Abstract
Homeobox genes (HOX genes) have been implicated in many tumors. As a member of HOX genes, HOXB5 is overexpressed in bladder cancer and contributes to the proliferation and invasion of breast cancer cells. However, functions of HOXB5 in retinoblastoma remain elusive. In this study, we found that HOXB5 expression is upregulated in retinoblastoma cell lines and tissues. Overexpression of HOXB5 promoted retinoblastoma cell migration and invasion, but knockdown of HOXB5 suppressed the migration and invasion. Moreover, HOXB5 induced the activation of ERK1/2 and upregulated the production of MMP-3 and MMP-13. In addition, ERK1/2 pathway was required for HOXB5-mediated retinoblastoma cell migration and invasion. Taken together, our study suggests that HOXB5 promotes the migration and invasion of retinoblastoma cells by inducing the activation of ERK1/2 and increasing the production of MMP-3 and MMP-13. Therefore, HOXB5 may represent an effective target for treatment of retinoblastoma.
Keywords: HOXB5, retinoblastoma, invasion, migration, ERK1/2, MMPs
Introduction
Retinoblastoma is the most common malignant tumor of the eye in the world [1,2]. Poor prognosis of retinoblastoma is closely related to the invasive and metastatic tendencies [3], which account for the major reasons of tumor-related death. Therefore, it is urgent to uncover the molecular mechanisms involved in the invasion and metastasis of retinoblastoma.
Homeobox genes (HOX genes) are first characterized in the fruit fly Drosophila melanogaster, and are further identified in other species, including mammals [4,5]. HOX genes are 183-bp sequences that encode highly conserved 61-amino-acid homeodomains. HOX proteins are able to bind specific DNA sites and subsequently regulate the key pathways in the organogenesis and oncogenesis [6]. In human, 39 HOX genes are identified that are further grouped into four clusters: HOXA, B, C, and D. Each cluster contains 9 to 11 members that located on four different chromosomes [7]. Many studies have reported that HOX genes are involved in the progression of tumor. Belonging to HOX genes, HOXB cluster plays an important role in many tumors. It is reported that HOXB9 promotes epithelial-to-mesenchymal transition in gastric and hepatocellular carcinoma cells [8,9]. Many studies have found that HOXB13 is frequently overexpressed in prostate and cervical cancers [10,11], and promotes the invasion of cervical cancer cells [12]. HOXB5, a member of HOXB cluster, has been reported to be amplified in oral squamous cell carcinoma [13]. However, functions of HOXB5 in retinoblastoma are still not clear.
In this work, we detected the expression of HOXB5 in retinoblastoma cells. We also tried to uncover the molecular functions of HOXB5 in retinoblastoma cell invasion and migration.
Materials and methods
Cell culture and reagents
Cell lines were obtained from Cell Resource Center of the Chinese Academy of Medical Science (Beijing, China). The retinoblastoma cell lines, including Y79, SO-RB50, and WERI-RB1 cells, were grown in RPMI-1640 with 10% FBS. Cells were allowed to culture at 37°C in a humidified atmosphere of 5% CO2. Antibodies against HOXB5, ERK1/2, and GAPDH were purchased from Santa Cruz (Camarillo, CA, USA). Antibody against phospho-ERK1/2 was purchased from Cell Signaling Technology (Danvers, MA, USA). ERK1/2 specific inhibitor U0126 was purchased from Sigma (St Louis, MO, USA).
Patients and tissue samples
Our study was approved by the Human Scientific Ethics Committee of Hangzhou Medical College. A total of 16 patients who were pathologically diagnosed as retinoblastoma at the Zhejiang Provincial People’s Hospital between 2010 and 2016 were recruited. Tissue samples were collected from 16 primary human retinoblastoma tissues and 8 normal pediatric retinas.
Western blotting
Cells were harvested and cellular protein was extracted with RIPA lysis buffer containing protease and phosphatase inhibitors (Applygen Technologies Inc, Beijing, China). BCA protein assay kit (Applygen Technologies Inc) was used to measure the concentration. Equal amounts of protein were separated electrophoretically on SDS-polyacrylamide gel and transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon P, Millipore, Bedford, MA). The membranes were blocked for 1 h at room temperature in 5% BSA, and then were immunoblotted with the antibodies of HOXB5, GADPH, ERK1/2 and p-ERK1/2 respectively, overnight at 4°C. Next, the membranes were incubated with secondary antibodies conjugated to horseradish peroxidase (HRP). Finally, the membranes were detected by enhanced chemiluminescence (Pierce, Rockford, IL).
Real-time PCR
Total RNA was isolated with TRIzol reagent (Invitrogen). The cDNA was prepared by using RNA of 2 μg, dNTPs, oligo dT and M-MLV reverse transcriptase. PCR was performed using SYBR® Green Real-Time PCR Master and primers of MMP-3 (forward: 5’-CTGGACTCCGACACTCTGGA-3’, reverse: 5’-CAGGAAAGGTTCTGAAGTGACC-3’), MMP-13 (forward: 5’-CCAGACTTCACGATGGCATTG-3’, reverse: 5’-GGCATCTCCTCCATAATTTGGC-3’) and GAPDH (forward: 5’-GAGTCAACGGATTTGGTCGT-3’, reverse: 5’-GACAAGCTTCCCGTTCTCAG-3’). Real-time PCR analysis was carried out as follows: 95°C for 1 min, 40 cycles of 95°C for 15 s, 60°C for 1 min. Finally, the threshold cycle number for MMP-3 and MMP-13 in each sample was normalized to that of GAPDH.
Migration assay
Cells were plated on the upper chambers (1 × 105 cells in 200 μl) of the 24-well Transwell plates cell culture inserts (BD Biosciences, NJ, USA). DMEM culture medium plus 20% FBS was added into the lower chambers to serve as a chemoattractant. After 18 h of culture, cells that migrated through the filter were fixed with methanol for 10 min and stained with eosin for 20 min. Finally, the stained cells were counted under a light microscope in seven random fields.
Invasion assay
The upper chambers of the 24-well Transwell plates cell culture inserts (BD Biosciences, NJ, USA) was coated with Matrigel basement membrane matrix (BD) before the experiment was performed. Cells at a density of 5 × 105 cells/ml suspended in 200 μl DMEM were added into the upper chambers, whereas the DMEM culture medium plus 20% FBS was added into the lower chambers. After 18 h of culture, cells that invaded through the filter were fixed with methanol for 10 min and stained with eosin for 20 min. Finally, the stained cells were counted under a light microscope in seven random fields.
ELISA assay
Cell supernatant was collected and stored at -80°C. MMP-3 and MMP-13 ELISA KIT were used to measure the protein level of MMP-3 and MMP-13 (Boster, Wuhan, China), according to the manufacturer’s instructions.
Cell transfection
Small interference sequence (siRNA) of HOXB5 (siHOXB5) was designed and purchased from Invitrogen. A si-scramble RNA (siNC) was used as a negative control. A HOXB5 open reading frame was subcloned into the pcDNA3.1 vector (vHOXB5), whereas a scrambled sequence was subcloned into the pcDNA3.1 vector as negative control (vNC). Cells were transfected with siRNA or pcDNA vector using Lipofectamine 2000 reagent. 36 h later, the expression of HOXB5 was detected by western blotting.
Statistical analysis
All experiments were performed and repeated independently at least three times. The results are expressed as means ± standard deviation (SD). For differences between groups, analysis was performed using Student’s t-test (two groups) or one-way analysis of variance (ANOVA) test (multiple groups). All data were estimated with the software of SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P<0.05.
Results
HOXB5 is significantly expressed in retinoblastoma cell lines and tissues
Using western blotting, the expression of HOXB5 in Y79, SO-RB50, and WERI-RB1 cells was detected. The results showed that HOXB5 expression was upregulated in retinoblastoma cells (Figure 1A). Further, the mRNA expression of HOXB5 in 16 tumor tissues and 8 normal pediatric retina were detected by real-time PCR. The results showed that the expression of HOXB5 was increased in tumor tissues (Figure 1B). These data imply that HOXB5 may play a positive role in retinoblastoma. Y79 and WERI-RB1 cells were used in further study, because that relatively lower HOXB5 expression in Y79 cells, but higher HOXB5 level in WERI-RB1 cells was observed as compared to that in SO-RB50 cells.
Figure 1.
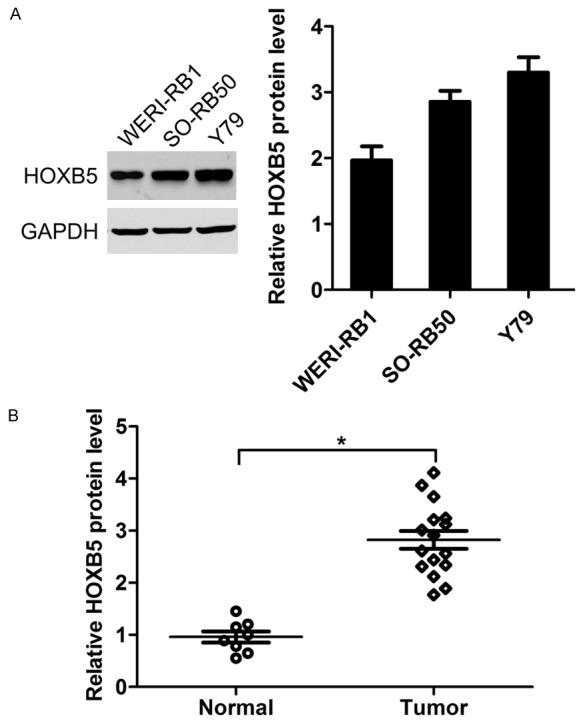
Expression of HOXB5 in retinoblastoma cells and tissues. A. Protein level of HOXB5 in retinoblastoma Y79, SO-RB50, and WERI-RB1 cell lines. B. Protein level of HOXB5 in retinoblastoma tissues. *P<0.05.
Knockdown of HOXB5 inhibits the migration and invasion of retinoblastoma cells
Then the role of HOXB5 in retinoblastoma cell migration and invasion was examined. The expression of HOXB5 in Y79 cells was greatly inhibited after transfection of siHOXB5 (Figure 2A). Cell migration ability was detected through migration assay, while cell invasion ability was determined by invasion assay. After knockdown of HOXB5 in Y79 cells using siRNA, the siNC and siHOXB5 cells were subjected to migration and invasion assays, respectively. The results showed that knockdown of HOXB5 suppressed the migration of Y79 cells (Figure 2B). Similarly, invasion assay showed that knockdown of HOXB5 inhibited the invasion of Y79 cells (Figure 2C). However, knockdown of HOXB5 did not affect the growth of Y79 cells (Data not shown).
Figure 2.
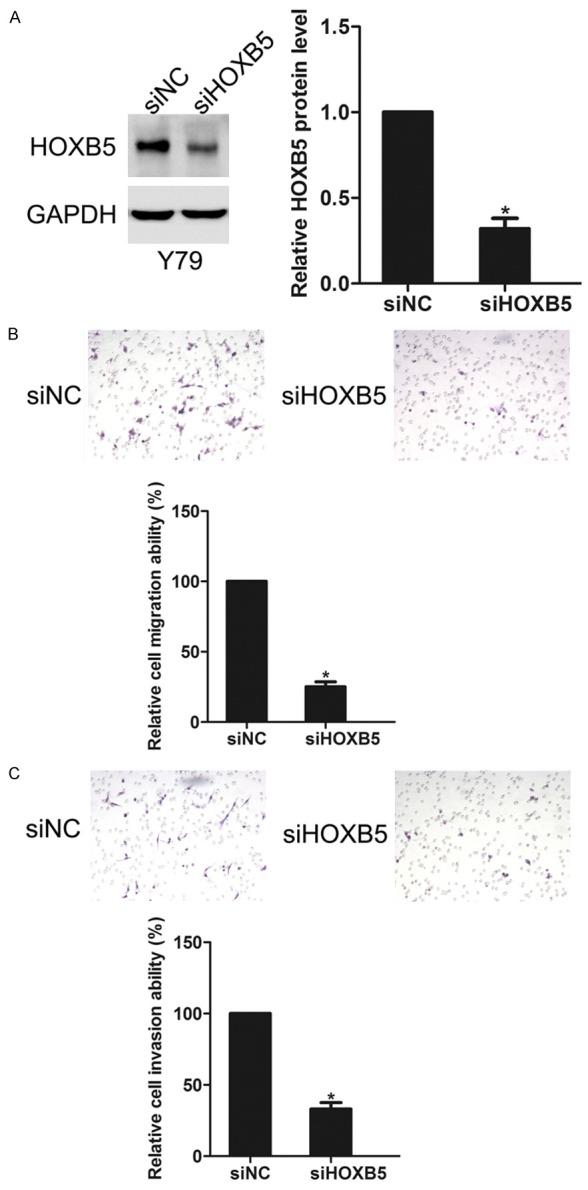
Effects of HOXB5 knockdown on Y79 cell migration and invasion. A. Y79 cells were transfected with siNC or siHOXB5 respectively, HOXB5 expression was detected by western blotting. B. Effect of HOXB5 knockdown on cell migration. C. Effect of HOXB5 knockdown on cell invasion. *P<0.05.
Overexpression of HOXB5 promotes the migration and invasion of retinoblastoma cells
Next, a pcDNA3.0-HOXB5 vector was transfected into WERI-RB1 cells. As shown in Figure 3A, transfection of vHOXB5 markedly increased the expression of HOXB5 in WERI-RB1 cells. Next, vNC and vHOXB5 cells were allowed to invade or migrate for 18 h. Using migration assay and invasion assay, we found that as compared with vNC cells, overexpression of HOXB5 promoted the migration and invasion of WERI-RB1 cells, suggesting that HOXB5 is involved in the migration and invasion of retinoblastoma cells (Figure 3B and 3C). However, overexpression of HOXB5 had little effect on the proliferation of WERI-RB1 cells (Data not shown).
Figure 3.
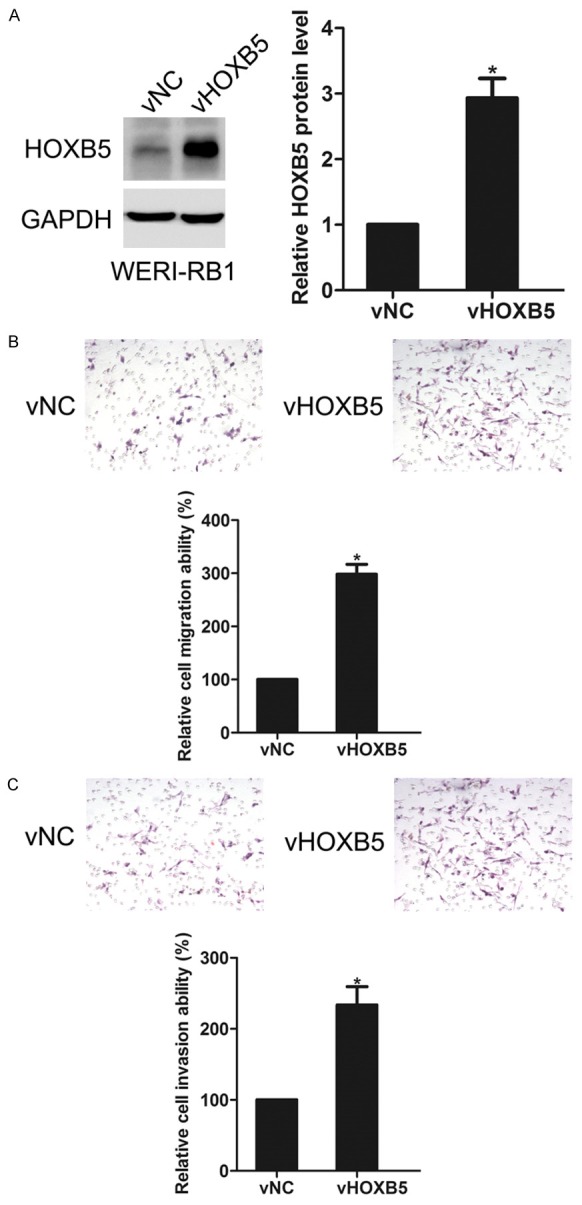
Effects of HOXB5 overexpression on WERI-RB1 cell migration and invasion. A. WERI-RB1 cells were transfected with vNC or vHOXB5 respectively, HOXB5 expression was detected by western blotting. B. Effect of HOXB5 overexpression on cell migration. C. Effect of HOXB5 overexpression on cell invasion. *P<0.05.
HOXB5 affects the activation of ERK1/2 in retinoblastoma cells
ERK1/2 pathway plays an important role in tumor development. EGF, a pivotal cytokine in tumor progression, was used to activate ERK1/2 activation in retinoblastoma cells. In siNC cells, we found that EGF stimulation for 10 min greatly induced ERK1/2 activation. However, the EGF-mediated ERK1/2 activation was attenuated in siHOXB5 cells (Figure 4A). Also, overexpression of HOXB5 in WERI-RB1 cells enhanced the EGF-induced activation of ERK1/2 (Figure 4B). These data suggest that HOXB5 can affect the activation of ERK1/2 in retinoblastoma cells.
Figure 4.
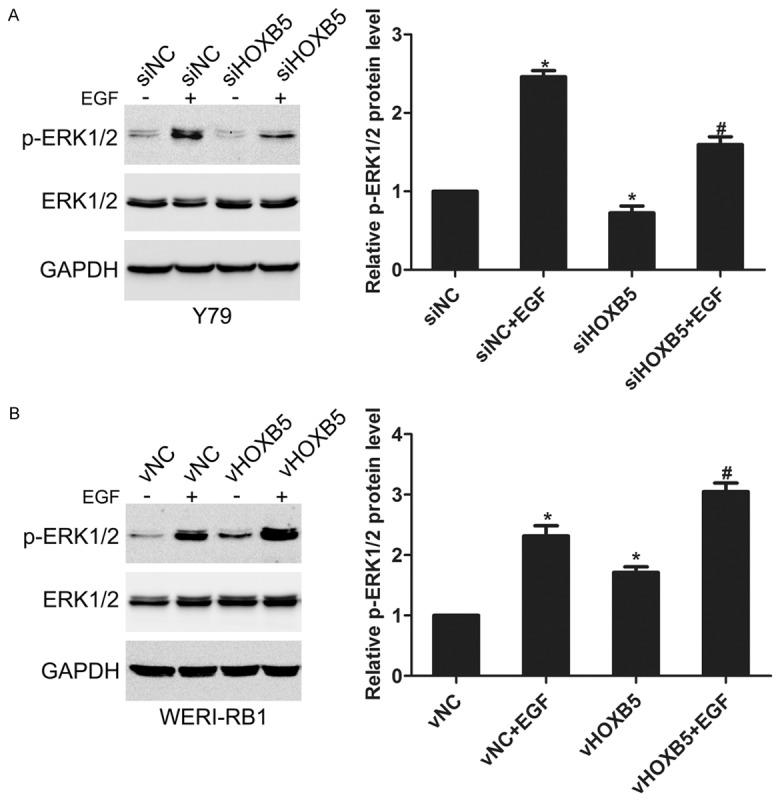
HOXB5 regulated EGF-induced activation of ERK1/2 in retinoblastoma cells. A. Y79 siNC and siHOXB5 cells were stimulated with 100 nM EGF for 15 min, ERK1/2 activation was detected by western blotting. *P<0.05 vs. siNC. #P<0.05 vs. siNC+EGF. B. WERI-RB1 vNC and vHOXB5 cells were stimulated with 100 nM EGF for 15 min, ERK1/2 activation was detected by western blotting. *P<0.05 vs. vNC. #P<0.05 vs. vNC+EGF.
Involvement of HOXB5 in regulating MMPs production in retinoblastoma cells
Matrix metalloproteinases (MMPs) are a group of zinc-dependent endopeptidases that are crucial for tumor invasion and metastasis [14]. By performing real-time PCR, we found that overexpression of HOXB5 increased the mRNA levels of MMP-3 and MMP-13 in WERI-RB1 cells, whereas knockdown of HOXB5 decreased MMP-3 and MMP-13 mRNA level in Y79 cells (Figure 5A and 5B). Similarly, ELISA assay also showed that MMP-3 and MMP-13 protein level was upregulated in WERI-RB1 vHOXB5 cells as compare to WERI-RB1 vNC cells, but was downregulated in Y79 siHOXB5 cells compared with Y79 siNC cells (Figure 5C and 5D). These results confirm that the production of MMP-3 and MMP-13 can be regulated by HOXB5 in retinoblastoma cells.
Figure 5.
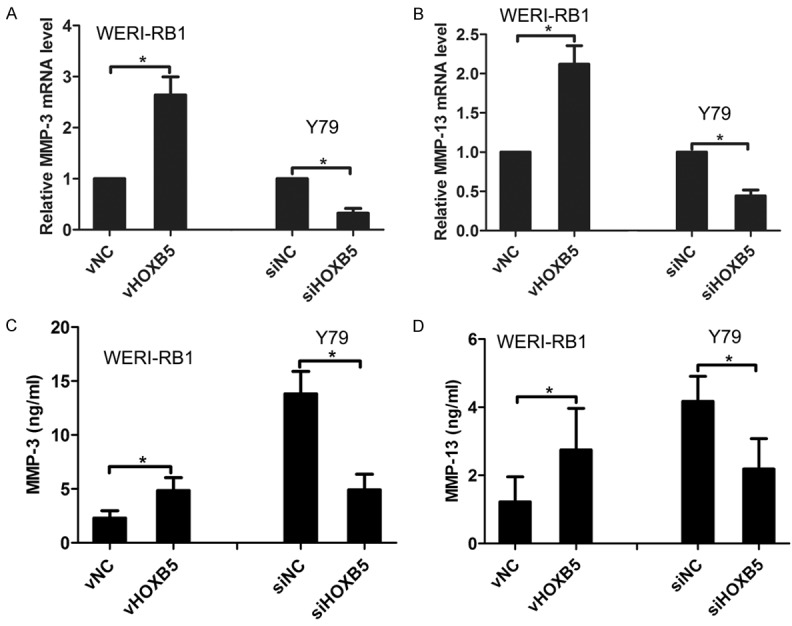
Effects of HOXB5 expression on MMP-3 and MMP-13 production. A. Effect of HOXB5 expression on MMP-3 mRNA level. B. Effect of HOXB5 expression on MMP-13 mRNA level. C. Effect of HOXB5 expression on MMP-3 protein level. D. Effect of HOXB5 expression on MMP-13 protein level. *P<0.05.
ERK1/2 pathway is required for HOXB5-mediated retinoblastoma cell migration and invasion
To investigate the role of ERK1/2 pathway in HOXB5-mediated retinoblastoma cell migration and invasion, an ERK1/2 inhibitor U0126 (100 nM) was used to suppress ERK1/2 activation in WERI-RB1 vNC and vHOXB5 cells. Migration assay and invasion assay showed that overexpression of HOXB5 promoted the migration and invasion in DMSO-treated cells, but inhibition of ERK1/2 activation blocked the HOXB5-mediated migration and invasion (Figure 6A and 6B), implying that ERK1/2 pathway is involved in HOXB5-mediated retinoblastoma cell migration and invasion. Further, we found that overexpression of HOXB5 promoted the expression of MMP-3 and MMP-13 at mRNA and protein levels in DMSO-treated cells, whereas suppression of ERK1/2 activation attenuated MMP-3 and MMP-13 production (Figure 6C-F), suggesting that HOXB5 affects MMP-3 and MMP-13 production via ERK1/2 pathway.
Figure 6.
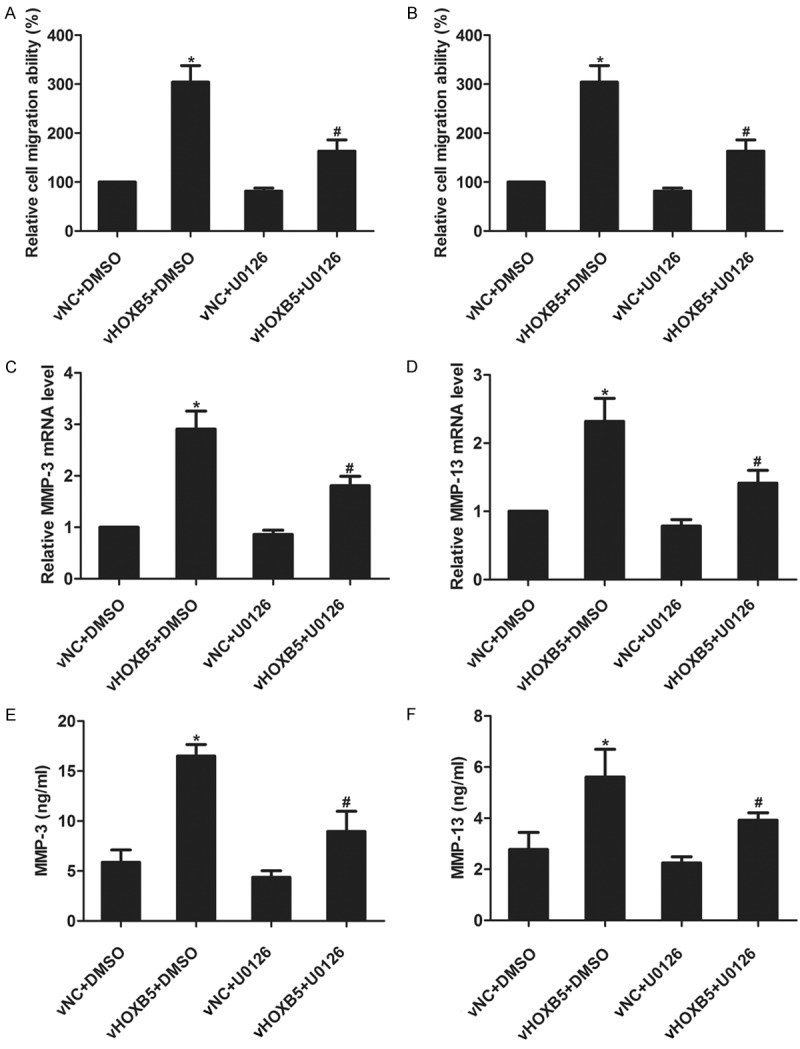
Effects of ERK1/2 pathway on HOXB5-mediated migration, invasion, and MMPs production. A and B. WERI-RB1 vNC and vHOXB5 cells were stimulated with 100 nM U0126 for 1 h, and then were subjected to cell migration assay and invasion assay. C and D. WERI-RB1 vNC and vHOXB5 cells were stimulated with 100 nM U0126. MMP-3 and MMP-13 mRNA level was detected by real-time PCR 12 h later. E and F. WERI-RB1 vNC and vHOXB5 cells were stimulated with 100 nM U0126. MMP-3 and MMP-13 protein level was detected by ELISA assay 12 h later. *P<0.05 vs. vNC+DMSO. #P<0.05 vs. vHOXB5+DMSO.
Discussion
The HOXB cluster has been implicated in many disease processes, including tumor cell invasion and metastasis [15,16]. The functions of HOXB cluster have been reported by many researchers, in particular, HOXB9 and HOXB13 [17]. However, the role of HOXB5 in tumor, especially in retinoblastoma, is still unclear. The major findings of this study are that HOXB5, which is overexpressed in retinoblastoma cell lines and tissues, promotes the invasion and migration of retinoblastoma cells through enhancing ERK1/2 pathway-mediated MMP-3 and MMP-13 production.
Abnormal expression of HOXB5 has been observed in a number of tumors. It is reported that HOXB5 is upregulated in bladder and ovarian cancers [18,19], and HOXB5 was amplified in 93.3% oral squamous cell carcinomas [13]. The expression of HOXB5 in retinoblastoma is elusive. Thus we here detected its protein level in retinoblastoma cell lines and tissues. We found that HOXB5 expression was overexpressed in both retinoblastoma cell lines and tissues, indicating a pivotal role of HOXB5 in retinoblastoma.
HOXB cluster has been reported to play an important role in tumor development. Lee et al. have found that HOXB5 acts as a positive modulator by promoting cell growth and invasiveness in ER-positive breast cancer [20]. Hong et al. have shown that HOXB5 induces invasion and migration through direct transcriptional up-regulation of β-catenin in human gastric carcinoma [21]. However, the role of HOXB5 in retinoblastoma cell migration and invasion is unknown. In our study, we found that HOXB5 could promote the migration and invasion of retinoblastoma cells. To our knowledge, it is the first time that the functions of HOXB5 are identified in retinoblastoma cells.
MMPs, a group of enzymes that account for the degradation of extracellular matrix proteins, play critical roles in tumor invasiveness [22]. HOXB cluster has been found to be able to regulate MMPs expression in tumor progression [23], but whether HOXB5 can induce MMPs expression is unclear. Here, we found that overexpression of HOXB5 increased MMP-3 and MMP-13 production, whereas knockdown of HOXB5 decreased the production of MMP-3 and MMP-13.
There are several cell signaling pathways that can be activated by HOX family, including PI3K/AKT, MAPK/ERK1/2, Wnt/β-catenin and Notch pathways [21,24-27]. ERK1/2 is a well-known kinase that can be phosphorylated by many cytokines such as EGF and interferon family, and then lead to the activation of ERK1/2 pathway [28]. ERK1/2 pathway is involved in the progression of tumor. In our study, we found that knockdown of HOXB5 inhibited the EGF-induced ERK1/2 activation of retinoblastoma cells, whereas overexpression of HOXB5 enhanced the activation of ERK1/2. Further, we found that inhibition of ERK1/2 pathway attenuated HOXB5-mediated migration and invasion of retinoblastoma cells, and suppressed HOXB5-induced MMP-3 and MMP-13 production, suggesting ERK1/2 pathway participates in retinoblastoma cell migration and invasion mediated by HOXB5 expression.
In conclusion, our study demonstrates that HOXB5 is upregulated in retinoblastoma cells and tissues. Further, HOXB5 induces MMP-3 and MMP-13 through activating ERK1/2 pathway, which finally promotes retinoblastoma cell migration and invasion. Thus, targeting HOXB5 may have a therapeutic potential against the progression of retinoblastoma.
Acknowledgements
This work was supported by grants from the science and technology program of experimental animal, science and technology department of Zhejiang Province (2015C37132) and the Medical scientific Research Foundation of Zhejiang Province of China (2018273482).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kamihara J, Bourdeaut F, Foulkes WD, Molenaar JJ, Mosse YP, Nakagawara A, Parareda A, Scollon SR, Schneider KW, Skalet AH, States LJ, Walsh MF, Diller LR, Brodeur GM. Retinoblastoma and neuroblastoma predisposition and surveillance. Clin Cancer Res. 2017;23:e98–e106. doi: 10.1158/1078-0432.CCR-17-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teixo R, Laranjo M, Abrantes AM, Brites G, Serra A, Proenca R, Botelho MF. Retinoblastoma: might photodynamic therapy be an option? Cancer Metastasis Rev. 2015;34:563–573. doi: 10.1007/s10555-014-9544-y. [DOI] [PubMed] [Google Scholar]
- 4.Lewis EB. A gene complex controlling segmentation in drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 5.Gehring WJ, Hiromi Y. Homeotic genes and the homeobox. Annu Rev Genet. 1986;20:147–173. doi: 10.1146/annurev.ge.20.120186.001051. [DOI] [PubMed] [Google Scholar]
- 6.Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. J Mol Med. 2014;92:811–823. doi: 10.1007/s00109-014-1181-y. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Fernandez J. The genesis and evolution of homeobox gene clusters. Nat Rev Genet. 2005;6:881–892. doi: 10.1038/nrg1723. [DOI] [PubMed] [Google Scholar]
- 8.Sha L, Dong L, Lv L, Bai L, Ji X. HOXB9 promotes epithelial-to-mesenchymal transition via transforming growth factor-beta1 pathway in hepatocellular carcinoma cells. Clin Exp Med. 2015;15:55–64. doi: 10.1007/s10238-014-0276-7. [DOI] [PubMed] [Google Scholar]
- 9.Chang Q, Zhang L, He C, Zhang B, Zhang J, Liu B, Zeng N, Zhu Z. HOXB9 induction of mesenchymal-to-epithelial transition in gastric carcinoma is negatively regulated by its hexapeptide motif. Oncotarget. 2015;6:42838–42853. doi: 10.18632/oncotarget.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zabalza CV, Adam M, Burdelski C, Wilczak W, Wittmer C, Kraft S, Krech T, Steurer S, Koop C, Hube-Magg C, Graefen M, Heinzer H, Minner S, Simon R, Sauter G, Schlomm T, Tsourlakis MC. HOXB13 overexpression is an independent predictor of early PSA recurrence in prostate cancer treated by radical prostatectomy. Oncotarget. 2015;6:12822–12834. doi: 10.18632/oncotarget.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Herrera A, Salgado-Bernabe M, Velazquez-Velazquez C, Salcedo-Vargas M, Andrade-Manzano A, Avila-Moreno F, Pina-Sanchez P. Increased expression of HOXB2 and HOXB13 proteins is associated with HPV infection and cervical cancer progression. Asian Pac J Cancer Prev. 2015;16:1349–1353. doi: 10.7314/apjcp.2015.16.4.1349. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H, Kajiyama H, Ito S, Chen D, Shibata K, Hamaguchi M, Kikkawa F, Senga T. HOXB13 and ALX4 induce SLUG expression for the promotion of EMT and cell invasion in ovarian cancer cells. Oncotarget. 2015;6:13359–13370. doi: 10.18632/oncotarget.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tucci R, Campos MS, Matizonkas-Antonio LF, Durazzo M, Pinto Junior Ddos S, Nunes FD. HOXB5 expression in oral squamous cell carcinoma. J Appl Oral Sci. 2011;19:125–129. doi: 10.1590/S1678-77572011000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong L, Wu D, Zou J, Chen J, Chen L, Chen Y, Ni C, Yuan H. Prognostic impact of serum and tissue MMP-9 in non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:18458–18468. doi: 10.18632/oncotarget.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhan J, Song J, Wang P, Chi X, Wang Y, Guo Y, Fang W, Zhang H. Kindlin-2 induced by TGF-beta signaling promotes pancreatic ductal adenocarcinoma progression through downregulation of transcriptional factor HOXB9. Cancer Lett. 2015;361:75–85. doi: 10.1016/j.canlet.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Kim IJ, Kang TW, Jeong T, Kim YR, Jung C. HOXB13 regulates the prostate-derived Ets factor: implications for prostate cancer cell invasion. Int J Oncol. 2014;45:869–876. doi: 10.3892/ijo.2014.2485. [DOI] [PubMed] [Google Scholar]
- 17.Platais C, Hakami F, Darda L, Lambert DW, Morgan R, Hunter KD. The role of HOX genes in head and neck squamous cell carcinoma. J Oral Pathol Med. 2016;45:239–247. doi: 10.1111/jop.12388. [DOI] [PubMed] [Google Scholar]
- 18.Luo J, Cai Q, Wang W, Huang H, Zeng H, He W, Deng W, Yu H, Chan E, Ng CF, Huang J, Lin T. A microRNA-7 binding site polymorphism in HOXB5 leads to differential gene expression in bladder cancer. PLoS One. 2012;7:e40127. doi: 10.1371/journal.pone.0040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kar SP, Tyrer JP, Li Q, Lawrenson K, Aben KK, Anton-Culver H, Antonenkova N, Chenevix-Trench G, Baker H, Bandera EV, Bean YT, Beckmann MW, Berchuck A, Bisogna M, Bjorge L, Bogdanova N, Brinton L, Brooks-Wilson A, Butzow R, Campbell I, Carty K, Chang-Claude J, Chen YA, Chen Z, Cook LS, Cramer D, Cunningham JM, Cybulski C, Dansonka-Mieszkowska A, Dennis J, Dicks E, Doherty JA, Dork T, du Bois A, Durst M, Eccles D, Easton DF, Edwards RP, Ekici AB, Fasching PA, Fridley BL, Gao YT, Gentry-Maharaj A, Giles GG, Glasspool R, Goode EL, Goodman MT, Grownwald J, Harrington P, Harter P, Hein A, Heitz F, Hildebrandt MA, Hillemanns P, Hogdall E, Hogdall CK, Hosono S, Iversen ES, Jakubowska A, Paul J, Jensen A, Ji BT, Karlan BY, Kjaer SK, Kelemen LE, Kellar M, Kelley J, Kiemeney LA, Krakstad C, Kupryjanczyk J, Lambrechts D, Lambrechts S, Le ND, Lee AW, Lele S, Leminen A, Lester J, Levine DA, Liang D, Lissowska J, Lu K, Lubinski J, Lundvall L, Massuger L, Matsuo K, McGuire V, McLaughlin JR, McNeish IA, Menon U, Modugno F, Moysich KB, Narod SA, Nedergaard L, Ness RB, Nevanlinna H, Odunsi K, Olson SH, Orlow I, Orsulic S, Weber RP, Pearce CL, Pejovic T, Pelttari LM, Permuth-Wey J, Phelan CM, Pike MC, Poole EM, Ramus SJ, Risch HA, Rosen B, Rossing MA, Rothstein JH, Rudolph A, Runnebaum IB, Rzepecka IK, Salvesen HB, Schildkraut JM, Schwaab I, Shu XO, Shvetsov YB, Siddiqui N, Sieh W, Song H, Southey MC, Sucheston-Campbell LE, Tangen IL, Teo SH, Terry KL, Thompson PJ, Timorek A, Tsai YY, Tworoger SS, van Altena AM, Van Nieuwenhuysen E, Vergote I, Vierkant RA, Wang-Gohrke S, Walsh C, Wentzensen N, Whittemore AS, Wicklund KG, Wilkens LR, Woo YL, Wu X, Wu A, Yang H, Zheng W, Ziogas A, Sellers TA, Monteiro AN, Freedman ML, Gayther SA, Pharoah PD. Network-based integration of GWAS and gene expression identifies a HOX-centric network associated with serous ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 2015;24:1574–1584. doi: 10.1158/1055-9965.EPI-14-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JY, Hur H, Yun HJ, Kim Y, Yang S, Kim SI, Kim MH. HOXB5 promotes the proliferation and invasion of breast cancer cells. Int J Biol Sci. 2015;11:701–711. doi: 10.7150/ijbs.11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong CS, Jeong O, Piao Z, Guo C, Jung MR, Choi C, Park YK. HOXB5 induces invasion and migration through direct transcriptional up-regulation of beta-catenin in human gastric carcinoma. Biochem J. 2015;472:393–403. doi: 10.1042/BJ20150213. [DOI] [PubMed] [Google Scholar]
- 22.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237:273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 23.Care A, Felicetti F, Meccia E, Bottero L, Parenza M, Stoppacciaro A, Peschle C, Colombo MP. HOXB7: a key factor for tumor-associated angiogenic switch. Cancer Res. 2001;61:6532–6539. [PubMed] [Google Scholar]
- 24.Wang H, Liu G, Shen D, Ye H, Huang J, Jiao L, Sun Y. HOXA1 enhances the cell proliferation, invasion and metastasis of prostate cancer cells. Oncol Rep. 2015;34:1203–1210. doi: 10.3892/or.2015.4085. [DOI] [PubMed] [Google Scholar]
- 25.Roux M, Laforest B, Capecchi M, Bertrand N, Zaffran S. Hoxb1 regulates proliferation and differentiation of second heart field progenitors in pharyngeal mesoderm and genetically interacts with Hoxa1 during cardiac outflow tract development. Dev Biol. 2015;406:247–258. doi: 10.1016/j.ydbio.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Sengupta A, Banerjee D, Chandra S, Banerji SK, Ghosh R, Roy R, Banerjee S. Deregulation and cross talk among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic myeloid leukemia progression. Leukemia. 2007;21:949–955. doi: 10.1038/sj.leu.2404657. [DOI] [PubMed] [Google Scholar]
- 27.Zhang B, Li N, Zhang H. Knockdown of homeobox B5 (HOXB5) inhibits cell proliferation, migration, and invasion in non-small cell lung cancer cells through inactivation of the Wnt/beta-Catenin pathway. Oncol Res. 2018;26:37–44. doi: 10.3727/096504017X14900530835262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samatar AA, Poulikakos PI. Targeting RASERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]


