enriched environment and physical exercise have been shown to promote neurogenesis and are associated with improved performance on hippocampus-dependent tasks.11,12 In contrast, acute and chronic diseases, and genetic or epigenetic mutations, reduce adult neurogenesis and are associated with impaired performance on tests of hippocampus-dependent learning and memory.12–15 Extracellular signaling molecules have also been shown to regulate neurogenesis in the adult DG including γ-Aminobutyric acid (GABA), WNT, insulin growth factor (IGF), and the neurohormone melatonin.2,3
Melatonin (N-acetyl-5-methoxytryptamine) was first discovered as the hormone of the pineal gland and has now been shown to be produced by many organs.16 It has pleiotropic neurobiological functions (reviewed in Hardeland et al.17) including modulation of adult hippocampal neurogenesis and mitochondrial function. Moreover, melatonin modulates proliferation in the DG of early postnatal rats,18 promotes neurogenesis in the DG of pinealectomized rats,19 and enhances cell survival and dendrite maturation of new neurons in the hippocampus of adult mice.20,21 In vitro studies also confirmed that melatonin influenced proliferation and differentiation of embryonic and adult rat midbrain neural stem cells (NSCs).22,23 Due to the wide array of functions that melatonin displays,24 pharmaceutical research has focused on the development of new agents derived from melatonin.25 Recently, we developed a new family of melatonin-based compounds in which the acetamido group of melatonin was replaced by several bioisosteric groups and we tested their neurogenic effects in vitro.26 Among these melatonin derivatives, 2-(2-(5-methoxy-1H-indol-3-yl)ethyl)-5-methyl-1,3,4-oxadiazole (IQM316) was chosen for further preclinical studies, namely, modulation of adult neurogenesis, recognition memory, and mitochondrial function, to delineate its therapeutic usefulness.
Materials and Methods
Materials
5-Bromo-2′-deoxyuridine (BrdU), dimethyl sulfoxide (DMSO), free fatty acid bovine serum albumin (BSA), poly-d-Lysine (PDL), laminin, 4′,6-diamidino-2-phenylindole (DAPI), all-trans-retinoic acid, melatonin, and luzindole were purchased from Sigma-Aldrich (Madrid, Spain). IQM316 was synthesized as previously described26 (IQM316 is referred as compound 16).
Animals
The 3-mo-old C57BL/6j male mice were obtained from Janvier (France). A total of 48 mice were divided into 6 groups (8 mice per group). They were housed in standard laboratory cages under 12-h light/12-h dark cycles. The animals had access to food and water ad libitum. All animals were handled and cared for according to the Council Directive 2010/63/UE of September 22, 2010.
Treatments
IQM316 and melatonin stock solutions were initially dissolved in a minimum amount of DMSO. Working solutions at a concentration of 2 mg/kg body weight were prepared by diluting stock solutions in phosphate buffered saline (PBS) (pH 7.2). The final volume of DMSO in working solutions was less than 1%. Vehicle solution was prepared with an equivalent amount of the PBS/DMSO fluid only. Tubes containing the solutions were wrapped in aluminum foil to prevent light-induced degradation.
Animals were injected intraperitoneally (i.p.) with IQM316, melatonin, or vehicle at zeitgeber time ZT11 (ZT0 corresponds to the beginning of the light phase of the daily cycle). For acute treatment, animals were administered 2 mg/kg body weight of either IQM316 or melatonin or vehicle solution for 7 d, whereas for chronic treatment, animals were administered for 28 d. The thymidine analog BrdU (50 mg/kg body weight) was also injected i.p. daily for the first 7 d. All animals were sacrificed 29 d after the beginning of treatment.
Novel Object Recognition Test
The novel object recognition test is based on the innate tendency of rodents to explore novel objects over familiar ones; thus, a rodent that remembers the familiar object will spend more time exploring the novel object. Mice were placed into an open-field (OF) box consisting of a quadrangular area (60 cm wide × 60 cm long × 60 cm high) and were habituated for 15 min. The next day, the training trial (familiarization phase) was performed by placing the animals in the same OF box with the first object (object A; yellow marble) for a period of 10 min. The testing trial (the test phase) was performed either 3 or 24 h later, by including a novel object (object B, red dice) placed together with the first object (object A), and the animals were left in the OF box for 10 min. The exploration of the objects was considered when sniffing or deliberate contact occurred with the objects or when the animal’s snout was directed toward the object at a distance <1 cm. The exploration time for the familiar (TF) or the novel object (TN) during the test phase was recorded by hand by an observer blind to the treatment status of the mice, and the discrimination index (DI) was calculated as the difference in exploration time between novel and familiar objects, dividing this value by the total amount of exploration of both objects, DI = (TN − TF)/(TN + TF).27
Tissue Preparation
After completion of the novel object recognition test, animals were deeply anesthetized with isoflurane, transcardially perfused with 0.9% saline, and brains were immediately removed. One hemisphere of the brain fixed in phosphate-buffered 4% paraformaldehyde, pH 7.4, at 4 °C for immunohistochemical studies. The hippocampus from the other hemisphere was dissected and immediately stored at −80 °C until its use for further analysis.
Immunohistochemistry and Imaging
Immunohistochemistry for BrdU and NeuN was done on free-floating sections. Briefly, fixed brains were cut on a vibratome (Leica Microsystems) at 30 µm, and tissue sections were collected in cold phosphate buffer (PB) (0.1 M). DNA was denatured by incubation with 2 N HCl for 30 min at room temperature (RT). Nonspecific staining was blocked with 10% horse serum and 0.5% Triton X-100 in (0.1 M PB) for 1 h at RT and then incubated overnight with primary antibodies at 4 °C. Primary antibodies were mouse anti-BrdU (1:15,000, Hybridoma Bank, Iowa City, IA, USA) and rabbit anti-NeuN (1:1,000, Merck Millipore, Darmstadt, Germany). Then, secondary fluorescent antibodies Donkey Anti-Mouse Alexa Fluor 488 Conjugate and Goat Anti-Mouse and Goat Anti-Rabbit Texas Red Conjugate (both 1:1,000, Thermo Fisher Scientific, Madrid, Spain) were incubated for 1 h at RT. Sections were mounted with FluorSave reagent (Merck Millipore) onto gelatinized slides. The estimated total number of BrdU-positive cells or BrdU/NeuN-positive cells per granule cell layer of the DG was counted in a 1-in-6 series of sections (180 μm apart) from each mouse. The resulting numbers were multiplied by 6. The entire DG was scanned under a Zeiss confocal microscope at a magnification of 40× and Z-stacks were acquired to confirm the double-positive cells, as previously described.28
RNA, DNA, and Protein Extraction
Mouse hippocampi were homogenized in TRIzol (Thermo Fisher Scientific) and total RNA, DNA, and protein were extracted according to the manufacturer’s instructions. RNA integrity was verified by 260/280 optical density ratios. DNA was further precipitated by sodium acetate/ethanol, and DNA integrity was verified by 260/280 optical density ratios. For increased efficiency of protein extraction, the last step of protein elution was slightly modified as described in Simoes et al.’s study.29 Briefly, protein pellets from TRIzol extraction were solubilized in 1% SDS/8 M urea, sonicated 5 times for 15 s, with 30 s pauses in ice and centrifuged at 3,200g for 10 min. Protein concentration was measured using the Pierce BCA Protein Assay Kit (Thermo Fisher) and 6-fold dilutions to prevent BCA reaction inhibition by urea and SDS. NSCs cultured in an adherent monolayer under differentiation for 12 d were homogenized in TRIzol, and total RNA was extracted according to the manufacturer’s instructions.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Mitochondrial DNA (mtDNA) was quantified in triplicates using 1 ng of DNA extracted from the hippocampus and performed with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Madrid, Spain). mtDNA was amplified using primers for murine 12S ribosomal RNA gene (mtDNA), whereas nuclear DNA (nDNA) was amplified using primers for murine 18S ribosomal RNA gene.30 The values of mtDNA levels were normalized by nDNA, and the data expressed relative to vehicle-treated mice.
Mitochondrial biogenesis genes RT-qPCR was run in triplicates using 50 ng of RNA extracted from the hippocampus and performed with an iTaq Universal SYBR Green One-Step Kit (Bio-Rad). The mRNA expression levels were normalized using the hypoxanthine guanine phosphoribosyltransferase gene as the reference gene.
The relative expression levels were calculated with the 2−ΔΔCT equation.31 Calibrators consisting of standard controls were run in the same reaction to verify amplification efficiencies of each experiment as well as melting curve analysis to confirm the specificity of amplification and lack of primer dimers. All real-time PCR was performed in a LightCycler 480 II (Roche Life Science, Barcelona, Spain) using the primers listed in Table 1.
Table 1.
Primers Used for Real-Time Polymerase Chain Reaction.
| Gene | Accession Number | Sequence (5′-3′) |
|---|---|---|
| 12S Ribosomal RNA | NC_005089.1 | CTAGCCACACCCCCACGGGA |
| CGTATGACCGCGGTGGCTGG | ||
| 18S Ribosomal RNA | NR_003278.3 | ACCTGGTTGATCCTGCCA |
| GCCATTCGCAGTTTCACTGT | ||
| PGC-1α | NM_008904.2 | TCTCAGTAAGGGGCTGGTTG |
| AGCAGCACACTCTATGTCACTC | ||
| Nrf1 | NM_001164226.1 | CATGGGCGGGAGGATCTTTT |
| TACCAACCTGGATGAGCGAC | ||
| Tfam | NM_009360.4 | GGGAATGTGGAGCGTGCTAA |
| GACAAGACTGATAGACGAGGGG | ||
| Hprt | NM_013556.2 | GTTGGGCTTACCTCACTGCT |
| TAATCACGACGCTGGGACTG | ||
| Dcx | NM_001110222.1 | TCGTAGTTTTGATGCGTTGCT |
| GCTTTCCCCTTCTTCCAGTTC | ||
| NeuN | NM_001039167.1 | GCGTTCCCACCACTCTCTTG |
| TAGCCTCCATAAATCTCAGCACC |
Western Blot
Protein samples were separated by polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore). Blots were blocked with 5% nonfat dry milk in tris-buffered saline containing 0.05% Tween-20 (TBST) for 1 h and then incubated with primary antibodies. The following antibodies were used: mouse anti-MTCO1 (1:2,000, Abcam, Cambridge, UK), mouse anti-complex IV subunit (COX IV; 1:2,000, Abcam), mouse anti-ATPB (1:2,000, Abcam), Total Oxidative Phosphorylation (OXPHOS) Human WB Antibody Cocktail (1:200, Abcam), mouse anti-VDAC1/Porin (1:1,000, Abcam). Peroxidase-conjugated goat antimouse IgG (1:10,000, Bio-Rad) and antirabbit IgG (1:5,000, Bio-Rad) were used as secondary antibodies. To control for the amount of protein loaded, we used a mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-GAPDH-loading control antibody (1:10,000, Abcam). Immunoreactive proteins were visualized using an enhanced chemiluminescence detection system (Clarity ECL, Bio-Rad), and bands were detected with an ImageQuant LAS 4000 Imaging System (GE Healthcare Life Sciences, Barcelona, Spain).
Isolation of Functional Hippocampal Mitochondria
Functional mitochondria for respiratory measurements were isolated from the hippocampus using “method C” described in Sims and Anderson’s study.32 The mitochondrial pellet was gently resuspended in respiration medium (see below) with 1% fatty acid free-BSA and kept on ice for up to 2 h until the experiments were performed. The hippocampus from 3 mice in a given group was combined for a single homogenate and then assayed in triplicate.
Measurement of Mitochondrial Respiratory Activity
Oxygen consumption was measured polarographically at 25 °C using 1.0 to 2.5 mg protein from the hippocampal mitochondrial fraction in respiration medium consisting of 20 mM KCl, 5 mM MgCl2, 10 mM KH2PO4, 10 mM tris–HCl, 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 225 mM sucrose, at pH 7.4, using a Clark-type electrode. Five mM glutamate and malate were added as the respiratory substrates, and the mitochondrial respiration was initiated by adding 50 nmol adenosine-5-diphospate (ADP). Oxygen consumption measured in the presence of added ADP was defined as state III respiration, while that measured following the consumption of ADP was defined as state IV respiration. The respiration control ratio (RCR) was calculated as the ratio of state III respiration to state IV respiration and used as a marker of mitochondrial respiratory activity. The ADP/O ratio was calculated as the ratio of the added ADP concentration to the consumption of oxygen during state III respiration. Mitochondrial respiration was calculated as nanomoles of O2 per min per milligram of protein. Mitochondrial protein was determined by the Pierce BCA Protein Assay Kit using BSA as standard and 10-fold dilutions to prevent reaction inhibition.
Determination of Mitochondrial Complex Activities
After sacrifice, mouse hippocampi were immediately removed, excised, washed with cold saline, and processed as described for mitochondrial preparation.33 The activities of mitochondrial complexes II, III, and IV were measured on mitochondrial pellets as previously described.34
Mouse NSC Culture
Neurospheres isolated from mouse embryonic day 14 cortices (Stem Cell Technologies, Grenoble, France) were expanded in adherent monolayer cultures. NSCs were plated on PDL (100 µg/mL)/laminin (10 µg/mL)-coated tissue culture dishes and cultured in proliferation medium: Neurobasal medium supplemented with B27 and Glutamax (Thermo Fisher Scientific), containing 20 ng/mL of human epidermal growth factor (EGF) and 20 ng/mL of human basic fibroblast growth factor (hbFGF; PeproTech, London, UK). Half of the medium was changed every 2 to 3 d with fresh medium and growth factors added to a final concentration of 20 ng/mL each. Cells were maintained in a humidified incubator at 37 °C and 5% CO2.
Cell Proliferation Assays
For proliferation studies, NSCs were plated at a density of 2 × 104 cells/cm2 in adherent monolayer and treated for 48 h with different concentrations of IQM316, melatonin, or vehicle. Cell proliferation was measured with the Cell Proliferation Kit II (XTT; Roche Life Science) according to the manufacturer’s instructions. Cell proliferation was also measured by DAPI staining and cells were counted using an inverted microscope with a 20× objective. All measurements were from 3 independent experiments and performed in duplicate.
Cell Differentiation Assays
For differentiation studies, NSCs were plated on PDL (100 µg/mL)/laminin (15 µg/mL)-coated tissue culture dishes at a density of 4 × 104 cells/cm2 and maintained in proliferation medium. After 48 h, differentiation was initiated by switching the medium to Neurobasal medium supplemented with B27 and Glutamax, containing 5 ng/mL of hbFGF and 0.5 µM all-trans-retinoic acid. After 48 h (differentiation day 2), the medium was changed to Neurobasal medium supplemented with B27 and Glutamax (no growth factors). Half of the medium was changed every 2 to 3 d until day 12 of differentiation. To study the effect of IQM316, melatonin, or vehicle on differentiation, different concentrations were added to the medium and replaced every 2 to 3 d.
Immunocytochemistry and Imaging
After 12 d of differentiation, NSCs were briefly rinsed in PBS and fixed in 4% paraformaldehyde (PFA) at 4 °C for 20 min. Cells were permeabilized in 0.3% Triton X-100 for 30 min and blocked for in 1% BSA/PBS for 1 h at RT. Incubation with primary antibody goat anti-Doublecortin (1:250, Santa Cruz, Sc-8066) diluted in 0.1% BSA/0.2% Triton X-100 was performed overnight at 4 °C. The secondary antibody Alexa Fluor antigoat-555 (1:1,000; Thermo Fisher Scientific) was incubated for 1 h at RT followed by DAPI staining (1:20,000) for 10 min. Coverslips were mounted on glass slides using FluorSave reagent (Merck Millipore). Images were acquired using a 63 oil immersion objective (numerical aperture 1.4) on a Zeiss confocal microscope. The number and length of neuronal processes were measured using the NeuronJ plugin of ImageJ (ImageScience).
Statistical Analysis
Results are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software Inc.). The statistical significance of differences among multiple groups was assessed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests.
Results
Effect of IQM316 and Melatonin on Adult Hippocampal Neurogenesis
We first tested whether melatonin or IQM316 administration at a concentration of 2 mg/kg body weight was able to induce adult neurogenesis in vivo. Adult mice were administered i.p. with either IQM316 or melatonin daily for 7 (acute) or 28 (chronic) d, and brains were examined 29 d after the first injection. New neurons that were able to differentiate into their mature phenotypes were identified by BrdU and NeuN double labeling. Acute IQM316 administration significantly increased the number of BrdU+/NeuN+ cells per DG section when compared to vehicle-treated mice (∼349%, P < 0.001; Fig. 1A; from 150.0 ± 20.5 to 577.5 ± 84.0 in the entire DG, ∼385%). Acute melatonin administration also increased significantly the number of BrdU+/NeuN+ cells per DG section when compared to vehicle-treated mice (∼243%, P < 0.001; Fig. 1A; from 150.0 ± 20.5 to 465.0 ± 37.8 in the entire DG, ∼310%). When comparing IQM316 and melatonin, IQM316-treated mice showed a significantly higher number of BrdU+/NeuN+ cells per DG section (P < 0.01). Representative photomicrographs are shown in Fig. 1B. Chronic IQM316 administration also increased significantly the number of BrdU+/NeuN+ cells per DG section (∼259%, P < 0.001; from 214.3 ± 39.0 to 480.0 ± 78.6 in the entire DG, ∼224%) as well as chronic melatonin administration (∼194%, P < 0.01; from 214.3 ± 39.0 to 437.1 ± 42.9 in the entire DG, ∼204%) when compared to vehicle-treated mice (Fig. 1C). When comparing IQM316 and melatonin, although IQM316-treated mice showed a higher number of BrdU+/NeuN+ cells, this difference did not reach statistical significance (P = 0.06), indicating that they have similar chronic efficacies. These data show that both IQM316 and melatonin administrations promote adult hippocampal neurogenesis by stimulating neuronal differentiation.
Fig. 1.
Effect of acute and chronic 2-(2-(5-methoxy-1H-indol-3-yl)ethyl)-5-methyl-1,3,4-oxadiazole (IQM316) or melatonin administration on adult hippocampal neurogenesis. Animals were treated with vehicle (Vhc), IQM316 (IQM), or melatonin (Mel) for 7 (acute) or 28 d (chronic). (A) Quantification of the percentage of 5-bromo-2′-deoxyuridine (BrdU) and neuronal nuclei (NeuN) double-labeling positive cells per dentate gyrus (DG) section upon acute administration. Both IQM316 and melatonin acute administrations induce neurogenesis, with being IQM316 more potent than melatonin. (B) Representative confocal images of BrdU (green) and NeuN (red) immunohistochemistry of the DG quantified in A. BrdU and NeuN double labeling is shown in yellow (merged images). Higher magnification insets of double labeled neurons are displayed on the right. Scale bar is 25 µm. (C) Quantification of the percentage of BrdU and NeuN double-labeling positive cells per DG section upon chronic administration. Both IQM316 and melatonin chronic administrations induce neurogenesis with similar efficacies. Data are mean ± SEM, n = 7 animals per group. **P < 0.01, ***P < 0.001, significantly different from vehicle, Bonferroni post hoc test.
Effect of IQM316 and Melatonin on Memory
Recent reports have shown that hippocampal neurogenesis in adulthood induces loss of established memories.8,9 Therefore, we tested whether neurogenesis induced by chronic administration of either IQM316 or melatonin could interfere with existing memories. We performed the novel object recognition test at the beginning (day 1) and at the end of treatment (day 29; Fig. 2A), this is, prior to the commencement of treatment stimulating neurogenesis. We found that the DI at day 29 of IQM316-treated mice was significantly increased (∼2 fold) when compared to the vehicle-treated group (0.51 ± 0.07 vs. 0.22 ± 0.06; P < 0.05; Fig. 2B), indicating that they recalled the familiar object and therefore explored the novel object more. The DI of melatonin-treated mice was slightly increased when compared to vehicle (0.39 ± 0.04 vs. 0.22 ± 0.06), but it did not reach statistical significance. Then, we analyzed the ratio of time spent exploring the object during the familiarization phase at day 29 comparing it to day 1, and we did not find any differences between the vehicle and melatonin-treated mice (ratio: 1.18 ± 0.17 and 0.87 ± 0.15, respectively; Fig. 2C). In contrast, IQM316-treated mice had a significantly smaller ratio when compared to the vehicle-treated mice (0.46 ± 0.07 vs. 1.18 ± 0.17; P < 0.001), indicating that they spent approximately half of the time exploring the object at day 29 (Fig. 2C). The average time spent exploring the objects was not different across treatments (vehicle vs. IQM316 vs. melatonin; P > 0.05), indicating that the treatments did not alter exploratory interest. These data show that IQM316-treated mice were able to recall the object from day 1. Taken together, these results suggest that adult neurogenesis induced by IQM316 occurs at a sustainable and healthy rate, allowing for newly generated neurons to integrate into the hippocampus without disrupting previously acquired memories.
Fig. 2.
Effect of acute and chronic 2-(2-(5-methoxy-1H-indol-3-yl)ethyl)-5-methyl-1,3,4-oxadiazole (IQM316) or melatonin administration on long-term recognition memory. Animals were treated with vehicle (Vhc), IQM316 (IQM), or melatonin (Mel) for 7 (Acute) or 28 d (Chronic). (A) Experimental design of drug administration and novel object recognition test analyzed in B and C. (B) Discrimination index (DI) quantified at day 29. Chronic IQM316 administration increased the DI, indicating that the animals recalled the object, whereas Fig. 2. (continued). melatonin administration did not alter it (C) Ratio of object exploration time as day 29 versus day 1, during the familiarization phase. IQM316-treated mice spent less time exploring at day 29, suggesting that they recalled the object from day 1. Melatonin-treated mice displayed similar exploration times at both days 1 and 29. (D) Experimental design of drug administration and novel object recognition test analyzed in E. (E) DI quantified at day 29, after acute and chronic administration. Neither acute nor chronic administration of IQM316 or melatonin modified short-term recognition memory. Data are mean ± SEM, n = 8 animals per group. *P < 0.05, **P < 0.01, significantly different from vehicle, Bonferroni post hoc test.
We also performed the novel object recognition test, involving a retention interval of 24 h between the familiarization and testing phase, a time frame typically used to evaluate rodent long-term memory, at day 29 (Fig. 2D) to study newly acquired memories (after neurogenesis has occurred). There were no significant differences in the DI between vehicle-treated mice and IQM316 or melatonin, in either acute (0.32 ± 0.06 vs. 0.39 ± 0.05 or 0.34 ± 0.09, respectively) nor chronic treatments (0.43 ± 0.07 vs. 0.41 ± 0.10 or 0.46 ± 0.08, respectively; Fig. 2E). These data indicate that neither IQM316 nor melatonin administration (acute or chronic) alter long-term memory of newly acquired memories.
Effect of IQM316 and Melatonin on Mitochondria
Recent studies have shown that mitochondrial function is important in neuroplasticity, including neural proliferation and differentiation.35–37 Given that mitochondrial function has been shown to be regulated by melatonin (reviewed by Hardeland et al.17), we studied the effect of IQM316 and melatonin on hippocampal mitochondrial function.
Effect on OXPHOS Proteins
We measured the levels of OXPHOS proteins in the hippocampus by Western blot analysis. We found that when compared to vehicle, acute administration of both IQM316 and melatonin significantly increased the protein levels of succinate dehydrogenase complex, subunit B of complex II (SDHB; 192 ± 19%, P < 0.001 and 178 ± 16%, P < 0.05, respectively) and cytochrome c oxidase I, a COX I (181 ± 19%, P < 0.01 and 191 ± 34%, P < 0.05, respectively). However, IQM316 and melatonin administrations significantly reduced protein levels of COX IV (68 ± 10% and 61 ± 10%, P < 0.05, respectively) and ATP-5β (64 ± 6% and 53 ± 5%, P < 0.001, respectively; Fig. 3A and B). On the contrary, chronic IQM316 or melatonin administration significantly increased the protein levels of NDUFB8 when compared to the vehicle (177 ± 20%, P < 0.01, and 159 ± 12%, P < 0.05, respectively; Fig. 3C and D).
Fig. 3.
Effect of acute and chronic 2-(2-(5-methoxy-1H-indol-3-yl)ethyl)-5-methyl-1,3,4-oxadiazole (IQM316) or melatonin administration on hippocampal oxidative phosphorylation (OXPHOS) protein levels. Animals were treated with vehicle (Vhc), IQM316 (IQM), or melatonin (Mel). Quantitative analysis of the effect of acute (A) and chronic (C) administrations on hippocampal OXPHOS protein levels. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used for normalization. Representative Western blots for acute (B) and chronic (D) administrations quantified in A and C, respectively. Acute administration of either IQM316 or melatonin significantly increased subunit B of complex II and complex I subunit (COX I) protein levels but reduced COX IV and ATP-5β. Nonetheless, chronic IQM316 or melatonin administration only increased the protein levels of NDUFB8. Data are mean ± SEM, n = 8 animals per group. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from vehicle, Bonferroni post hoc test.
Effect on mtDNA Copy Number
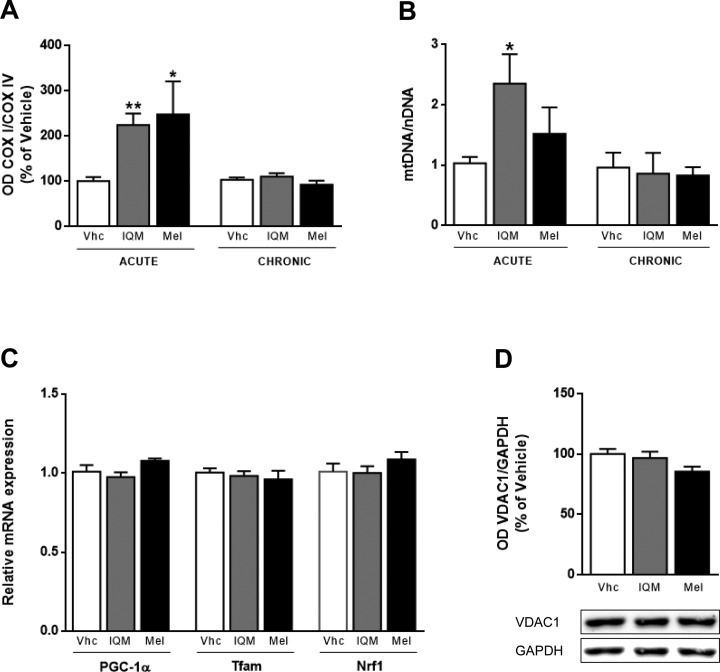
We analyzed the COX I/COX IV ratio and found that it was significantly increased by IQM316 (224 ± 25%, P < 0.01) and melatonin (247 ± 73%, P < 0.05) acute administration when compared to vehicle-treated mice (Fig. 4A). Chronic administration did not alter the COX I/COX IV ratio (Fig. 4A). Since COX I is an mtDNA-encoded subunit whereas COX IV is an nDNA-encoded subunit, these data suggest that IQM316 and melatonin acute administration activates mtDNA replication or translation. So, we next quantified the mtDNA copy number by quantitative PCR, in DNA extracted from mouse hippocampi IQM316 acute administration significantly increased by 228% the mtDNA copy number when compared to the vehicle (2.35 ± 0.48 vs. 1.03 ± 0.10, P < 0.05). Acute melatonin administration slightly increased the ratio when compared to vehicle (1.52 ± 0.44 vs. 1.03 ± 0.10), but it did not reach statistical significance (Fig. 4B). Chronic administration of either compound did not alter the mtDNA/nDNA ratio when compared to the control (Fig. 4B).
Fig. 4.
Effect of acute 2-(2-(5-methoxy-1H-indol-3-yl)ethyl)-5-methyl-1,3,4-oxadiazole (IQM316) or melatonin administration on mitochondrial biogenesis. Animals were treated with vehicle (Vhc) or IQM316 (IQM) or melatonin (Mel). (A) Quantitative analysis of the complex I subunit (COX I)/COX IV protein level ratio upon acute and chronic administrations. Acute administration of either IQM316 or melatonin increased the COXI/COXIV ratio, suggesting activation of mitochondrial DNA replication or translation. Chronic administration had no effect on the ratio. (B) Quantification of the mitochondrial DNA/nuclear DNA ratio upon acute and chronic administrations. Acute administrations increased the ratio, whereas chronic administrations did not. (C) Quantification of relative mRNA expression levels of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α), mitochondrial transcription factor A (Tfam), and nuclear respiratory factor 1 (NRF-1) upon acute administration. Expression levels were normalized to hypoxanthine guanine phosphoribosyltransferase and relative to vehicle. Acute administration of either compound did not activate the expression mitochondrial biogenesis genes. (D) Quantitative analysis and representative Western blots of Voltage-dependent anion-selective channel 1 (VDAC1) protein levels upon acute administration. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the normalization control. Acute administration of either compounds did not alter the mitochondrial protein levels, indicating that mitochondrial biogenesis was not affected. Data are mean ± SEM, n = 8 animals per group. *P < 0.05, **P < 0.01, significantly different from vehicle, Bonferroni post hoc test.
Effect on Mitochondrial Biogenesis
To ascertain whether the increase in mtDNA copy number was due to activation of mitochondrial biogenesis, we measured mRNA levels of key players of this process: the peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), nuclear respiratory factor 1 (Nrf1), and mitochondrial transcription factor A (Tfam).38 We found that acute administration of either compound did not alter the expression of PGC-1α, nor Tfam, or Nrf1 (Fig. 4C), indicating that mitochondrial biogenesis was not activated at the time point studied. We also measured the voltage-dependent anion-selective channel protein 1 (VDAC1) levels, an outer mitochondrial membrane protein. We did not find any changes on VDAC1 levels after IQM316 or melatonin acute administration (Fig. 4D), indicating that mitochondrial biogenesis was not changed.
Effect on Mitochondrial Respiratory Activity
In order to evaluate whether the change on the levels of OXPHOS proteins (Fig. 3) affected mitochondrial function, we measured the activities of respiratory complexes II, III, and IV of the mitochondrial respiratory chain in isolated hippocampal mitochondria fractions obtained from mice administered acute and chronically with each compound (data not shown). We did not observe any significant changes in the activity of these complexes meaning that despite the change in the proteins levels, overall the complexes remained active.
Additionally, we performed respiratory measurements and found that state III respiration and state IV respiration levels remained unchanged between IQM316 and melatonin and vehicle-treated mice. Furthermore, no significant differences between treatments were observed in the RCR meaning that the tightness of the coupling between respiration and phosphorylation was not altered. Also, the ADP/O ratio which measures the efficiency of the mitochondrial phosphorylative system remained unchanged between treatments (data not shown).
Effect of IQM316 and Melatonin on NSCs
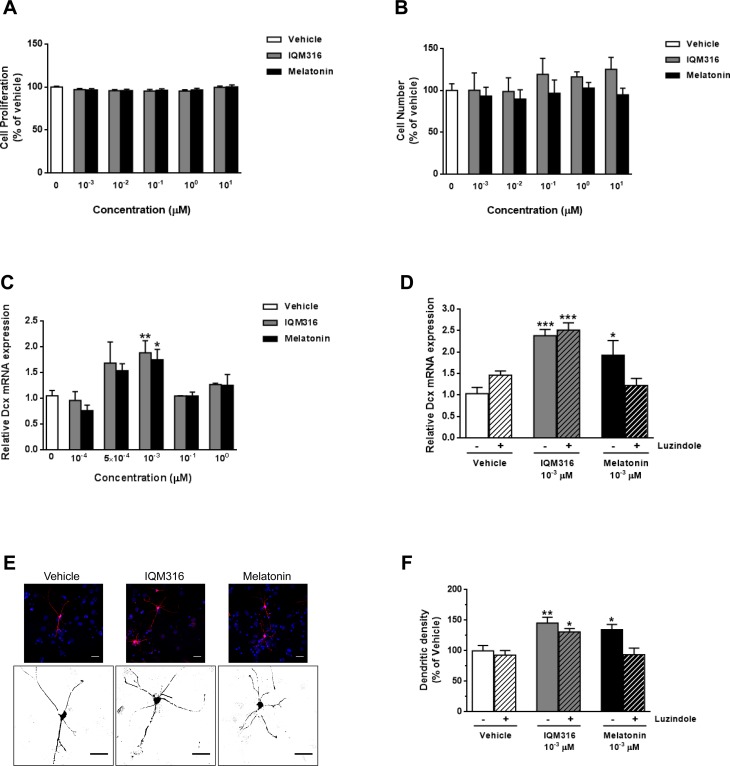
We performed in vitro studies using mouse embryonic NSCs to study the influence of IQM316 or melatonin on cell proliferation and differentiation. First, under proliferating conditions (in the presence of growth factors) we performed a dose–response curve (101, 100, 10−1, 10−2, and 10−3 μM) for both compounds. Measurement of cell proliferation using a XTT-based kit showed that neither compound altered cell proliferation (Fig. 5A) at the concentrations tested. These results were further confirmed by DAPI staining and cell counting (Fig. 5B). Next, in order to evaluate the neurogenic potential of each compound, we performed a dose–response curve (100, 10−1, 10−3, 5 × 10−4, and 10−4 μM) for both compounds under differentiation conditions and measured the relative mRNA levels of doublecortin (Dcx), a marker for immature neurons, and NeuN, a marker for mature neurons. We found that at a 10−3 µM concentration, IQM316 and melatonin were able to significantly increase Dcx mRNA expression when compared to the vehicle (from 1.05 ± 0.10 to 1.88 ± 0.23, P < 0.01 and 1.75 ± 0.20, P < 0.05, respectively; Fig. 5C). However, NeuN mRNA expression remained unaltered for all concentrations tested (data not shown). To ascertain whether the neurogenic effect of IQM316 depended on melatonergic receptors (MTRs) MT1 and MT2, we induced differentiation of NSCs with vehicle, or 10−3 µM IQM316 or 10−3 µM melatonin, in the presence or absence of the MTR antagonist luzindole (0.2 µM). We found that luzindole was able to completely block the increase in Dcx mRNA expression induced by melatonin, whereas the IQM316-induced increase in Dcx was not altered, when compared to vehicle-treated cells (Fig. 5D). Finally, we performed immunocytochemistry to label NSCs that were able to differentiate into Dcx+ expressing cells, in the presence of vehicle, or 10−3 µM IQM316 or 10−3 µM melatonin (Fig. 5E). When analyzing morphological features of Dcx+ cells, we found that both IQM316 and melatonin significantly increased the number of dendrites when compared to the vehicle (from 100.0 ± 8.5 to 145.2 ± 9.5, P < 0.01 and 134.6 ± 8.5, P < 0.05, respectively; Fig. 5F). In agreement with the previous data, luzindole completely blocked the effect of melatonin, whereas the IQM316-induced increase in dendritic density was not blocked (Fig. 5F). Other features such as dendritic length, axonal length, and number of axonal branches were not modulated (data not shown). Taken together, these results reinforce the finding that both IQM316 and melatonin induce direct differentiation of neural precursor cells without affecting cell proliferation and more importantly that IQM316 is neurogenic through a melatonin receptors MT1 and MT2 independent pathway.
Fig. 5.
Effect of 2-(2-(5-methoxy-1H-indol-3-yl)ethyl)-5-methyl-1,3,4-oxadiazole (IQM316) or melatonin on embryonic neural stem cell (NSC) proliferation and differentiation. (A, B) Dose–response effect of IQM316 or melatonin on cell proliferation, showing no effect of either compound. (A) Cell proliferation quantified by XTT assay. (B) Cell proliferation quantified by 4′,6-diamidino-2-phenylindole (DAPI) staining and cell counting. (C, D) Quantification of doublecortin (Dcx) relative mRNA expression levels under differentiation conditions. Expression levels are normalized to hypoxanthine guanine phosphoribosyltransferase and relative to the vehicle. (C) Dose–response effect of IQM316 or melatonin on Dcx relative mRNA expression levels. Both IQM316 and melatonin treatments increase Dcx mRNA levels, indicating activation of neuronal differentiation and therefore neurogenic potential. (D) Cells were differentiated and treated with either IQM316 or melatonin 10−3 µM, in the presence or absence of luzindole (0.2 µM). The MT1 and MT2 antagonist luzindole was able to fully block neuronal differentiation induced by melatonin but did not block the effect of IQM316. (E) Representative confocal images of Dcx (red) and DAPI (blue) immunocytochemistry, with higher inset below showing the detailed morphology (Dcx, black) of NSCs differentiated in the presence of vehicle, IQM316 or melatonin 10–3 µM. Scale bar is 20 µm. (F) Cells under the same conditions as described in D. Quantification of dendritic density. NSCs differentiated in the presence of either IQM316 or melatonin display a higher number of dendritic processes. The effect of melatonin is completely blocked by luzindole, whereas the IQM316-induced effect is not. IQM316 induces neuronal differentiation independently of melatonergic receptors MT1 and MT2. Data are mean ± SEM, n = 3 independent experiments. *P< 0.05, **P < 0.01, ***P < 0.001, significantly different from vehicle, Bonferroni post hoc test.
Discussion
The present study demonstrates that melatonin and the melatonin analog, IQM316, increase neurogenesis in the DG of healthy adult mice. Melatonin has been previously shown to promote adult hippocampal neurogenesis, but usually at a range of 8 to 10 mg/kg.18–21 Here, we used C57BL/6J mice, which are considered to be “melatonin depleted” due to the low level of pineal melatonin synthesis when compared to other mouse strains39,40 and lower drug concentrations, (2 mg/kg). The finding that both acute (7 d) and chronic (28 d) administrations were able to significantly increase the number of BrdU and NeuN double-labeled positive cells on the DG, a marker of adult neurogenesis, indicates that IQM316 and melatonin stimulate precursor cells to differentiate into neurons. It is worth noting that acute IQM316 administration was more potent than melatonin, whereas chronic administration of either compound displayed similar efficacies. These data suggest that IQM316 and melatonin induce neurogenesis through different mechanisms, since at a short period (acute −7 d) IQM316 is more potent and at a longer period (chronic −28 d), they are similar.
Consistent with in vivo results, proliferation and differentiation studies using NSCs showed that IQM316 and melatonin induce direct differentiation of neural precursors without altering their proliferative activity. We found that at the 10−3 µM concentration, both IQM316 and melatonin significantly increased Dcx mRNA expression. Our results are in agreement with previous studies that found that physiological concentrations of melatonin did not affect proliferation of embryonic NSCs but enhanced neuronal differentiation.20,22,23 During neuronal differentiation, neuroblasts committed to the neuronal lineage express Dcx but as neuronal maturation occurs, Dcx expression is downregulated as NeuN is upregulated.41,42 Therefore, we also measured NeuN mRNA levels to ascertain the level of neuronal maturation but found no significant differences between treatments. This suggests that at the time point studied (12 d of in vitro differentiation), NSC differentiation induced by either IQM316 or melatonin, only reached the stage of immature neurons.
Adult neurogenesis can be considered a unique form of circuit plasticity. The hippocampus is able to adapt to environmental changes by generating new adult-born neurons that will establish synaptic connections within preexisting neuronal networks where they participate in hippocampus-dependent learning and memory recall processes. Previous studies aimed at examining the role of new adult-born neurons in new hippocampus-dependent memories have shown that neurogenesis stimulation can enhance spatial pattern separation in mice5,43 as well as various forms of contextual and spatial learning.12 However, the role of neurogenesis in old memories seems contradictory. Theoretical computational models44,45 and recent reports8,9,46 have suggested that when new neurons integrate into mature hippocampal network, where they either coexist with, or even replace by competing with established synapses and thus contribute to loss of previously acquired memories and forgetting. We have found that chronic IQM316 administration, but not melatonin, was able to preserve previously acquired memories as tested by the object recognition paradigm. We support the hypothesis that neurogenesis and forgetting are correlated and hence, the right rate of neurogenesis would not interfere with memory.47 Also, we believe that IQM316 is capable of inducing a healthy rate of neurogenesis, contrary to melatonin, precisely by not acting on melatonin receptors MT1 and MT2. In fact, IQM316 has a very low binding affinity when compared to melatonin for MT1 (709 ± 10 nM vs. 0.09 ± 0.01 nM) and MT2 (190 ± 8 nM vs. 0.15 ± 0.07 nM) receptors, and it is barely able to stimulate the activation of melatonin receptors (≤30% relative intrinsic activity) as measured by iodomelatonin displacement assays.26 Furthermore, our in vitro results showed that luzindole, a melatonergic antagonist, did not block neuronal differentiation of NSCs induced by IQM316, whereas it fully blocked melatonin action. Since IQM316 is a melatonin analog, it is very lipophilic and able to cross biological barriers 26 and possibly subcellular compartments. This means that it could be acting directly at the nucleus regulating transcription factors or epigenetic regulators; binding to cytosolic proteins or activating signaling pathways that have been implicated in adult neurogenesis, such as Wnt/β-catenin, Notch, and Sonic hedgehog.3 We have previously reported that IQM316 did not interact with several receptors such as cannabinoid receptors 1 and 2, serotonin receptors subtype 1A, 2A, 2B, 2C (5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C), serotonin transporter, retinoic acid receptor-α, a, peroxisome proliferator–activated receptor gamma (PPAR-γ), and glycogen synthase kinase 3β (GSK-3β), thereby excluding the implications of these receptors in the IQM316 neurogenic pathways.26 However, further studies are needed to ascertain the mechanism of IQM316-induced adult neurogenesis, and how it allows the preservation of old established memories. In addition, neither IQM316 nor melatonin altered long-term memory of newly acquired memories, which is in agreement with other reports where mice with neurogenesis stimulated by running displayed normal object recognition.5 Despite previous studies reporting that melatonin administration improves learning and memory impairments, these are effects induced under pathological conditions, such as in mouse models of Down syndrome 48 or Alzheimer’s disease.49–51 Furthermore, chronic (30 d) administration of melatonin (0.1–10 mg/kg, subcutaneously) to young mice (3 mo of age) had no effect on either short-term working memory nor on spatial long-term memory.52 These observations together with our present work show that in young and healthy animals, melatonin or IQM316 administration does not improve memory.
Here, we also studied the effect of IQM316 or melatonin administration on mitochondrial function. Neurons are high-energy, demanding cells that critically depend on mitochondria for ATP production through OXPHOS and regulate mtDNA copy number in order to maintain cellular energy requirements.53,54 We found that acute IQM316 or melatonin administration increased mtDNA copy number, suggesting either that the number of mitochondria was increased or that mtDNA replication was being activated. Mitochondrial biogenesis was not altered (no changes in mRNA relative expression of key factors of this process: PGC1-α, Nrf1, and Tfam) nor the mitochondrial protein VDAC1, indicating that the number of mitochondria was not changed, and therefore, hippocampal mitochondria have more copies of mtDNA by local activation of mtDNA replication. Neuronal differentiation has been associated with increased mitochondrial mass,35,37 and despite mitochondrial number not being altered, mitochondrial morphology (volume or length) could be increased with the net result of increased mass. However, the finding that mitochondrial function (respiratory measurements and complex activities) remained stable suggests that mitochondrial mass is not affected. Therefore, we infer that upregulation of mtDNA replication and transcription, as evidenced by the increased in mtDNA copy number and COX I protein levels, was indeed a mechanism to counterbalance the decrease in the OXPHOS proteins COX IV and ATP-5β observed upon IQM316 or melatonin acute administrations. Furthermore, SDHB, an enzyme that participates in both the respiratory chain and the citric acid cycle, was also increased probably as a compensatory mechanism to provide further energy. Given that neurogenesis is critically regulated by the redox balance in the neurogenic microenvironment,55–57 we hypothesize that IQM316 or melatonin acute administration for 7 d, followed by withdrawal of an antioxidant drug and reactive oxygen species (ROS)/radical scavenger such as melatonin,58 for 3 wk, activated adult neurogenesis mechanisms but left neurons unprotected from the subsequent increase in ROS levels. This could explain the observed disturbance in OXPHOS proteins levels. This hypothesis was further supported by the finding that chronic administration for 28 d prevented the disruption of OXPHOS proteins observed with acute treatment. Nonetheless, we found that chronic administration increased NDUFB8 protein levels. NDUFB8 is a nuclear-encoded accessory subunit of the mitochondrial membrane respiratory chain NADH dehydrogenase (complex I), that is believed not to be involved in catalysis.59 However, it has been shown to be necessary for mitochondrial function in endothelial cells.60 In the present work, despite the increase in NDUFB8 levels, we did not observe any significant changes in hippocampal mitochondria respiratory measurements using complex I substrates. Overall, we can only speculate that the changes in OXPHOS proteins are the result of a compensatory mechanism, so that mitochondrial function remains stable. Furthermore, the differences observed between acute and chronic treatments suggest that chronic treatment allows for better compensation and global energetic balance and stability between newly generated neurons and the existing hippocampal network. Moreover, it suggests that adult neurogenesis is energetically healthier if induced and maintained, in the presence of IQM316 or melatonin.
An important issue that should always be kept in mind is to ensure comparable levels of brain bioavailability of IQM316 and melatonin. Future experiments are needed to address this issue since in the present study, despite all our efforts, we were not able to reliably quantify their hippocampal levels. Since we administered the animals with a very low amount of IQM316 or melatonin (approximately 50 micrograms), when we tried to quantify them by high-performance liquid chromatography followed by fluorescence emission or mass spectrometry, the levels were below 1 pg/mL and therefore not reliable for quantification or publication.
These findings open the possibility of using IQM316 as a pharmacological agent to stimulate adult hippocampal neurogenesis in neurodegenerative diseases, where the generation of new neurons is especially important due to the gradual loss of different neuronal populations associated with these pathologies.
Footnotes
Ethical Approval: This study was approved by (Comite Etico de Investigacion, CEI).
Statement of Human and Animal Rights: This article contains animal studies approved by the local CEI.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This study was supported by grants from the Instituto de Salud Carlos III (FIS2015/00780), FEDER, and CIBERNED and awarded to E. Carro. M. I. Rodríguez-Franco gratefully acknowledges the financial support of the Spanish Ministry of Economy and Competitiveness (MINECO, Grants SAF2015-64948-R and SAF2012-31035, partially financed by FEDER funds) and CSIC (Grant PIE-201580E109). M.F.R. thanks the JAE-Predoctoral Contract (Grant JAE-Pre-2009-106) cofinanced by the CSIC and the European Social Fund. D. Acuña-Castroviejo also acknowledges grants from the Instituto de Salud Carlos III, Spain (RD12/0043/0005, PI13-981) and from the Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía, Spain (P07-CTS-03135).
References
- 1. Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013, August 15;153(6):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830(2):2435–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70(4):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31(42):15113–15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598–602. [DOI] [PubMed] [Google Scholar]
- 10. Rolando C, Taylor V. Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease. Curr Top Dev Biol. 2014;107:183–206. [DOI] [PubMed] [Google Scholar]
- 11. van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16(3):296–304. [DOI] [PubMed] [Google Scholar]
- 14. Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3(4): e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4(5): e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, Rosales-Corral S, Tan DX, Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350–384. [DOI] [PubMed] [Google Scholar]
- 18. Kim MJ, Kim HK, Kim BS, Yim SV. Melatonin increases cell proliferation in the dentate gyrus of maternally separated rats. J Pineal Res. 2004;37(3):193–197. [DOI] [PubMed] [Google Scholar]
- 19. Rennie K, De Butte M, Pappas BA. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J Pineal Res. 2009;47(4):313–317. [DOI] [PubMed] [Google Scholar]
- 20. Ramirez-Rodriguez G, Klempin F, Babu H, Benitez-King G, Kempermann G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology. 2009;34(9):2180–21891. [DOI] [PubMed] [Google Scholar]
- 21. Ramirez-Rodriguez G, Ortiz-Lopez L, Dominguez-Alonso A, Benitez-King GA, Kempermann G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res. 2011;50(1):29–37. [DOI] [PubMed] [Google Scholar]
- 22. Moriya T, Horie N, Mitome M, Shinohara K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J Pineal Res. 2007;42(4):411–418. [DOI] [PubMed] [Google Scholar]
- 23. Kong X, Li X, Cai Z, Yang N, Liu Y, Shu J, Pan L, Zuo P. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell Mol Neurobiol. 2008;28(4):569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology (Bethesda). 2014;29(5):325–333. [DOI] [PubMed] [Google Scholar]
- 25. Carocci A, Catalano A, Sinicropi MS. Melatonergic drugs in development. Clin Pharmacol. 2014;6(1179–1438 (Electronic)): 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de la Fuente Revenga M, Fernandez-Saez N, Herrera-Arozamena C, Morales-Garcia JA, Alonso-Gil S, Perez-Castillo A, Caignard DH, Rivara S, Rodriguez-Franco MI. Novel N-acetyl bioisosteres of melatonin: melatonergic receptor pharmacology, physicochemical studies, and phenotypic assessment of their neurogenic potential. J Med Chem. 2015;58(12):4998–5014. [DOI] [PubMed] [Google Scholar]
- 27. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13(2):93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murai K, Qu Q, Sun G, Ye P, Li W, Asuelime G, Sun E, Tsai GE, Shi Y. Nuclear receptor TLX stimulates hippocampal neurogenesis and enhances learning and memory in a transgenic mouse model. Proc Natl Acad Sci U S A. 2014;111(25):9115–9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simoes AE, Pereira DM, Amaral JD, Nunes AF, Gomes SE, Rodrigues PM, Lo AC, D’Hooge R, Steer CJ, Thibodeau SN, et al. Efficient recovery of proteins from multiple source samples after TRIzol((R)) or TRIzol((R))LS RNA extraction and long-term storage. BMC Genomics. 2013;14:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Podlesniy P, Figueiro-Silva J, Llado A, Antonell A, Sanchez-Valle R, Alcolea D, Lleo A, Molinuevo JL, Serra N, Trullas R. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol. 2013;74(5):655–668. [DOI] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 32. Sims NR, Anderson MF. Isolation of mitochondria from rat brain using percoll density gradient centrifugation. Nat Protoc. 2008;3(7):1228–1239. [DOI] [PubMed] [Google Scholar]
- 33. Lopez A, Garcia JA, Escames G, Venegas C, Ortiz F, Lopez LC, Acuna-Castroviejo D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res. 2009;46(2):188–198. [DOI] [PubMed] [Google Scholar]
- 34. Carretero M, Escames G, Lopez LC, Venegas C, Dayoub JC, Garcia L, Acuna-Castroviejo D. Long-term melatonin administration protects brain mitochondria from aging. J Pineal Res. 2009;47(2):192–200. [DOI] [PubMed] [Google Scholar]
- 35. Kathleen Baxter K, Uittenbogaard M, Yoon J, Chiaramello A. The neurogenic basic helix-loop-helix transcription factor NeuroD6 concomitantly increases mitochondrial mass and regulates cytoskeletal organization in the early stages of neuronal differentiation. ASN Neuro. 2009;1(4). pii: e00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheng A, Hou Y, Mattson MP. Mitochondria and neuroplasticity. ASN Neuro. 2010;2(5):e00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steib K, Schaffner I, Jagasia R, Ebert B, Lie DC. Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. J Neurosci. 2014;34(19):6624–6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88(2):611–638. [DOI] [PubMed] [Google Scholar]
- 39. Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin “knockdown” in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63(1):189–197. [DOI] [PubMed] [Google Scholar]
- 40. Gomez-Corvera A, Cerrillo I, Molinero P, Naranjo MC, Lardone PJ, Sanchez-Hidalgo M, Carrascosa-Salmoral MP, Medrano-Campillo P, Guerrero JM, Rubio A. Evidence of immune system melatonin production by two pineal melatonin deficient mice, C57BL/6 and Swiss strains. J Pineal Res. 2009;47(1):15–22. [DOI] [PubMed] [Google Scholar]
- 41. Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. [DOI] [PubMed] [Google Scholar]
- 42. Zhang J, Jiao J. Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. Biomed Res Int. 2015;2015:727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107(5):2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fusi S, Drew PJ, Abbott LF. Cascade models of synaptically stored memories. Neuron. 2005;45(4):599–611. [DOI] [PubMed] [Google Scholar]
- 45. Weisz VI, Argibay PF. Neurogenesis interferes with the retrieval of remote memories: forgetting in neurocomputational terms. Cognition. 2012;125(1):13–25. [DOI] [PubMed] [Google Scholar]
- 46. Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10(6):727–734. [DOI] [PubMed] [Google Scholar]
- 47. Mongiat LA, Schinder AF. Neuroscience. A price to pay for adult neurogenesis. Science. 2014;344(6184):594–595. [DOI] [PubMed] [Google Scholar]
- 48. Corrales A, Martinez P, Garcia S, Vidal V, Garcia E, Florez J, Sanchez-Barcelo EJ, Martinez-Cue C, Rueda N. Long-term oral administration of melatonin improves spatial learning and memory and protects against cholinergic degeneration in middle-aged Ts65Dn mice, a model of down syndrome. J Pineal Res. 2013;54(3):346–358. [DOI] [PubMed] [Google Scholar]
- 49. Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, Wang L, Zhang C, Lin X, Zhang G, et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009;47(1):82–96. [DOI] [PubMed] [Google Scholar]
- 50. Garcia-Mesa Y, Gimenez-Llort L, Lopez LC, Venegas C, Cristofol R, Escames G, Acuna-Castroviejo D, Sanfeliu C. Melatonin plus physical exercise are highly neuroprotective in the 3xTg-AD mouse. Neurobiol Aging. 2012;33(6):1124 e13–e29. [DOI] [PubMed] [Google Scholar]
- 51. Peng CX, Hu J, Liu D, Hong XP, Wu YY, Zhu LQ, Wang JZ. Disease-modified glycogen synthase kinase-3beta intervention by melatonin arrests the pathology and memory deficits in an Alzheimer’s animal model. Neurobiol Aging. 2013;34(6):1555–1563. [DOI] [PubMed] [Google Scholar]
- 52. Raghavendra V, Kulkarni SK. Possible antioxidant mechanism in melatonin reversal of aging and chronic ethanol-induced amnesia in plus-maze and passive avoidance memory tasks. Free Radic Biol Med. 2001;30(6):595–602. [DOI] [PubMed] [Google Scholar]
- 53. Pinto M, Moraes CT. Mitochondrial genome changes and neurodegenerative diseases. Biochim Biophys Acta. 2014;1842(8):1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rugarli EI, Langer T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J. 2012;31(6):1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10(4):385–394. [DOI] [PubMed] [Google Scholar]
- 56. Huang TT, Zou Y, Corniola R. Oxidative stress and adult neurogenesis—effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol. 2012;23(7):738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walton NM, Shin R, Tajinda K, Heusner CL, Kogan JH, Miyake S, Chen Q, Tamura K, Matsumoto M. Adult neurogenesis transiently generates oxidative stress. PLoS One. 2012;7(4): e35264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57(2):131–146. [DOI] [PubMed] [Google Scholar]
- 59. Loeffen JL, Triepels RH, van den Heuvel LP, Schuelke M, Buskens CA, Smeets RJ, Trijbels JM, Smeitink JA. cDNA of eight nuclear encoded subunits of NADH: ubiquinone oxidoreductase: human complex I cDNA characterization completed. Biochem Biophys Res Commun. 1998;253(2):415–422. [DOI] [PubMed] [Google Scholar]
- 60. Davis CW, Hawkins BJ, Ramasamy S, Irrinki KM, Cameron BA, Islam K, Daswani VP, Doonan PJ, Manevich Y, Madesh M. Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Radic Biol Med. 2010;48(2):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]