Abstract
Nuclear receptor-related factor 1 (Nurr1) has a crucial role in the development and maturation of mesencephalic dopamine (DA) neurons and also plays a protective role in maintenance of DA neurons by inhibiting the activation of microglia and astrocyte. Moreover, the mutations in Nurr1 gene are associated with familial Parkinson’s disease (PD), suggested that Nurr1 modulation is a potential therapeutic target for PD. This study examines the therapeutic effects of transplantation of Nurr1 gene-modified bone marrow mesenchymal stem cells (MSCs) on 6-hydroxydopamine (6-OHDA)-induced PD rat models. MSCs were transduced with lentivirus expressing Nurr1 gene and then intrastriatally transplanted into PD rats. Our results showed that Nurr1 gene-modified MSCs overexpress and secrete Nurr1 protein in vitro and also survive and migrate in the brain. Four weeks after transplantation Nurr1 gene-modified MSCs dramatically ameliorated the abnormal behavior of PD rats and increased the numbers of tyrosine hydroxylase (TH)-positive cells in the substantia nigra (SN) and TH-positive fibers in the striatum, inhibited the activation of glial cells, and reduced the expression of inflammatory factors in the SN. Taken together, these findings suggest that intrastriatal transplantation of lentiviral vector mediated Nurr1 gene-modified MSCs has notable therapeutic effect for PD rats.
Keywords: Mesenchymal stem cells, Nurr1, gene therapy, lentivirus vectors, Parkinson’s disease, intrastriatal transplantation
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, characterized by the progressive degeneration of dopaminergic neurons (DA neurons) in the substantia nigra (SN), resulting in severe motor dysfunction, including tremor, rigidity, slowness of movement, and postural instability [1,2]. The cause of PD is not clear. The main strategy for PD treatment is to restore the level of DA and compensate the functional changes of the basal ganglia caused by the loss of DA. However, all current therapeutic approaches for PD do not slow down or halt the progress of the disease, because the missing DA neurons cannot be restored. There is an urgent need for new drugs and new methods of treating PD [3]. Cell replacement therapy is considered to be one of the best candidate approaches for cellular therapies because of the pathological features of PD’s single cell damage [4]. In recent years, the research of stem cells in the treatment of PD by us and other researchers is increasing [5-9]. In principle, the efficacy of cell-based transplantation for PD animals and patients would be apparent, but this therapeutic strategy does not become a routine treatment for PD because of the limited donor, long term efficacy, tumorigenicity and ethical issues.
Mesenchymal stem cells (MSCs) have the advantages of easy acquirement, self-renewing ability, multilineage potential [10-12], immunosuppressive properties and low immunogenicity, and secretory function [13,14]. Furthermore, they possess a non-tumorigenic potential and are free of ethical restriction for cell transplantation [15]. Without doubt, MSCs hold great promise for clinical applications. Previous studies already exhibited their neural differentiation abilities [10,16,17], and confirmed their potential use in the treatment of PD [5,18,19]. Although there are a number of reports on the differentiation of bone marrow MSCs into DA neurons, there still exist some obstacles to the application, such as low directional differentiation rate of MSCs and long-term viability of MSCs-derived DA neurons. Therefore, it is necessary to improve the methods for inducing bone marrow MSCs to DA neurons in vivo and to search for a more favorable strategy of cell-based transplantation.
Nuclear receptor-related factor 1 (Nurr1), a transcription factor, belongs to the orphan nuclear receptor family. It is important for DA neuron development and maturation [20]. Nurr1 governs DA formation by inducing expression of tyrosine hydroxylase (TH), vesicular monoamine transporter (VMAT2), dopamine transporter (DAT), and aromatic amino acid decarboxylase (AADC) [21-25]. Nurr1 deficiency in developing or mature DA neurons resulted in loss of striatal DA, loss of expression of mDA neuron-specific marker gene, and neuronal degeneration, and associated with PD or exaggerated PD severity [20,26], suggested that Nurr1 might be a potential therapeutic target for PD [27]. Moreover, Nurr1 also plays a protective role in DA neuron maintenance by inhibiting the activation of microglia and astrocyte [28,29]. There were some reports that over-expression of Nurr1 induced neural precursor cells to differentiate into DA neurons [30-33].
In view of the distinctive features of MSCs and the crucial roles of Nurr1 in development differentiation and maintenance of DA neurons, intrastriatal transplantation of MSCs engineered to overexpress Nurr1 via recombinant lentivirus was used to investigate effects on 6-hydroxydopamine (6-OHDA) induced PD rats in respect to survival, differentiation and the therapeutical effect of such cells. Our results showed that Nurr1 gene-modified MSCs can overexpress and secrete Nurr1 protein in vitro and can survive and migrate in the brain. Intrastriatal transplantation of Nurr1 gene-modified MSCs improved the rotational behavior of PD rats and increased the numbers of TH-positive cells in SN and TH-positive fibers in the striatum, inhibited the activation of glial cells, and reduced the expression of inflammatory factors in the SN. These results provide proof that Nurr1 gene-modified MSCs intrastriatal transplantation has a promising prospect for application in PD.
Materials and methods
Culture and determination of rat MSCs
All experiments associated with rats were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the Animal Ethics Committee of Weifang Medical University. Rat MSCs were isolated from the bone marrow of 6-week-old Sprague-Dawley (SD) rats as described [5]. Briefly, the rats were anesthetized with chloral hydrate, and whole bone marrow was extracted from the tibias and femurs and was suspended in low-glucose Dulbecco’s Modified Eagle’s Medium (L-DMEM) (Gibco, USA), containing 10% fetal bovine serum (Gibco, USA), and maintained in a HERA Cell 150 CO2 incubator (Heraeus, Germany) at 37°C under 5% CO2. The final concentration of cells in culture flasks (Corning, USA) was at 5 × 106/mL. After 24 h, the medium was changed to remove the nonadherent cells, and later the medium was changed every 3 days. When the cells reached 80% confluence, they were harvested using 0.25% trypsin (Sigma, USA) and subcultured at a ratio of 1:3. At passage 3, cells were characterized by immunocytochemistry and used for gene transfection and transplantation.
By staining the cell surface antigen markers, the features of the bone marrow-derived MSCs were determined by immunocytochemical methods [10]. The isolated bone marrow-derived MSCs were approximately 100% positive for CD44 known mesenchymal stem cell markers, but negative for CD34 regarded as hematopoietic stem cell markers [34,35], indicating no contamination with hematopoietic stem cells.
Plasmids and virus vector GV287-Nurr1 production
Recombinant lentivirus containing rat Nurr1 gene was provided by Shanghai GeneChem. The gene of Nurr1 was amplified by PCR after designed primers. The primers of Nurr1 are: forward, 5’ GAG GAT CCC CGG GTA CCG GTC GCC ACC ATG CCT TGT GTT CAG GCG 3’, reverse, 5’ TCC TTG TAG TCC ATA CCG AAA GGT AAG GTG TCC AGG 3’, The size of the amplified Nurr1 gene was 1838 bp. Then GFP-expressing GV287 lentivirus vector inserted by Age I/Age I digestion to construct the plasmids GV287-Nurr1. The clone obtained was confirmed by DNA sequencing. Then the vector mixture of GV287-Nurr1 20 μg, pHelper 1.0 15 μg and pHelper 2.0 10 μg was used for packaging lentivirus vector. The lentivirus vectors were used for the transient transfection of 293T cells by liposome transfection method.
Transfection of MSCs
The third passages of MSCs were used in the following experiments. MSCs were transduced with LV-Nurr1 (multiplicity of infection, MOI: 50) lentivirus vectors in the presence of polybrene (5-10 µg/mL) for 12 h. The titer of lentivirus vector GV287-Nurr1 was 2 × 108 transforming units (TU)/mL. Packaging and production of the GV287 was performed. The empty backbone vectors (GV287) were used as negative controls. Expression of Nurr1 protein in the cells and supernatant of MSCs was examined by Western blot after viral infection 72 h.
Animal grouping and treatment strategy
Male Sprague Dawley (SD) rats (200-250 g) received unilateral stereotaxic injections of 15 μg 6-OHDA (Sigma, USA) (2.5 μg/μL, dissolved in 0.2% ascorbate-saline) into the right striatum with two-point injection of 6-OHDA in two spots. In accordance with the atlas of Paxinos and Watson [36], the right caudate putamen and right globus pallidus coordinate system were determined as following: caudate putamen: AP + 0.7 mm, ML 2.6 mm, DV -4.5/-5.5 mm; globus pallidus: AP + 4.4 mm, ML 1.2 mm, DV -4.5/-5.5 mm. The injection rate was 1.0 μL/min, and the needle was left in place for 10 min after each injection and then slowly withdrawn at 1.0 mm/min.
All PD rats (n = 45) were randomly divided into three groups based on the table of random numbers. Saline group (PD + normal saline, n = 15): PD rats that received normal saline 6 μL; Lv-MSCs group (PD + GV287-MSCs, n = 15): PD rats that received 3-5 × 104/µL GV287-MSCs; Lv-Nurr1-MSCs group (PD + GV287-Nurr1-MSCs, n = 15): PD rats that received 3-5 × 104/µL GV287-Nurr1-MSCs.
PD rats were anesthetized by intraperitoneal (i. p.) injection of chloral hydrate and placed in a stereotaxic apparatus. Cells (GV287-MSCs or GV287-Nurr1-MSCs) were harvested by trypsinization, washed twice, and resuspended in serum-free medium at 3-5 × 104/μL. A total of 2-3 × 105 cells/5 μL MSCs were injected into striatum ipsilateral to the unilateral 6-OHDA injection. Cell suspensions were implanted into the striatum of the 6-OHDA-injected side hemisphere with a 25 µL Hamilton syringe (Hamilton, USA) at the following coordinates: AP + 0.2 mm, ML 3.0 mm, DV -4.5/-5.5 mm. The syringe was inserted to the deepest position (5.5 mm), and 3 μL cell suspension was injected. The needle was left in place for 2 min, withdrawn to 4.5 mm, and another 3 μL cell suspension was injected. The syringe was left in place for 10 minutes before slowly extracting completely. In place of cell suspensions, normal saline was injected into the rats in the saline group. The animals’ treatment strategies see Figure 1.
Figure 1.
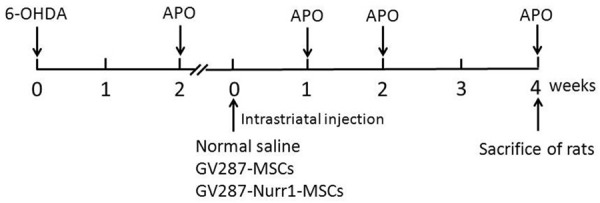
In vivo study chronology. Rats received 6-OHDA injection (left “0”), and post-lesion impairment was evaluated by intraperitoneal injection of apomorphine at the end of 2 weeks. One day later, PD rats were divided into 3 groups (Saline group, Lv-MSCs group and Lv-Nurr1-MSCs group). The PD rats in each group were intrastriatal injection of normal saline, GV287-MSCs and GV287-Nurr1-MSCs, respectively. The number of animals in each group was five. 1, 2 and 4 weeks after cell transplantation the rats in each group were examined the behavioral changes. Rats were sacrificed for immunohistochemical, RT-PCR and Western blot studies at the end of 4 weeks post-cell transplantation.
Apomorphine-induced rotations
Two weeks after 6-OHDA injection or 1, 2 and 4 weeks after the cells intrastriatal injection, rotational behavior of rats were examined as previously described [5]. Briefly, rats were injected intraperitoneally with apomorphine (Sigma, USA) (0.5 mg/kg), and only those rats exhibiting ≥7 r/min during a 30 min period toward the contralateral side after apomorphine treatment were regarded as successful PD rat models and used for further experiments. Rat behavioral analysis has been assessed by two experimenters in a blinded fashion to minimize bias.
Immunohistochemical detection
Four weeks after cell transplantation, PD rats were deeply anesthetized with chloral hydrate and perfused transcardially with 0.1 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde fixative. The brains transferred to a 4% paraformaldehyde solution, post-fixed for 24 h at 4°C and then immersed in 30% sucrose until they sank. Five rat brains in each group were embedded in paraffin and cut into 5 μm sections.
To investigate the expression of Nurr1 and TH in both substantia nigra and striatum, and the expression of CD11b, GFAP, COX-2, TNF-α in substantia nigra, immunohistochemical staining was performed. The paraffin-embedded sections were deparaffinized and rehydrated, followed by washes in PBS and 0.01 mol/L citrate buffer for antigen retrieval. Brain sections were blocked in 10% normal goat serum for 30 min, and then incubated overnight at 4°C with the following primary antibodies: Nurr1 (1:200, rabbit, Abcam, ab93332, RRID: AB_10563537); TH (1:1000, mouse, Sigma, T2928, RRID: AB_477569); DAT (1:150, rabbit, Santa Cruz, sc-14002, RRID: AB_2190287); CD11b (1:300, mouse, Chemicon, CBL1512, RRID: AB_93253); GFAP (1:300, rabbit, Santa Cruz, sc-9065, RRID: AB_641022); COX-2 (1:100, goat, Santa Cruz, sc-1746, RRID: AB_631310); TNF-α (1:70, goat, Santa Cruz, sc-1350, RRID: AB_2204365). After a thorough rinse, sections were incubated with biotinylated secondary antibodies (Invitrogen, USA) at 37°C for 30 min, followed by 30 min of incubation in avidin-biotinylated peroxidase complex (Invitrogen, USA) at 37°C. After being rinsed in PBS, all sections were visualized with DAB.
Western blot
Western blot was performed to assess whether Nurr1 was expressed and secreted by MSCs infected with GV287-Nurr1 virus and to examine the expression changes of Nurr1, TH and DAT in SN tissue after the Nurr1 gene-modified MSCs transplantation.
The MSCs infected with GV287-Nurr1 virus were cultured for 72 h and total proteins were extracted using RIPA lysis buffer (Takara). Protein concentrations were estimated using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Pierce, Rockford, Illinois, United States). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, Massachusetts, United States). The membranes were blocked using 5% non-fat dry milk in Tris-buffered saline and Tween 20 (TBST) and then incubated with primary antibodies directed against Nurr1 (1:300, Abcam, Cambridge, United Kingdom), β-actin (1:1000, Santa Cruz, USA, sc-69879, RRID: AB_1119529) at 4°C overnight. The secondary antibodies were applied for 2 h at room temperature. The immunoblots were visualized using enhanced chemiluminescence (ECL; Thermo Scientific). The absorbances were quantified by using Quantity One 1D Analysis Software (Bio-Rad), and the relative level of Nurr1 protein was determined as the ratio of Nurr1 to β-actin protein level.
Four weeks after cell transplantation, protein extracts were prepared from the midbrain of each group, and the Western blot procedure the same as above. The primary antibodies are rabbit anti-Nurr1 (1:400, Abcam, Cambridge, United Kingdom), mouse anti-TH (1:2000, Sigma, USA), rabbit anti-DAT (1:200, Santa Cruz). β-actin (1:1000, Santa Cruz) served as a loading control.
Reverse transcription-PCR
Total RNA was extracted from the lesion side of the substantia nigra of each group using TRIzol reagent (Sangon Biotech (Shanghai) Co., Ltd.) following the manufacturer’s protocols. About 2 μg of total RNA were reversely transcribed in a 20 μL reaction using the AMV reverse transcription system (Sangon Biotech (Shanghai) Co., Ltd.). Polymerase chain reaction (PCR) was performed in a 50 μL reaction solution containing 25 μL of Premix TaqTM (TaKaRa), 1 μL of forward primer, 1 μL of reverse primer, 2 μL of template cDNA, and 21 μL RNase-free ddH2O. Sequences of the primers are shown in Table 1. The PCR products were separated by electrophoresis on 1% agarose gel, stained with ethidium bromide. The gels were scanned and qualified using Image J software. The mRNA level relative to that of GAPDH was calculated.
Table 1.
Primer sequences used in reverse transcription polymerase chain reaction
| Gene | Sequences of primers | Annealing condition (°C) | Size (bp) | |
|---|---|---|---|---|
| Nurr1 | Forward primer | 5’-GCTAGCTGAAGCCATGCCTTGTGT-3’ | 58.7 | 1835 |
| Reverse primer | 5’-CTCGAGACGTGCATGGGAGAAAGT-3’ | |||
| TH | Forward primer | 5’-TTATGGTGCAGGGCTGCTGTCTT-3’ | 60 | 130 |
| Reverse primer | 5’-CACAGGCTGGTAGGTTTGATCTTGG-3’ | |||
| DAT | Forward primer | 5’-TGGGTTTGGAGTGCTGATTGCC-3’ | 62 | 130 |
| Reverse primer | 5’-GAAGGAGAAGACGACGAAGCCAGAG-3’ | |||
| CD11b | Forward primer | 5’-GCGAGAAGGAGACATCCAGAGCA-3’ | 58.3 | 290 |
| Reverse primer | 5’-CTGTGAAGATCCTCTGAGCCTCCAT-3’ | |||
| GFAP | Forward primer | 5’-GTTGTGAAGGTCTATTCCTGGTTGCTC-3’ | 59 | 208 |
| Reverse primer | 5’-CAAACTTTCAAACAGGATGGACGCT-3’ | |||
| COX-2 | Forward primer | 5’-AGTGGGATGACGAGCGACTGTTC-3’ | 58 | 210 |
| Reverse primer | 5’-AGCAGCGGATGCCAGTGATAGAG-3’ | |||
| TNF-α | Forward primer | 5’-CACCCACACCGTCAGCCGAT-3’ | 59 | 266 |
| Reverse primer | 5’-GATGAACACGCCAGTCGCCTC-3’ | |||
| GAPDH | Forward primer | 5’-TGTCGTGGAGTCTACTGGCGTCTT-3’ | 62 | 278 |
| Reverse primer | 5’-TTCAGCTCTGGGATGACCTT-3’ | |||
Statistical analysis
Data (such as cell counting, average optical densities detection) were analyzed by one-way ANOVA or two-way ANOVA. Statistical analysis was performed with SPSS 13.0 software (SPSS, Chicago, IL, USA). All data were presented as mean ± SEM. Statistical significances were calculated using F-test. Statistical significance was defined at P<0.05.
Results
Morphological characteristics of rat MSCs
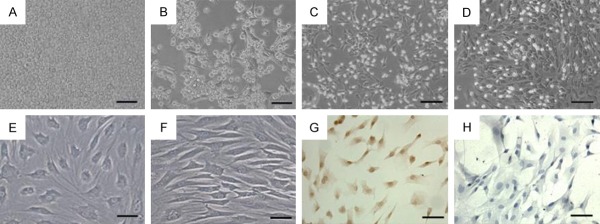
Under the inverted microscope, the bone marrow cells were small and round and suspended in culture fluid just after inoculation (Figure 2A). After 24 h some cells adhering in the flask were visible (Figure 2B). After plating for 72 h, the attached cells were proliferating and dividing. These cells showed a spindle or polygonal shape (Figure 2C). After about 10 days, the cells reached 80% confluence before being subcultured (Figure 2D). After 3-4 passages, the cells presented a relatively homogeneous spindle shape (Figure 2E), and were arranged in parallel or spiral (Figure 2F). The adhering cells were positive for CD44 (Figure 2G) but negative for CD34 (Figure 2H) by immunocytochemical staining, indicating that they are bone marrow MSCs.
Figure 2.
Morphology and surface antigen of MSCs. The bone marrow cells were small and round and suspended in culture fluid just after inoculation (A). After 24 h some cells adhering in the flask were visible (B). After plating for 72 h, the attached cells were proliferating and dividing, and the cells showed a spindle or polygonal shape (C). After about 10 days, the cells reached 80% confluence before being subcultured (D). After 3-4 passages, the cells presented a relatively homogeneous spindle shape (E), and were arranged in parallel or spiral (F). Cells were positive for CD44 (G) and negative for CD34 (H). Scale bar: (A-D) = 100 μm; (E-H) = 50 μm.
Expression of Nurr1 gene in transduced rat MSCs
When MOI = 50, the GV287-Nurr1 infection efficiency was the highest, and the efficiency of infection reached about 90% after 72 h. MSCs differentiated into neuron-like cells with monopolar or multipolar processes under fluorescence microscope (Figure 3A-C), but the MSCs transduced with the blank vector underwent no obvious morphological changes (Figure 3D). In order to determine the effect of Nurr1 expression in MSCs transduced with GV287-Nurr1, Western-blot analysis was performed to detect the effectiveness of transgene expression. The results showed that Nurr1 specific protein bands were detected in MSCs and supernatant before and after GV287-Nurr1 infection, but after GV287-Nurr1 infection, MSCs produced more Nurr1 protein (Figure 3E). The average density of Nurr1 and β-actin specific bands was assessed and the relative levels of Nurr1 protein after normalizing to β-actin increased more than ten times in MSCs after GV287-Nurr1 infection that were significantly increased compared with control MSCs. The Nurr1 protein was almost undetectable in the supernatant of MSCs, while it was observed clearly in that of GV287-Nurr1 transduced MSCs (Figure 3F). The results indicated that lentivirus GV287 mediated Nurr1 gene-modified MSCs over-express and secrete Nurr1.
Figure 3.
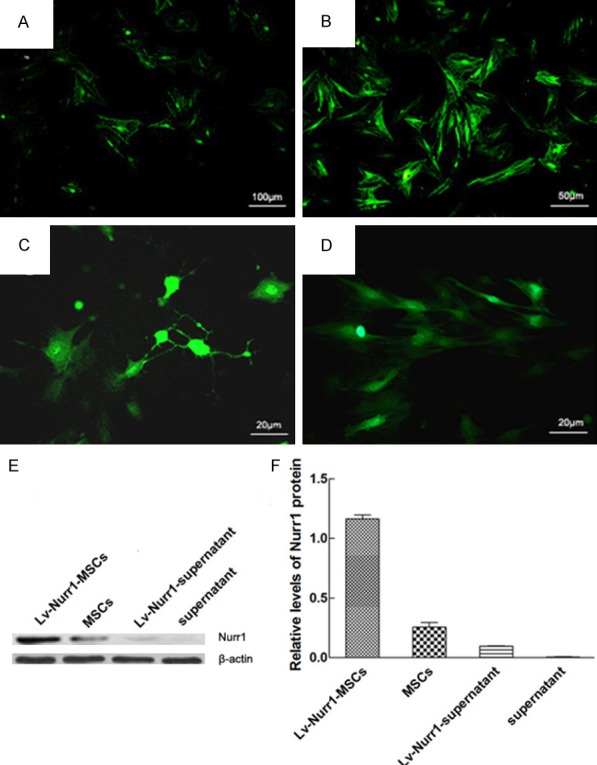
Expression of Nurr1 gene in transduced rat MSCs. The morphological features of MSCs infected by lentivirus GV287-Nurr1 (MOI = 50) at different times (A-C) and MSCs transduced by lentivirus GV287 (D). MSCs infected by lentivirus GV287-Nurr1 after 24 h (A); MSCs infected by lentivirus GV287-Nurr1 after 48 h (B); MSCs infected by lentivirus GV287-Nurr1 after 72 h, and differentiated into neuron-like cells with monopolar or multipolar processes (C); MSCs infected by lentivirus GV287 after 72 h (D). Representative Western blot of Nurr1 protein in cell extracts and supernatant from MSCs after transduction with or without GV287-Nurr1. β-actin was used as an internal control (E). The relative intensity of Nurr1 protein was analyzed by Western blot (F). Data are presented as mean ± SEM. Scale bar: (A) = 100 μm; (B) = 50 μm; (C, D) = 20 μm.
Expression of Nurr1, TH and DAT in the substantia nigra of PD rats
The microphotographs of Nurr1 immunoreactivity (ir) showed that Nurr1-ir products located in the neuronal nucleus of the substantia nigra as Chu Y reported [37]. Nurr1-positive cells were found not only in the substantia nigra, but also in the other brain regions. 6-OHDA resulted in the decrease of Nurr1-, TH- and DAT-positive cells. There were a lot of Nurr1-positive cells in the substantia nigra of the normal rats (0.912 ± 0.031, the ratio of the number of positive cells in bilateral substantia nigra), but less positive cells in the substantia nigra of PD rats (0.339 ± 0.023) (Figure 4A). TH-immunoreactive cells in the substantia nigra compacta pars displayed polygonal or pyramidal shapes, and DAT-immunoreactive cells were oval or triangular. There were many TH- and DAT-positive cells in the substantia nigra of control rats (0.908 ± 0.021) and (0.889 ± 0.029), but only a few TH-positive cells (0.279 ± 0.033) and a small number of DAT-positive cells in the substantia nigra of PD rats (0.346 ± 0.033) (Figure 4A). Compared with the normal rats, the number of the Nurr1-, TH- and DAT-positive cells in the substantia nigra of PD rats was significantly decreased (P<0.01).
Figure 4.
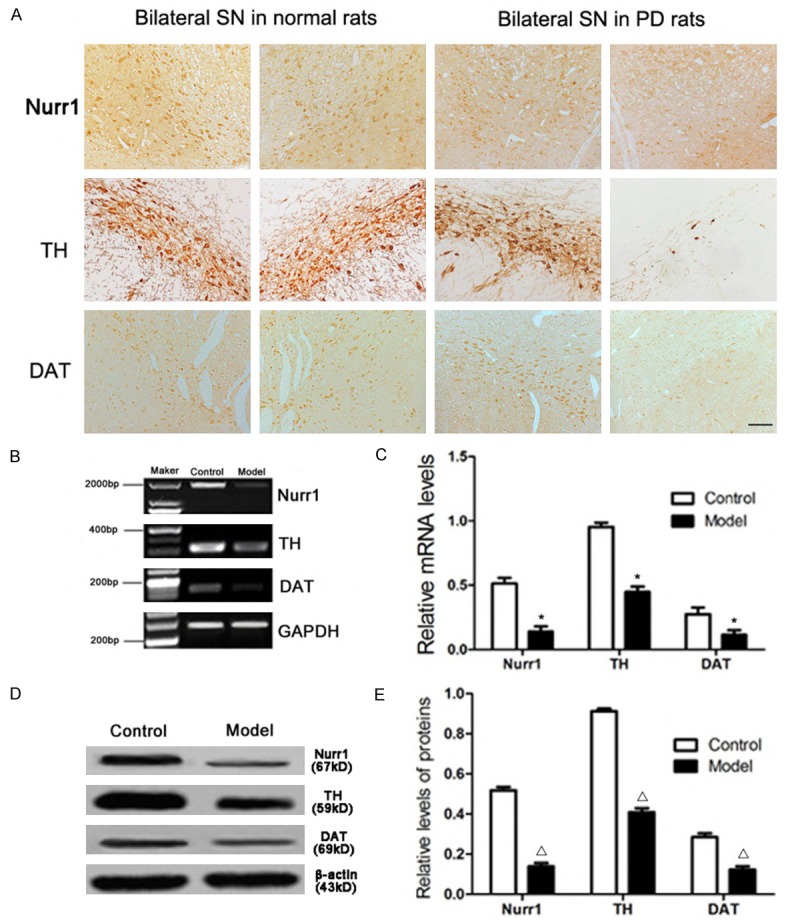
Expression of Nurr1, TH and DAT in the substantia nigra of PD rats detected by immunohistochemistry, RT-PCR and Western blot. Microphotographs showed the Nurr1, TH and DAT immunoreactive neurons (A). Representative RT-PCR of Nurr1, TH and DAT in normal and PD rats. GAPDH was used as an internal control (B). The relative mRNA level of Nurr1, TH and DAT was analyzed by RT-PCR (C). Representative Western blot of Nurr1, TH and DAT protein in normal and PD rats. β-actin was used as an internal control (D). The relative intensity of Nurr1, TH and DAT protein was analyzed by Western blot (E). Data are presented as mean ± SEM. *P<0.01, Δ P<0.01 versus PD rat. The number of rats in each group was as follows: control (n = 3), model (n = 5). Scale bar = 50 μm.
Message RNA expression analysis of Nurr1, TH and DAT was carried by reverse transcription PCR. The mRNA expression level of Nurr1, TH and DAT were reduced in PD rats compared with the normal rats (Figure 4B, 4C, *P<0.01). The similar pattern was also observed for the protein levels of Nurr1, TH and DAT in the substantia nigra in normal and PD rats by Western-blot (Figure 4D, 4E, Δ P<0.01).
Survival and migration and differentiation of the Lv-Nurr1-MSCs
Nurr1 is essential for early differentiation of midbrain DA neurons [19]. Nurr1 over-expression improved survival rate of MSCs and promote fetal bone marrow MSCs migration [38]. Four weeks post-injection, the transplanted cells could be easily observed by immunohistochemical staining in paraffin sections of the striatum, which was used as the target structure for the injection. The number of Nurr1-positive cells in the right striatum was significantly more than that in the left side. Lv-Nurr1-MSCs were detected at other parts of the brain, such as cerebral cortex and the contralateral brain. Some cells even have morphological characteristics similar to neurons (Figure 5A, 5B). Double immunofluorescence showed Nurr1/TH-positive cells (Figure 5C-E), and no Nurr1/DAT-positive cells were found.
Figure 5.
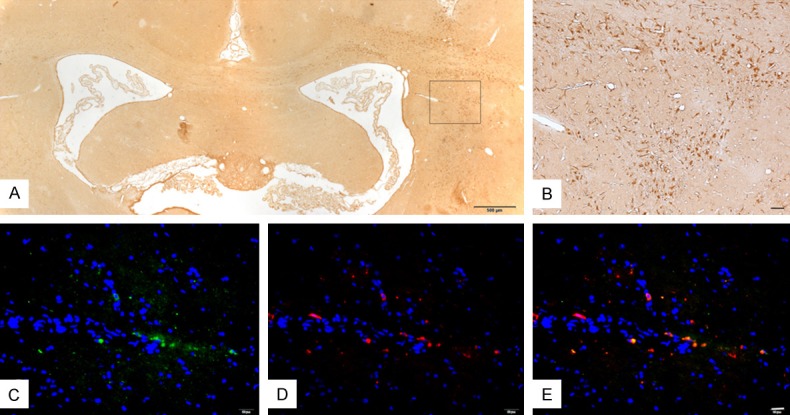
Survival and migration and differentiation of implanted cells in the rat brain of the Lv-Nurr1-MSCs group four weeks after transplantation by immunohistochemical and double immunofluorescence staining. Lv-Nurr1-MSCs were injected into the striatum with microsyringe. The number of Nurr1-positive cells in the right striatum was significantly more than that in the left side, and cell migration was visible. Nurr1-positive cells were in the different part of the brain. (A). Nurr1-positive cells were fusiform or polygonal, and some like neurons. (B) is the enlarged view of (A) (B). Double immunofluorescence staining of TH (red) and Nurr1 (green) and nucleus (blue) (C-E). The number of rats in LV-Nurr1-MSCs group was five. Scale bar: (A) = 500 μm; (B) = 50 μm; (C-E) = 20 μm.
Effects of Lv-Nurr1-MSCs on the survival of nigral DA neurons and their axons
There were many dense-distributed TH-immunoreactive cells in the bilateral SN of the normal rats (Figure 6A1). But only a few in the SN of lesion side of the saline group (Figure 6A2). Four weeks after cell transplantation, the ratio of the cell number of lesion side/normal side in the Lv-Nurr1-MSCs group (0.903 ± 0.037) (Figure 6A4) is significantly greater than that of the saline group (0.279 ± 0.033) (P<0.01) and Lv-MSCs group (0.446 ± 0.029) (Figure 6A3) (P<0.05). Similar difference of TH-immunoreactive fiber in the striatum, which is the projecting site of DA neurons from the SN, was observed (Figure 6B1-B4). The ratio of the average optical density of TH-positive terminals in the striatum in the lesion and normal sides of the Lv-Nurr1-MSCs group (0.049 ± 0.014) was significantly higher than that of the saline group (0.026 ± 0.009) (P<0.01) and the Lv-MSCs group (0.046 ± 0.012) (P<0.05). These results indicated that Lv-Nurr1-MSCs transplantation significantly improved the functions of the nigrastriatal system.
Figure 6.
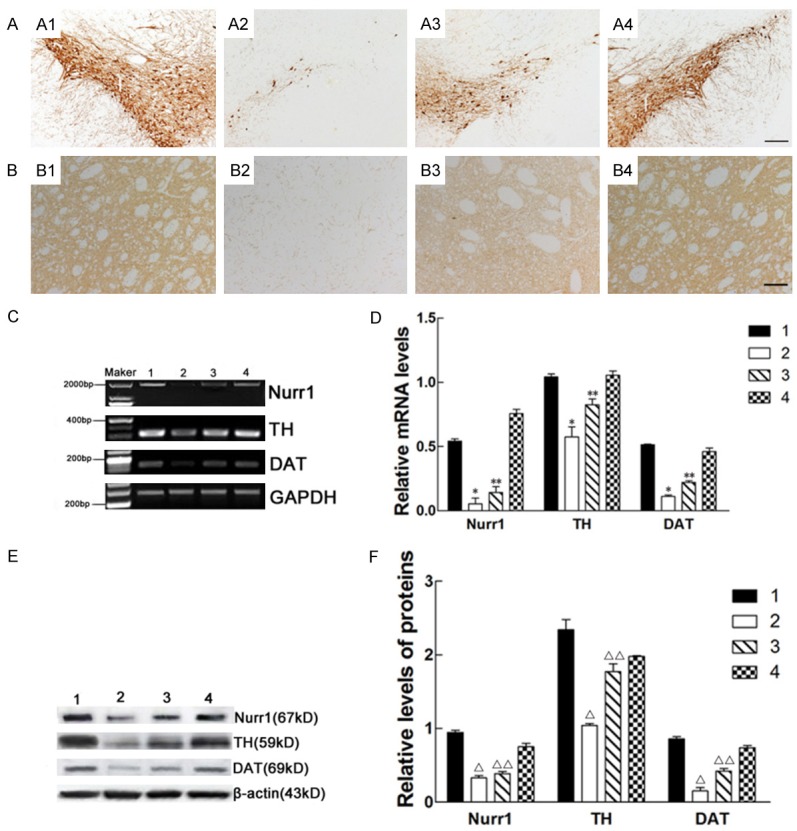
Effects of Lv-Nurr1-MSCs on the number of nigral TH-positive neurons, the density of striatal TH-positive fibers, and the expression of Nurr1, TH and DAT in mRNA and protein level of the substantia nigra. The number of nigral TH-positive neurons and the density of striatal TH-positive fibers in the group of Lv-Nurr1-MSCs-injected were more than other group. TH-positive cells in the SN (A1-A4); TH-positive fiber in the striatum (The data has been shown in the text) (B1-B4); Representative RT-PCR of Nurr1, TH and DAT in the SN. GAPDH was used as an internal control (C). The relative mRNA level of Nurr1, TH and DAT was analyzed by RT-PCR (*P<0.01, **P<0.05) (D). Representative Western blot of Nurr1, TH and DAT protein in the striatum. β-actin was used as an internal control (E). The relative intensity of Nurr1, TH and DAT protein was analyzed by Western blot (Δ P<0.01, ΔΔ P<0.05) (F). (A) Substantia nigra; (B) Striatum; 1: normal side of the saline group; 2: saline-injected side of the saline group; 3: Lv-MSCs-injected side of the Lv-MSCs group; 4: Lv-Nurr1-MSCs-injected side of the Lv-Nurr1-MSCs group. The number of rats in each group was five. Scale bar: (A1-A4) = 100 μm; (B1-B4) = 50 μm.
Effects of Lv-Nurr1-MSCs on the expression of Nurr1, TH and DAT mRNA and protein levels in substantia nigra
Nurr1 is critical for the development and maintenance of midbrain dopaminergic neurons [39], and the expression of Nurr1 is initiated in post-mitotic midbrain dopaminergic neuron precursors, before the expression of TH [40,41] and DAT [42]. RT-PCR results showed there was a constitutive expression of Nurr1, TH and DAT mRNA in SN, and the expression of these mRNA in Lv-Nurr1-MSCs group was significantly greater than that in the saline group (P<0.01) and the Lv-MSCs group (P<0.05) (Figure 6C, 6D). Western blots confirmed that significant levels of Nurr1, TH and DAT protein were produced in the SN of rats in the Lv-Nurr1-MSCs group, more than that of the saline group (P<0.01) and the Lv-MSCs group (P<0.05) (Figure 6E, 6F). These data suggest that the MSCs had therapeutic effect for PD in the Lv-MSCs group and the Lv-Nurr1-MSCs group, and the Lv-Nurr1-MSCs transplantation had a better effect.
Effects of Lv-Nurr1-MSCs on rotational asymmetry
Behavioral tests were carried out to assess locomotor activity 2 weeks after 6-OHDA injection and 1, 2, 4 weeks after cells transplantation. The rotational behaviors induced by apomorphine of all groups were showed as Figure 7. Intrastriatal injection of Lv-Nurr1-MSCs resulted in a significant decrease of the number of rotations in Lv-Nurr1-MSCs group (7.43 ± 1.14 rotation/min at 1 week, 6.14 ± 1.48 at 2 weeks and 5.58 ± 1.16 at 4 weeks) when compared with rats in the Lv-MSCs group (10.57 ± 1.86 at 1 week, 7.64 ± 1.56 at 2 weeks and 6.71 ± 1.87 at 4 weeks) (P<0.05) or in the saline group (10.92 ± 1.69 at 1 week, 10.94 ± 1.67 at 2 weeks, and 10.83 ± 1.59 at 4 weeks) (P<0.01). Moreover, the rotational asymmetry in the Lv-MSCs group at 2 weeks and 4 weeks (P<0.05), not at 1 week, was significantly decreased compared to the saline group. Data are expressed as mean ± SEM. The improvement of PD rat behavior suggests that although both LV-MSCs and LV-Nurr1-MSCs injections may have prevented striatal dopamine depletion, leading to neuroprotective effect of the nigrostriatal passway in PD rats, LV-Nurr1-MSCs is better than LV-MSCs.
Figure 7.
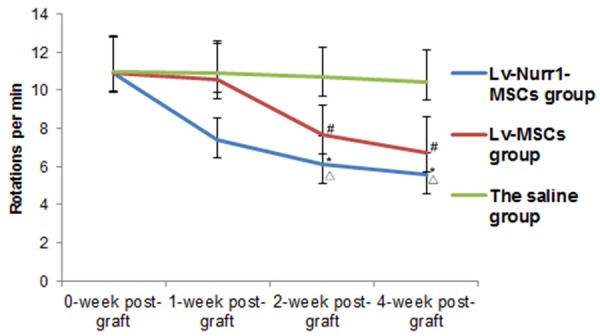
Effects of intrastriatal injection of Lv-Nurr1-MSCs on apomorphine-induced rotation asymmetry in rats after 6-hydroxydopamine (6-OHDA) lesion. Average rotational turns/min are showed at different time points (1, 2 and 4 weeks, n = 5 for each group). The average rotational rates of PD rats in the Lv-MSCs group (10.57 ± 1.86 at 1 week, 7.64 ± 1.56 at 2 weeks and 6.71 ± 1.87 at 4 weeks) significantly decreased (# P<0.05), and the greatest decrease was observed in the Lv-Nurr1-MSCs group (7.43 ± 1.14 rotation/min at 1 week, 6.14 ± 1.48 at 2 weeks and 5.58 ± 1.16 at 4 weeks) compared with the saline group (10.92 ± 1.69 at 1 week, 10.94 ± 1.67 at 2 weeks, and 10.83 ± 1.59 at 4 weeks) (*P<0.01). Moreover, the Lv-Nurr1-MSCs group showed significantly greater behavioral improvement than that of the Lv-MSCs group (Δ P<0.01).
Effects of Lv-Nurr1-MSCs on the activation of neuroglial cells and the expression of pro-imflammatory factors in the SN
Nurr1 acts in microglia and astrocytes to suppress the production of inflammatory mediators associating with the death of dopaminergic neurons [28,43]. We investigated the effect of Lv-MSCs and Lv-Nurr1-MSCs on microglia activation in the midbrain 4 weeks after the transplantation. Microglia proliferation was visualized by CD11b immunohistochemical staining, and it was sparsely in normal side of SN in the saline group rats (Figure 8A1). A large number of activated microglia with thick processes and enlarged cell bodies, also known as ameba-like, was observed in the lesion side of SN in the saline group rats (Figure 8A2). Lv-MSCs transplantation could reduce the number and the intensity of CD11b immunostaining in SN in the Lv-MSCs group (Figure 8A3). A significantly less increase in CD11b immunostaining was observed in lesion side of SN in the Lv-Nurr1-MSCs group (Figure 8A4). Similar results were seen in the activation of another neuroglia cell, astrocyte, observed by GFAP-immunoreactive staining (Figure 8B1-B4). We also detected the expression levels of pro-imflammatory factors in the SN. The results showed that the integrated absorbance (IA) of COX-2 and TNF-α positive cell in the SN in Lv-MSCs group and Lv-Nurr1-MSCs group was less, and the staining was lighter than that of the saline group. The results suggested that cell transplantation alleviates the increased expression of COX-2 and TNF-α induced by 6-OHDA (Figure 8C1-C4, 8D1-D4), and the effect of transplantation of Lv-Nurr1-MSCs is more obvious. RT-PCR displayed the expression levels of CD11b, GFAP, COX-2 and TNF-α mRNA in the SN, and the results of RT-PCR were same as the trend of the immunohistochemistry. Taken together, it indicates that MSCs transplantation inhibits 6-OHDA induced neuroinflammatory response, and Lv-Nurr1-MSCs transplantation exerts a greater effect.
Figure 8.
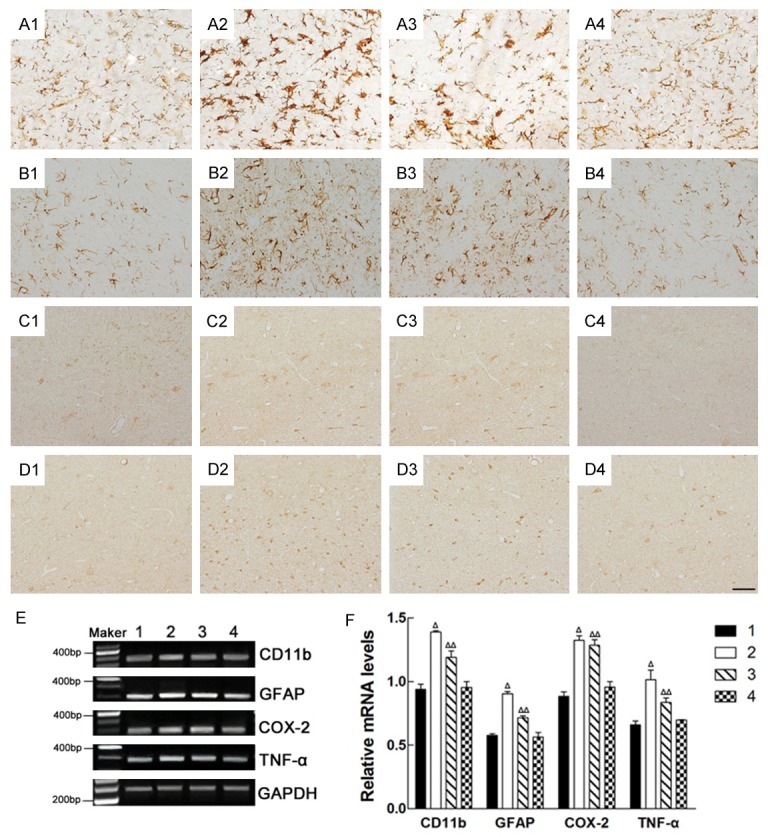
Effects of intrastriatal injection of Lv-Nurr1-MSCs on the morphology of the CD11b-, GFAP-, COX-2- and TNF-α-positive cells and the integrated absorbance (IA) of CD11b, GFAP, COX-2 and TNF-α in mRNA level of the substantia nigra. CD11b-positive cells (A1-A4); GFAP-positive cells (B1-B4); COX-2-positive cells (C1-C4); TNF-α-positive cells (D1-D4); Representative RT-PCR of CD11b, GFAP, COX-2 and TNF-α in the SN. GAPDH was used as an internal control (E). The relative mRNA level of CD11b, GFAP, COX-2 and TNF-α was analyzed by RT-PCR (Δ P<0.01, ΔΔ P<0.05) (F). 1: normal side of the saline group; 2: saline-injected side of the saline group; 3: Lv-MSCs-injected side of the Lv-MSCs group; 4: Lv-Nurr1-MSCs-injected side of the Lv-Nurr1-MSCs group. The number of rats in each group was five. Scale bar = 20 μm.
Discussion
So far there is no effective way to cure PD. Stem cell therapy has great promise for PD as these cells have self-renewal and multi-differential potential. Bone marrow MSCs are the important candidates for cell therapy, regarding their easy acquirement, low immunogenicity, high transfection efficiency and long-term expression of exogenous gene [44]. The DA precursor cells of the substantia nigra are derived from the embryonic neural epithelium. Many cytokines and transcription factors are involved in the development and differentiation of DA neurons in the central nervous system. Nurr1 plays important roles in neuronal neurotransmitter phenotype determination, neuronal survival and functional maintenance [20,45]. The midbrain DA neurons of Nurr1 gene knockout mice are agenesis, and their phenotypic markers, such as TH, ADCC, DA, are undetectable in the nigrostriatal pathway. However, DA neurons in other brain regions were intact. These findings indicated that the role of Nurr1 gene is specific for the development of midbrain DA cells. Nurr1 also regulates midbrain DA neuron markers such as TH, DAT, ADCC, VMAT2 [20], and these proteins are closely related with DA synthesis, vesicle packaging, axonal transport and DA reuptake and vesicle storage [46].
Nowadays cell and gene therapy has brought hope to the treatment of PD. MSCs are considered as a potent tool for regenerative cell therapy [47]. Our preliminary study proved bone marrow MSCs can differentiation into neurons and glial cells in vivo and in vitro, further more when MSCs were transplanted into the striatum of the PD rats, the implanted cells can differentiate into MAP+-positive neurons and promote the regeneration of neurons and axons in the striatum, improve the abnormal behavior of PD rat models. But in previous studies, it was not found that the transplanted MSCs differentiated into TH (the rate limiting enzyme in the synthesis of dopamine) positive cells [5,10]. We speculate that this may be because the ability of MSCs to naturally differentiate into mature DA neurons in the striatum is low as a pluripotent stem cell. Therefore, in order to promote the differentiation of MSCs into DA cells and ensure a sufficient number of DA cells to meet the needs of PD treatment, one of the approaches is to transduce the key transcription factors that determine the development and maturation of DA neurons from MSCs. Since Nurr1 plays a crucial role in the development and maturation of DA neurons, in the present study, we represent the efficient transduction of rat MSCs with a lentiviral vector encoding the complete cDNA of the rat nurr1 gene. The immunofluorescent staining showed that the Nurr1-transducted MSCs revealed strong fluorescence, and Western blot showed that both the Nurr1-transducted MSCs and its culture medium expressed Nurr1 protein. These results indicated that lentivirus mediated Nurr1 gene-modified MSCs could over-express and secret Nurr1.
Gene therapy brings hope to the treatment of PD. In the field of gene therapy, the lentiviral vector has been widely used because it has the advantages of high transduction efficiency, long-term, stable transgene expression in the CNS [48-50] and low cytotoxicity of human and rat MSCs [51,52]. Some of the previous studies on the lentiviral vector-based gene therapy for PD model displayed that the vector containing the target gene is injected directly into the brain of the rat [53-55]. The result of clinical application displayed that ProSavin, a lentiviral vector-based gene therapy that was produced by a triple transient transfection of HEK293T cells [51], was good in safety and tolerability in patients with advanced PD, and the motor behavior of all patients was improved [51]. While others exhibited that lentivirus-mediated genetic engineering mesenchymal stem cells were transplanted for PD model [56-59]. Transduction genes encode growth factors, such as GDNF [56,57,60], neurturin [58], Persephin (PSPN) [59], sonic hedgehog [54], CDNF, MANF [61] or enzymes, for example, TH [62], G protein coupled receptor kinase 6 [55] or transcription factor, such as LMX1a [63] and so on. Gene transduction not only improves the aberrant behavior, dramatically increases the number of TH-positive cells of the SN, stimulates axon regeneration and increases the density of TH-positive terminals, but also raises DA level in the striatum [52,56,58,60,61]. In this study, we prepared lentivirus-mediated Nurr1 genetic engineering bone marrow MSCs, and transplanted the LV-Nurr1-MSCs into the striatum of PD rats. The rotational behavior of rats was significantly improved in 2 weeks after cell transplantation, and in 4 weeks, the improvement of animal behavior is more obvious.
After 4 weeks of transplantation, the implanted Lv-Nurr1-MSCs cells could survive in the striatum and migrate to the cortex and the contralateral brain tissue along the corpus callosum. Compared with the saline group, the number of Nurr1-positive cells and the density of TH-positive terminals in the lesion side of the striatum and the number of TH- and DAT-positive cells in the lesion side of the SN in the Lv-Nurr1-MSCs group and the Lv-MSCs group were increased significantly, especially the Lv-Nurr1-MSCs group. Although only a small amount of Nurr1/TH double positive cells were found in the striatum, the number of Nurr1/TH positive cells in the SN increased significantly. These results suggested that although the possibility of the transplanted Lv-Nurr1-MSCs differentiated into functional DA neurons is still to be confirmed further, there are a lot of Nurr1-positive cells in the striatum, and these cells may act on the neighboring cells by secreting Nurr1 proteins, which is beneficial to the repair of the injured DA neurons in the SN.
Neural inflammation is an important cascade event of neuronal degeneration in PD [64], including microglia activation, astrocyte gliosis and cytokine release, which aggravated neurodegenerative process and finally led to neuronal death. Postmortem analyses reported that there were some microglia and astrocytes around the degenerated neurons of the SN [65]. The microglia activation and astrocyte response were also found in various animal models of PD. Microglia activation promotes the release of reactive oxygen species (H2O2, O2-, NO) and toxic cytokines (TNF-α, IL-1β, IL-6, IFN-γ), then gives rise to oxidative stress and mitochondrial dysfunction, and finally leads to neuronal damage. Astrocytes play important and critical roles in the initiation and progression of PD [66]. The changes of microglia and other microenvironment in the brain are able to trigger the function of astrocytes that produces plenty of neurotoxic factors and lack of growth factors to the neurons, eventually lead to neuronal death [28,67]. Recently, the neuroprotective effects of Nurr1 have been increasingly considered [29]. The research evidences presented that the reduction of Nurr1 expression resulted in the release of IL-1β and TNF-α from microglia, and the production of hazardous factors, such as NO and ROS, could further magnified the inflammatory reaction, which are the factors that leads to the death of DA neurons. So Nurr1 expressed in glia acts as an inhibitor of inflammatory cytokines secreted by activated microglia, and plays a key role in inhibiting of expression of pro-inflammatory neurotoxic molecules in microglia and astrocytes [28,67]. Therefore, we studied the effects of nurr1 gene-modified bone marrow MSCs on the changes of glial cells and the expression of the inflammatory factors in the SN of PD rats. The results showed that compared with the LV-MSCs group and the saline group, the numbers of activated glial cells and COX-2- and TNF-α-positive cells of the lesion side of the SN in the LV-Nurr1-MSCs group were reduced. The expression of CD11b, GFAP, COX-2 and TNF-α mRNA were reduced significantly. These results suggested that the transplantation of LV-Nurr1-MSCs may inhibit the activation of glial cells and the expression of the inflammatory factors of the SN, and plays an important role in anti-inflammatory and neuroprotective effects on DA neurons.
In summary, we verified that Nurr1 gene-modified MSCs secrete Nurr1 protein in vitro, and Nurr1 gene-modified MSCs transplanted into the striatum survives and migrates in the brain and some differentiate into TH-positive cells, which results dramatically ameliorated the abnormal behavior of PD rat and increased the numbers of DA neurons in the substantia nigra. The protective effect on DA neurons of the intrastriatal transplantation of Nurr1 gene-modified MSCs is closely related with the inhibition activation of glial cells and the reduction of expression of inflammatory factors. Thus, intrastriatal transplantation of the Nurr1 gene-modified MSCs achieves the purpose of treating PD by two ways of secreting Nurr1 protein and differentiating into DA neurons.
Acknowledgements
This work was supported by Natural Science Foundation of Shandong Province (Y2008C129; ZR2014HL043); Shandong Province Taishan Scholar Project; The Brigham and Women’s Hospital BRI Fund to Sustain Research Excellence (X.W.), (S.Z.); Key Project of Science and Technology Innovation Research Fund of Weifang Medical University (K1301002).
Disclosure of conflict of interest
None.
References
- 1.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 2.Beitz JM. Parkinson’s disease: a review. Front Biosci (Schol Ed) 2014;6:65–74. doi: 10.2741/s415. [DOI] [PubMed] [Google Scholar]
- 3.Fu W, Zhuang W, Zhou S, Wang X. Plant-derived neuroprotective agents in Parkinson’s disease. Am J Transl Res. 2015;7:1189–1202. [PMC free article] [PubMed] [Google Scholar]
- 4.Petit GH, Olsson TT, Brundin P. The future of cell therapies and brain repair: Parkinson’s disease leads the way. Neuropathol Appl Neurobiol. 2014;40:60–70. doi: 10.1111/nan.12110. [DOI] [PubMed] [Google Scholar]
- 5.Fu W, Zheng Z, Zhuang W, Chen D, Wang X, Sun X, Wang X. Neural metabolite changes in corpus striatum after rat multipotent mesenchymal stem cells transplanted in hemiparkinsonian rats by magnetic resonance spectroscopy. Int J Neurosci. 2013;123:883–891. doi: 10.3109/00207454.2013.814132. [DOI] [PubMed] [Google Scholar]
- 6.Arenas E, Denham M, Villaescusa JC. How to make a midbrain dopaminergic neuron. Development. 2015;142:1918–1936. doi: 10.1242/dev.097394. [DOI] [PubMed] [Google Scholar]
- 7.Barker RA, Drouin-Ouellet J, Parmar M. Cellbased therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol. 2015;11:492–503. doi: 10.1038/nrneurol.2015.123. [DOI] [PubMed] [Google Scholar]
- 8.Lindvall O. Clinical translation of stem cell transplantation in Parkinson’s disease. J Intern Med. 2016;279:30–40. doi: 10.1111/joim.12415. [DOI] [PubMed] [Google Scholar]
- 9.Chen D, Fu W, Zhuang W, Lv C, Li F, Wang X. Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson’s disease. J Neurosci Res. 2017;95:907–917. doi: 10.1002/jnr.23879. [DOI] [PubMed] [Google Scholar]
- 10.Fu W, Lv C, Zhuang W, Chen D, Lv E, Li F, Wang X. An effective inducer of dopaminergic neuron-like differentiation. Neural Regen Res. 2013;8:427–434. doi: 10.3969/j.issn.1673-5374.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu S, Luan J, Xin M, Wang Q, Xiao R, Gao Y. Fate of adipose-derived stromal vascular fraction cells after co-implantation with fat grafts: evidence of cell survival and differentiation in ischemic adipose tissue. Plast Reconstr Surg. 2013;132:363–373. doi: 10.1097/PRS.0b013e31829588b3. [DOI] [PubMed] [Google Scholar]
- 12.Bao J, Wu Q, Wang Y, Li Y, Li L, Chen F, Wu X, Xie M, Bu H. Enhanced hepatic differentiation of rat bone marrow-derived mesenchymal stem cells in spheroidal aggregate culture on a decellularized liver scaffold. Int J Mol Med. 2016;38:457–465. doi: 10.3892/ijmm.2016.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:375–392. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 16.Trzaska KA, King CC, Li KY, Kuzhikandathil EV, Nowycky MC, Ye JH, Rameshwar P. Brainderived neurotrophic factor facilitates maturation of mesenchymal stem cell-derived dopamine progenitors to functional neurons. J Neurochem. 2009;110:1058–1069. doi: 10.1111/j.1471-4159.2009.06201.x. [DOI] [PubMed] [Google Scholar]
- 17.Czarnecka J, Porowińska D, Bajek A, Hołysz M, Roszek K. Neurogenic differentiation of mesenchymal stem cells induces alterations in extracellular nucleotides metabolism. J Cell Biochem. 2017;118:478–486. doi: 10.1002/jcb.25664. [DOI] [PubMed] [Google Scholar]
- 18.Venkataramana NK, Pal R, Rao SA, Naik AL, Jan M, Nair R, Sanjeev CC, Kamble RB, Murthy DP, Chaitanya K. Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson’s disease: a pilot clinical study. Stem Cells Int. 2012;2012:931902. doi: 10.1155/2012/931902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan M, Sun M, Zhou Y, Wang W, He Z, Tang D, Lu S, Wang X, Li S, Wang W, Li H. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopamine neurons mediated by the Lmx1a and neurturin in vitro: potential therapeutic application for Parkinson’s disease in a rhesus monkey model. PLoS One. 2013;8:e64000. doi: 10.1371/journal.pone.0064000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, Muramatsu S, Sumi-Ichinose C, Nomura T, Metzger D, Chambon P, Lindqvist E, Larsson NG, Olson L, Björklund A, Ichinose H, Perlmann T. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurada K, Ohshima-Sakurada M, Palmer TD, Gage FH. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development. 1999;126:4017–4026. doi: 10.1242/dev.126.18.4017. [DOI] [PubMed] [Google Scholar]
- 22.Sacchetti P, Mitchell TR, Granneman JG, Bannon MJ. Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism. J Neurochem. 2001;76:1565–1572. doi: 10.1046/j.1471-4159.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- 23.Hermanson E, Joseph B, Castro D, Lindqvist E, Aarnisalo P, Wallen A, Benoit G, Hengerer B, Olson L, Perlmann T. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Exp Cell Res. 2003;288:324–334. doi: 10.1016/s0014-4827(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 24.Kim KS, Kim CH, Hwang DY, Seo H, Chung S, Hong SJ, Lim JK, Anderson T, Isacson O. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem. 2003;85:622–634. doi: 10.1046/j.1471-4159.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 25.García-Yagüe ÁJ, Rada P, Rojo AI, Lastres-Becker I, Cuadrado A. Nuclear import and export signals control the subcellular localization of Nurr1 protein in response to oxidative stress. J Biol Chem. 2013;288:5506–5517. doi: 10.1074/jbc.M112.439190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadkhodaei B, Alvarsson A, Schintu N, Ramsköld D, Volakakis N, Joodmardi E, Yoshitake T, Kehr J, Decressac M, Björklund A, Sandberg R, Svenningsson P, Perlmann T. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc Natl Acad Sci U S A. 2013;110:2360–2365. doi: 10.1073/pnas.1221077110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong J, Li S, Mo JL, Cai HB, Le WD. Nurr1-Based therapies for Parkinson’s disease. CNS Neurosci Ther. 2016;22:351–359. doi: 10.1111/cns.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volakakis N, Kadkhodaei B, Joodmardi E, Wallis K, Panman L, Silvaggi J, Spiegelman BM, Perlmann T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc Natl Acad Sci U S A. 2010;107:12317–12322. doi: 10.1073/pnas.1007088107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JY, Koh HC, Lee JY, Chang MY, Kim YC, Chung HY, Son H, Lee YS, Studer L, McKay R, Lee SH. Dopaminergic neuronal differentiation from rat embryonic neural precursors by Nurr1 overexpression. J Neurochem. 2003;85:1443–1454. doi: 10.1046/j.1471-4159.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 31.Park CH, Kang JS, Kim JS, Chung S, Koh JY, Yoon EH, Jo AY, Chang MY, Koh HC, Hwang S, Suh-Kim H, Lee YS, Kim KS, Lee SH. Differential actions of the proneural genes encoding Mash1 and neurogenins in Nurr1-induced dopamine neuron differentiation. J Cell Sci. 2006;119:2310–2320. doi: 10.1242/jcs.02955. [DOI] [PubMed] [Google Scholar]
- 32.Park CH, Kang JS, Shin YH, Chang MY, Chung S, Koh HC, Zhu MH, Oh SB, Lee YS, Panagiotakos G, Tabar V, Studer L, Lee SH. Acquisition of in vitro and in vivo functionality of Nurr1-induced dopamine neurons. FASEB J. 2006;20:2553–2555. doi: 10.1096/fj.06-6159fje. [DOI] [PubMed] [Google Scholar]
- 33.Shim JW, Park CH, Bae YC, Bae JY, Chung S, Chang MY, Koh HC, Lee HS, Hwang SJ, Lee KH, Lee YS, Choi CY, Lee SH. Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using Nurr1 overexpression. Stem Cells. 2007;25:1252–1262. doi: 10.1634/stemcells.2006-0274. [DOI] [PubMed] [Google Scholar]
- 34.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 35.Hussain I, Magd SA, Eremin O, El-Sheemy M. New approach to isolate mesenchymal stem cell (MSC) from human umbilical cord blood. Cell Biol Int. 2012;36:595–600. doi: 10.1042/CBI20110336. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C. The rat brain stereotaxic coordinates. Academic Press Sydney. 1985;25:87–92. [Google Scholar]
- 37.Chu Y, Kompoliti K, Cochran EJ, Mufson EJ, Kordower JH. Age-related decreases in Nurr1 immunoreactivity in the human substantia nigra. J Comp Neurol. 2002;450:203–214. doi: 10.1002/cne.10261. [DOI] [PubMed] [Google Scholar]
- 38.Maijenburg MW, Gilissen C, Melief SM, Kleijer M, Weijer K, Ten Brinke A, Roelofs H, Van Tiel CM, Veltman JA, de Vries CJ, van der Schoot CE, Voermans C. Nuclear receptors Nur77 and Nurr1 modulate mesenchymal stromal cell migration. Stem Cells Dev. 2012;21:228–238. doi: 10.1089/scd.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 40.Wallén A, Perlmann T. Transcriptional control of dopamine neuron development. Ann N Y Acad Sci. 2003;991:48–60. doi: 10.1111/j.1749-6632.2003.tb07462.x. [DOI] [PubMed] [Google Scholar]
- 41.Martinat C, Bacci JJ, Leete T, Kim J, Vanti WB, Newman AH, Cha JH, Gether U, Wang H, Abeliovich A. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci U S A. 2006;103:2874–2879. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smits SM, Ponnio T, Conneely OM, Burbach JP, Smidt MP. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur J Neurosci. 2003;18:1731–1738. doi: 10.1046/j.1460-9568.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- 43.Bensinger SJ, Tontonoz P. A Nurr1 pathway for neuroprotection. Cell. 2009;137:26–28. doi: 10.1016/j.cell.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Glavaski-Joksimovic A, Bohn MC. Mesenchymal stem cells and neuroregeneration in Parkinson’s disease. Exp Neurol. 2013;247:25–38. doi: 10.1016/j.expneurol.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Bae EJ, Lee HS, Park CH, Lee SH. Orphan nuclear receptor Nurr1 induces neuron differentiation from embryonic cortical precursor cells via an extrinsic paracrine mechanism. FEBS Lett. 2009;583:1505–1510. doi: 10.1016/j.febslet.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Alavian KN, Scholz C, Simon HH. Transcriptional regulation of mesencephalic dopaminergic neurons: the full circle of life and death. Mov Disord. 2008;23:319–328. doi: 10.1002/mds.21640. [DOI] [PubMed] [Google Scholar]
- 47.Tanna T, Sachan V. Mesenchymal stem cells: potential in treatment of neurodegenerative diseases. Curr Stem Cell Res Ther. 2014;9:513–521. doi: 10.2174/1574888x09666140923101110. [DOI] [PubMed] [Google Scholar]
- 48.Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, Buch P, MacLaren RE, Anderson PN, Barker SE, Duran Y, Bartholomae C, von Kalle C, Heckenlively JR, Kinnon C, Ali RR, Thrasher AJ. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- 49.Kantor B, Bayer M, Ma H, Samulski J, Li C, McCown T, Kafri T. Notable reduction in illegitimate integration mediated by a PPT-deleted, nonintegrating lentiviral vector. Mol Ther. 2011;19:547–556. doi: 10.1038/mt.2010.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kantor B, McCown T, Leone P, Gray SJ. Clinical applications involving CNS gene transfer. Adv Genet. 2014;7:71–124. doi: 10.1016/B978-0-12-800149-3.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S, Iwamuro H, Lefaucheur JP, Thiriez C, Fenelon G, Lucas C, Brugières P, Gabriel I, Abhay K, Drouot X, Tani N, Kas A, Ghaleh B, Le Corvoisier P, Dolphin P, Breen DP, Mason S, Guzman NV, Mazarakis ND, Radcliffe PA, Harrop R, Kingsman SM, Rascol O, Naylor S, Barker RA, Hantraye P, Remy P, Cesaro P, Mitrophanous KA. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383:1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 52.Chen SS, Yang C, Hao F, Li C, Lu T, Zhao LR, Duan WM. Intrastriatal GDNF gene transfer by inducible lentivirus vectors protects dopaminergic neurons in a rat model of parkinsonism. Exp Neurol. 2014;261:87–96. doi: 10.1016/j.expneurol.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 53.McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A, Tansey MG. Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Dong W, Guo S, Zhao S, He S, Zhang L, Tang Y, Wang H. Lentivirus-mediated delivery of sonic hedgehog into the striatum stimulates neuroregeneration in a rat model of Parkinson disease. Neurol Sci. 2014;35:1931–1940. doi: 10.1007/s10072-014-1866-6. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed MR, Bychkov E, Kook S, Zurkovsky L, Dalby KN, Gurevich EV. Overexpression of GRK6 rescues L-DOPA-induced signaling abnormalities in the dopamine-depleted striatum of hemiparkinsonian rats. Exp Neurol. 2015;266:42–54. doi: 10.1016/j.expneurol.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biju K, Zhou Q, Li G, Imam SZ, Roberts JL, Morgan WW, Clark RA, Li S. Macrophagemediated GDNF delivery protects against dopaminergic neurodegeneration: a therapeutic strategy for Parkinson’s disease. Mol Ther. 2010;18:1536–1544. doi: 10.1038/mt.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi D, Chen G, Lv L, Li L, Wei D, Gu P, Gao J, Miao Y, Hu W. The effect of lentivirus-mediated TH and GDNF genetic engineering mesenchymal stem cells on Parkinson’s disease rat model. Neurol Sci. 2011;32:41–51. doi: 10.1007/s10072-010-0385-3. [DOI] [PubMed] [Google Scholar]
- 58.Biju KC, Santacruz RA, Chen C, Zhou Q, Yao J, Rohrabaugh SL, Clark RA, Roberts JL, Phillips KA, Imam SZ, Li S. Bone marrow-derived microglia-based neurturin delivery protects against dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. Neurosci Lett. 2013;535:24–29. doi: 10.1016/j.neulet.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin X, Xu H, Jiang Y, Deng W, Wu Z, Xiang H, Sun P, Xie J. The effect of lentivirus-mediated PSPN genetic engineering bone marrow mesenchymal stem cells on Parkinson’s disease rat model. PLoS One. 2014;9:e105118. doi: 10.1371/journal.pone.0105118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu-Nguyen NB, Broadstock M, Schliesser MG, Bartholomae CC, von Kalle C, Schmidt M, Yáñez-Muñoz RJ. Transgenic expression of human glial cell line-derived neurotrophic factor from integration-deficient lentiviral vectors is neuroprotective in a rodent model of Parkinson’s disease. Hum Gene Ther. 2014;25:631–641. doi: 10.1089/hum.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cordero-Llana Ó, Houghton BC, Rinaldi F, Taylor H, Yáñez-Muñoz RJ, Uney JB, Wong LF, Caldwell MA. Enhanced efficacy of the CDNF/MANF family by combined intranigral overexpression in the 6-OHDA rat model of Parkinson’s disease. Mol Ther. 2015;23:244–254. doi: 10.1038/mt.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakata Y, Yasuda T, Mochizuki H. Recent progress in gene therapy for Parkinson’s disease. Curr Mol Med. 2012;12:1311–1318. doi: 10.2174/156652412803833580. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez-Danés A, Consiglio A, Richaud Y, Rodríguez-Pizà I, Dehay B, Edel M, Bové J, Memo M, Vila M, Raya A, Izpisua Belmonte JC. Efficient generation of A9 midbrain dopaminergic neurons by lentiviral delivery of LMX1A in human embryonic stem cells and induced pluripotent stem cells. Hum Gene Ther. 2012;23:56–69. doi: 10.1089/hum.2011.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niranjan R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: foc on astrocytes. Mol Neurobiol. 2014;49:28–38. doi: 10.1007/s12035-013-8483-x. [DOI] [PubMed] [Google Scholar]
- 65.Deleidi M, Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol Life Sci. 2013;70:4259–4273. doi: 10.1007/s00018-013-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rappold PM, Tieu K. Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics. 2010;7:413–423. doi: 10.1016/j.nurt.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Decressac M, Volakakis N, Björklund A, Perlmann T. NURR1 in Parkinson disease from pathogenesis to therapeutic potential. Nat Rev Neurol. 2013;9:629–636. doi: 10.1038/nrneurol.2013.209. [DOI] [PubMed] [Google Scholar]