Abstract
Objective: This study aims to investigate the role of three-dimensional visualization technique in the diagnosis and treatment of progressive hilar cholangiocarcinoma. Methods: From January 2014 to February 2017, a three-dimensional visualization model was set up in 23 patients with progressive hilar cholangiocarcinoma. The distributions and variations of the hepatic portal ducts were observed. The tumors were classified based on Bismuth classification. The simulation operation was performed and the operation plan was established. Results: All 23 patients revealed a clear relationship between the intrahepatic and extrahepatic ducts, as well as the tumors and ducts. An individualized surgery program was established through the accurate calculation of liver volume and residual liver volume. Among these patients, 13 patients completed radical resection of hilar cholangiocarcinoma combined with massive hepatectomy. No bile leakage occurred and no operative death was found. Conclusion: For patients with progressive hilar cholangiocarcinoma, the optimized three-dimensional visualization technique can accurately demonstrate the dilated biliary tract system, provide a new standard to determine the presence of tumor and peripheral vascular invasion, help in establishing a reasonable individualized operation plan, reduce the incidence of bile leakage and liver failure after the operation, and improve the success rate of operation.
Keywords: Progressive hilar cholangiocarcinoma, three-dimensional visualization technique, hepatectomy
Introduction
Hilar cholangiocarcinoma has occult symptoms, its vast majority of patients have been in the progressive stage on medical visit, and at that time, the caudate lobe and hepatic portal duct are often invaded [1]. Since hilar cholangiocarcinoma is not sensitive to present chemoradiotherapy protocols, radical resection of hilar cholangiocarcinoma combined with hepatectomy has become the only effective approach for the treatment of progressive hilar cholangiocarcinoma [2-5].
However, these patients are often accompanied by severe jaundice before surgery, and impairment of liver and kidney function and coagulation; and radical surgery requires high technique and a long operation time, which results in significantly increased postoperative complications and mortality [6]. In order to reduce operation risk and improve the cure rate, the preoperative evaluation of the patient’s tumor progress is critical. In particular, determining the presence of anatomic variations in the hepatic hilum, the Bismuth classification, the extent of the tumor invasion, and the residual hepatic volume are important [7,8].
As a new evaluation method, the role of three-dimensional (3D) visualization in liver surgery has become increasingly important, and has been gradually promoted [9-13]. However, few studies have been reported on the application of this technique in progressive hilar cholangiocarcinoma. This is mainly due to obvious biliary dilatation in patients with hilar cholangiocarcinoma. Dilated bile ducts causes poor 3D computed tomography (CT) image reconstruction, and the accurate image reconstruction of the dilated bile duct and occupied space cannot be carried out in the arterial phase, portal vein phase, venous phase and delayed phase. Thus, it seriously affects the classification of tumors and the judgment of the scope of invasion. In the present study, the 3D visualization software Intelligent Precise Service (IPS, Shenzhen Xudong Digital Medical Imaging Technology Co. Ltd.) was used. Through technical optimization, the multi-phase fusion visualization of each phase of the blood vessel and occupied space was realized, and a 3D visualization model of bile duct expansion was established for the first time, worldwide. IPS was used to perform the preoperative evaluation and surgical planning for 23 patients with progressive hilar cholangiocarcinoma, and the imaging features of the tumor invasion to the surrounding duct were observed under the 3D visualization model. Details are reported as follows.
Data and methods
General information
From January 2014 to February 2017, a total of 23 patients with hilar cholangiocarcinoma of Bismuth grades III and IV underwent image reconstruction by 3D visualization model before surgery. Among these patients, 13 patients underwent surgical resection, in which seven patients were male and six patients were female. Furthermore, among these patients, nine patients were Bismuth grade III and four patients were Bismuth grade IV. All patients provided a signed informed consent, and this protocol was approved by the Ethics Committee of our hospital.
Three-dimensional visualization model reconstruction
In the present study, the 64-slice spiral CT (Philips, Holland) was used to scan the hepatic hilum and its surrounding structures. Thin slice CT data were collected during the non-enhanced phase, arterial phase, portal vein phase and hepatic vein phase, and stored in a CD disc in Digital Imaging and Communications in Medicine 3.0 (DICOM 3.0) format. The above data was imported into the 3D reconstruction software IPS (Xudong, Shenzhen, China), and a 3D visualization model of the liver was established by combining surface rendering and volume rendering.
(1) The courses, distributions and variations of the hepatic artery, portal vein and biliary tract system were observed by IPS. (2) According to the distribution of the portal vein, hepatic vein and the topological relation of blood flow, Couinaud’s liver segmentation was carried out, and the volume of whole liver and volumes of the liver segments were calculated. (3) Bismuth classification was performed according to the scope of the tumors invading bile duct. (4) Through rotation, magnification and transparency in the 3D visualization model, tumors were classified, and the invasion relationship between tumors and the hepatic artery and portal vein was determined. Furthermore, whether the radical resection was successful was determined by comparison ofextensive liver resection points (U, P). The pre-resection line was delineated, and the residual liver volume and the percentage of the residual liver volume were calculated. The biliary stump of the residual liver section was accurately displayed. (5) The operation plan or abandonment of the operation were finally determined based on the preoperative general situation, Child-Pugh grade, degree of cirrhosis, indocyanine green test result, and the percentage of residual liver volume.
Results
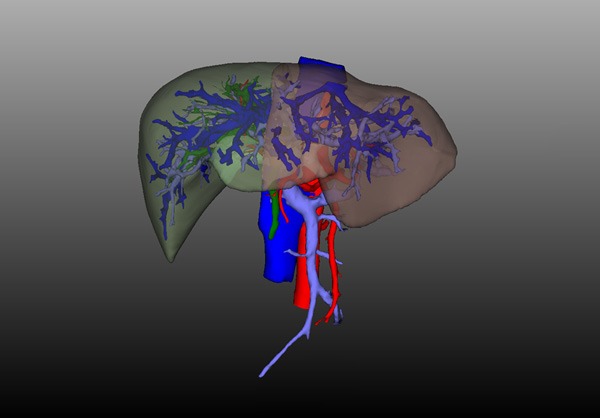
All 23 patients completed the 3D visualization model analysis. The distributions, courses and variations of the hepatic artery, portal vein and bile duct were clearly observed (Figure 1). (1) Patients with common hepatic arteries accounted for 70% (16/23), patients with right hepatic artery originating from the superior mesenteric artery accounted for 13% (3/23), patients with left hepatic artery originating from the left gastric artery accounted for 13% (3/23), and patients with common hepatic artery originating from the superior mesenteric artery accounted for 4% (1/23). (2) Patients with common portal vein accounted for 84% (19/23), patients with trident type portal veins accounted for 8% (2/23), and patients with right anterior portal vein originating from the left branch of the portal vein accounted for 8% (2/23). (3) Patients with common bile ducts accounted for 52% (12/23), patients with right posterior hepatic duct veins originating from the left hepatic duct accounted for 26% (6/23), patients with trident type bile ducts accounted for 14% (3/23); and patients with right anterior bile duct originating from the left hepatic duct accounted for 8% (2/23).
Figure 1.
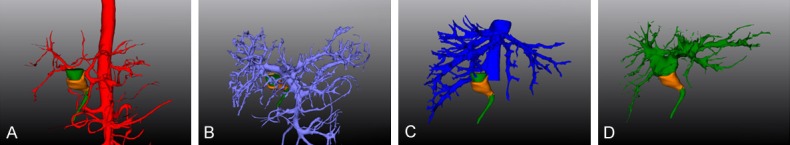
Liver 3D visualization model. A. Hepatic artery 3D reconstruction, the proper hepatic artery is divided into the left hepatic artery and the right hepatic artery before it travels into the liver; B. Portal vein 3D reconstruction, the portal vein is divided into the left portal vein and the right portal vein before it travels into the liver; C. Hepatic vein 3D reconstruction, it can be observed that the thick right inferior hepatic vein enters the inferior vena cava alone; D. Bile duct 3D reconstruction, the common bile duct is divided into the left hepatic duct and the right hepatic duct before it travels into the liver.
The divided liver segments were distinguished using different colors, and the total hepatic volume was 1,016-1,725 ml (Figure 2).
Figure 2.
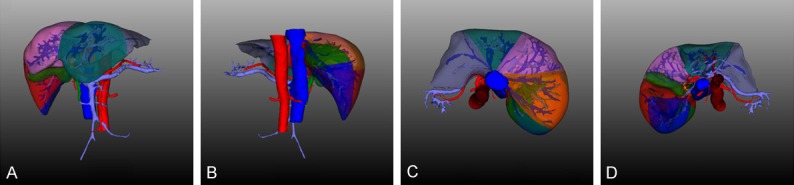
Divided liver segments. A. Front of divided liver segments, revealing segments II, III, IV, V and VIII; B. Back of divided liver segments, revealing segments II, III, XI and XII; C. Head side of divided liver segments, revealing segments II, IV, VII and VIII; D. Tail side of divided liver segments, revealing segments I, III, IV, V, VI and VIII.
In these 23 patients, the 3D images of the dilated bile duct were accurately displayed, and the tumors were localized using the IPS dilation biliary tract optimization technique. The tumors were also classified based on the Bismuth classification: seven patients were grade IIIa, two patients were grade IIIb, and 14 patients were grade IV (Figure 3).
Figure 3.
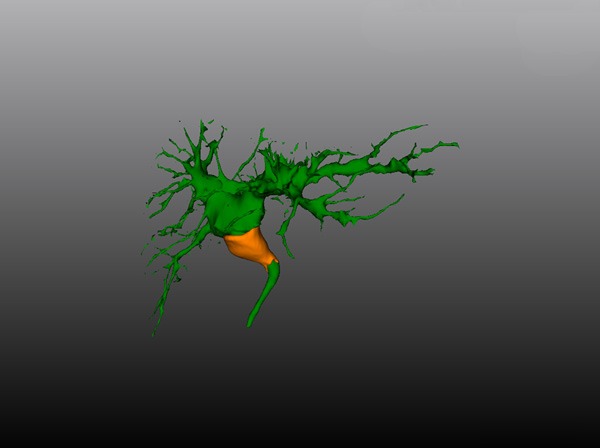
The 3D images of the dilated bile duct: The tumor invades the confluence of the right and left hepatic ducts, but does not invade the left hepatic duct, right anterior hepatic duct and right posterior hepatic duct.
Among these 13 patients, it was found that the attachment area of the tumor to the surrounding hepatic artery was greater than 1/3 of the circumferential area of the hepatic artery in seven patients, and the attachment area of the tumor to the surrounding portal vein was greater than 1/3 of the circumferential area of the portal vein in three patients. All these attachments were tumor invasions instead of inflammatory adhesions, which were verified during the operation (Figure 4).
Figure 4.
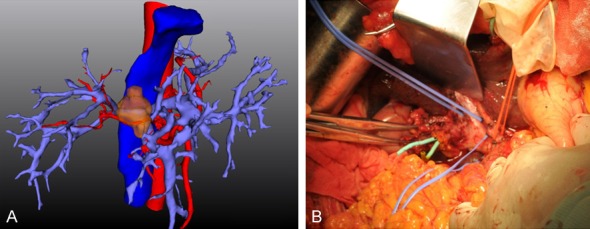
The extent of tumor invasion of portal vein. A. The attachment area of the tumor to the surrounding right hepatic artery was greater than 1/3 of the circumferential area of the hepatic artery. B. All these attachments to the right hepatic artery were tumor invasions instead of inflammatory adhesions, which were verified during the operation.
(1) After undergoing the simulation operation, the percentage of residual liver volume of seven patients with Bismuth grade IIIa was greater than 40% (Figure 5). Among these patients, five patients underwent right hemihepatectomy plus caudate lobectomy, and two patients underwent extended right hemihepatectomy plus caudate lobectomy. (2) After undergoing the simulation operation, the percentage of residual liver volume in two patients at Bismuth grade IIIb was greater than 40%. Both patients underwent left hemihepatectomy plus caudate lobectomy. (3) In 10 patients at Bismuth grade IV, the range of the tumor invasion exceeded the extensive liver resection point; and these patients were unable to undergo radical surgery, but underwent percutaneous transhepatic biliary drainage (PTBD) to reduce jaundice instead. The percentage of residual liver volume in six patients at Bismuth grade IV was 21%, 31%, 37% and 45%, respectively. For two patients with greater than 35% of the residual liver volume, adequate drainage was performed by preoperative PTBD. After bilirubin was lowered to the normal level, right trisegmentectomy plus caudate lobectomy were performed directly. For two patients who had <35% of the residual liver volume, after undergoing PTBD combined with portal vein embolization (PVE), CT reexamination and the reconstruction of the 3D visualization model was performed; and residual liver volume increased to 37% and 42%, respectively. One patient underwent right trisegmentectomy plus caudate lobectomy, and one patient underwent extended right hemihepatectomy plus caudate lobectomy.
Figure 5.
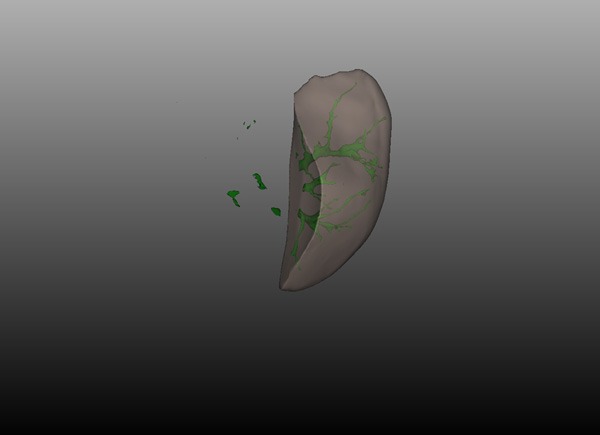
The percentage of residual liver volume calculation: the residual liver volume was 45.30%.
The broken ends of the biliary tract in the cross-section of the residual liver after the operation were clearly demonstrated in the 13 operated patients by simulation operation (Figure 6). The electronic portable device with the 3D visualization model of the liver was brought into the operation room, which was used for real-time observation and contrast during the operation. The intraoperative condition was confirmed to coincide with the 3D model. After mutilation of the liver, the liver section and bile duct opening were accurately matched, and bile duct anastomosis was performed. The operation time was 365-740 minutes, and intraoperative blood loss was 200-1,500 ml. No bile leakage was found after the operation. No perioperative death was found.
Figure 6.
The broken ends of the biliary tract in the cross-section of the residual liver: the branches of the bile duct in the 2nd and 3rd segments of residual liver were observed.
Discussion
Combined hepatectomy has become the standard surgical procedure for hilar cholangiocarcinoma. At present, the number of patients who underwent combined hepatectomy accounts for 69-98% of the total number of operations for hilar cholangiocarcinoma, and the radical resections performed on these patients account for 78-95% of the total number of operations. After radical resection, the 5-year survival rate was approximately 40% [14,15]. Neuhaus et al. reported that the 5-year survival rate of the combination of right trisegmentectomy and portal vein reconstruction reached up to 72% [16].
Hilar cholangiocarcinoma primarily occurs in the biliary tract. Therefore, the courses, distributions and variations of the intrahepatic and extrahepatic biliary tract system, and the range of tumor invasion in the biliary tract, are of great significance for operation planning. At present, the vast majority of software is targeted at the visual reconstruction of the hepatic arteriovenous system [17-19], and biliary tract system imaging has poor results due to biliary tract dilatation. IPS software pioneered the optimization of the biliary tract system in the world, which can clearly reveal the 3D anatomical structures of the dilated bile duct. Therefore, through the single display of the 3D visualization model of the biliary tract system, the biliary tract system type and variation can be accurately revealed, and the limit point of bile duct separation can be accurately determined. It can also prevent accidental injury to the vital bile duct in the retention side of the liver during the operation, establishing a solid foundation for the design of the operation plan. In addition, after the delineation of the pre-resection line, by hiding the resected side of the liver in IPS, the cross-section of broken ends of the biliary tract of the retention side of liver can clearly be demonstrated. During the operation, the liver was excised as scheduled; and the surgeons could find the bile duct openings on the cross-section of the residual liver when performing the anastomosis by repeatedly comparing with the 3D visualization model of the biliary tract on a portable electronic device, avoiding omission, and effectively preventing the occurrence of bile leakage after the operation.
For progressive hilar cholangiocarcinoma, hepatic artery and portal vein invasion often occurs in the early stage. Accurately determining the presence of the tumor invasion to peripheral vessels is of great significance for the development of the operation plan. On two-dimensional images, this is difficult to determine due to the absence of a definite standard, and its determination often relies on the clinician’s experience. We revealed on the 3D visualization model that if the attachment area of the tumor to its surrounding vessels was more than 1/3 of the total area of corresponding vessels, it could be confirmed that this tumor invasion is difficult to separate during the operation, and a corresponding vascular resection is required. Thus, 3D visualization models provide a new clue for the diagnosis of tumor invasion in progressive hilar cholangiocarcinoma, ensuring the accurate and effective establishment of the operation plan. Thus, it reduces intraoperative bleeding, and avoids hepatic insufficiency and hepatic failure caused by the poor blood supply of the residual liver after the operation, reducing risks from the operation.
In addition, in order to achieve the R0 radical resection criteria of progressive hilar cholangiocarcinoma, it usually requires the extended resection of half of the liver or even the hepatic trefoil. Postoperative complications and mortality are closely related to the volume of liver resection in patients with hilar cholangiocarcinoma. Gerhards et al. reported that for patients with hilar cholangiocarcinoma, the total incidence of complications was 65% after resection, and the operative mortality of combined hepatectomy was 25% [20]. Therefore, the calculation of the residual liver volume and the percentage of residual liver volume are essential for the surgical planning of patients with progressive hilar cholangiocarcinoma. The IPS system can not only routinely calculate the volume of each segment of the liver to provide accurate information for routine hepatectomy, but also accurately calculate the volume of irregular residual livers according to the patient’s individualized surgical plan. It greatly increases the flexibility of the operation plan, provides more possibility for surgical design for many patients that require massive hepatectomy, improves the resection rate, and effectively reduces the incidence of postoperative complications.
In summary, for patients with progressive hilar cholangiocarcinoma, the optimized 3D visualization technique can accurately demonstrate the dilated biliary tract system, provide a new clue to determine the presence of tumor invasion to peripheral vessels, help in establishing a reasonable individualized operation plan, and improve the success rate of the operation. In addition, this technique can reduce the incidence of operative complications, such as bile leakage. Furthermore, on the basis of radical resection, it retains the liver volume as far as possible, and reduces the risk of postoperative liver failure. Therefore, 3D visualization is of great significance for the diagnosis and treatment of progressive hilar cholangiocarcinoma.
Acknowledgements
We are particularly grateful to all the people who have given us help on our article.
Disclosure of conflict of interest
None.
References
- 1.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy wih caudate lobe resection for bile duce carcinoma of the hepatic hilus. World J Surg. 1990;14:535–544. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]
- 2.Baton O, Azoulay D, Adam DV, Castating D. Major hepatectomy for hilar chlangiocacinoma type 3 and 4: prognostic factors and long-time outcomes. J Am Coll Surg. 2007;204:250–260. doi: 10.1016/j.jamcollsurg.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Ramos E. Principles of surgical resection in hilar choloangiocarcinoma. World J Gastrointest Oncol. 2013;5:139–146. doi: 10.4251/wjgo.v5.i7.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Evolution of surgical technique for perihilar cholangiocarcinoma: a single-centre 34 year review of 574 consecutive resections. Ann Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectablility, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhards MF, Vangulik TM, Dewit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma-a single center experience. Surgery. 2000;127:395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 7.Paik KY, Choi DW, Chung JC, Kang KT, Kim SB. Improved survival following right trisectionectomy with caudate lobectomy without operative mortality: surgical treatment for hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12:1268–74. doi: 10.1007/s11605-008-0503-1. [DOI] [PubMed] [Google Scholar]
- 8.Seyama Y, Kubota K, Sano K, Takayama T, Kosuge T, Makuuchi M. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mise Y, Tani K, Aoki T, Hasegawa K, Sugawara Y, Kokudo N. Virtual liver resection: computer-assisted operation planning using a three-dimensional liver representation. J Hepatobiliary Pancreat. 2013;20:157–164. doi: 10.1007/s00534-012-0574-y. [DOI] [PubMed] [Google Scholar]
- 10.Simpson AL, Geller DA, Hemming AW, Jarnaqin WR, Clements LW, Dangelica MI, Dumpuri P, Gonen M, Zendeias I, Miqa MI, Stefansic JD. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 2014;219:199–207. doi: 10.1016/j.jamcollsurg.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang G, Zhou XJ, Zhu CZ, Dong Q, Su L. Usefulness of three-dimensional(3D) simulation software in hepatectomy for pediatric hepatoblastoma. Surg Oncol. 2016;25:236–243. doi: 10.1016/j.suronc.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Tian F, Wu JX, Rong WQ, Wang LM, Wu F, Yu WB, An SL, Liu FQ, Feng L, Bi C, Liu YH. Three-dimensional morphometric analysis of hepatectomy of centrally located hepatocellular carcinoma: a pilot study. World J Gastroenterol. 2015;21:4607–4619. doi: 10.3748/wjg.v21.i15.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallet J, Gayet B, Tsung A, Wakabayashi G, Pessaux P. 2nd international Consensus Conference on Laparoscopic Liver Resection Group. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: current status and future perspectives. J Hepatobiliary Pancreat. 2015;22:353–362. doi: 10.1002/jhbp.220. [DOI] [PubMed] [Google Scholar]
- 14.Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol. 2007;13:1505–15. doi: 10.3748/wjg.v13.i10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto N, Ebata T, Yokoyama Y, Iqami T, Suqawara G, Shimoyama Y, Naqino M. Role of anatomical riht hepatic trisectionectomy for perihilar cholangiocarcinoma. Br J Surg. 2014;101:261–268. doi: 10.1002/bjs.9383. [DOI] [PubMed] [Google Scholar]
- 16.Neuhaus P, Jonas S, Settmacher U, Thelen A, Benckert C, Lopez-Hänninen E, Hintze RE. Surgical management of proximal bile duct cancer extended right lobe resection increases respectability and radicality. Langenbecks Arch Surg. 2003;388:194–200. doi: 10.1007/s00423-003-0383-5. [DOI] [PubMed] [Google Scholar]
- 17.Oshiro Y, Yano H, Mitani J, Kim S, Fukunaga K, Ohkohchi N. Novel 3-dimensional virtua hepatectomy simulation combined with real-time deformation. World J Gastroenterol. 2015;21:9982–9992. doi: 10.3748/wjg.v21.i34.9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson AL, Geller DA, Hemming AW, Jarnagin WR, Clements LW, Dangelica MI, Dumpuri P, Gonen M, Zendehas I, Miga MI, Stefansic JD. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 2014;219:199–207. doi: 10.1016/j.jamcollsurg.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshiro Y, Mitani J, Okada T, Ohkohchi N. A novel three-dimensional print of liver vessels and tumors in hepatectomy. Surg Today. 2017;47:521–524. doi: 10.1007/s00595-016-1383-8. [DOI] [PubMed] [Google Scholar]
- 20.Gerhards MF, Vangulik TM, Dewit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma-a single center experience. Surgery. 2000;127:395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]


