Abstract
Background: Glypican-3 (GPC3) is one of the key tissue markers that could discriminate malignant precancerous lesions from benign hepatic lesions in cirrhotic patients. We aimed to develop a GPC3 cancer vaccine to induce specific T cells to intervene in hepatocellular carcinoma (HCC) development. Methods: Synthesizing mannosylated liposomes (LPMan) as vaccine delivery system, incorporating one Toll-like receptor (TLR)-7/8 agonist CL097 as adjuvant, we prepared a GPC3 nanovaccine, LPMan-GPC3/CL097. We injected 25 mg/kg diethylnitrosamine intraperitoneally to induce autochthonous HCC in HBV-transgenic mice, which persistently express hepatitis B surface antigen in hepatocytes. Starting from week 8 after diethylnitrosamine injection when malignant hepatocytes generated, we immunized the mice subcutaneously every 2 weeks 4 times with LPMan-GPC3/CL097 containing 5 µg of GPC3 plus 5 µg of CL097. Results: The vaccine efficiently targeted draining lymph nodes where naïve T cells reside and enhanced the expression of molecules involved in antigen presentation in migratory dendritic cells (DCs). Antigen was professionally processed in endoplasmic reticulum-Golgi system of DCs, subsequently priming both CD4+ and CD8+ T cells. The LPMan-GPC3/CL097 immunization generated significantly more GPC3-specific CD4+ IFNγ- and CD8+ IFNγ-producing T cells in mice spleens and livers, which specifically eliminated GPC3-expressing tumor cells. One week after last immunization (week 15 after diethylnitrosamine), 5/5 un-immunized, 5/5 sham (LPMan-CL097) and 1/5 LPMan-GPC3/CL097-immunized mice developed HCC. By week 20 after diethylnitrosamine, significantly less HCC developed in LPMan-GPC3/CL097-immunized mice than in sham-immunized mice (P<0.01). Conclusions: LPMan-GPC3/CL097 immunization induced de novo generation of specific T cells against tumor-associated antigen GPC3 that could prevent HCC development in cirrhotic liver.
Keywords: Cancer vaccine, murine model, T cells, tumor-associated antigen, hepatocellular carcinoma
Introduction
Preclinical and clinical evidences have demonstrated that early neoplastic cells (transformed cells that initiate cancer formation) express antigens that enable the immune system to distinguish them from normal cells. Cancer immunoprevention by modulating the host immune response was considered a better strategy to control the initiation or development of cancer. Cancer vaccine is one of the promising approaches [1,2]. Hepatocellular carcinoma (HCC) arising from cirrhosis is usually preceded by the appearance of malignant precancerous lesion nodules [3]. At this stage, disease progression could be interfered if the malignant progenitors in cirrhosis were eliminated by HCC vaccine-elicited specific immunity.
Neoantigens resulting from coding gene mutations in tumors are considered as optimal personalized cancer vaccine targets because they bypass thymic selection [4]. Disease progression was well controlled after immunization with personalized neoantigens in melanoma patients [5,6]. However, we recently found that the mutations of coding genes and numbers of candidate neoantigens were much fewer in HCC, except for those of aflatoxin-related HCC [7]. Tumor-associated antigen, which is re-expressed in tumors and not/lowly expressed in normal tissues, might be an appropriate target [8]. Previous studies have documented that inducing specific T cells to alpha-fetoprotein (AFP), which is re-expressed in most HCC, led to tumor regression [8], and prevented carcinogen-induced murine autochthonous HCC [9]. However, significant hepatocyte damage was observed in the regenerating mouse liver [10].
Glypican-3 (GPC3) was identified as a new HCC-associated antigen [11]. Different from AFP, it is undetectable in the cirrhotic liver or even in benign hepatic lesions. Tissue expression of GPC3 was used to discriminate the nature of a <2 cm hepatocellular lesion lacking HCC radiological features detected in a cirrhotic patient [11]. Up to 60% of early HCC showed immunoreactivity to GPC3, either as membrane and/or cytoplasmic staining in the biopsy materials [12]. Therefore, we hypothesized that eliciting the host’s own specific T-cell immunity against GPC3 could interfere with disease progression in cirrhosis patients.
Both CD4+ and CD8+ antigen-specific T cells are required to reject established tumor cells [13]. Normally, protein vaccines hardly gain access to the subcellular compartments of dendritic cells (DC) to be monitored by the MHC-I antigen presentation pathway, and hardly prime naïve CD8+ T cells [14]. When vaccine protein was conjugated with some Toll-like receptor (TLR) agonists, mainly TLR7/8 agonists, the magnitude and quality of T-cell responses were significantly improved by activation of multiple DC subsets [15,16]. In addition, the T-cell repertoire of tumor-associated antigens is limited and shows low-avidity because of the thymus selection [4]. Therefore, it is crucial to deliver the antigen signals into secondary lymphoid organs and activate DCs properly when tumor-associated antigen was used as a cancer vaccine [14,17].
Polymeric nanoparticulate carriers may enhance antigen stability, immunogenicity and immunostimulatory effects with sustained and controlled release of the antigen to the target sites [18], which are efficient for vaccine system development, especially for prophylactic and therapeutic cancer vaccines. Using the model protein vaccine, we previously reported that mannosylated liposomes (LPMan) could be a good vaccine delivery system for lymph node targeting [19]. In addition, we found that, when the antigens were conjugated with chemically synthesized TLR7/8 agonists, such as CL097, they induced the generation of antigen-specific Th1 responses in an immune tolerant state [20,21]. Here, we prepared a GPC3 nanovaccine, LPMan-GPC3/CL097, by synthesizing LPMan as vaccine delivery system, one TLR7/8 agonist CL097 as adjuvant. The results showed that LPMan-GPC3/CL097 nanovaccine could target draining lymph nodes in vivo. In the mice, LPMan-GPC3/CL097 immunization induced GPC3-specific immunity, which prevented the development of premalignant hepatic lesions to cancer.
Materials and methods
Supplementary Materials and Methods provide detailed information.
Analysis of molecule expressions in migratory DCs
Bone marrow-derived dendritic cells (BMDCs) were generated using standard laboratory protocols [22], stimulated with 5 µg/ml GPC3 (Sino-Biological, Beijing; endotoxin level <1 EU/µg protein) or 5 µg/ml GPC3 plus 0.5 µg/ml CL097 (Invivogen, CA) for 24 h. The molecular expression levels in BMDCs were determined using SYBR Premix Ex-Taq on an Applied Bio-systems 7500 Real-Time PCR system, with GAPDH as the control and represented as 2-ΔΔCT [23]. Primers used are provided in Supplementary Table 1.
Preparation and characterization of the GPC3 nanovaccine containing CL097 using mannosylated liposomes
According to our previous report, DSPE-PEG-Man was synthesized, and LPMan was prepared by thin-film hydration method by mixing 5% DSPE-PEG-Man with 95% DOTAP [19]. To prepare LPMan-GPC3, we added different concentrations of purified GPC3 solution. To prepare LPMan-GPC3/CL097, different concentrations of CL097 were added into the LPMan-GPC3 solution.
The particle size and zeta potential were measured using Zetasizer Nano ZS (Malvern, Worcs, UK). The LPMan encapsulation efficiency of GPC3 and CL097 was determined by measuring percentage of liposome-bound components after removing free GPC3 and CL097 through dialysis (molecule weight cut-off: 100 kD). Liposome-bound components were then measured as we described previously [19,21]. Presence of mannosylate residuals was determined using the concanavalin A agglutination assay [19].
Analysis of antigen uptake and lymph node trafficking in vivo
Alexa Flour-488 labeled BSA (AF-BSA, Invitrogen, CA) was used as a model antigen and was encapsulated with LPMan as performed for GPC3. BMDCs (3×106/ml) were stimulated with LPMan-BSA/CL097 containing 5 μg/ml AF-BSA plus 0.5 μg/ml or plus 5 μg/ml CL097 for 30 min. Treatment with same amount of LPMan-BSA or free AF-BSA was used as controls. FCM analyzed the antigen uptake efficacy. To analyze antigen trafficking into lymph nodes in vivo, 2.5 μg of AF-BSA in different formulations was injected into a mouse forepaw, and brachial and axillary lymph nodes were collected 2 days later and analyzed by FCM.
Immunofluorescent microscopy
BMDCs were stimulated with LPMan-BSA/CL097 containing 5 μg/ml AF-BSA plus 0.5 μg/ml CL097, or the same amount LPMan-BSA or free AF-BSA for 3 h. The cells were then placed on poly-L-lysine-coated slides, fixed and permeabilized. After incubation with goat anti-EEA1 (N-19), or anti-Erp78 (N-20), or anti-Giantin (N-18; Santa Cruz, CA) or anti-LAMP1 (Sigma-Aldrich, MO) overnight at 4°C, the cells were stained with Cy3-labeled donkey anti-goat IgG for 1 h. Image acquisitions and analysis were performed using a Leica microscope and software (Wentzler, Germany).
Flow cytometry (FCM) analysis
Immunofluorescent-labeled antibodies were all purchased from eBioscience (San Diego, CA). For intracellular staining, cells were firstly stained with antibodies against their surface markers, followed by fixation and permeabilization and stained with PE/Cy7-conjugated anti-mouse IFNγ. Data were acquired in LSR-II and analyzed using Flowjo software (Tree Star, OR).
Mice and murine autochthonous liver cancer model
Study protocols (NCC2015A011) involving mice were approved by the Institutional Animal Care and Use Committee at National Cancer Center, Chinese Academy of Medical Sciences (NCC-CAMS). All the mice were maintained under specific pathogen-free conditions at the Laboratory Animal Services Center of NCC-CAMS. C57BL/6 mice were purchased from Vital River Laboratory Animal Technology, Beijing. HBV transgenic (HBV-Tg) mice, C57BL/6J-TgN(AlblHBV)44Bri/J, which persistently express large and small HBV surface antigen within the hepatocytes and secrete small HBV surface antigen from hepatocytes [24], were purchased from Health Science Center, Peking University and used for breeding mice. For liver cancer induction, diethylnitrosamine (DEN; Sigma) was injected intraperitoneally at 25 mg/kg into male littermates at 2 weeks old [25].
Detection of serum alanine aminotransferase (ALT) and anti-GPC3 antibodies
Two days before each immunization, the serum ALT level was measured within 2 h after sample collection using the reagents from Biosino Bio-Technology (Beijing, China). To determine anti-GPC3 antibodies, the serum samples were stored at -20°C and detected at the same time using a method established in laboratory, which is provided in the Supplementary Materials and Methods.
Assay of antigen-specific cytotoxicity on liver cancer cells
Mouse liver was removed, and intrahepatic lymphocytes (IHL) were prepared as reported previously [26]. Syngeneic murine hepatoma cell line, GPC3-expressing Hepa/GPC3 was constructed (Supplementary Figure 2) and labeled with 2 μM CFSE (Invitrogen, CA). Two cell populations were co-cultured at a ratio of 2000 CFSE-labeled Hepa/GPC3 with 80,000 IHLs for 4 h, followed by FCM analysis [9].
Statistical analysis
Un-paired t-tests were used to compare differences between groups. A P-value less than 0.05 was considered to be statistically significant.
Results
CL097 stimulation augments molecular expression involved in MHC-I antigen presentation in migratory DCs
We stimulated BMDCs with purified GPC3 or GPC3 plus CL097 to validate the CL097 effect on molecules involved in MHC-I antigen presentation, which is required to prime naïve T cells for CD8+ T cell generation [14]. Analysis by qRT-PCR showed that the mRNA expression levels of TAP1, TAP2 and TAPBP, which transport cytosolic antigenic peptides across the membrane of endoplasmic reticulum (ER) (Figure 1A), BiP, calreticulin and ERp57, which ensure correct folding of proteins in ER, and ERAP, which trim transported long peptides in ER to the right size for MHC-I binding (Figure 1B), were all enhanced significantly after the cells were stimulated with GPC3 plus CL097. The stimulation also enhanced the expression levels of Sec22b, CD74, GILT, HSP90, and EDEM1, EDEM2, which are involved in recruiting ER proteins to phagosomes and translocating antigen from phagosomes into cytosol, accelerating ER-associated degradation of misfolded polypeptides (Figure 1C).
Figure 1.
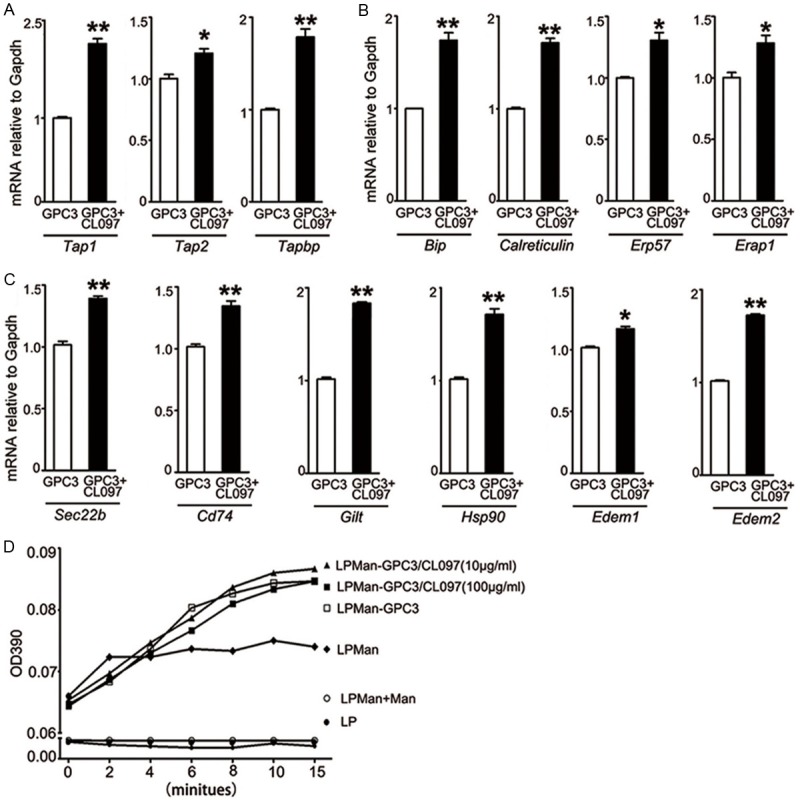
Effect of CL097 stimulation on molecules involved in the MHC-I antigen presentation pathway. BMDCs (3×106/ml) were stimulated with 5 μg/ml GPC3, or 5 μg/ml GPC3 plus 0.5 μg/ml CL097 for 24 h. A-C. Determined by qRT-PCR assay, the mRNA expression levels of TAP1, TAP2, TAPBP, Bip, calreticulin, ERp57, ERAP, Sec22b, CD74, GILT, HSP90, EDEM1 and EDEM2 were calculated based on GAPDH. Bars show fold changes, with the GPC3-treated sample arbitrarily set as 1 (n=3). Data are presented as mean ± SE, Paired t-test was conducted between two groups. *P<0.05, **P<0.01. D. The concanavalin A agglutination assay was used to determine mannosylate residues (n=3).
GPC3 nanovaccine containing CL097 promotes migratory DC antigen uptake, maturation and migration to draining lymph nodes
For better targeting lymph nodes where naïve T-cells reside, we synthesized positively charged cationic LPMan according to our previous report [19]. Using a previously defined molar ratio of 95% DOTAP and 5% DSPE-PEG-Man, we identified that the optimal encapsulation efficiency was 1 µmol/ml LPMan with 100 µg/ml GPC3 (Supplementary Table 2). At this ratio, the maximal CL097 inclusion was 50-100 µg/ml. The particles showed no variation in the diameter but had decreased the surface charge with an increasing CL097 concentration (Supplementary Table 3). In the presence of the maximal concentration of mannosylate residues, no significant difference was found after CL097 was added into LPMan-encapsulated GPC3 (LPMan-GPC3/CL097) (Figure 1D). We prepared the GPC3 nanovaccine at the ratio of 1 µmol LPMan with 100 µg of GPC3 plus 10 µg of CL097 for in vitro, and 1 µmol LPMan with 100 µg of GPC3 plus 100 µg of CL097 for in vivo experiments.
We stimulated BMDCs with LPMan-GPC3/CL097 containing 5 μg/ml GPC3 plus 0.5 μg/ml CL097, or LPMan-GPC3 containing 5 μg/ml GPC3, or 5 μg/ml purified GPC3. FCM analysis showed that LPMan-GPC3/CL097 significantly increased the surface expression of CD83, MHC-I and MHC-II (Figure 2A). CCR7 expression, which is required for DC migration to draining lymph organs, was also significantly augmented (Figure 2A).
Figure 2.
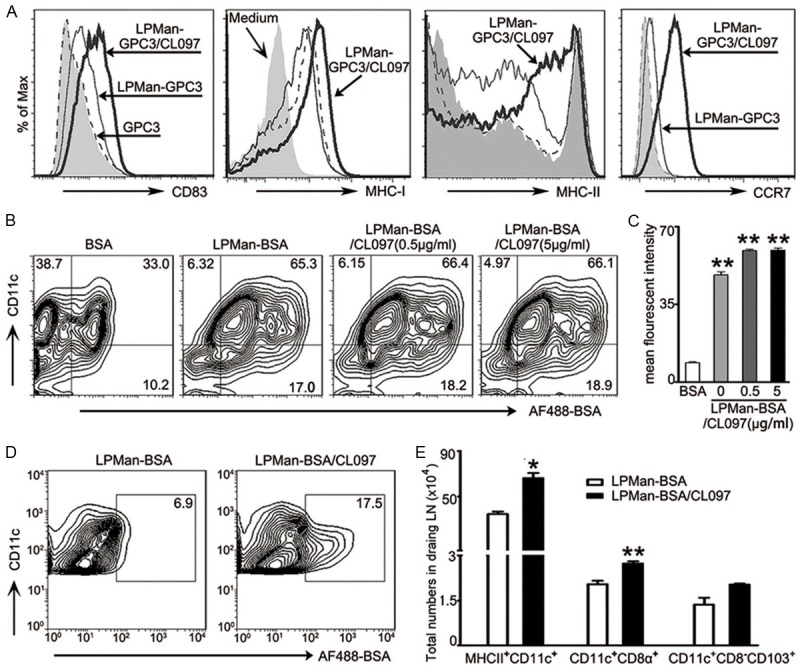
Effect of LPMan-GPC3/CL097 on migratory DCs and draining lymph nodes in vivo. A. FCM analysis of cell surface markers on BMDCs (3×106/ml) by staining with antibody to CD83, MHC-I, MHC-II and CCR7 after they were stimulated for 24 h with LPMan-GPC3/CL097 containing 5 μg/ml GPC3 plus 0.5 μg/ml CL097. The same amount of LPMan-GPC3 containing 5 μg/ml GPC3, or free GPC3 (5 μg/ml) were used as control. Profiles show representative of three independent experiments. B, C. AF488-labeled BSA (BSA) was used as the model antigen and encapsulated with LPMan as performed as GPC3. BMDCs (3×106/ml) were stimulated with LPMan-BSA/CL097 containing 5 μg/ml-BSA plus 0.5 μg/ml-CL097, or LPMan-BSA/CL097 containing 5 μg/ml BSA plus 5 μg/ml CL097, or LPMan-BSA containing 5 μg/ml BSA, or 5 μg/ml free BSA for 30 min. The percentage (B) and amount (C, as indicated by mean fluorescent intensity) of BSA in CD11c+ DCs was determined. **P<0.01 compared with the group treated with free BSA. D, E. Each mouse received 5 μg of AF-BSA in the form of LPMan-BSA (n=5) or received 5 μg of AF-BSA plus 5 μg of CL097 in the form of LPMan-BSA/CL097 via subcutaneous injection (n=5). raining lymph nodes were removed 48 h later. D. Profiles show the presence of AF-BSA in CD11c+ cells of the draining lymph nodes. E. Bars show the total numbers of MHCII+CD11c+ cells, CD11c+CD8α+ cells and CD11c+CD8α-CD103+ in draining lymph nodes of each mouse. Data are shown as mean ± SE, and un-paired t test was conducted between the two groups. *P<0.05; **P<0.01.
We used AF-BSA as a model antigen to confirm antigen uptake and delivery in lymph nodes. FCM analysis showed that 33% of CD11c+ cells were AF-BSA positive when free AF-BSA added, but more than 65% of CD11c+ cells were positive for AF-BSA when BMDCs were conditioned with LPMan-BSA containing 5 μg/ml BSA (Figure 2B). Adding different concentration of CL097 into LPMan-BSA did not alter the antigen-internalization capacity (Figure 2C). We injected 50 μl of LPMan-BSA/CL097 containing 5 μg of AF-BSA plus 5 μg of CL097, or the same amount of LPMan-BSA subcutaneously into mice. The encapsulated antigens were well drained to lymph nodes and taken up by CD11c+ cells 2 days later (Figure 2D). In the presence of CL097, the total numbers of MHCII+CD11c+, particularly CD11c+CD8α+ and CD103+ DCs cells which were potent for priming CD8+ T cells, increased significantly in the draining lymph nodes (Figure 2E).
GPC3 nanovaccines containing CL097 are efficiently processed in ER-Golgi system of migratory DCs for activating CD4+ T and CD8+ T cells
BMDCs were conditioned with LPMan-BSA/CL097 containing 5 μg/ml AF-BSA plus 0.5 μg/ml CL097, or LPMan-BSA containing 5 μg/ml AF-BSA or free AF-BSA for 3 h. Immunofluorescence microscopy analysis showed that the internalized antigen delivered by LPMan was mainly co-localized with early endosomes (EEA-1), inside the ER (Grp78) and Golgi apparatus (Giantin), particularly in the presence of CL097 (Figure 3Aa-c). Compared with free AF-BSA-treated BMDCs, internalized antigen delivered by LPMan was hardly detected within lysosomes (as indicated by LAMP-1) (Figure 3Ad).
Figure 3.
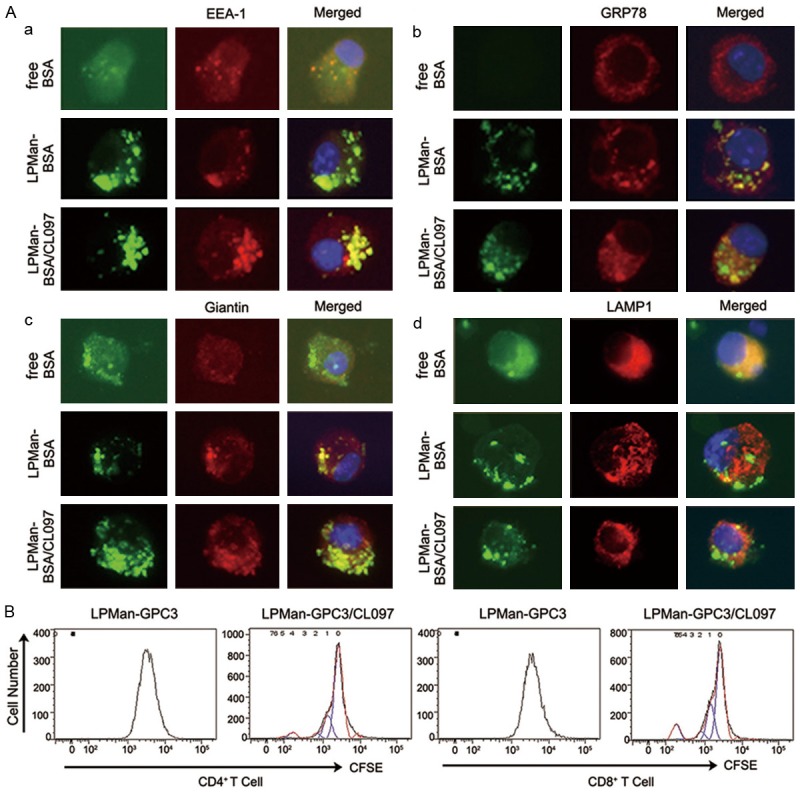
Fate of internalized antigens in DCs delivered by mannosylated liposomes. A. AF488-labeled BSA (BSA, green) was used a model antigen. BMDCs were treated for 3 h with 5 μg/ml BSA alone, or LPMan-BSA containing 5 μg/ml BSA, or LPMan-BSA/CL097 containing 5 μg/ml-BSA plus 0.5 μg/ml-CL097. The cells were washed and placed on poly-L-lysine-coated slides and then were fixed with 2% paraformaldehyde for 10 min. The cells were stained with Cy3-labeled (red) antibodies of anti-EEA-1 (a), anti-Grp78 (b), anti-Giantin (c) or anti-LAMP1 (d). One representative experiment of five is shown. B. BMDCs were treated with LPMan-GPC3/CL097 containing 5 μg/ml GPC3 plus 0.5 μg/ml CL097, or same amount of LPMan-GPC3 containing 5 μg/ml GPC3 for 24 h. Untouched T cells were isolated from naïve mice and labeled with 5 μM CFSE. The two cell populations were co-cultured at the ratio of 1-BMDC to 20-T cells for 5 days. CD4+ T cell (left panel) and CD8+ T cell (right panel) proliferation levels were determined by FCM (one representative experiment out of three).
To verify the effect of LPMan-GPC3/CL097-conditioned migratory DCs in priming naïve T cells for the generation of GPC3-specific T cells, we treated BMDCs with 5 μg/ml of LPMan-GPC3/CL097, or LPMan-GPC3, or free GPC3 for 24 h. Negatively selected T cells were labeled with CFSE and co-cultured with the above conditioned BMDCs for 5 days. T-cell proliferation (both CD8+ T and CD4+ T cells) was only observed when the cells were stimulated with BMDCs, which were conditioned with LPMan-GPC3/CL097 (Figure 3B), indicating the antigen in LPMan-GPC3/CL097 could be processed professionally.
LPMan-GPC3/CL097 immunization prevents DEN-induced premalignant hepatocytes to tumor development in an autochthonous liver cancer murine model
A single postnatal injection of DEN induces hepatocyte DNA damage and results in mouse liver cancer, the development course of which is similar to human HCC [25]. The tumor progenitors have been identified to highly express GPC3, AFP and other malignant markers 2-3 months after DEN and develop to an HCC mass in the inflamed liver [27]. Because HCC mainly develops in an HBV background [28], we adopted the method to induce murine autochthonous liver cancer by injecting one dose of DEN to an HBV-transgenic mouse at 2 weeks old (Figure 4A). By wk6 after DEN, clusters of premalignant hepatocytes were observed with GPC3 over-expression (Figure 4B). Liver cancer nodules were firstly observed by wk14, and many cancer nodules developed by wk20 after DEN, with more than 90% of the tumor cells expressing GPC3 (Figure 4B).
Figure 4.
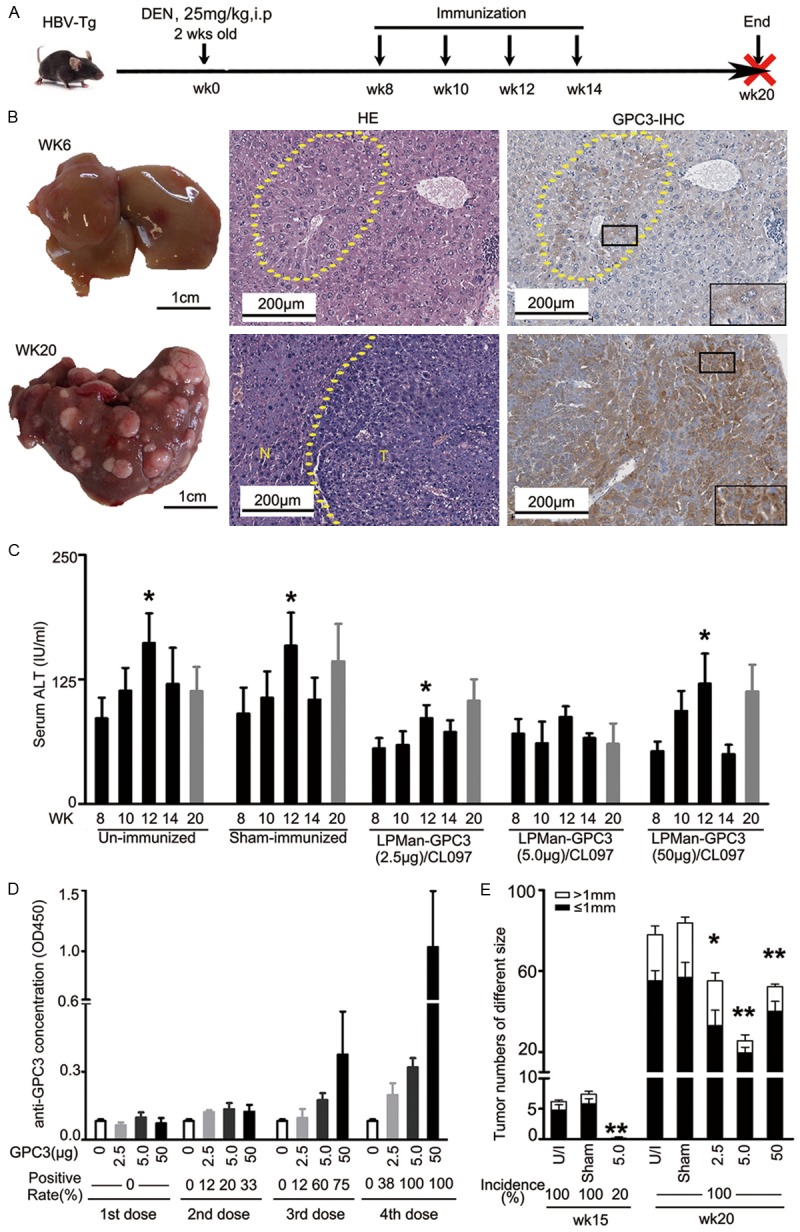
Effect of LPMan-GPC3/CL097 immunization on liver cancer development in a DEN-induced murine autochthonous liver cancer model. A. Experimental scheme. Male HBV-transgenic mice (HBV-Tg) received 25 mg/kg of DEN intraperitoneally at 2 weeks old. They were immunized 4 times with LPMan-GPC3/CL097. At each time point, each mouse received 2.5 μg (n=5), 5 μg (n=15) or 50 μg (n=5) of GPC3 plus 5 μg of CL097 starting 8 weeks after DEN injection (wk8). Sham-immunized mice received 5 μg of CL097/mouse in the form of LPMan-CL097 at the same time. The livers of DEN-administered mice were sampled at wk6, wk8, wk14, wk16 and wk20 after DEN. B. H&E staining, and anti-GPC3 immunohistochemistry staining of the mouse liver sampled at wk6 and wk20 after DEN administration. C. Variation of serum ALT determined at different time points, **P<0.05 compared with serum ALT determined at wk8. D. Serum anti-GPC3 antibody concentration at different time points. E. Tumor numbers of different sizes counted at wk15 and wk20 in the mice immunized with different dose of GPC3. Un-paired t test was conducted. *P<0.05 and **P<0.01 compared with sham-immunized mice.
Starting at wk8 after DEN, when GPC3 over-expressing premalignant hepatocyte clusters generated, we immunized the DEN-treated HBV-transgenic mice 4 times with LPMan-GPC3/CL097 every 2 weeks (Figure 4A). At the indicated time point, each mouse received 2.5 μg, 5 μg, or 50 μg of GPC3 with 5 μg of CL097. Sham-immunized mice were given LPMan/CL097 containing 5 μg of CL097 in the same way. Compared with un-immunized and sham-immunized mice, the elevation of serum ALT, which reflects the status of hepatocyte damage related to the accumulation of HBV surface antigens [24], was alleviated, particularly in the group of mice immunized with 5 μg of GPC3 plus 5 μg of CL097 (Figure 4C). Anti-GPC3 antibody began to be detectable after immunization for 2 doses (Figure 4D).
By wk20 after DEN all mice were sacrificed and all the mice developed liver cancer. However, compared with sham-immunized mice, the tumor burden in LPMan-GPC3/CL097 immunized mice was reduced significantly, with a maximal decrease in the mice that received 5 μg of GPC3 plus 5 μg of CL097 (Figure 4E). We repeated our experiments in another batch of DEN-treated HBV-transgenic mice to confirm the effect by administration 4 doses of LPMan-GPC3/CL097 containing 5 μg of GPC3 plus 5 μg of CL097. In each group, 5 mice were sacrificed 1 week after the last immunization (wk15 after DEN). All unimmunized (5/5) and sham-immunized (5/5) mice developed liver tumor nodules. Only 1/5 mice immunized with LPMan-GPC3/CL097 developed one small-volume tumor nodule (Figure 4E). Consistent with the previous observation, LPMan-GPC3/CL097-immunized mice developed significantly fewer tumor numbers than sham-immunized mice by wk20. No significant difference was observed in the tumor burden between sham group and LPMan-GPC3 or GPC3 immunized mice (Supplementary Figure 1).
LPMan-GPC3/CL097 immunization-induced GPC3-specific T cells eliminate GPC3-expressing tumor cells
Mouse splenocytes were collected 1 week after last immunization (wk15) and re-stimulated with 5 μg/ml purified GPC3 protein. The numbers of GPC3-specific CD4+ IFNγ-producing and CD8+ IFNγ-producing cells were all significantly increased in LPMan-GPC3/CL097-immunized than in sham-immunized mice. Among spleen CD3+ T cells, ~1.5% were GPC3-specific CD4+ IFNγ-producing cells, and ~0.25% were GPC3-specific CD8+ IFNγ-producing cells (Figure 5A). After the cells were stimulated with GPC3, IFNγ was increased by ~2.5 fold and Granzyme B was increased by ~1.6 fold in the LPMan-GPC3/CL097-immunized mice (Figure 5B).
Figure 5.
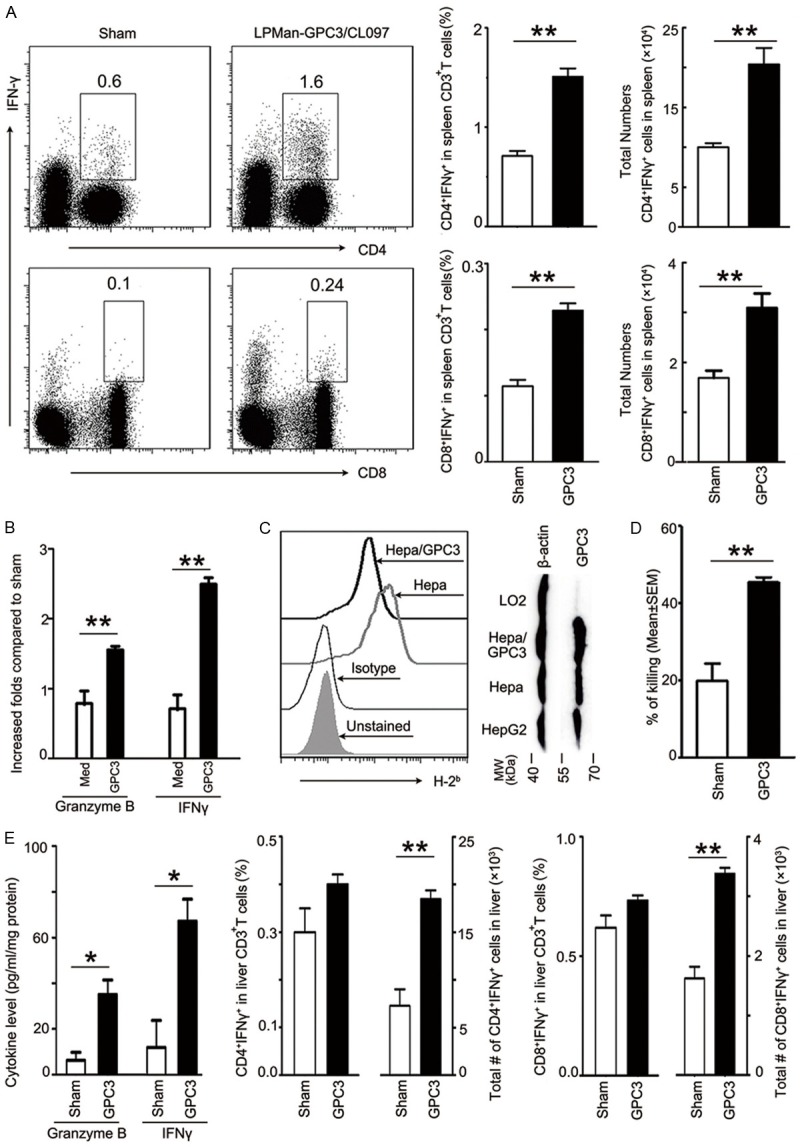
Immunization of LPMan-GPC3/CL097 induces potent immunity, eliminating GPC3-expressed tumor cells. A, B. Splenocytes (5×106/ml) were isolated from sham- or LPMan-GPC3/CL097-immunized mice and stimulated with 5 μg/ml GPC3 protein for 72 h. A. The percentage of IFNγ-producing CD4+ T and CD8+ T cells (based on CD3+ T gating) and absolute numbers in each spleen were presented. Dot plot profile is one representative of the five mice in each group. B. The increased folds of IFNγ and Granzyme B in the GPC3-stimulated splenocyte supernatants from LPMan-GPC3/CL097-immunized mice. C. Confirmation of GPC3 expression by western blotting and MHC-I surface expression by FCM using PE-labeled anti-H2Kb/H2Db-specific antibody on Hepa and Hepa/GPC3 cells which were constructed by transfecting Hepa1-6 with plasmid (Supplementary Figure 2). D. IHLs (as effector) were isolated from LPMan-GPC3/CL097-immunized or sham-immunized mice and co-cultured with CFSE-labeled Hepa/GPC3 cells (as target) for 4 h at the ratio of 40 effectors to 1 target. Percentage of double positivity for CFSE and PI cells was calculated. E. All mice were sacrificed at wk20 after DEN administration. The concentration of IFNγ and Granzyme B in the livers of sham- or LPMan-GPC3/CL097-immunized mice was measured using commercialized ELISA kits. Percentage and total numbers of IFN-γ+CD4+ T cells or IFN-γ+CD8+ T cells in the livers of sham- or LPMan-GPC3/CL097-immunized mice are presented. Un-paired t test was conducted. *P<0.05. **P<0.01.
To confirm the T-cell activity in eradicating GPC3-expressing tumor cells, we over-expressed GPC3 in Hepa1-6 cells (Hepa/GPC3) (Supplementary Figure 2) derived from C57BL/6 mice [8] and confirmed the H-2Kb/H2-Db expression in the cells (Figure 5C). IHLs were isolated and co-cultured with Hepa/GPC3 for 4 h. The antigen-specific cytotoxicity was increased significantly in the IHLs from LPMan-GPC3/CL097-immunized compare with that in sham-immunized mice (Figure 5D).
We collected liver interstitial fluid and quantified IFNγ and Granzyme B produced locally 6 weeks after the last immunization. Significantly higher amounts of these two cytokines were presented in LPMan-GPC3/CL097-immunized mice compared with that in sham-immunized mice (Figure 5E). After the IHLs were stimulated with GPC3, ~0.4% of CD4+ T cells and ~0.7% CD8+ T cells were GPC3-specific IFNγ-producing cells (Figure 5E).
Discussion
By synthesizing LPMan as a vaccine delivery system and incorporating TLR7/8 agonist CL097 as the adjuvant, we prepared the GPC3 nanovaccine, LPMan-GPC3/CL097. The vaccine efficiently targeted draining lymph nodes where naïve T cells reside, promoting DC maturation with increased surface expressions of MHC-I and MHC-II, and CCR7. The delivered antigen in DCs escaped from lysosomes and was processed professionally into the MHC-I antigen presentation pathway. Consequently, naïve T cells were primed and GPC3-specific CD4+ T and CD8+ T cells were de novo generated that could eradicate GPC3-expressing tumor cells. Immunization with 4 doses of LPMan-GPC3/CL097 containing 5 µg of GPC3 plus 5 µg of CL097 could prevent carcinogen-induced premalignant/malignant hepatic lesion development to cancer in HBV-transgenic mice. GPC3-specific T cells generated after immunization could eliminate GPC3-expressing tumor cells to prevent HCC. The current study provided a possible approach for cirrhotic patients with malignant precancerous lesion nodules.
DCs have been found to play fundamental roles in cell-based vaccination immunotherapy [29,30]. Our current results displayed that many key molecules involved in MHC-I presentation were significantly augmented when BMDCs were stimulated with GPC3 plus CL097. These results provided the molecular basis for TLR7/8 agonists in activating migratory DCs to induce antigenic peptide presentation on MHC-I molecules to prime naïve CD8+ T cells. Because naïve T cells do not directly patrol peripheral tissues, it is crucial to deliver antigen to draining lymph nodes to generate antigen-specific CD4+ and CD8+ T cells, which are both required to reject established tumor cells [13]. Previous studies have reported that mannosylated antigens without the addition of adjuvants could be processed in both MHC-I- and MHC-II-presenting pathways and induce the generation of antigen-specific CD4+ T and CD8+ T cells [19,31]. The addition of some TLR agonists is helpful to cross-present protein vaccines that are in a cell’s external environment to prime naïve CD8+ T cells [14]. Our functional assay validated that only the DCs conditioned with LPMan-GPC3/CL097, not LPMan-GPC3, could stimulate T-cell proliferation. Notably, the vaccine with CL097 increased the numbers of DCs in draining lymph nodes, particularly cells that have potent capacity of antigen cross-presentation i.e., CD11c+CD8α+ and CD11c+CD8α-CD103+ cells [14]. The current study indicated that the addition of CL097 in the vaccine formula was necessary to induce the de novo generation of GPC3-specific CD4+ and CD8+ T cells.
Promising results from clinical trials were observed in advanced HCC patients receiving GPC3-peptide vaccines [32] but not in anti-GPC3 specific antibody therapy [33]. Peptide vaccine needs the exact MHC-I molecule to induce monoclonal or oligoclonal T-cell populations. In addition, some of the synthetic short peptides predicted by computer may not be presented by the targeted malignant cells. The whole GPC3-protein vaccine could induce multiple clones of T cells when the protein is processed and presented by potent DCs in appropriate ways. Our results showed that LPMan-encapsulated GPC3 containing CL097 was processed professionally in migratory DCs, subsequently inducing the GPC3-specific CD4+ T and CD8+ T cell generation.
We injected DEN into HBV-transgenic mice to possibly simulate HCC development clinically in the HBV background. Dysplastic hepatocytes and GPC3-positive cell clusters were detected 6 weeks after DEN injection, which was recently confirmed as liver cancer progenitors [27]. Our results showed that immunization beginning at the stage when premalignant/malignant hepatocytes generated could prevent liver cancer development. Although the mice immunized with the GPC3 nanovaccine developed liver cancer, the tumor burden reduced significantly in the GPC3-immunized mice compared with sham-immunized mice. The HCC-associated antigen GPC3 proves as a useful target. Still better strategy is required to be developed in order to eradicate the established tumors, including induction of more potent GPC3-specific CD4+ T and CD8+ T cells, or in combination with the immune check-point inhibitors.
In summary, the GPC3 nanovaccine LPMan-GPC3/CL097 efficiently targeted draining lymph nodes where naïve T cells reside. When precancerous hepatocytes generated, immunization with LPMan-GPC3/CL097 induced specific T-cell immunity, eliminating GPC3-expressing tumor progenitors or tumor cells. Hence, when the malignant precancerous lesion nodules were presented in the cirrhotic liver, immunization with LPMan-GPC3/CL097 could prevent HCC development. The benefit of the vaccine must be evaluated in high-risk patients.
Acknowledgements
Current address for Yifan Ma: HRYZ BIO TECH Co., Shenzhen 518057, China. This work was supported by National Natural Science Foundation of China (No. 81172888, 81161120495 to CQ), CAMS Innovative Medicine (No. 2016-I2M-1-007 to CQ); State Key Projects Specialized for Infectious Diseases (No. 2012ZX10002008-001 to CQ), PUMC Innovation Fund for PhD students (No. 2014-1001-1014, 3332015061 to KC) and Shenzhen Science and Technology program (No. CYZZ20170331150956189 to YM). The sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- AF-BSA
alexa flour-488 labeled bovine serum albumin
- AFP
alpha-fetoprotein
- BMDC
bone marrow-derived dendritic cells
- BSA
bovine serum albumin
- CFSE
5-(and -6)-carboxyfluorescein diacetate succinimidyl ester
- DC
dendritic cells
- DEN
diethylnitrosamine
- ER
endoplasmic reticulum
- FCM
Flow cytometry
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GPC3
Glypican-3
- IHL
intrahepatic lymphocytes
- IFNγ
interferon gamma
- LPMan
mannosylated liposomes
- MHC
major histocompatibility complex
- TAP
transporter associated with antigen processing
- TAPBP
TAP binding protein
Supporting Information
References
- 1.Umar A. Cancer immunoprevention: a new approach to intercept cancer early. Cancer Prev Res (Phila) 2014;7:1067–1071. doi: 10.1158/1940-6207.CAPR-14-0213. [DOI] [PubMed] [Google Scholar]
- 2.Finn OJ, Beatty PL. Cancer immunoprevention. Curr Opin Immunol. 2016;39:52–58. doi: 10.1016/j.coi.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Tommaso L, Sangiovanni A, Borzio M, Park YN, Farinati F, Roncalli M. Advanced precancerous lesions in the liver. Best Pract Res Clin Gastroenterol. 2013;27:269–284. doi: 10.1016/j.bpg.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 5.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Muller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Bruck AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Holler C, Utikal J, Huber C, Loquai C, Tureci O. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, He H, Zang M, Wu Q, Zhao H, Lu Ll, Ma P, Zheng H, Wang N, Zhang Y, He S, Chen X, Wu Z, Wang X, Cai J, Liu Z, Sun Z, Zeng YX, Qu C, Jiao Y. Genetic features of aflatoxin-associated hepatocellular carcinoma. Gastroenterology. 2017;153:249–262. e242. doi: 10.1053/j.gastro.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Grimm CF, Ortmann D, Mohr L, Michalak S, Krohne TU, Meckel S, Eisele S, Encke J, Blum HE, Geissler M. Mouse alpha-fetoproteinspecific DNA-based immunotherapy of hepatocellular carcinoma leads to tumor regression in mice. Gastroenterology. 2000;119:1104–1112. doi: 10.1053/gast.2000.18157. [DOI] [PubMed] [Google Scholar]
- 9.Hong Y, Peng Y, Guo ZS, Guevara-Patino J, Pang J, Butterfield LH, Mivechi NF, Munn DH, Bartlett DL, He Y. Epitope-optimized alphafetoprotein genetic vaccines prevent carcinogen-induced murine autochthonous hepatocellular carcinoma. Hepatology. 2014;59:1448–1458. doi: 10.1002/hep.26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geissler M, Mohr L, Weth R, Kohler G, Grimm CF, Krohne TU, von Weizsacker F, Blum HE. Immunotherapy directed against alpha-fetoprotein results in autoimmune liver disease during liver regeneration in mice. Gastroenterology. 2001;121:931–939. doi: 10.1053/gast.2001.28019. [DOI] [PubMed] [Google Scholar]
- 11.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280:2471–2476. doi: 10.1111/febs.12126. [DOI] [PubMed] [Google Scholar]
- 12.Di Tommaso L, Destro A, Seok JY, Balladore E, Terracciano L, Sangiovanni A, Iavarone M, Colombo M, Jang JJ, Yu E, Jin SY, Morenghi E, Park YN, Roncalli M. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol. 2009;50:746–754. doi: 10.1016/j.jhep.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 14.Cruz FM, Colbert JD, Merino E, Kriegsman BA, Rock KL. The biology and underlying mechanisms of cross-presentation of exogenous antigens on MHC-I molecules. Annu Rev Immunol. 2017;35:149–176. doi: 10.1146/annurev-immunol-041015-055254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kastenmuller K, Wille-Reece U, Lindsay RW, Trager LR, Darrah PA, Flynn BJ, Becker MR, Udey MC, Clausen BE, Igyarto BZ, Kaplan DH, Kastenmuller W, Germain RN, Seder RA. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest. 2011;121:1782–1796. doi: 10.1172/JCI45416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero P, Banchereau J, Bhardwaj N, Cockett M, Disis ML, Dranoff G, Gilboa E, Hammond SA, Hershberg R, Korman AJ, Kvistborg P, Melief C, Mellman I, Palucka AK, Redchenko I, Robins H, Sallusto F, Schenkelberg T, Schoenberger S, Sosman J, Tureci O, Van den Eynde B, Koff W, Coukos G. The human vaccines project: a roadmap for cancer vaccine development. Sci Transl Med. 2016;8:334ps9. doi: 10.1126/scitranslmed.aaf0685. [DOI] [PubMed] [Google Scholar]
- 18.Kalam MA, Khan AA, Alshamsan A. Non-invasive administration of biodegradable nanocarrier vaccines. Am J Transl Res. 2017;9:15–35. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Liu P, Zhuang Y, Li P, Jiang B, Pan H, Liu L, Cai L, Ma Y. Lymphatic-targeted cationic liposomes: a robust vaccine adjuvant for promoting long-term immunological memory. Vaccine. 2014;32:5475–5483. doi: 10.1016/j.vaccine.2014.07.081. [DOI] [PubMed] [Google Scholar]
- 20.Qu C, Nguyen V, Merad M, Randolph G. MHC class I/peptide transfer between dendritic cells overcomes poor cross-presentation by monocyte-derived APCs that engulf dying cells. J Immunol. 2009;182:3650–3659. doi: 10.4049/jimmunol.0801532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Chen K, Wu Z, Liu Y, Liu S, Zou Z, Chen SH, Qu C. Immunizations with hepatitis B viral antigens and a TLR7/8 agonist adjuvant induce antigen-specific immune responses in HBV-transgenic mice. Int J Infect Dis. 2014;29:31–36. doi: 10.1016/j.ijid.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colonystimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Chisari FV, Pinkert CA, Milich DR, Filippi P, McLachlan A, Palmiter RD, Brinster RL. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science. 1985;230:1157–1160. doi: 10.1126/science.3865369. [DOI] [PubMed] [Google Scholar]
- 25.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Du J, Liu L, Li Q, Rong W, Wang L, Wang Y, Zang M, Wu Z, Zhang Y, Qu C. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS One. 2012;7:e50035. doi: 10.1371/journal.pone.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He G, Dhar D, Nakagawa H, Font-Burgada J, Ogata H, Jiang Y, Shalapour S, Seki E, Yost SE, Jepsen K, Frazer KA, Harismendy O, Hatziapostolou M, Iliopoulos D, Suetsugu A, Hoffman RM, Tateishi R, Koike K, Karin M. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013;155:384–396. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology. 2014;60:1767–1775. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palucka K, Banchereau J. Dendritic-cellbased therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q, Cui XX, Liang PF, Dou JX, Liu ZY, Sun WW. Immunotherapy with dendritic cells and cytokine-induced killer cells for MDAMB-231 breast cancer stem cells in nude mice. Am J Transl Res. 2016;8:2947–2955. [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng KC, Kalkanidis M, Pouniotis DS, Esparon S, Tang CK, Apostolopoulos V, Pietersz GA. Delivery of antigen using a novel mannosylated dendrimer potentiates immunogenicity in vitro and in vivo. Eur J Immunol. 2008;38:424–436. doi: 10.1002/eji.200737578. [DOI] [PubMed] [Google Scholar]
- 32.Sayem MA, Tomita Y, Yuno A, Hirayama M, Irie A, Tsukamoto H, Senju S, Yuba E, Yoshikawa T, Kono K, Nakatsura T, Nishimura Y. Identification of glypican-3-derived long peptides activating both CD8+ and CD4+ T cells; prolonged overall survival in cancer patients with Th cell response. Oncoimmunology. 2016;5:e1062209. doi: 10.1080/2162402X.2015.1062209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW, Ross P, Peron JM, Ebert O, Chan S, Poon TP, Colombo M, Okusaka T, Ryoo BY, Minguez B, Tanaka T, Ohtomo T, Ukrainskyj S, Boisserie F, Rutman O, Chen YC, Xu C, Shochat E, Jukofsky L, Reis B, Chen G, Di Laurenzio L, Lee R, Yen CJ. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol. 2016;65:289–295. doi: 10.1016/j.jhep.2016.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


