Abstract
Although an increasing number of findings have proven that glutathione peroxidase 3 (GPX3) is methylated and down-regulated in various cancers, the underlying mechanism of its occurrence in esophageal squamous cell carcinoma (ESCC) remains unknown. In the present study, we found that the methylation rate in advanced cancers was significantly higher than that in early stage cancers by a methylation-specific polymerase chain reaction. Furthermore, the proliferation and migration capacities of KYSE-510 cells were inhibited after up-regulating GPX3 expression by GPX3 lentivirus transfection. As expected, the proliferation and migration capacities of KYSE-150 cells were promoted after down-regulating GPX3 expression with siRNA interfering. Moreover, we found that GPX3 might have deactivated the FAK/AKT signaling pathway to lower the expression of MMP-9 to suppress the migration and invasive capacities of KYSE-150 and KYSE-510 cells. Our findings suggested that GPX3 played a pivotal role in the suppression of carcinogenesis and progression in ESCC, and GPX3 has the potential as a novel biomarker in the diagnosis of ESCC.
Keywords: Esophageal squamous cell carcinoma, glutathione peroxidase 3, FAK/AKT pathway, methylation, migration, invasion
Introduction
Esophageal cancer is a common digestive tract malignancy that has the sixth highest mortality rate worldwide. There are two pathology patterns of esophageal cancer, namely, esophageal adenocarcinoma and esophageal squamous carcinoma. Esophageal adenocarcinoma is prevalent in western countries. In eastern countries, there are more esophageal squamous carcinoma patients, particularly in China. More than 150 thousand unfortunate people died from the torture of esophageal cancer. With the progress in multidisciplinary treatment, the 5-year survival rate of early esophageal cancer has improved dramatically, but the risk of death from advanced esophageal cancer remains high. The prognosis of advanced esophageal cancer is not as good as that of early esophageal cancer because of the distant metastasis of tumors or recurrence after treatment. Therefore, a novel biomarker that can detect early esophageal cancer needs to be identified.
Accumulating evidences has demonstrated that the silencing of tumor suppressor genes by methylation is closely related to the occurrence and development of malignant tumors. Glutathione peroxidase (GPX) is an important peroxide that is widely involved in the decomposing of enzymes, the reduction of toxic peroxides into hydroxyl compounds and nontoxic compounds, and the promotion of the decomposition of the H2O2 interference structure that functions to protect the cell membrane from the oxides and damage [1]. Therefore, GPX reduces the occurrence and development of tumors [2]. GPX3, which is a member of the GPX family, as a tumor suppressor gene, has been shown to increase the incidence of liver, breast, ovarian cancer and many other malignancies greatly when silenced by methylation [2], indicating that GPX3 plays a crucial role in anti-carcinogenesis. Nevertheless, the mechanism of GPX3 in the carcinogenesis and progression of esophageal cancer remains largely unknown.
In the present study, we first examined the clinical role of GPX3 in ESCC patients and found that the down-regulation of GPX3 was significantly related to tumor T-stage and N-stage. Second, we explored the biological role of GPX3 in esophageal squamous cell lines by up-regulating and down-regulating GPX3 expression. Thirdly, we further illustrated the biological role of GPX3 in vivo. Finally, we elucidated the mediated function of GPX3 in the FAK/AKT pathway. Taken together, the present findings indicated that GPX3 may play a critical role in tumorigenesis and the progression of tumors.
Materials and methods
Tissue samples
A total of 24 cancer tissue samples from esophageal squamous carcinoma, along with matched samples of adjacent tissue and 6 of non-tumor esophageal epithelial samples were collected from Zhujiang Hospital, Guangdong province, China, from June to September 2012. After the operation, tissue specimens were frozen in nitrogen immediately, then stored at -80°C until use. None of the patients had been treated with radiotherapy or chemotherapy. All the patients and controls were informed of the questionnaire and signed the informed consent. The diagnosis of esophageal squamous carcinoma was confirmed by two experienced pathologists. Staging of the tumor was assessed according to the TNM classification of the American Joint Committee on Cancer (AJCC).
Cells lines and cell culture
Human ESCC cell lines Eca-109, KYSE-150, KYSE-450 and KYSE-510 were donated by the Central Laboratory of Southern Medical University. All of them were grown in RPMI 1640 medium (Gibco; Invitrogen, Carlsbad, California, USA), supplied with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin in humid atmosphere containing 5% CO2 at 37°C.
Plasmids and transfection
The KYSE-150 cell line was transiently transfected with siRNA and a negative control (NC, Ribobio, Guangzhou, China) using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) following the manufacturer’s instructions, and the sequence of interference is as follows: GTGGCACCATTTACGAGTA. RNA and proteins were harvested at 48 h post-transfection. For stable transfection of KYSE-510, an overexpression Lentivirus vector and a control vector were purchased from Ribobio (Guangzhou, China). Instructions of the manufacturer were followed.
RNA extraction and qRT-PCR
The total RNA was obtained from the cell lysates using the Trizol reagent (Invitrogen, Carlsbad, California, USA), and reverse-transcription reactions were performed using 1 µg of total RNA with the Reverse Transcription System (Takara, Dalian, China). GPX3 mRNA level was measured by RT-PCR with the PrimeScript RT reagent kit (Takara, Dalian, China), and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference. The primers used for GPX3 were GPX3-F: 5’-CTTCCTACCCTCAAGTATGTCCG-3’ and GPX3-R: 5’-GAGGTGGGAGGACAGGAGTTCTT-3’ [8]. The RT-PCR consisted of 50 cycles with an annealing temperature of 60°C.
Bisulfite modification of genomic DNA and methylation-specific PCR (MSP)
Tissue DNA was extracted using a QIAamp DNA Mini Kit (Qiagen), and cell DNA was extracted by QIAamp DNA Blood Mini Kit (Qiagen). Genomic DNA was bisulfited-modified with a Zymo DNA Modification Kit (Zymo Research, Orange, CA, USA) following the manufacturer’s instructions. The bisulfite-modified genomic DNA was amplified using either primer-methylated or primer-unmethylated in a total volume of 10 µl containing 0.25 µl of hot start Taq-polymerase (Takara) per reaction. Methylation-primers were as follows: GPX3-MF: CGTTCGTTTTTGAAATTTTAGTC, and GPX3-MR: CTACCTAATCCCTAACCACCGT. Unmethylated primers were as follows: GPX3-UF: TGTTTGTTTTTGAAATTTTAGTTGT, GPX3-UR: CTACCTAATCCCTAACCACCATC. Finally, the MSP products were separated electrophoretically on 2% agarose gels.
Western blot assay and antibodies
Cell and tissue proteins were extracted from the cell lysate using RIPA (Biyotime Biotechnology), then the concentration was tested with a BCA Kit (Biyotime Biotechnology), and equal masses of protein were separated by SDS-PAGE (10% or 8%) and then transferred onto 0.45 µm PVDF membranes (Milipore, Billerica, MA, USA). The PVDF membrane were incubated in TBST blocking solution containing 5% skimmed milk at room temperature for 1 h to block nonspecific proteins. After incubation with primary antibodies at 4°C overnight, the membrane was washed in TBST for 3×10 minutes, followed by incubation with secondary antibodies at room temperature for 1 h, and then once again with TBST, washing 3 times. A chemiluminescence substrate solution (Pierce Biotechnology; Rockford, IL, USA) was used to visualize the protein signals on the membrane. Rabbit antibodies against human FAK, phosphorylation-FAK, PI3K, MMP-9, phosphorylation-AKT and RAS were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Mouse antibodies against GPX3 were obtained from Abcam (USA), and mouse antibodies against human AKT and rabbit antibodies against human GAPDH were obtained from Proteintech (Wuhan, Hubei, China).
Wound-healing assay
After successful transfection with the lentivirus and siRNA, the KYSE-510 and KYSE-150 cells were seeded at 3×105 cells/well in a 6-well plate, respectively. When the cell confluence reached approximately 90%, a sterile pipette tip was used to make a scratch, scraped cells were cleaned out with PBS, and then replaced with 2% culture medium. The width of the scratches was photographed at 200× using an optical microscope at 0 h and 24 h after scratching.
Transwell cell migration and invasion assay
As in previous assays, in the Transwell cell invasion assay, we first coated it with Matrigel matrix (BD, Biosciences, CA, USA) in the upper chamber. Soon afterwards, 5×104/100 µl transfected KYSE-510 cells suspended in 100 µl RPMI 1640 containing 5% FBS were added into the upper chamber, RPMI 1640 containing 15% FBS was placed into the bottom, and then the cells were allowed to invade for 48 h. In the Transwell cell migration assay, 5×104/100 µl transfected KYSE-510 suspended in RPMI 1640 containing 5% FBS were placed into the upper chamber without Matrigel matrix, and the cells were allowed to migrate for 24 h. For the transfected KYSE-150 cells, the experiments were the same as the KYSE-510, other than the concentration of KYSE-150 cells were 8×104/100 ul.
Cell viability assay
The KYSE-150 cells were seeded at 1500 cells/well. After transient transfection with siRNA or a negative control for 24 h, the cellular proliferation of the cells was measured following stable transfection with a lentivirus-expression vector and control, with the cells seeded in 96-well plates at 2000 cells/well. After incubation for 24 h, according to the manufacturer’s protocol, 10 µl of CCK-8 solution was added into 100 µl of culture medium, then incubated at 37°C for 2 h. The optical density at 450 nm (OD450) was measured. Each experiment was performed in triplicate.
Colony formation assay
Transfected KYSE-510 and KYSE-150 cells were seeded at 200-300 cells/well, and for transiently transfected KYSE-150 cells, owing to the short silencing effect, transient transfection of KYSE-150 needed to be performed again during the experiment. After 14 days, the cells were fixed with 75% ethanol for 30 min and stained with 0.2% crystal violet. The number of clones was counted. Each experiment was repeated 3 times.
Immunohistochemistry
The tumor tissues were immersed in 4% paraformaldehyde for 4 h and transferred to 70% ethanol. Individual lobes of tissue biopsy material were placed in processing cassettes, dehydrated through a serial alcohol gradient, and embedded in paraffin wax blocks. Before immunostaining, 5-µm-thick tissue sections were dewaxed in xylene, rehydrated through decreasing concentrations of ethanol, and washed in PBS, and then stained with hematoxylin and eosin (H&E). After staining, sections were dehydrated through increasing concentrations of ethanol and xylene.
Tail vein metastasis assay
Twelve male BALB/c nude mice were purchased from the animal experiment center of Southern Medical University. GPX3-KYSE-510 cells and control-KYSE-510 cells were suspended in 200 µl of PBS. The mice were injected intravenously with 3.0×106 GPX3-KYSE-510 and control-KYSE-510 cells, respectively. After 1 month, the two groups of mice were visualized by live imaging. Soon afterwards, animals were sacrificed and their lungs were removed and fixed in 10% formalin for metastatic nodule counting and further histopathological analysis. The protocols of the animal studies were approved by the Institutional Animal Care and Use Committee of Southern Medical University.
Statistical analysis
For TMA, the relationship between the clinicopathological parameters of ESCC patients and GPX3 methylation was assessed by X2 tests. The differences between tumor tissues and their corresponding normal tissues were also evaluated by X2 tests. Students t-test or one-way analysis of variance (ANOVA) was used to calculate the P values with SPSS 22.0. P<0.05 was considered statistically significant.
Results
Correlation between the clinicopathological parameters of ESCC patients and GPX3 methylation
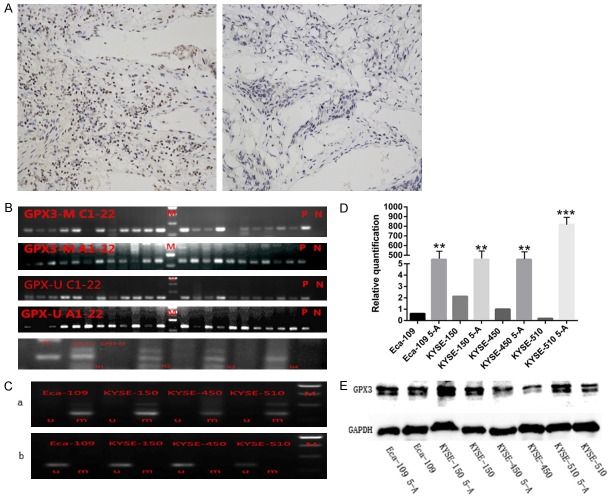
An MSP analysis showed that 22 out of 24 ESCC tumor tissues had GPX3 methylation, and 20 out of 24 ESCC adjacent tumor tissues had gained GPX3 methylation (Figure 1B). However, only 1 in 6 paired normal tissues had it. The difference between GPX3 methylation of tumor tissues and that of paired normal tissues was statistically significant (P=0.001) (Table 2), but there was no significant difference (P=0.333) between the rate of GPX3 methylation of the tumor tissues and adjacent-tumor tissues. Immunohistochemistry results also showed that GPX3 expression in tumor tissues with hypermethylation were significantly lower than that of normal tissues without hypermethylation (Figure 1A). In the relationship between GPX3 methylation and clinicopathology parameters, it was found that GPX3 methylation was significantly associated with M-stage (P=0.014) and N-stage (P<0.01) of ESCC, but had no relationship with sex, age, family history, T-stage or tumor size (Table 1).
Figure 1.
GPX3 expression was lower in ESCC tissues due to higher DNA methylation rate and could be reversed by demethylation. (A) Representative images of strong immunostaining of GPX3 expression in normal esophageal tissues (left) and weak immunostaining of GPX3 expression in ESCC tissues (right). (B) MSP was performed to detect the level of GPX3 methylation in ESCC tissues, adjacent tumor tissues and normal tissues. (C) A represents the GPX3 methylation state of four ESCC cell lines, and methylation states are present in all cell lines. B represents the GPX3 methylation state of four ESCC cell lines after demethylating by 5-aza-dc, the methylation state disappeared after demethylation treatment. Moreover, RT-PCR (D) and Western-blot (E) were performed to examine the demethylation efficiency. Similar to previous reports, the expression level of RNA and protein have been significantly increased after gene demethylation (*P<0.05).
Table 2.
Frequency of methylation in ESCC and normal tissues
| Gene | Frequency of methylation (%) | P value | |
|---|---|---|---|
|
| |||
| ESCC | Normal | ||
| GPX3 | 91.70% | 16.70% | 0.01* |
significant difference.
Table 1.
The relationship between GPX3 methylation and clinicopathological features in ESCC patients
| Clinical data | Number | GPX3 methylation status | Positive rate (%) | X2 | P | |
|---|---|---|---|---|---|---|
|
| ||||||
| Positive | Negative | |||||
| Gender | ||||||
| Male | 13 | 12 | 1 | 0.923 | 0.167 | 0.683 |
| Female | 11 | 10 | 1 | 0.909 | ||
| Age (year) | ||||||
| <60 | 8 | 7 | 1 | 0.727 | 2.667 | 0.102 |
| ≥60 | 16 | 15 | 1 | 0.938 | ||
| Family history | ||||||
| Yes | 8 | 8 | 0 | 1 | 0.167 | 0.683 |
| No | 16 | 14 | 2 | 0.875 | ||
| Tumor size | ||||||
| <5 | 11 | 10 | 1 | 0.909 | 0.167 | 0.683 |
| ≥5 | 13 | 10 | 3 | 0.769 | ||
| T-stage | ||||||
| T1-2 | 10 | 9 | 1 | 0.9 | 0.667 | 0.414 |
| T3 | 14 | 13 | 1 | 0.929 | ||
| N-stage | ||||||
| N0-1 | 21 | 19 | 2 | 0.905 | 13.5 | 0.0001* |
| N2 | 3 | 3 | 0 | 1 | ||
| Tumor-stage | ||||||
| I-II | 18 | 17 | 1 | 0.944 | 6 | 0.014* |
| III | 6 | 6 | 0 | 1 | ||
significant difference.
Frequency of GPX3 methylation and down-regulation in ESCC cell lines
All of the ESCC cell lines (Eca-109, KYSE-150, KYSE-450, KYSE-510) showed a methylation status as being detected by MSP (Figure 1C). To elucidate whether the DNA methylation state was reversible, 5-zza-2’-deoxycytidine (5-A, Sigma, St. Louis, MO, USA) was added to cell cultures for demethylation. The methylation status of the ESCC cell lines were disappeared after 5-zza-2’-deoxycytidine treatment, and expression of GPX3 mRNA and protein increased significantly (Figure 1D, 1E).
Successful construction of stable GPX3-overexpression KYSE-510 and transfection of KYSE-150 with siRNA
As the RT-PCR and WB results showed (Figure 1D, 1E), the expression of GPX3 mRNA and protein in KYSE-150 was higher than those in other cells; while the KYSE-510 cells had the lowest expression of GPX3 among all. Subsequently, KYSE-510 was successfully transfected with overexpression-lentivirus vector and a control vector. GPX3 mRNA expression in GPX3-KYSE-510 group was 250 times higher compared with that of NC-KYSE-510 group (P<0.001, Figure 2A). Expression of GPX3 in KYSE-150 was also successfully knocked down by transient transfection of GPX3-siRNA, meanwhile, transfection of negative control siRNA was also performed for control. The expression of GPX3 mRNA in siRNA-KYSE-150 group was significantly lower than that of NC-KYSE-150 group (0.44±0.04 vs 1, P=0.0013, Figure 2B). Consistent with the mRNA expression change, we further confirmed the expression of GPX3 protein was increased or decreased accordingly (Figure 2A, 2B).
Figure 2.
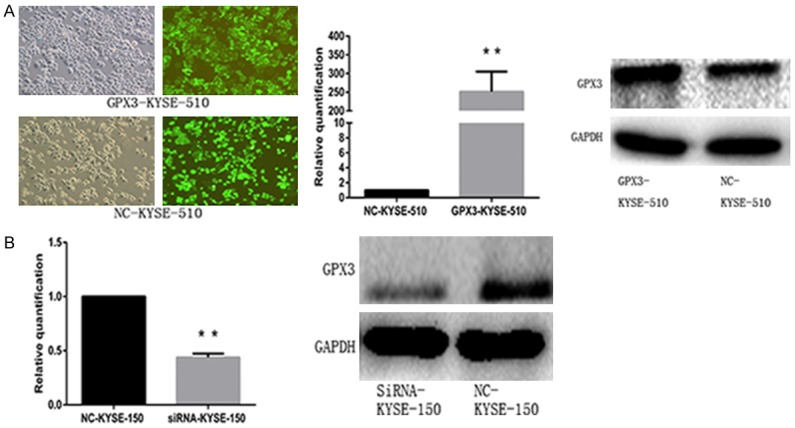
Successful transfection of lentivirus and SiRNA. A. After 48 hours of transfection with lentivirus overexpressing GPX3 and empty vector lentivirus in KYSE-510 cells, the successful transfection of more than 95% cells was observed under the fluorescence microscope (Left), and verified by RT-PCR (middle) and Western-blot (right); B. KYSE-150 cells were transfected with siRNA and negative control siRNA for 24 hours and 48 hours after transfection, respectively. RT-PCR (Left) and Western-blot (right) demonstrated the successful interference of GPX3 expression (*P<0.05).
GPX3 inhibited migration and invasion in vitro
Since down-regulation of GPX3 may be related to metastasis of tumors in patients with ESCC, wound-healing and transwell cell migration/invasion assays were performed to explore the relationship between GPX3 expression and migration and invasion function of ESCC cell lines. Comparing the scratch width at 0 h with that at 24 h after scratching, the results indicated that the healing ability of the GPX3-KYSE-510 group was worse than that of the NC-KYSE-510 group (Figure 4A). Moreover, we also found that the migration (Figure 4B) and invasion (Figure 4C) ability of KYSE-510 cells were significantly inhibited after up-regulation of GPX3 expression. However, the difference was not sufficient to completely demonstrate that GPX3 does have a significant inhibition effect on the cell migration and invasion. Thus, it was necessary to observe the changes of migration and invasion after down-regulation of GPX3. The opposite results were observed after down-regulation of GPX3, the healing width of the siRNA-KYSE-150 group was significantly wider than in the NC-KYSE-150 group (Figure 4A). We found that the migration and invasion ability of KYSE-510 cells were significantly promoted after down-regulation of GPX3 expression (Figure 4B, 4C).
Figure 4.
GPX3 suppresses migration and invasion on ESCC cells. A. Wound-healing assay showed that the healing ability of the GPX3-KYSE-510 group was worse than that of the NC-KYSE-510 group. B, C. Transwell migration and invasive assay showed that migration and invasion ability of KYSE-510 cells were significantly inhibited after up-regulation of GPX3 expression. On the contrary, the migration and invasion ability of KYSE-510 cells were significantly promoted after down-regulation of GPX3 expression (*P<0.05).
GPX3 suppressed proliferation in vitro
To study the effect of GPX3 on tumor growth, CCK-8 and colony formation assays were performed to examine the exact relationship between GPX3 and tumor growth. In the CCK-8 assay, GPX3-KYSE-510 showed a higher level of cellular proliferation speed than that of NC-KYSE-510. siRNA-KYSE-150 with lower G-PX3 expression showed a slower growth rate compared with NC-KYSE-150 (Figure 3A). In addition, compared with the NC-KYSE-510 group, the GPX3-KYSE-510 group had a 3-fold decrease in colony formation rate, while the siRNA-KYSE-150 group demonstrated 2.13-fold increase in colony formation rate (Figure 3B).
Figure 3.

GPX3 inhibits proliferation of KYSE-510 and KYSE-150 cells. A. In the CCK-8 assay, GPX3-KYSE-510 showed a higher level of cellular proliferation speed than that of NC-KYSE-510. siRNA-KYSE-150 with lower GPX3 expression showed a slower growth rate compared with NC-KYSE-150. B. colony formation study showed, compared with the NC-KYSE-510 group, the GPX3-KYSE-510 group had a 3-fold decrease in colony formation rate, while the siRNA-KYSE-150 group demonstrated 2.13-fold increase in colony formation rate (*P<0.05).
GPX3 down-regulated the expression of MMP-9 through deactivation of the FAK/AKT signaling pathway
To determine the underlying mechanism of GPX3 on migration and invasion, we investigated the expression of the related proteins in the FAK/AKT pathway, such as P-FAK, FAK, PI3K, P-AKT, AKT and RAS, via western blot analysis. After transfection with GPX3 lentivirus or control lentivirus, total FAK, PI3K and total AKT all remained unchanged. The expression of P-FAK, P-AKT, RAS and MMP-9 were reduced 4.36, 2.98, 3.57 and 2.37 folds respectively, in GPX3-KYSE-510 group compared with those in the NC-KYSE-510 group (Figure 5A). Moreover, we further investigated the alteration of related proteins in the FAK/AKT pathway after down-regulation of GPX3. The expression levels of P-FAK, P-AKT, RAS and MMP-9 were increased 3.24, 2.11, 1.59 and 1.58 folds in the siRNA-KYSE-150 group compared with those of the NC-KYSE-150 group (Figure 5B). In addition, we also found that the FAK/AKT signaling pathway was suppressed after up-regulating GPX3 expression, and the FAK/AKT signaling pathway was restored after the addition of P-AKT agonists SC-79 (Figure 5C). While the addition of P-AKT inhibitor AZD5363 suppressed the FAK/AKT pathway, which was activated by down-regulating GPX3 expression before (Figure 5D).
Figure 5.
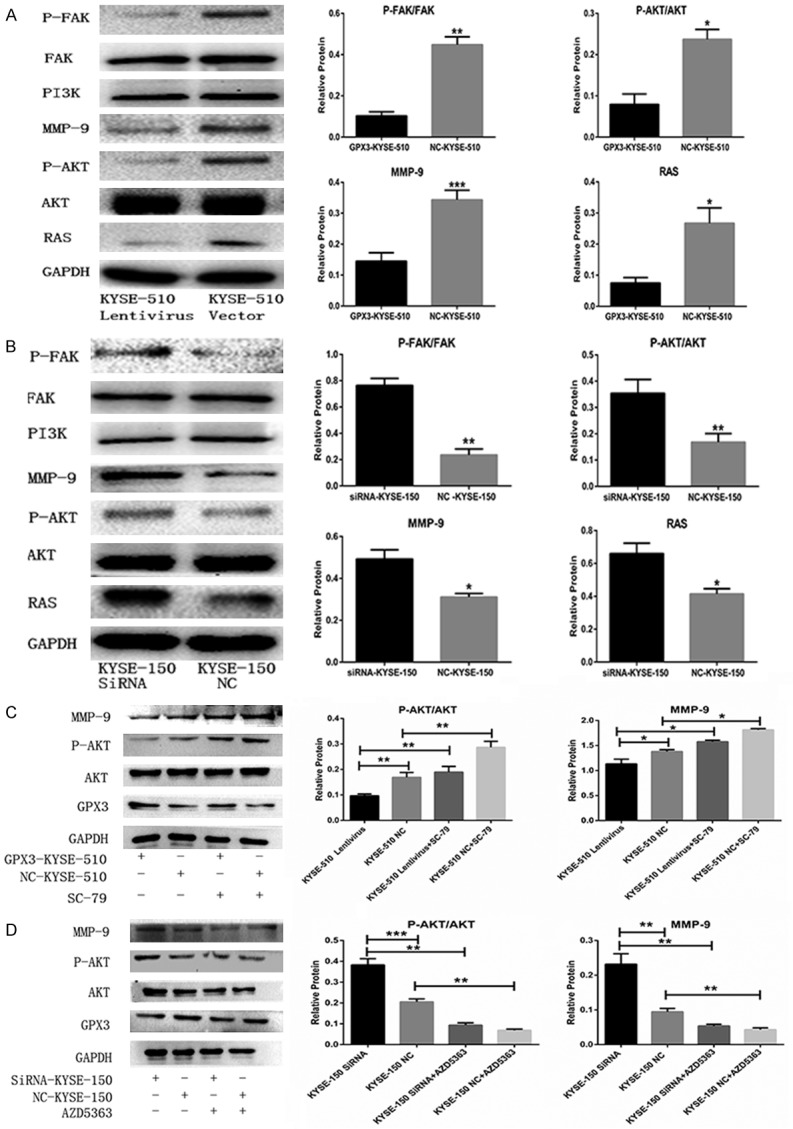
GPX3 deactivated the FAK/AKT signaling pathway. A, B. The related proteins in the FAK/AKT signaling pathway are detected by Western blot. GPX3 suppressed P-AKT, P-FAK, RAS and MMP-9 expression, while the expression of AKT and FAK were not affected. C. Transfected KYSE-510 cells were pre-treated with P-AKT agonist SC-79 for 24 h before harvesting, the detection of AKT, P-AKT, MMP-9 expression level was performed by western-blot. The expression of MMP-9 is suppressed with the inhibition of P-AKT expression. D. KYSE-150 cells were transfected with siRNA against GPX3 or non-targeting control siRNA, and then 48 h later cells were pre-treated with P-AKT inhibitor AZD5363 as described before, the expression of MMP-9 was up-regulated with the improvement of P-AKT expression (*P<0.05).
GPX3 inhibits metastasis in vivo
To further validate the biological role of GPX3 in vivo, we used BALB/c nude mice as animal models. We conducted a tail vein metastasis assay to study whether GPX3 has a biological function in inhibiting tumor metastasis in vivo. GPX3-KYSE-510 and NC-KYSE-510 cells (3.0×106) were injected through the tail vein to two groups of male nude mice (3 mice/per group). Thirty days after injection, a bioluminescence imaging assay was used to observe the metastasis of the tumor in vivo. The thermographs showed that the tumors produced by the GPX3-KYSE-510 cells were significantly smaller and fewer compared with those produced by the NC-KYSE-510 cells (Figure 6A). The lungs filled with metastatic tumors were removed for immunohistochemistry, and GPX3 and P-AKT were found to be markedly reduced in GPX3-KYSE-510 group compared with the NC-KYSE-510 group (Figure 6B). Taken together, our findings suggest that GPX3 inhibits metastasis in ESCC in vivo.
Figure 6.
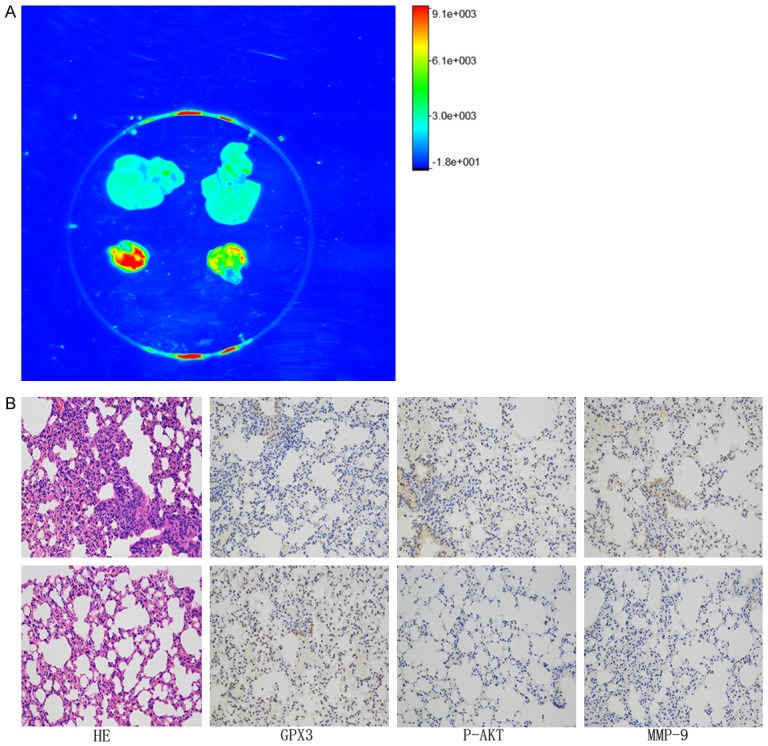
GPX3 inhibits distant metastasis in vivo. Stable transfected KYSE-510 cells were injected into nude mice through the caudal vein, and the two groups of mice were visualized by live imaging after 1 month. A. Living body image experiments showed that overexpression of GPX3 (Right) significantly inhibits distant metastasis to the lungs compared with the negative control (Left), while there have no sign of metastasis in liver. B. H&E staining and immunohistochemistry of the cancer tissues showed that the expression of P-AKT in the GPX3-KYSE-510 group (down) was significantly lower than that of the NC-KYSE-510 group (up).
Discussion
There are many complicated, undiscovered multi-factor and multi-steps in the tumorigenesis and progression of malignant tumors. It is known that biology, chemistry, physics and others are the common pathogenic causes accounting for tumorigenesis. Along with the advancement of treatment and diagnosis, the 5-year survival rate, prognosis and quality of life of ESCC patients has improved greatly, but this is only limited to early-stage cases. Although an increasing number of biomarkers have been discovered in past decades, a specific biomarker for esophageal cancer has not been identified. It is apparent that an early diagnosis of malignancy is the key to ensuring a better patient prognosis. Consequently, it is urgent to find promising biomarkers that possess both therapeutic and prognostic value for ESCC patients.
Reactive oxygen species (ROS) have played a crucial role in the development of various diseases by damaging DNA structure. ROS-induced DNA damage can not only initiate carcinogenesis but also boost its progression [9-11]. Glutathione peroxidase can induce the conversion of hydrogen peroxide and lipid peroxide to harmless hydroxy peroxide to protect the cell membrane from damage from the oxide [9-11]. Moreover, increasing evidence has elucidated that GPX3, a plasma antioxidant enzyme, has its expression lowered by hypermethylation and other potential causes to play a pivotal role in tumorigenesis [1,3,6,13-15]. Chen etc. hypothesized that GPX3 methylation may account for chemotherapy resistance [6]. In liver cancer, Qi etc. found that GPX3 methylation could be identified as an independent predictor after liver transplantation [16]. Similarly, in the present study, widespread methylation and down-regulation of GPX3 was confirmed in ESCC. Furthermore, we found that GPX3 can suppress ESCC cell proliferation, migration and invasion in vivo and in vitro. These results suggested that GPX3 could be a tumor suppressor gene in patients with ESCC.
In esophageal cancer, an Infinium Methylation 450 k array was used to analyze the differentiated methylation of ESCC tissues and paired adjacent-tumor tissues, and our group proved that GPX3 methylation frequency was higher in tumor tissues than in normal tissues [17]. Based on the research of GPX3 methylation frequency in the different stages of esophageal cancer, Lee etc. found that GPX3 methylation frequency increased during the development of esophageal cancer [17]. Consistent with our results, he also validated the lower GPX3 methylation frequency in normal tissues than in ESCC tumor tissues [19]. In addition to its role in tumorigenesis, its functional role in the migration and invasion of GPX3 has been frequently researched [20,21]. Qi etc. proved that GPX3 may play a critical role in the progression of hepatocellular carcinoma by mediating the Erk-NFκB-SIP1 pathway [22]. Although accumulating evidence has proven GPX3 may play an important role in the progression of ESCC, there has never been a meaningful study to demonstrate the concrete mechanism of the function of GPX3 in the progression of ESCC. In our study, we found GPX3 could suppress the level of MMP-9 expression via deactivating the FAK/AKT pathway to weaken the migratory and invasive capacity of KYSE-150 and KYSE-510 cells.
Focal adhesion kinase (FAK) is a protein tyrosine kinase involved in the regulation of cell migration and invasion in the majority of cancer cells. Previous studies have reported that the down-regulation of FAK could reduce human conventional renal cell carcinoma cell migration and invasion in vitro and in vivo. Their most important finding was that FAK has the ability to down-regulate the expression of AKT [23]. Shang and colleagues discovered that FAK is activated by MEK, which then induces the activation of the AKT/Erk pathway and finally mediates tumor proliferation [24]. The present study confirmed that GPX3 overexpression decreased the expression of P-FAK compared with that of negative controls, and similarly, the down-regulation of GPX3 increased P-FAK compared with that of negative controls. However, regardless of the level of expression of GPX3, the expression of FAK was not affected.
Accumulating findings have shown that P-FAK can regulate the expression of AKT to monitor the migration and invasion of cells. In gastric carcinoma, P-AKT expression was widespread in 78% of gastric tumor tissues, and as expected, P-AKT expression also had a strong relationship with the depth of invasion, number of lymph nodes and poor patient [25]. EGF-induced CCR1 expression could be caused by activating the AKT-mTOR-STAT3 pathway to promote the capacity of invasion and migration of breast cancer cells, according to Shin’s findings [26]. In our results, P-AKT was significantly down-regulated in the KYSE-510 lentivirus group compared with the KYSE-510 control group. Conversely, the interference of GPX3 could contribute to the increasing in P-AKT. However, the expression level of AKT remains unchanged.
There have been numerous studies reporting that distant metastasis of tumors results in the death of patients, and moreover, extracellular matrix is an important protective screen to prohibit tumor cells from invading to distant organs. Matrix metalloproteinases (MMPs) are the key mediators to remodel the extracellular matrix [27]. It has been reported that increasing the expression of MMP-9 is related to a poor prognosis in the majority of cancers [27]. This is consistent with our findings [28]; the higher the expression of MMP-9, the stronger the migration and invasive ability of KYSE-150 and KYSE-510 cells. To further verify that GPX3 activated the FAK/AKT signaling pathway and then mediated the expression of MMP-9 to alter the migration and invasion capacity, a P-AKT agonist (SC-79) and an inhibitor (AZD5363) were added to regulate the FAK/AKT pathway. The findings showed that the expression of MMP-9 changes along with the changes of the expression of P-AKT, while the expression of AKT had no change. Taken together, the present study illustrated that GPX3 might have deactivated the FAK/AKT signaling pathway to lower the expression of MMP-9 to suppress the migration and invasive capacities of KYSE-150 and KYSE-510 cells.
In summary, GPX3 expression was down-regulated in ESCC tissues and ESCC cell lines, and it played the role of a suppressor tumor gene in ESCC by mediating the FAK/AKT pathway. In addition, the detection of GPX3 methylation in blood might be a promising noninvasive biomarker, and GPX3 may become a potential novel therapeutic target.
Acknowledgements
This work is supported by Natural Science Foundation of Guangdong Province (No. 2015A030313273), and Science and Technology Development Project of Southern Medical University (No. C1033032).
Disclosure of conflict of interest
None.
References
- 1.Barrett CW, Ning W, Chen X, Smith JJ, Washington MK, Hill KE, Coburn LA, Peek RM, Chaturvedi R, Wilson KT, Burk RF, Williams CS. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 2013;73:1245–1255. doi: 10.1158/0008-5472.CAN-12-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takebe G, Yarimizu J, Saito Y, Hayashi T, Nakamura H, Yodoi J, Nagasawa S, Takahashi K. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J Biol Chem. 2002;277:41254–41258. doi: 10.1074/jbc.M202773200. [DOI] [PubMed] [Google Scholar]
- 3.Cao S, Yan B, Lu Y, Zhang G, Li J, Zhai W, Guo W, Zhang S. Methylation of promoter and expression silencing of GPX3 gene in hepatocellular carcinoma tissue. Clin Res Hepatol Gastroenterol. 2015;39:198–204. doi: 10.1016/j.clinre.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Agnani D, Camacho-Vanegas O, Camacho C, Lele S, Odunsi K, Cohen S, Dottino P, Martignetti JA. Decreased levels of serum glutathione peroxidase 3 are associated with papillary serous ovarian cancer and disease progression. J Ovarian Res. 2011;4:18. doi: 10.1186/1757-2215-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed MM, Sabet S, Peng DF, Nouh MA, El-Shinawi M, El-Rifai W. Promoter hypermethylation and suppression of glutathione peroxidase 3 are associated with inflammatory breast carcinogenesis. Oxid Med Cell Longev. 2014;2014:787195. doi: 10.1155/2014/787195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen B, Rao X, House MG, Nephew KP, Cullen KJ, Guo Z. GPx3 promoter hypermethylation is a frequent event in human cancer and is associated with tumorigenesis and chemotherapy response. Cancer Lett. 2011;309:37–45. doi: 10.1016/j.canlet.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Lee OJ, Schneider-Stock R, McChesney PA, Kuester D, Roessner A, Vieth M, Moskaluk CA, El-Rifai W. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett’s tumorigenesis. Neoplasia. 2005;7:854–861. doi: 10.1593/neo.05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jee CD, Kim MA, Jung EJ, Kim J, Kim WH. Identification of genes epigenetically silenced by CpG methylation in human gastric carcinoma. Eur J Cancer. 2009;45:1282–1293. doi: 10.1016/j.ejca.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Dreher D, Junod AF. Role of oxygen free radicals in cancer development. Eur J Cancer. 1996;32A:30–38. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- 10.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 11.Kanno SI, Tomizawa A, Yomogida S, Hara A. Glutathione peroxidase 3 is a protective factor against acetaminopheninduced hepatotoxicity in vivo and in vitro. Int J Mol Med. 2017;40:748–754. doi: 10.3892/ijmm.2017.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debelec-Butuner B, Ertunc N, Korkmaz KS. Inflammation contributes to NKX3.1 loss and augments DNA damage but does not alter the DNA damage response via increased SIRT1 expression. J Inflamm (Lond) 2015;12:12. doi: 10.1186/s12950-015-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou JD, Lin J, Zhang TJ, Ma JC, Yang L, Wen XM, Guo H, Yang J, Deng ZQ, Qian J. GPX3 methylation in bone marrow predicts adverse prognosis and leukemia transformation in myelodysplastic syndrome. Cancer Med. 2017;6:267–274. doi: 10.1002/cam4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu YP, Yu G, Tseng G, Cieply K, Nelson J, Defrances M, Zarnegar R, Michalopoulos G, Luo JH. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 2007;67:8043–8050. doi: 10.1158/0008-5472.CAN-07-0648. [DOI] [PubMed] [Google Scholar]
- 15.Chang SN, Lee JM, Oh H, Park JH. Glutathione peroxidase 3 inhibits prostate tumorigenesis in TRAMP mice. Prostate. 2016;76:1387–1398. doi: 10.1002/pros.23223. [DOI] [PubMed] [Google Scholar]
- 16.Qi X, Ng KT, Shao Y, Li CX, Geng W, Ling CC, Ma YY, Liu XB, Liu H, Liu J, Yeung WH, Lo CM, Man K. The clinical significance and potential therapeutic role of GPx3 in tumor recurrence after liver transplantation. Theranostics. 2016;6:1934–1946. doi: 10.7150/thno.16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Zhou F, Jiang C, Wang Y, Lu Y, Yang F, Wang N, Yang H, Zheng Y, Zhang J. Identification of a DNA methylome profile of esophageal squamous cell carcinoma and potential plasma epigenetic biomarkers for early diagnosis. PLoS One. 2014;9:e103162. doi: 10.1371/journal.pone.0103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee OJ, Schneider-Stock R, McChesney PA, Kuester D, Roessner A, Vieth M, Moskaluk CA, El-Rifai W. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett’s tumorigenesis. Neoplasia. 2005;7:854–861. doi: 10.1593/neo.05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Wang Y, Li P, Zhu S, Wang J, Zhang S. Identification of GPX3 epigenetically silenced by CpG methylation in human esophageal squamous cell carcinoma. Dig Dis Sci. 2011;56:681–688. doi: 10.1007/s10620-010-1369-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zheng Z, Yingji S, Kim H, Jin R, Renshu L, Lee DY, Roh MR, Yang S. Downregulation of glutathione peroxidase 3 is associated with lymph node metastasis and prognosis in cervical cancer. Oncol Rep. 2014;31:2587–2592. doi: 10.3892/or.2014.3152. [DOI] [PubMed] [Google Scholar]
- 21.Peng DF, Hu TL, Schneider BG, Chen Z, Xu ZK, El-Rifai W. Silencing of glutathione peroxidase 3 through DNA hypermethylation is associated with lymph node metastasis in gastric carcinomas. PLoS One. 2012;7:e46214. doi: 10.1371/journal.pone.0046214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi X, Ng KT, Lian QZ, Liu XB, Li CX, Geng W, Ling CC, Ma YY, Yeung WH, Tu WW, Fan ST, Lo CM, Man K. Clinical significance and therapeutic value of glutathione peroxidase 3 (GPx3) in hepatocellular carcinoma. Oncotarget. 2014;5:11103–11120. doi: 10.18632/oncotarget.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beraud C, Dormoy V, Danilin S, Lindner V, Bethry A, Hochane M, Coquard C, Barthelmebs M, Jacqmin D, Lang H, Massfelder T. Targeting FAK scaffold functions inhibits human renal cell carcinoma growth. Int J Cancer. 2015;137:1549–1559. doi: 10.1002/ijc.29522. [DOI] [PubMed] [Google Scholar]
- 24.Shang N, Arteaga M, Zaidi A, Stauffer J, Cotler SJ, Zeleznik-Le NJ, Zhang J, Qiu W. FAK is required for c-Met/beta-catenin-driven hepatocarcinogenesis. Hepatology. 2015;61:214–226. doi: 10.1002/hep.27402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du M, Wen G, Jin J, Chen Y, Cao J, Xu A. Mangiferin prevents the growth of gastric carcinoma by blocking the PI3K-Akt signalling pathway. Anticancer Drugs. 2017 doi: 10.1097/CAD.0000000000000583. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Shin SY, Lee DH, Lee J, Choi C, Kim JY, Nam JS, Lim Y, Lee YH. C-C motif chemokine receptor 1 (CCR1) is a target of the EGF-AKT-mTOR-STAT3 signaling axis in breast cancer cells. Oncotarget. 2017;8:94591–94605. doi: 10.18632/oncotarget.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan X, Qi C, Liu X, Wang Y, Liu S, Li S, Wang L, Wang Y. Regional specificity of matrix metalloproteinase-9 expression in the brain: voxel-level mapping in primary glioblastomas. Clin Radiol. 2018;73:283–289. doi: 10.1016/j.crad.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Cox G, Jones JL, O’Byrne KJ. Matrix metalloproteinase 9 and the epidermal growth factor signal pathway in operable non-small cell lung cancer. Clin Cancer Res. 2000;6:2349–2355. [PubMed] [Google Scholar]