Abstract
The current study aims to assess the efficacy of allogenic adipose-derived stem cells (ADSCs) together with platelet-rich fibrin (PRF) for the treatment of rabbit ear cartilage defects. For this study, 12 New Zealand white rabbits were randomly allocated into 4 groups. Two full-thickness cartilage defects were created in the rabbit ears. Group 1 was left untreated; Group 2 was treated with allogenic ADSCs, Group 3 was treated with PRF; Group 4 was treated with allogenic ADSCs and PRF. Macroscopic observation, hematoxylin and eosin staining, and Alcian blue staining after 3 months suggested that the allogenic ADSCs/PRF significantly accelerated cartilage regeneration compared to other groups and this was associated with increased expression of collagen II relative to the other groups. Expression of genes associated with immune response such as cluster of differentiation 4 and 8 (CD4, CD8), and interleukin 2 and 4 (IL-2, IL-4) displayed no significant statistical difference compared to Group 1. In conclusion, these results suggest that allogenic ADSCs in combination with PRF can accelerate regeneration in full-thickness cartilage defects in the rabbit ear model without causing a significant immune response. The results suggest that allogenic ADSCs with PRF could successfully be used for cartilage regeneration.
Keywords: Adipose-derived stem cells, platelet-rich fibrin, cartilage defects, immune response
Introduction
Cartilage injuries take a long time to repair because the cartilage tissue is avascular and comprises few cells with low mitotic activity [1,2]. Cartilage injuries are treated mainly through the use of drugs, cells, and joint distraction. However, these methods suffer from several drawbacks. None of the drugs have been formally approved for use in patients for cartilage repair. The use of cells as a treatment modality is not effective on its own as structural properties also need to be considered. Furthermore, most studies on joint distraction focus on the ankle and tend to cause joint motion [3]. The most promising approach to treat cartilage defects is tissue engineering [2,4]. Adipose-derived stem cells (ADSCs) are readily available and can be expanded compared to stem cells derived from other sources, making them an ideal candidate for the repair of cartilage defects [5,6]. Moreover, since the cartilage tissue is avascular, it is possible to treat these tissues with allogenic ADSCs. Tissue repair and regeneration are complex processes that require the participation of cells, but also need additional growth factors [7]. Studies have demonstrated that a variety of growth factors are associated with cartilage repair, including transforming growth factor β [8], insulin-like growth factor [9], and also platelet-rich plasma (PRP) [10,11]. Platelet-rich fibrin (PRF) is a second-generation preparation of platelet concentrates with many advantages over the PRP. These include their simple preparation, growth factor abundance, and their slow release [12-14]. These advantages have resulted in the widespread use and application of the PRP for research.
We have previously demonstrated that PRF can decrease adipose implantation resorption rates from 49.39% to 36.41%. PRF, together with autologous ADSCs, also improved angiogenesis and remodeling [15,16]. In the event of salivary gland injury, we found that autologous ADSCs also protected the salivary gland against damage [17]. Above studies focused on the role of soft tissues for autologous ADSCs, but autologous ADSCs can be difficult to isolate thus inhibiting its utility. In the current study, we hypothesized that we could promote the repair of full-thickness cartilage defects using allogenic ADSCs/PRF, thus shortening the duration between isolation and application. In order to facilitate direct observation, we generated a cartilage defect model in rabbit’s ear cartilage not in the knee. The efficacy of allogenic ADSCs/PRF in stimulating cartilage regeneration was evaluated by macroscopic and microscopic analysis and the immune response was assessed using markers such as cluster of differentiation 4 and 8 (CD4, CD8), and interleukin 2 and 4 (IL-2, IL-4).
Materials and methods
All experimental protocols using animals were approved by the Institutional Animal Care and Use Committee at the Fourth Military Medical University, Xi’an, China.
Isolation and expansion of rabbit ADSCs
ADSCs were isolated from rabbits as previously reported [18]. Briefly, New Zealand white rabbits weighing 2 to 3 kg each and between 4 and 6 months of age were housed in the Fourth Military Medical University of Stomatology animal center, After performing general anesthesia, the scapular area of adipose tissue was removed, rinsed with phosphate buffered saline (PBS), followed by removal of the fat capsule and small blood vessels and chopping of the fat to a size of about 1 mm3. An equal volume of 1% type I collagenase was added and digestion was performed on a thermostat shaker (37°C, 180 rpm, 40 min). The resulting digest was then centrifuged (1000 r/min, 5 min) and then filtered through a 200 μm mesh screen filter and then centrifuged again. Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 medium containing 100 μg/ml penicillin/streptomycin and 10% FBS was used to resuspend the cells after adjusting the cell concentration to 1 × 105 cells/ml. Cells were then inoculated in a 25 cm2 flask and cultured under a humid atmosphere of 95% air, 5% CO2 at 37°C. The medium was changed every three days, until the cells reached 80-90% confluence. Cells were then passaged using a 0.25%/0.02% trypsin/EDTA solution.
ADSCs from passage 3 were characterized according to their adipogenic and osteogenic differentiation properties in vitro by staining with Oil Red O, Alizarin red, and Alkaline phosphatase.
Allogenic ADSCs/PRF were implanted directly into normal cartilage
PRF was prepared by centrifugation as previously described [19]. Briefly, the blood was isolated from rabbits and centrifuged immediately (3000 rpm, 10 min). The yellow segment in the third layer is the PRF. A total of 1-2 × 106 ADSCs from passage 3 were labeled with fluorescent Dil dye (Sigma, USA) was mixed with PRF (0.3 ml) and injected into the rabbit ear cartilage area. Euthanasia was performed 1 month after implantation for histological research.
Allogenic ADSCs/PRF repair of rabbit ear cartilage defects
Twelve rabbits were randomly divided into three pairs. Eight ears of each pair were subjected to full-thickness cartilage defects (5 × 5 × 1 mm). Each of ears were treated and assigned to the following groups: Control group (G1), Allogenic ADSCs group (G2), PRF group (G3), Allogenic ADSCs/PRF group (G4). The rabbits were sacrificed using an intravenous overdose of pentobarbital and the bilateral defective ears were harvested and subjected to digital photographs (Nikon-D7000, Japan), hematoxylin and eosin (HE) staining and Alcian blue staining. To test the immune response of the introduced allogenic ADSCs, 2 ml of blood before and after sacrifice from the superficial ear vein of each rabbit was saved 1, 2, and 3 months after surgery for the determination of CD4/CD8 and IL-2 and IL-4 levels.
Quantitative RT-PCR
For evaluation of collagen II (col-II) levels in cartilage repair region, total RNA was extracted using real-time PCR (Takara, Japan) and processed according to the manufacturer’s instructions. The Primer sequences for GAPDH and col II are shown in Table 1.
Table 1.
List of primers used for quantitative real-time PCR
| Gene | Primers Sequences |
|---|---|
| GAPDH forward | 5’-AGACACGATGGTGAAGGTCG-3’ |
| GAPDH reverse | 5’-TGCCGTGGGTGGAATCATAC-3’ |
| Collagen II forward | 5’-GCACCCATGGACATTGGAGG-3’ |
| Collagen II reverse | 5’-AGCCCCGCACGGTCTTGCTT-3’ |
Western blot analysis
After 3 months, total protein was collected and quantified according to the manufacturer’s instructions using the bicinchoninic acid (BCA) Protein Assay kit (Thermo Fisher Scientific) and the expression levels of col-II in the repaired area was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Statistical analysis
All quantitative data were expressed as mean ± standard deviation (S.D.). To compare the different groups undergoing repair, testing was performed using one-way analysis of variance (ANOVA) and then further analyzed by using Newman-Keuls method (SNK-q). Image-Pro Plus 6.0 (USA) was used to analyze the HE images to compute defect repair rate and graphs were generated using GraphPad Prism 6.0 (USA). The significance threshold was set at P < 0.05.
Results
ADSCs were isolated and expanded from rabbit scapular adipose tissue. ADSCs exhibited fibroblast-like morphology in culture (Figure 1A). We have previously characterized the phenotype of ADSCs in culture extensively [15]. At passage 3, the differentiation capacity of rabbit ADSCs was confirmed by staining with Oil Red O, Alizarin red, and Akaline phosphatase (Figure 1B-D).
Figure 1.
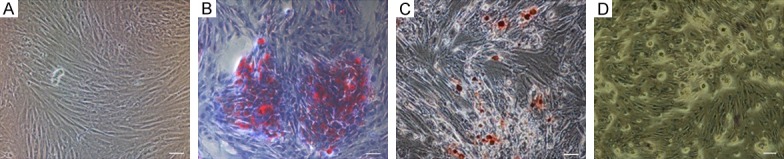
Identification of rabbit ADSCs (A) phase contrast imaging of living ADSCs, which exhibited fibroblast-like morphology. (B) Lipid drops were present in ADSCs after 2 weeks of adipogenic induction as identified by staining with Oil red O. (C) Calcium nodules were formed after 4 weeks of osteogenic induction as indicated by Alizarin red staining. (D) Alkaline phosphatase positive cells were observed after 1 week of osteogenic induction.
ADSCs at passage 3 were labeled with the fluorescent dye Dil and mixed with PRF. This mixture was injected into normal ear cartilage area for 1 month. The results demonstrated that compared to the controls (Figure 2A and 2B), a new cartilage region can be observed in the injection zone (Figure 2C) and expressed red fluorescence (Figure 2D), thus suggesting that the ADSCs can be directly induced to form cartilage in vivo in the presence of PRF.
Figure 2.
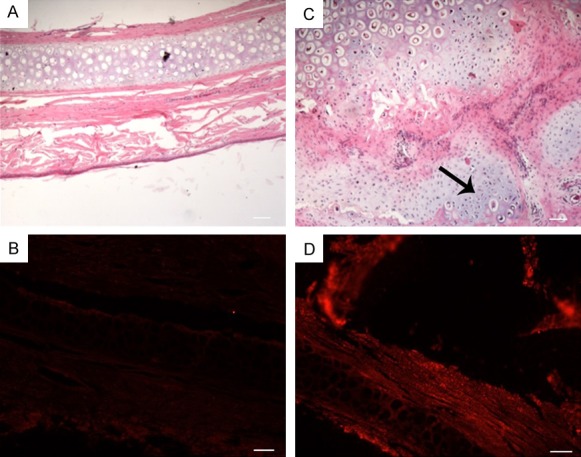
Growth and differentiation of ADSCs/PRF in the normal ear cartilage. A, B. Control group, HE and DiL labeled, respectively; C, D. HE and DiL labeled tissue at 1 month post-implantation showed new cartilage region (indicated by the arrow) Scale bar = 100 μm.
To study cartilage regeneration following ear defect, we followed the process delineated in Figure 3. After 1, 2, and 3 months, four rabbits each were sacrificed for general observation, and the samples were stained using HE and Alcian blue.
Figure 3.
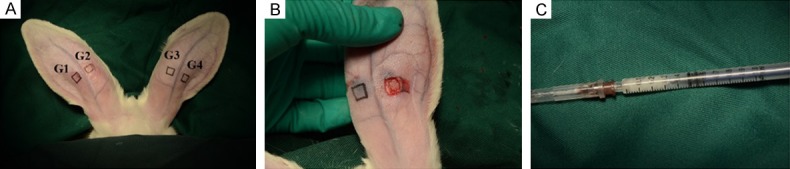
Stepwise procedure for repair of rabbit ear cartilage by ADSCs/PRF. A. G1, Control group; G2, allogenic ADSCs group; G3, PRF; G4, allogenic ADSCs/PRF group. B. Full-thickness cartilage defect (5 × 5 × 1 mm). C. Allogenic ADSCs/PRF (0.3 ml).
In the G1 control group, cartilage defect area can be identified as a significant depression with neat margins and clear cartilage tissue boundaries with little new cartilage tissue formation. The G2 group treated with allogenic ADSCs alone showed a thin layer of membrane-like tissue covering. The G3 group treated with PRF alone displayed blurry defect edges with a small amount of tissue-like newborn cartilage. The G4 group showed that the defective edge no longer exists and newborn cartilage tissue filled defect area (Figure 4B).
Figure 4.
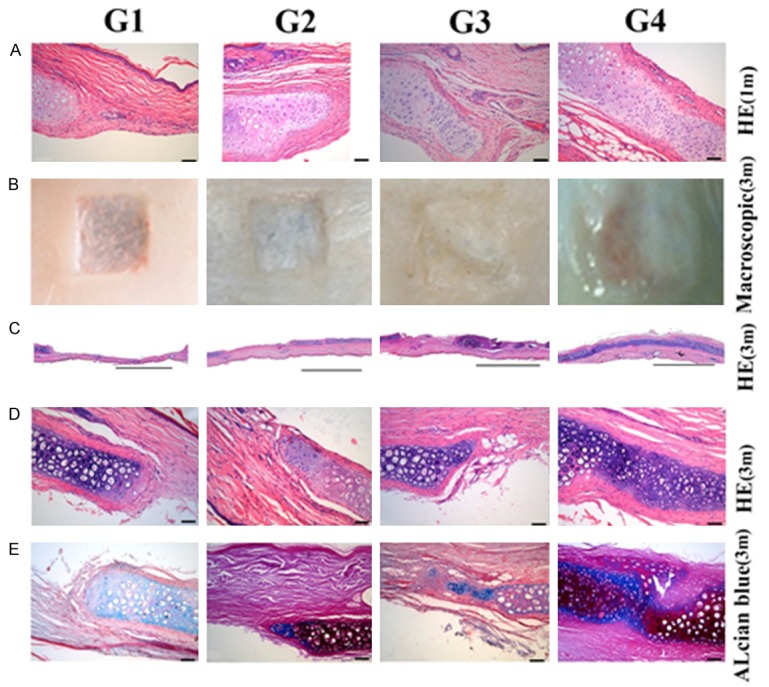
Cartilage defect after injection of cells. A. HE images after the operation 1 month later (Scale bar = 100 mm). B. Representative macroscopic images 3 months after implantation. C. Overall HE across 4 groups (Scale bar = 2 mm). D. HE of sections (Scale bar = 100 mm) used to determine the cartilage defect repair rate (%). E. Alcian blue images of sections (Scale bar = 100 mm). (G1 was untreated; G2 was treated with allogenic ADSCs, G3 was treated with PRF; G4 was treated with allogenic ADSCs/PRF).
Histological analysis of HE stained tissues showed that G2 and G4 displayed no obvious inflammatory cell recruitment compared to G1 at the boundary between the wound area and the normal tissue after 1 month (Figure 4A). G2 and G4 also showed no significant inflammatory response at the margin of the defect as compared to G1 after 3 months (Figure 4D). These observations were validated by HE staining (Figure 4C). Table 2 shows the rate of cartilage repair in the defect area. G4 group had the best rate of repair at all observation points and the repair rate was 90% greater than other groups at 3 months. There were significant statistical differences between the G1, G2, and G3 groups at several time points (P < 0.001).
Table 2.
Cartilage defect repair rate (%)
| Group | Post-implantation | ||
|---|---|---|---|
|
| |||
| 1 M | 2 M | 3 M | |
| G1 | 0 | 1.27±0.40 | 1.68±0.17 |
| G2 | 7.47±0.25 | 9.3±0.36 | 15.4±0.91 |
| G3 | 19.1±0.44 | 28.6±0.8 | 32.0±2.76 |
| G4 | 36.85±0.84a | 62.7±0.53b | 89.37±0.79c |
statistically significant difference between groups after 1 month surgery (P < 0.01).
statistically significant difference between groups after 1 month surgery (P < 0.01).
statistically significant difference between groups after 1 month surgery (P < 0.001).
All statistical analyses were performed by one-way ANOVA and data are represented as mean ± standard error of mean (SEM).
Alcian blue staining, which marks naïve chondrocytes, was observed in higher numbers within groups G2 and G3 compared to group G1 at the junction (Figure 4E). The cartilage defect area in G4 was almost completely filled by naïve chondrocytes. The above results that allogenic ADSCs or PRF alone can repair cartilage defects to a certain degree, but allogenic ADSCs together with PRF repairs cartilage defects much more efficiently.
Quantitative real-time PCR was performed for detecting the expression of the col-II gene in the new cartilage isolated from the different groups after 3 months. Col-II expression levels in G4 were significantly higher compared to G1, G2, and G3. Col-II expression levels in G3 were higher than in G2 (Figure 5A). Western blot results also suggest that PRF significantly increases col-II expression at the protein level (Figure 5B).
Figure 5.
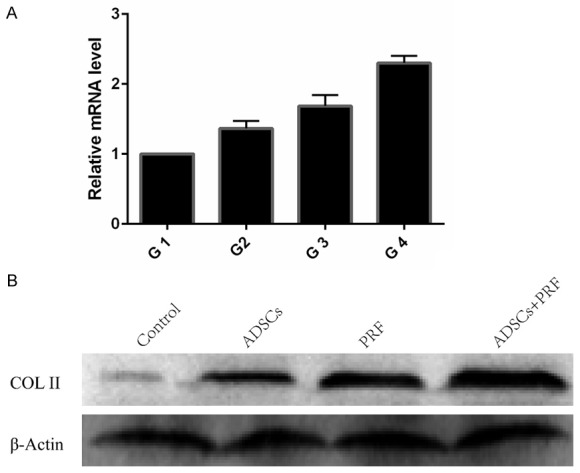
The expression of col-II in new cartilage. A. Total RNA was extracted from repaired tissues at 3 months post-injection. In group G4, expression of col-II was significantly higher than in the other groups. Expression in group G3 was higher than in G2. B. Western blot analysis demonstrated that the protein expression of col-II was significantly increased in the G4 group, compared with the other groups. (G1 was untreated; G2 was treated with allogenic ADSCs, G3 was treated with PRF; G4 was treated with allogenic ADSCs/PRF).
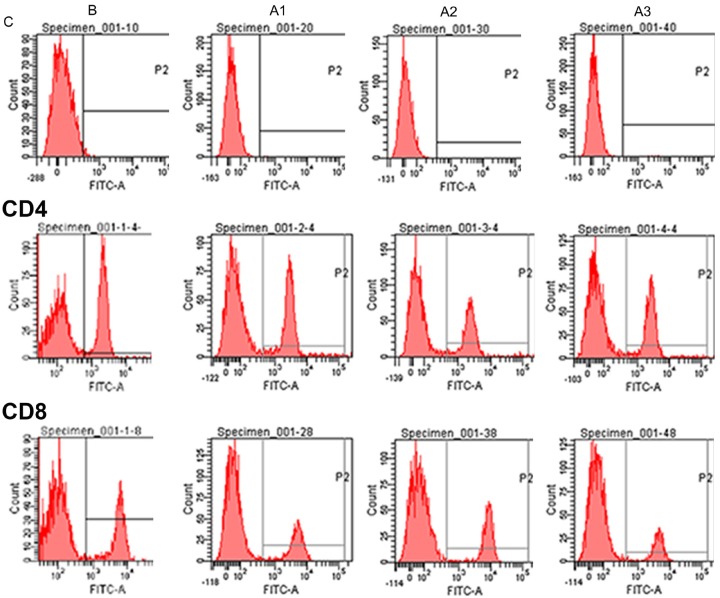
In order to further test whether there was an immune response to the exogenous introduction of ADSCs and PRF, we tested the relevant markers. The results of CD4, CD8, CD4/CD8, and IL-2 and IL-4 before and after allogenic ADSC implantation were compared with their levels at G1. No significant statistical differences were observed (Figure 6 and Table 3). These results indicate that allogenic ADSC transplantation does not induce a significant immune response.
Figure 6.
The results of CD4/CD8, IL-2 and IL-4 staining before and after allogenic ADSC implantation. B = before implantation, A1 = 1 month after implantation, A2 = 2 months after implantation, A3 = 3 months after implantation. C = Control group.
Table 3.
The results of CD4/CD8, IL-2, and IL-4 staining before and after allogenic ADSC implantation
| CD4/CD8 | IL-2 (pg/ml) | IL-4 (pg/ml) | |
|---|---|---|---|
| B | 1.80±0.072* | 169.7±9.31* | 63.56±7.35* |
| A1m | 1.93±0.145 | 168.5±10.52 | 64.45±5.34 |
| A2m | 1.82±0.124 | 170.9±8.47 | 65.67±6.42 |
| A3m | 1.94±0.124 | 169.6±10.35 | 65.69±6.34 |
P > 0.05, B = before implantation, A = after implantation.
Data are represented as mean ± standard error of mean (SEM).
Discussion
Mesenchymal stem cells have recently become a popular source of cells in the field of tissue engineering due to their proliferation and differentiation ability. ADSCs and bone marrow stem cells (BMSCs) are the most common mesenchymal stem cells used for this purpose. BMSCs are superior to ADSCs in terms of their osseointegration. However, ADSCs are more readily available, can be expanded in vitro, and are comparable to BMSCs in the context of allogenic transplantation [6,16,18]. Cartilage damage occurs mostly due to tumors, infections, and degenerative diseases. Cartilage tissue derives most of its nutrition from the surrounding tissues because it is avascular [1]. ADSCs are implanted directly into the cartilage defect area, often differentiating into osteogenic lineages rather than chondrogenic lineages, possibly due to the length of the blood vessels and endochondral ossification [1]. In this study however, allogenic ADSCs seeded with PRF differentiate into cartilage. This may suggest that PRF can regulate lineage decisions of ADSCs within the cartilage environment. PRF contains high levels of growth factors such as vascular platelet-derived growth factor (PDGF) and transforming growth factor-β1 (TGF-β1), which favor cartilage regeneration [8,20]. Col-II is an important indicator of cartilage regeneration [21]. The expression of the col-II gene was significantly increased in G3 compared to G2, suggesting that growth factors in the PRF can promote cartilage regeneration. Western blot results also demonstrated that PRF significantly increases col-II protein expression. This result is consistent with other studies about the effects of growth factors on cartilage differentiation [22,23]. After 3 months, the defects were repaired in the group where ADSCs were administered together with PRF compared to incomplete repair in other groups. Our study also showed that allogenic ADSCs and allogenic ADSCs/PRF repaired cartilage defects without mounting an immune response (Table 3), consistent with previous reports [24].
In conclusion, allogenic ADSCs/PRF can not only repair the cartilage, but also not induce an immune response. Despite our research findings on the advantages of ADSCs and PRF in promoting soft tissue regeneration, there is a need to study how PRF modulates the specific mechanisms by which allogenic ADSCs are transformed into the chondrogenic lineage. We believe that our method will help develop an allogenic ADSC bank that can be used for the regeneration of soft tissue.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31170942).
Disclosure of conflict of interest
None.
References
- 1.Xie A, Xue J, Shen G, Nie L. Thrombospondin-1 inhibits ossification of tissue engineered cartilage constructed by ADSCs. Am J Transl Res. 2017;9:3487–3498. [PMC free article] [PubMed] [Google Scholar]
- 2.Kazemi D, Fakhrjou A, Dizaji VM, Alishahi MK. Effect of autologous platelet rich fibrin on the healing of experimental articular cartilage defects of the knee in an animal model. Biomed Res Int. 2014;2014:1–10. doi: 10.1155/2014/486436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mastbergen SC, Saris DL, Lafeber FP. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol. 2013;9:277–290. doi: 10.1038/nrrheum.2013.29. [DOI] [PubMed] [Google Scholar]
- 4.Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13:295–307. doi: 10.2217/rme-2017-0152. [DOI] [PubMed] [Google Scholar]
- 5.Denkovskij J, Bagdonas E, Kusleviciute I, Mackiewicz Z, Unguryte A, Porvaneckas N, Fleury S, Venalis A, Jorgensen C, Bernotiene E. Paracrine potential of the human adipose tissue-derived stem cells to modulate balance between matrix metalloproteinases and their inhibitors in the osteoarthritic cartilage in vitro. Stem Cells Int. 2017;2017:9542702. doi: 10.1155/2017/9542702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riester SM, Denbeigh JM, Lin Y, Jones DL, De Mooij T, Lewallen EA, Nie H, Paradise CR, Radel DJ, Dudakovic A, Camilleri ET, Larson DR, Qu W, Krych AJ, Frick MA, Im HJ, Dietz AB, Smith J, van Wijnen AJ. Safety Studies for use of adipose tissue-derived mesenchymal stromal/stem cells in a rabbit model for osteoarthritis to support a phase I clinical trial. Stem Cell Transl Med. 2017;6:910–922. doi: 10.5966/sctm.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q, Huang Y, Lan Y, Zuo Q, Li C, Zhang Y, Guo R, Xue W. Acceleration of skin regeneration in full-thickness burns by incorporation of bFGF-loaded alginate microspheres into a CMCSPVA hydrogel. J Tissue Eng Regen Med. 2017;11:1562–1573. doi: 10.1002/term.2057. [DOI] [PubMed] [Google Scholar]
- 8.Ying J, Wang P, Zhang S, Xu T, Zhang L, Dong R, Xu S, Tong P, Wu C, Jin H. Transforming growth factor-beta1 promotes articular cartilage repair through canonical Smad and Hippo pathways in bone mesenchymal stem cells. Life Sci. 2018;192:84–90. doi: 10.1016/j.lfs.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Armakolas N, Dimakakos A, Armakolas A, Antonopoulos A, Koutsilieris M. Possible role of the Ec peptide of IGF1Ec in cartilage repair. Mol Med Rep. 2016;14:3066–3072. doi: 10.3892/mmr.2016.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spakova T, Amrichova J, Plsikova J, Harvanova D, Hornak S, Ledecky V, Rosocha J. A preliminary study comparing microfracture and local adherent transplantation of autologous adipose-derived stem cells followed by intraarticular injection of platelet-rich plasma for the treatment of chondral defects in rabbits. Cartilage. 2017:1947603517713816. doi: 10.1177/1947603517713816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, Fornasari P, Marcacci M, Kon E. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575–1582. doi: 10.1177/0363546515582027. [DOI] [PubMed] [Google Scholar]
- 12.Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015;473:1635–1643. doi: 10.1007/s11999-015-4192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohanty S, Pathak H, Dabas J. Platelet rich fibrin: a new covering material for oral mucosal defects. J Oral Biol Craniofac Res. 2014;4:144–146. doi: 10.1016/j.jobcr.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao YH, Zhang M, Liu NX, Lv X, Zhang J, Chen FM, Chen YJ. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials. 2013;34:5506–5520. doi: 10.1016/j.biomaterials.2013.03.079. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Li L, Zhao J, Wang Z, Tan X, Xu H, Au R, Liu Y. Platelet-rich fibrin and adipose-derived stem cells improve the efficacy of fat transplantation and soft tissue repair. J Biomater Tiss Eng. 2015;5:275–282. [Google Scholar]
- 16.Liu B, Tan X, Liu Y, Xu X, Li L, Xu H, An R, Chen F. The adjuvant use of stromal vascular fraction and platelet-rich fibrin for autologous adipose tissue transplantation. Tissue Eng Part C Methods. 2013;19:1–14. doi: 10.1089/ten.TEC.2012.0126. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Ju Z, He L, Li Z, Liu Y, Liu B. Intraglandular transplantation of adipose-derived stem cells for the alleviation of irradiation-induced parotid gland damage in miniature pigs. J Oral Maxil Surg. 2017;75:1784–1790. doi: 10.1016/j.joms.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Coelho de Faria AB, Chiantia FB, Teixeira ML, Aloise AC, Pelegrine AA. Comparative study between mesenchymal stem cells derived from bone marrow and from adipose tissue, associated with xenograft, in appositional reconstructions: histomorphometric study in rabbit calvaria. Int J Oral Maxillofac Implants. 2016;31:e155–e161. doi: 10.11607/jomi.4606. [DOI] [PubMed] [Google Scholar]
- 19.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Hanaoka K, Tanaka E, Takata T, Miyauchi M, Aoyama J, Kawai N, Dalla-Bona DA, Yamano E, Tanne K. Platelet-derived growth factor enhances proliferation and matrix synthesis of temporomandibular joint disc-derived cells. Angle Orthod. 2006;76:486–492. doi: 10.1043/0003-3219(2006)076[0486:PGFEPA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Ma N, Wang T, Bie L, Zhao Y, Zhao L, Zhang S, Gao L, Xiao J. Comparison of the effects of exercise with chondroitin sulfate on knee osteoarthritis in rabbits. J Orthop Surg Res. 2018;13:16. doi: 10.1186/s13018-018-0722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaissmaier C, Koh JL, Weise K. Growth and differentiation factors for cartilage healing and repair. Injury. 2008;39:88–96. doi: 10.1016/j.injury.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 23.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 24.Leijten JC, Georgi N, Wu L, van Blitterswijk CA, Karperien M. Cell sources for articular cartilage repair strategies: shifting from monocultures to cocultures. Tissue Eng Part B Rev. 2013;19:31–40. doi: 10.1089/ten.TEB.2012.0273. [DOI] [PubMed] [Google Scholar]